Differences in the Effects of Calcium and Magnesium Ions on the Anammox Granular Properties to Alleviate Salinity Stress
Abstract
:1. Introduction
2. Materials and Methods
2.1. Anammox Granules
2.2. Batch Test
2.3. Specific ANAMMOX Activity (SAA)
2.4. Analysis
2.5. Microbial Community Analysis
3. Results and Discussions
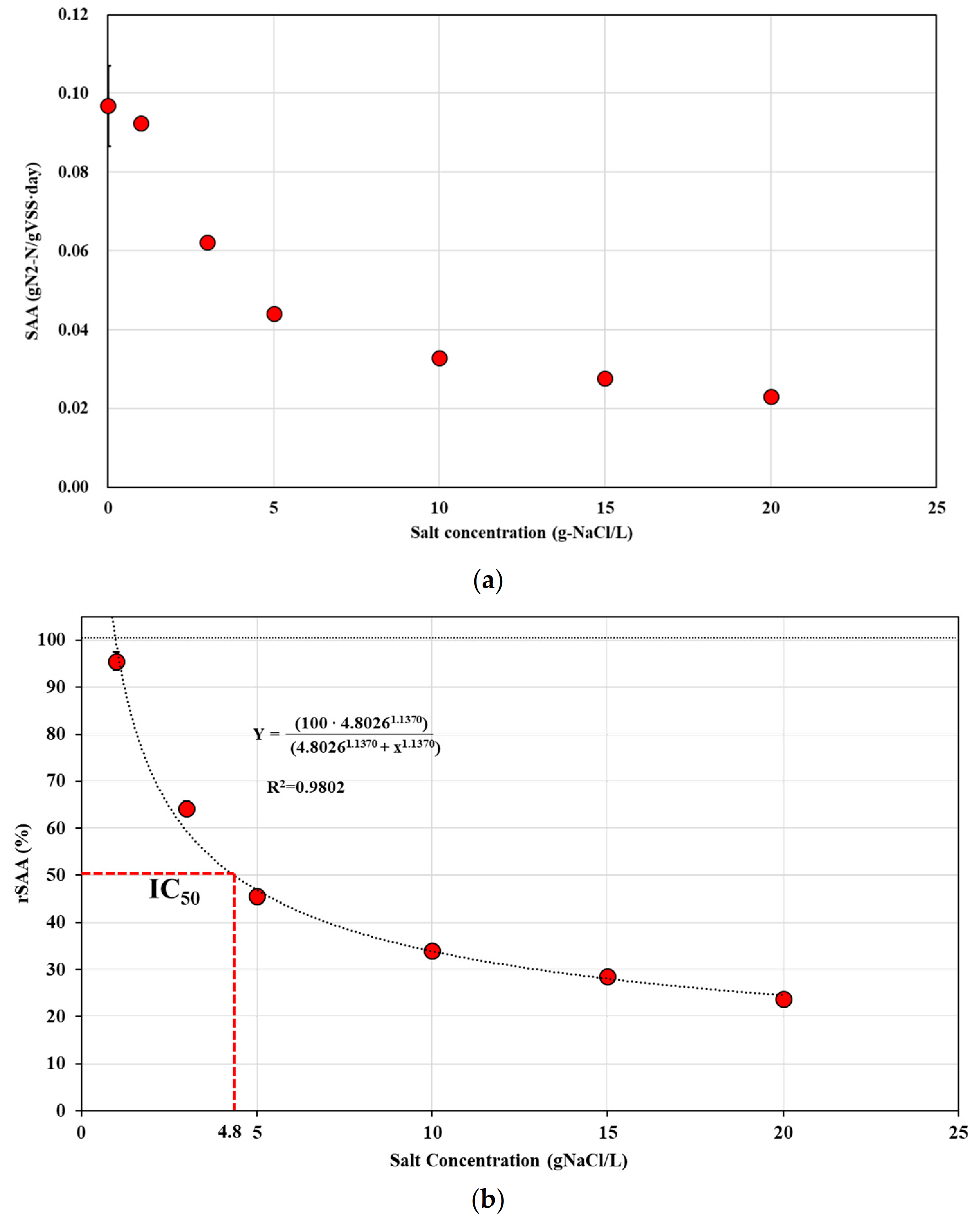
3.1. Inhibition of Specific Anammox Activity by Salinity Stress
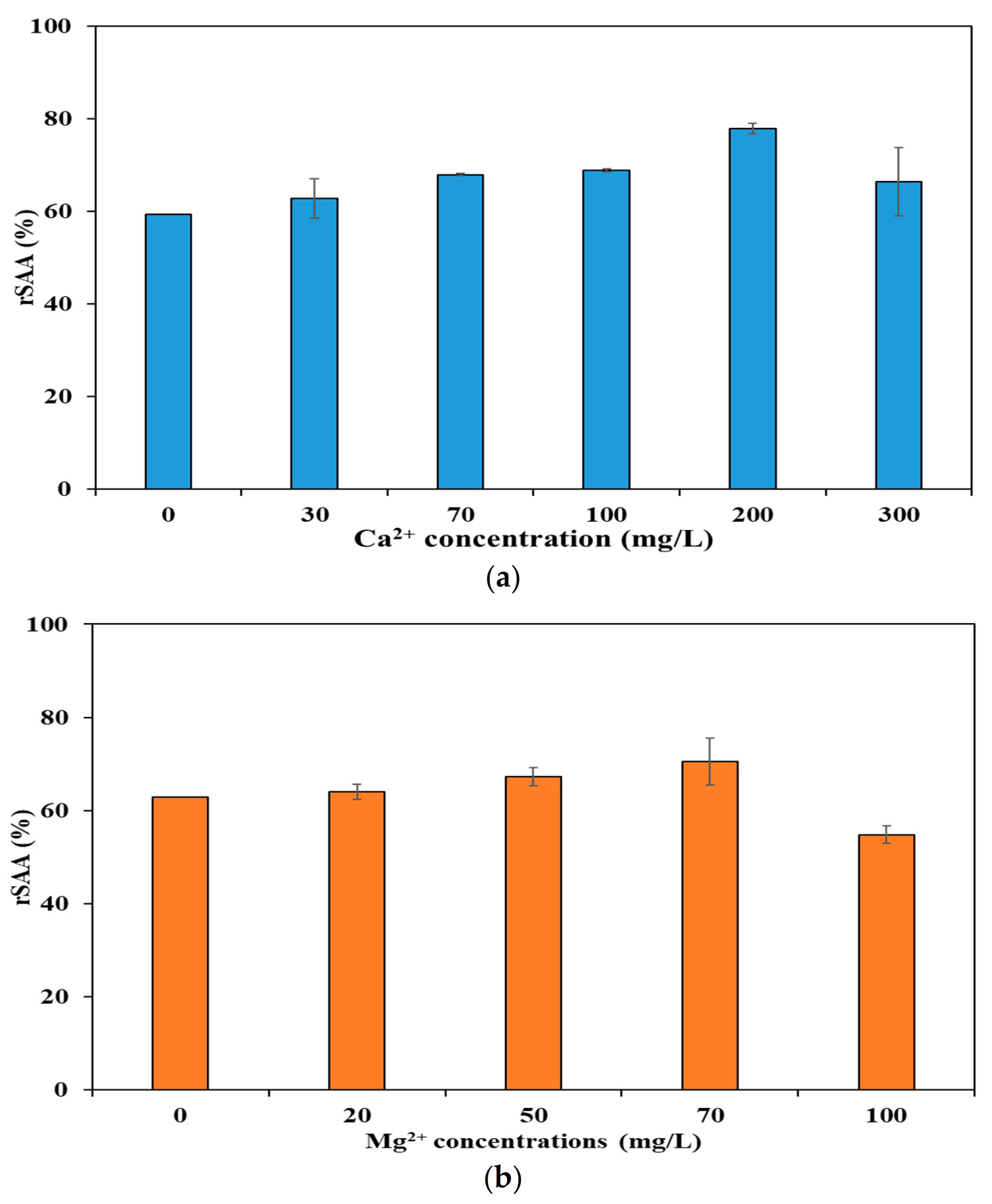
3.2. Effect of Divalent Cations on Alleviation of Salinity Stress
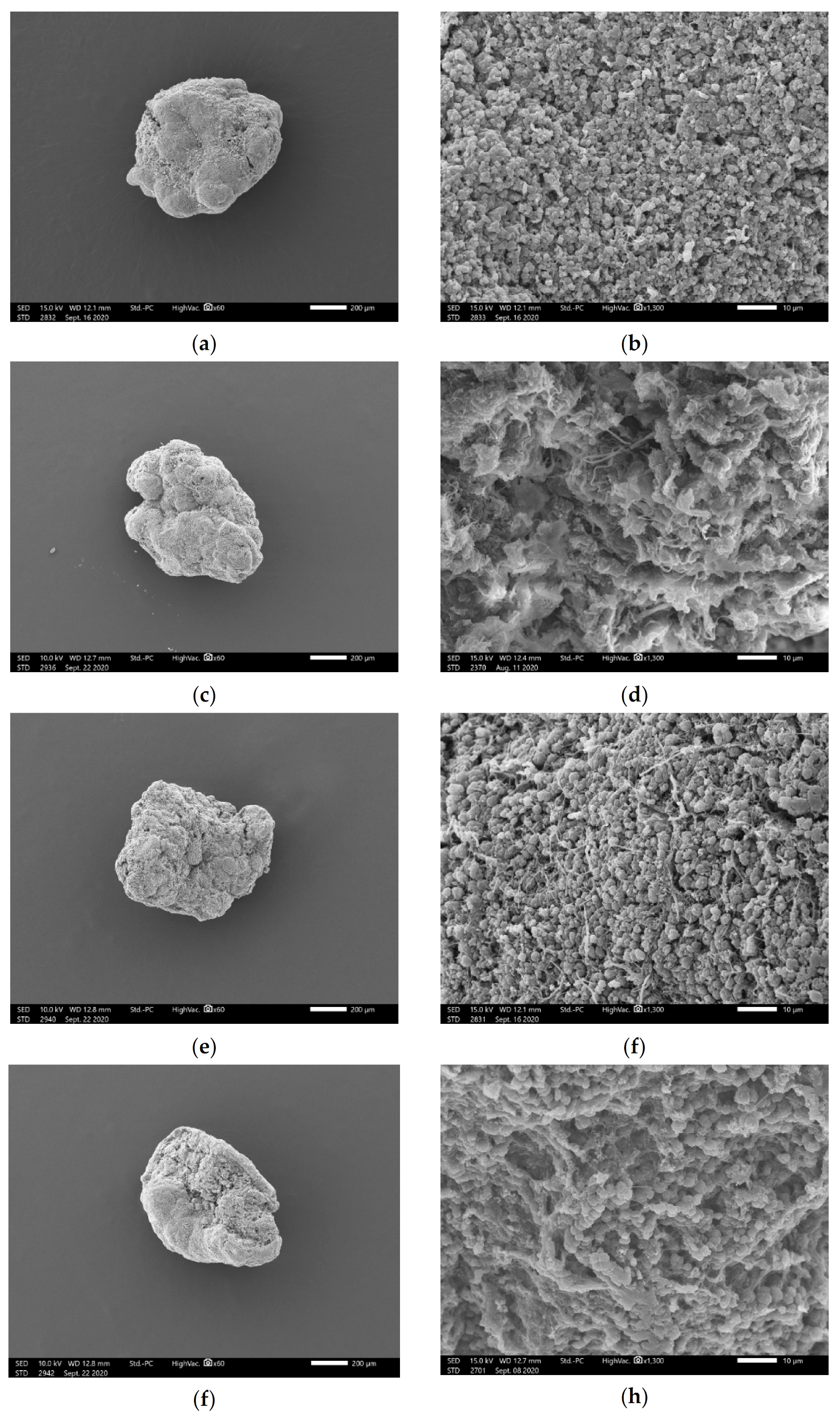
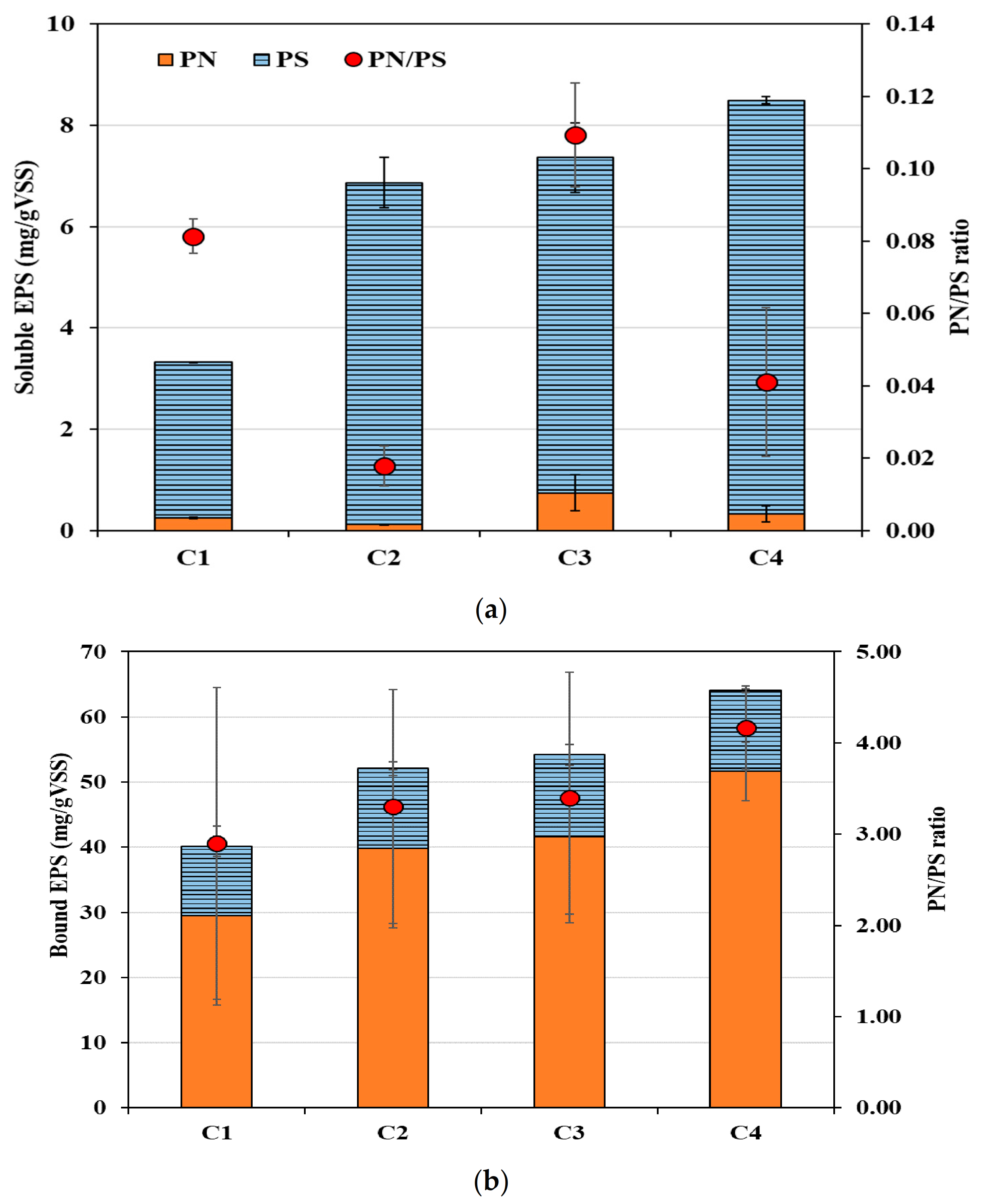
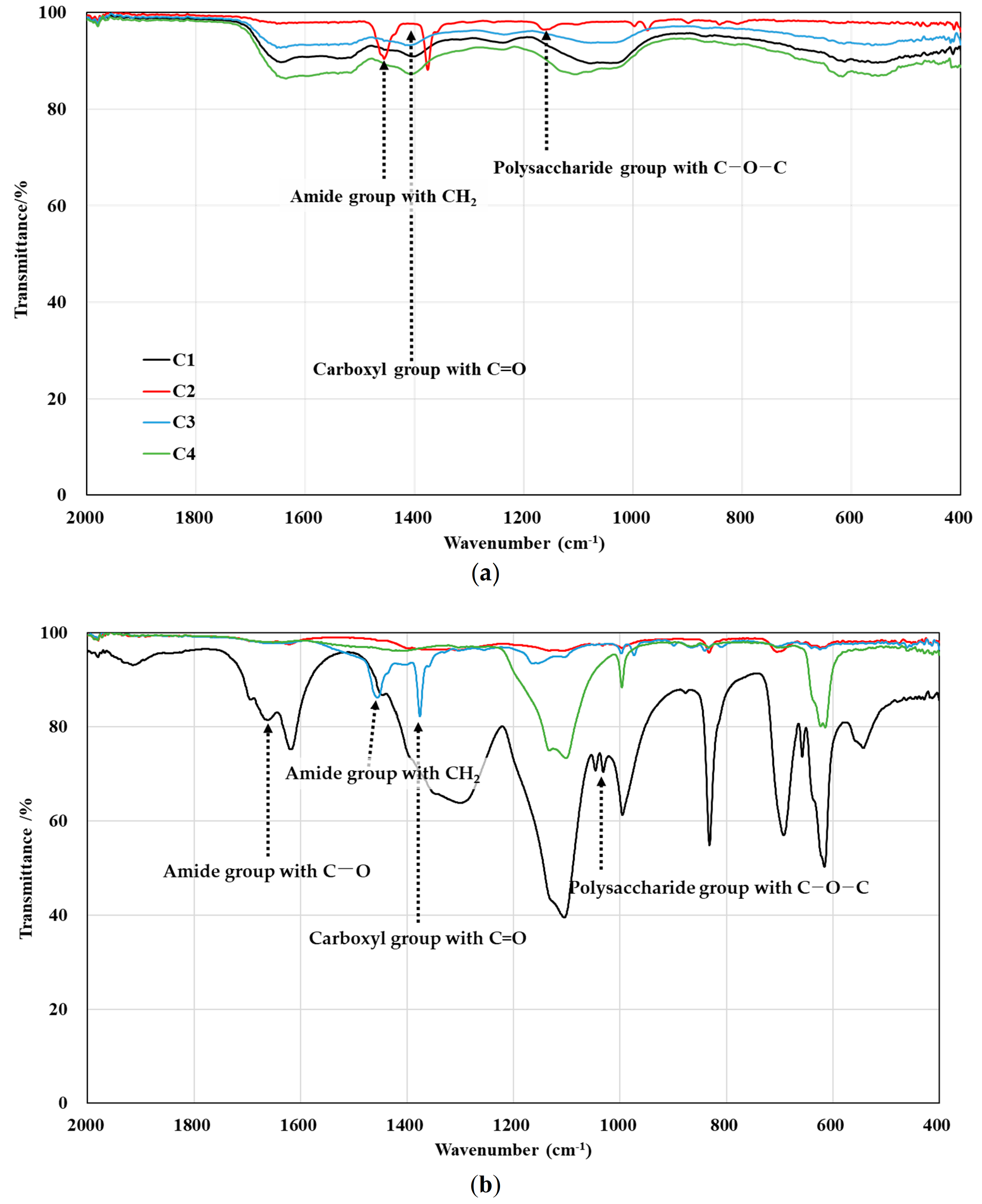
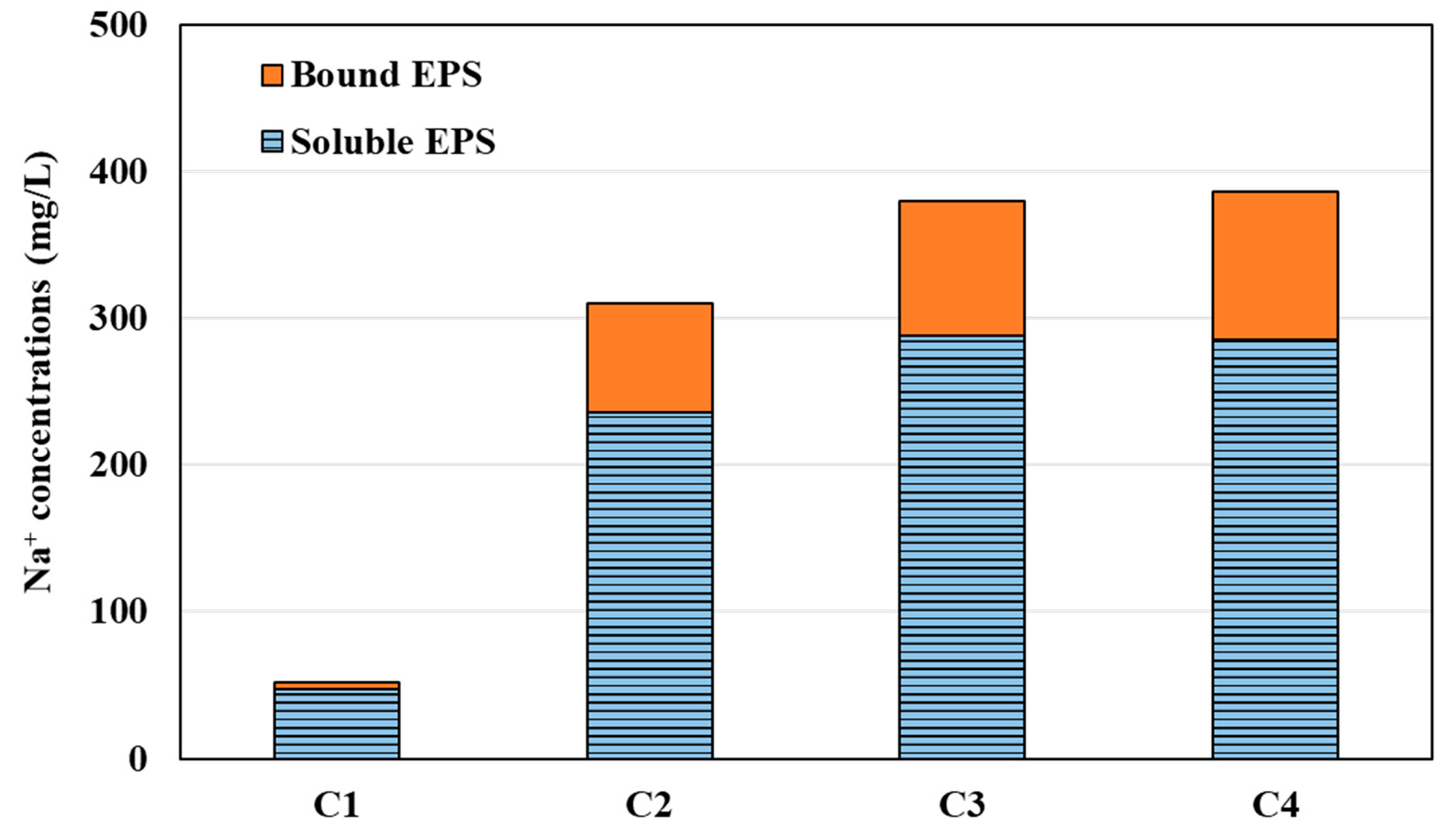
3.3. Effect of Divalent Cations on EPS Production and Compositions
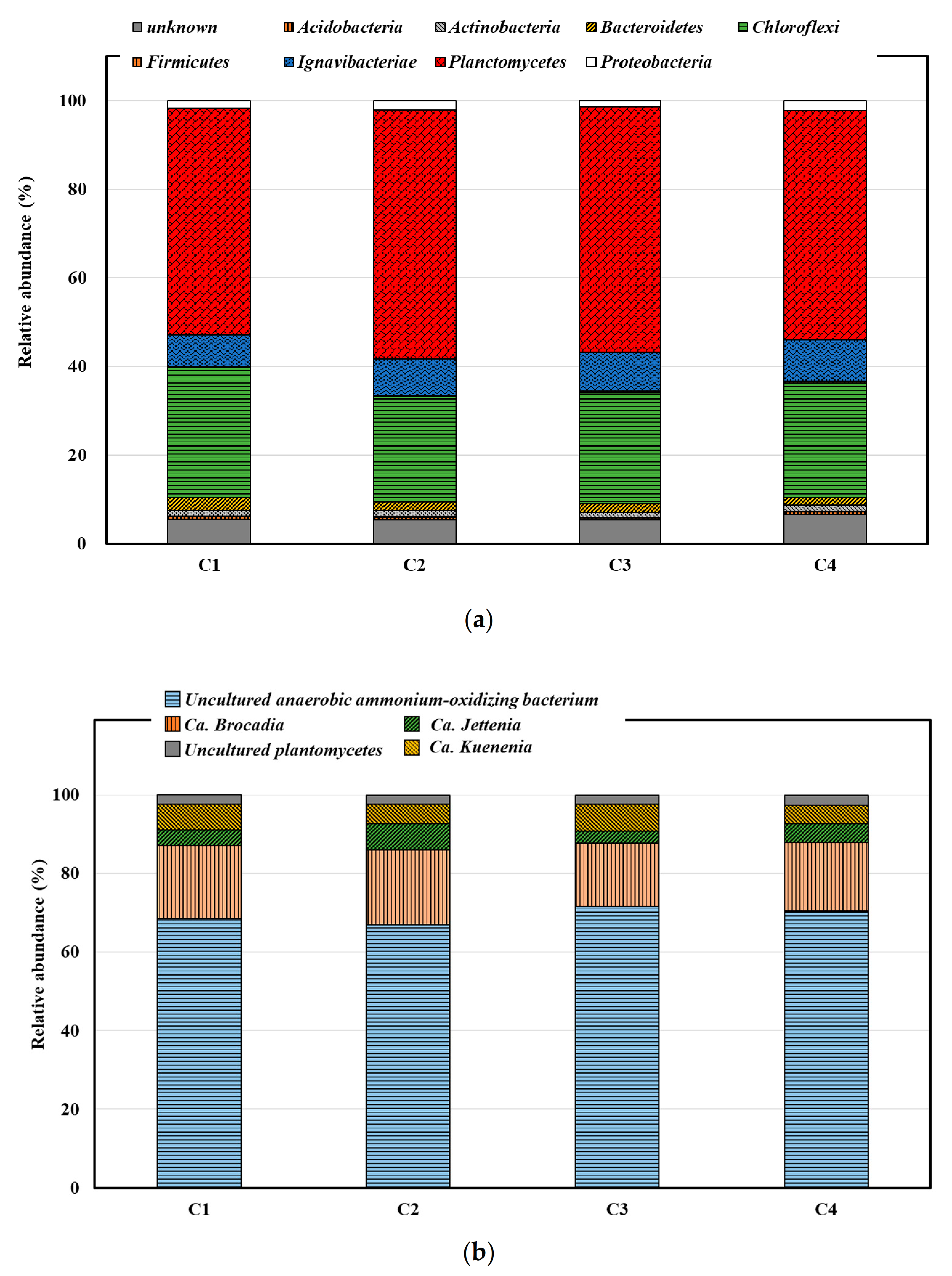
3.4. Effect of Divalent Cations on Microbial Community Compositions
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgement
Conflicts of Interest
References
- Li, J.; Qi, P.; Qiang, Z.; Dong, H.; Gao, D.; Wang, D. Is anammox a promising treatment process for nitrogen removal from nitrogen-rich saline wastewater? Bioresour. Technol. 2018, 270, 722–731. [Google Scholar] [CrossRef] [PubMed]
- Tan, X.; Acquah, I.; Liu, H.; Li, W.; Tan, S. A critical review on saline wastewater treatment by membrane bioreactor (MBR) from a microbial perspective. Chemosphere 2019, 220, 1150–1162. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Zhuang, X.; Ahmad, S.; Sung, S.; Ni, S. Biotreatment of high-salinity wastewater: Current methods and future di-rections. World J. Microbiol. Biotechnol. 2020, 36, 37. [Google Scholar] [CrossRef] [PubMed]
