Which Three-Dimensional Printing Technology Can Replace Conventional Manual Method of Manufacturing Oral Appliance? A Preliminary Comparative Study of Physical and Mechanical Properties
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparing the Test Specimen
2.2. Experiments
2.2.1. Water Absorption and Solubility
2.2.2. Color Stability
Δa = a2 − a1 (a2 = value after immersion, a1 = value before immersion (day0))
Δb = b2 − b1 (b2 = value after immersion, b1 = value before immersion (day0))
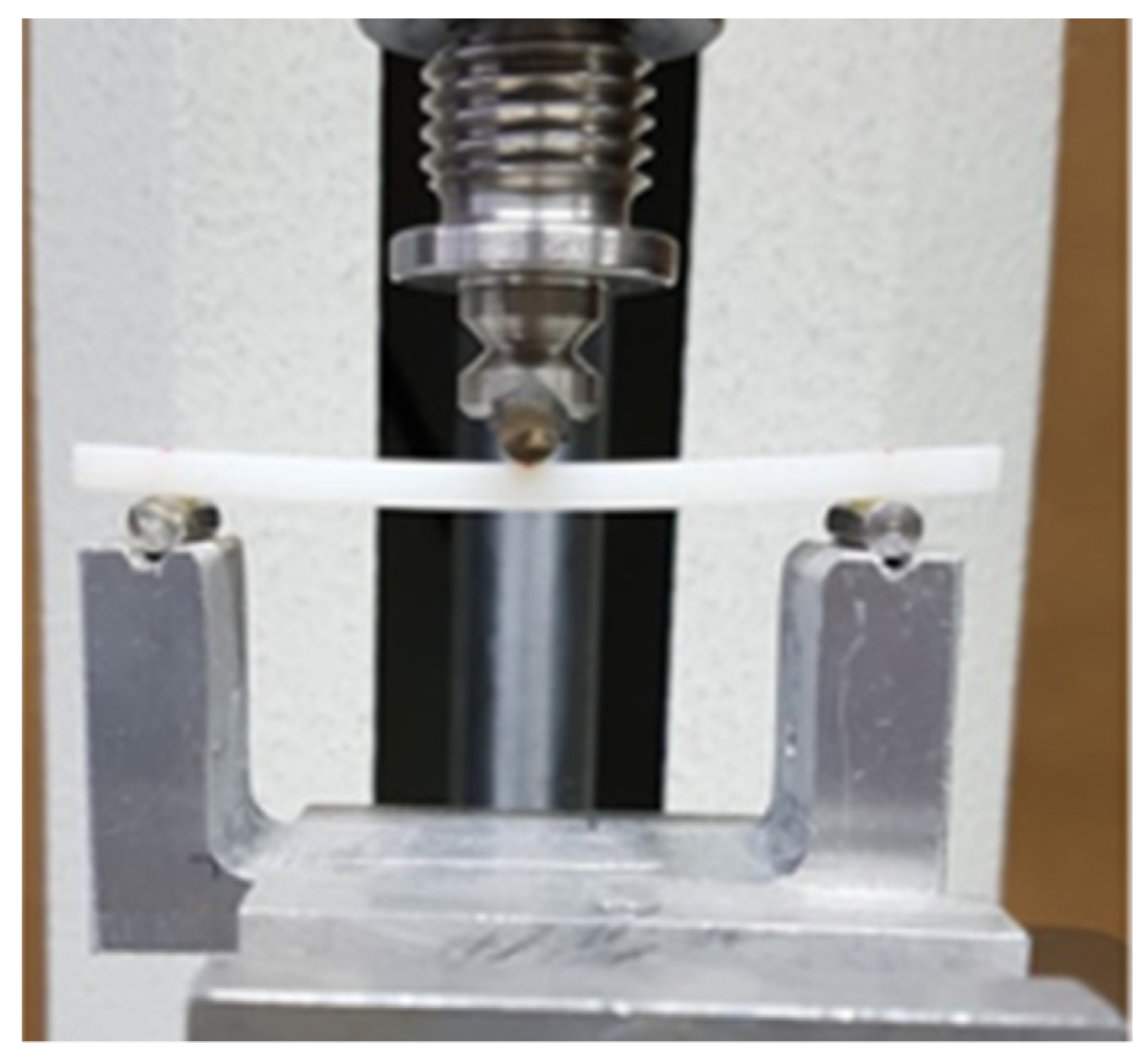
2.2.3. Flexural Strength
- P1: Force at the time of the destruction of the specimen (N)
- L: Spacing between supports
- B: Width of the specimen measured prior to the test
- H: The thickness of the specimen measured prior to the test
2.2.4. Surface Hardness
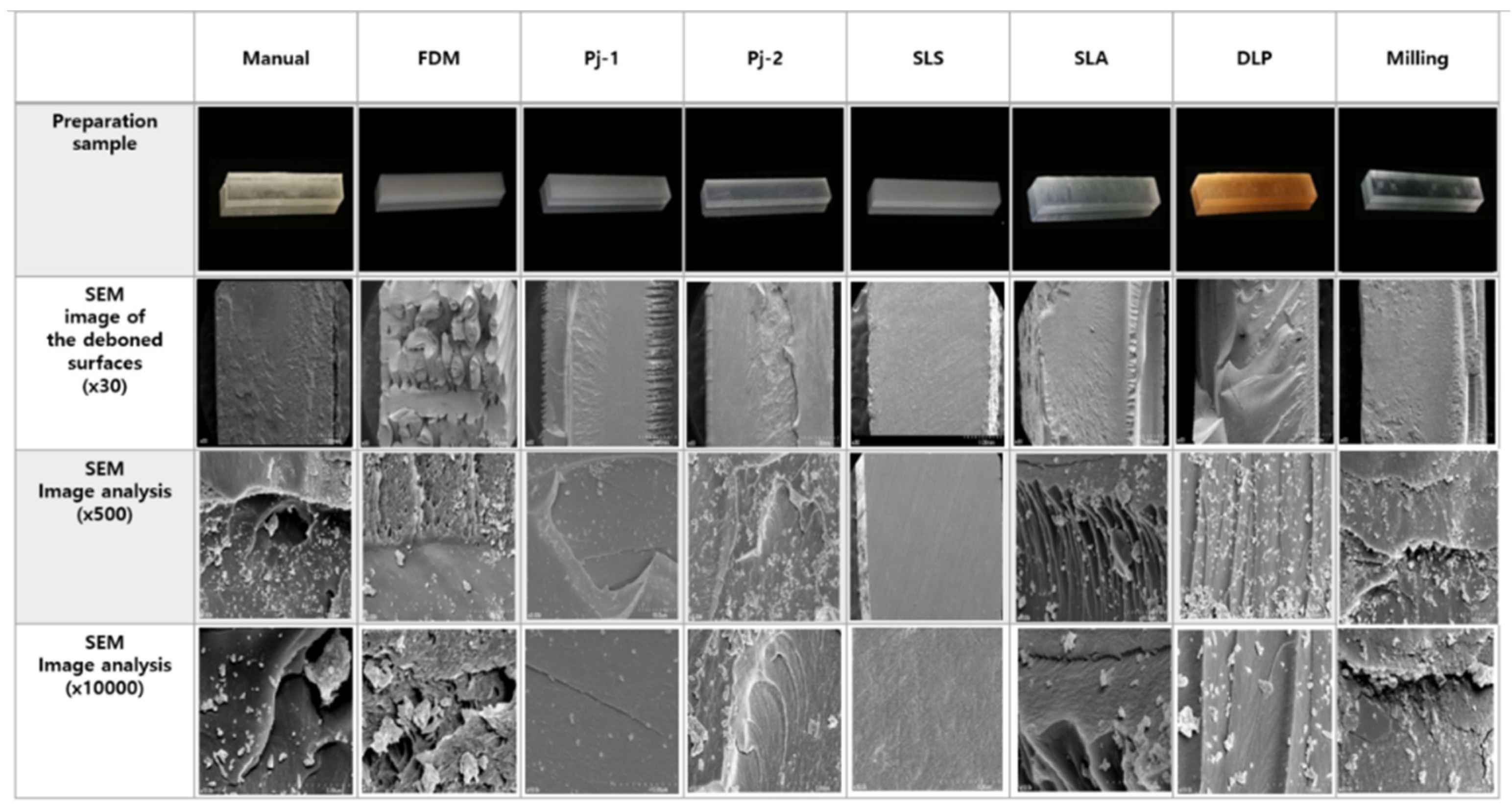
2.2.5. Fractured Surface Observation
2.3. Statistics Processing
3. Results
3.1. Experiments