- Ozturk, E.; Bal, N. Evaluation of ammonia–nitrogen removal efficiency from aqueous solutions by ultrasonic irradiation in short sonication periods. Ultrason. Sonochem. 2015, 26, 422–427. [Google Scholar] [CrossRef]
- Wei, Q.; Kawagoshi, Y.; Huang, X.; Hong, N.; van Duc, L.; Yamashita, Y.; Hama, T. Nitrogen removal properties in a contin-uous marine anammox bacteria reactor under rapid and extensive salinity changes. Chemosphere 2016, 148, 444–451. [Google Scholar] [CrossRef]
- He, S.; Yang, W.; Li, W.; Zhang, Y.; Qin, M.; Mao, Z. Impacts of salt shocking and the selection of a suitable reversal agent on anammox. Sci. Total Environ. 2019, 692, 602–612. [Google Scholar] [CrossRef]
- Jin, R.-C.; Zheng, P.; Mahmood, Q.; Hu, B.-L. Osmotic stress on nitrification in an airlift bioreactor. J. Hazard. Mater. 2007, 146, 148–154. [Google Scholar] [CrossRef]
- Lin, L.; Pratt, S.; Crick, O.; Xia, J.; Duan, H.; Ye, L. Salinity effect on freshwater Anammox bacteria: Ionic stress and ion com-position. Water Res. 2021, 188, 116432. [Google Scholar] [CrossRef]
- Liu, Y.; Ni, B.-J. Appropriate Fe (II) Addition Significantly Enhances Anaerobic Ammonium Oxidation (Anammox) Activity through Improving the Bacterial Growth Rate. Sci. Rep. 2015, 5, srep08204. [Google Scholar] [CrossRef]
- Mahoney, E.M.; Varangu, L.K.; Cairns, W.L.; Kosaric, N.; Murray, R.G.E. The Effect of Calcium on Microbial Aggregation during UASB Reactor Start-Up. Water Sci. Technol. 1987, 19, 249–260. [Google Scholar] [CrossRef]
- Teo, K.-C.; Xu, H.-L.; Tay, J.-H. Molecular Mechanism of Granulation. II: Proton Translocating Activity. J. Environ. Eng. 2000, 126, 411–418. [Google Scholar] [CrossRef]
- Dapena-Mora, A.; Fernandez, I.; Campos, J.; Mosquera-Corral, A.; Mendez, R.; Jetten, M. Evaluation of activity and inhibition effects on Anammox process by batch tests based on the nitrogen gas production. Enzym. Microb. Technol. 2007, 40, 859–865. [Google Scholar] [CrossRef]
- The Water Environment Federation; American Public Health Association (APHA). Standard Methods for the Examination of Water and Wastewater; American Public Health Association (APHA): Washington, DC, USA, 2005. [Google Scholar]
- Sheng, G.-P.; Yu, H.-Q. Formation of extracellular polymeric substances from acidogenic sludge in H2-producing process. Appl. Microbiol. Biotechnol. 2007, 74, 208–214. [Google Scholar] [CrossRef]
- Fang, P.; He, X.; Li, J.; Yang, G.; Wang, Z.; Sun, Z.; Zhang, X.; Zhao, C. Impact of sodium ion on multivalent metal ion content in extracellular polymeric substances of granular sludge from an expanded granular sludge bed. Environ. Technol. 2019, 40, 3105–3113. [Google Scholar] [CrossRef]
- Team RC. R: A Language and Environment for Statistical Computing; Team RC: Vienna, Austria, 2013. [Google Scholar]
- Cho, S.; Kambey, C.; Nguyen, V.K. Performance of Anammox Processes for Wastewater Treatment: A Critical Review on Effects of Operational Conditions and Environmental Stresses. Water 2019, 12, 20. [Google Scholar] [CrossRef] [Green Version]
- Scaglione, D.; Lotti, T.; Ficara, E.; Malpei, F. Inhibition on anammox bacteria upon exposure to digestates from biogas plants treating the organic fraction of municipal solid waste and the role of conductivity. Waste Manag. 2017, 61, 213–219. [Google Scholar] [CrossRef]
- Carvajal-Arroyo, J.M.; Sun, W.; Sierra-Alvarez, R.; Field, J. Inhibition of anaerobic ammonium oxidizing (anammox) enrichment cultures by substrates, metabolites and common wastewater constituents. Chemosphere 2013, 91, 22–27. [Google Scholar] [CrossRef]
- Liu, S.; Zhang, Z.; Ni, J. Effects of Ca2+ on activity restoration of the damaged anammox consortium. Bioresour. Technol. 2013, 143, 315–321. [Google Scholar] [CrossRef]
- Schmidt, J.E.; Ahring, B. Effects of magnesium on thermophilic acetate-degrading granules in upflow anaerobic sludge blanket (UASB) reactors. Enzym. Microb. Technol. 1993, 15, 304–310. [Google Scholar] [CrossRef]
- Yu, H.; Tay, J.; Fang, H.H. The roles of calcium in sludge granulation during uasb reactor start-up. Water Res. 2001, 35, 1052–1060. [Google Scholar] [CrossRef]
- Zhen, J.; Cui, Q.; Liu, X.; Yu, Z.; Wang, C.; Ni, S. Unravelling the importance of Ca2+ and Mg2+ as essential in anammox culture medium. Bioresour. Technol. 2021, 340, 125729. [Google Scholar] [CrossRef] [PubMed]
- Lu, H.; Li, Y.; Shan, X.; Abbas, G.; Zeng, Z.; Kang, D.; Wang, Y.; Zheng, P.; Zhang, M. A holistic analysis of ANAMMOX pro-cess in response to salinity: From adaptation to collapse. Sep. Purif. Technol. 2019, 215, 342–350. [Google Scholar] [CrossRef]
- Sheng, G.; Yu, H.; Li, X. Extracellular polymeric substances (EPS) of microbial aggregates in biological wastewater treatment systems: A review. Biotechnol. Adv. 2010, 28, 882–894. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Gao, D.-W.; Zhang, M.; Fu, Y. Comparison of Ca2+ and Mg2+ enhancing aerobic granulation in SBR. J. Hazard. Mater. 2010, 181, 382–387. [Google Scholar] [CrossRef]
- Hengge, R. Principles of c-di-GMP signalling in bacteria. Nat. Rev. Microbiol. 2009, 7, 263–273. [Google Scholar] [CrossRef]
- Guo, Y.; Zhao, Y.; Tang, X.; Na, T.; Pan, J.; Zhao, H.; Liu, S. Deciphering bacterial social traits via diffusible signal factor (DSF) -mediated public goods in an anammox community. Water Res. 2021, 191, 116802. [Google Scholar] [CrossRef]
- Flemming, H.; Neu, T.R.; Wingender, J. Microbial Extracellular Polymeric Substances: Characterization, Structure, and Function. Springer: Berlin/Heidelberg, Germany, 1999. [Google Scholar]
- Xing, B.-S.; Guo, Q.; Yang, G.-F.; Zhang, Z.-Z.; Li, P.; Guo, L.-X.; Jin, R.-C. The properties of anaerobic ammonium oxidation (anammox) granules: Roles of ambient temperature, salinity and calcium concentration. Sep. Purif. Technol. 2015, 147, 311–318. [Google Scholar] [CrossRef]
- Babiak, W.; Krzemińska, I. Extracellular Polymeric Substances (EPS) as Microalgal Bioproducts: A Review of Factors Affecting EPS Synthesis and Application in Flocculation Processes. Energies 2021, 14, 4007. [Google Scholar] [CrossRef]
- Hou, X.; Liu, S.; Zhang, Z. Role of extracellular polymeric substance in determining the high aggregation ability of anammox sludge. Water Res. 2015, 75, 51–62. [Google Scholar] [CrossRef]
- Liu, Y.; Tay, J.-H. The essential role of hydrodynamic shear force in the formation of biofilm and granular sludge. Water Res. 2002, 36, 1653–1665. [Google Scholar] [CrossRef]
- Ni, S.-Q.; Sun, N.; Yang, H.; Zhang, J.; Ngo, H.H. Distribution of extracellular polymeric substances in anammox granules and their important roles during anammox granulation. Biochem. Eng. J. 2015, 101, 126–133. [Google Scholar] [CrossRef]
- Fang, F.; Yang, M.; Wang, H.; Yan, P.; Chen, Y.; Guo, J. Effect of high salinity in wastewater on surface properties of anammox granular sludge. Chemosphere 2018, 210, 366–375. [Google Scholar] [CrossRef]
- Liu, Y.; Xu, W.; Bao, L.; Li, Y.; Liu, S.; Zhang, Q.; Alessi, D.S.; Konhauser, K.O.; Zhao, H. Cell surface characterization and trace metal adsorptive properties of anaerobic ammonium-oxidizing (anammox) consortia. Chemosphere 2019, 221, 11–20. [Google Scholar] [CrossRef]
- Feng, C.; Lotti, T.; Lin, Y.; Malpei, F. Extracellular polymeric substances extraction and recovery from anammox granules: Evaluation of methods and protocol development. Chem. Eng. J. 2019, 374, 112–122. [Google Scholar] [CrossRef]
- Ali, M.; Shaw, D.R.; Albertsen, M.; Saikaly, P.E. Comparative genome-centric analysis of freshwater and marine ANAMMOX cultures suggests functional redundancy in nitrogen removal processes. Front. Microbiol. 2020, 11, 1637. [Google Scholar] [CrossRef]
- Chu, Z.; Wang, K.; Li, X.; Zhu, M.; Yang, L.; Zhang, J. Microbial characterization of aggregates within a one-stage nitritation–anammox system using high-throughput amplicon sequencing. Chem. Eng. J. 2015, 262, 41–48. [Google Scholar] [CrossRef]
- Gao, D.; Liu, L.; Liang, H.; Wu, W. Aerobic granular sludge: Characterization, mechanism of granulation and application to wastewater treatment. Crit. Rev. Biotechnol. 2010, 31, 137–152. [Google Scholar] [CrossRef]
- Kindaichi, T.; Yuri, S.; Ozaki, N.; Ohashi, A. Ecophysiological role and function of uncultured Chloroflexi in an anammox reactor. Water Sci. Technol. 2012, 66, 2556–2561. [Google Scholar] [CrossRef] [Green Version]
- Kartal, B.; Koleva, M.; Arsov, R.; van der Star, W.; Jetten, M.S.; Strous, M. Adaptation of a freshwater anammox population to high salinity wastewater. J. Biotechnol. 2006, 126, 546–553. [Google Scholar] [CrossRef]
- Wang, J.-B.; Li, X.; Zhou, Z.-W.; Fan, X.-Y. Bacterial communities, metabolic functions and resistance genes to antibiotics and metals in two saline seafood wastewater treatment systems. Bioresour. Technol. 2019, 287, 121460. [Google Scholar] [CrossRef]
- Ali, M.; Oshiki, M.; Awata, T.; Isobe, K.; Kimura, Z.; Yoshikawa, H.; Hira, D.; Kindaichi, T.; Satoh, H.; Fujii, T. Physiological characterization of anaerobic ammonium oxidizing bacterium ‘Candidatus Jettenia caeni’. Environ. Microbiol. 2015, 17, 2172–2189. [Google Scholar] [CrossRef]
- Park, M.; Kim, J.; Lee, T.; Oh, Y.-K.; Nguyen, V.K.; Cho, S. Correlation of microbial community with salinity and nitrogen removal in an anammox-based denitrification system. Chemosphere 2021, 263, 128340. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, Y.; Yu, J.; Jeong, S.; Kim, J.; Park, S.; Bae, H.; Rhee, S.-K.; Unno, T.; Ni, S.-Q.; Lee, T. Differences in the Effects of Calcium and Magnesium Ions on the Anammox Granular Properties to Alleviate Salinity Stress. Appl. Sci. 2022, 12, 19. https://doi.org/10.3390/app12010019
Kim Y, Yu J, Jeong S, Kim J, Park S, Bae H, Rhee S-K, Unno T, Ni S-Q, Lee T. Differences in the Effects of Calcium and Magnesium Ions on the Anammox Granular Properties to Alleviate Salinity Stress. Applied Sciences. 2022; 12(1):19. https://doi.org/10.3390/app12010019
Chicago/Turabian StyleKim, Yeonju, Jaecheul Yu, Soyeon Jeong, Jeongmi Kim, Seongjae Park, Hyokwan Bae, Sung-Keun Rhee, Tatsuya Unno, Shou-Qing Ni, and Taeho Lee. 2022. "Differences in the Effects of Calcium and Magnesium Ions on the Anammox Granular Properties to Alleviate Salinity Stress" Applied Sciences 12, no. 1: 19. https://doi.org/10.3390/app12010019
APA StyleKim, Y., Yu, J., Jeong, S., Kim, J., Park, S., Bae, H., Rhee, S.-K., Unno, T., Ni, S.-Q., & Lee, T. (2022). Differences in the Effects of Calcium and Magnesium Ions on the Anammox Granular Properties to Alleviate Salinity Stress. Applied Sciences, 12(1), 19. https://doi.org/10.3390/app12010019







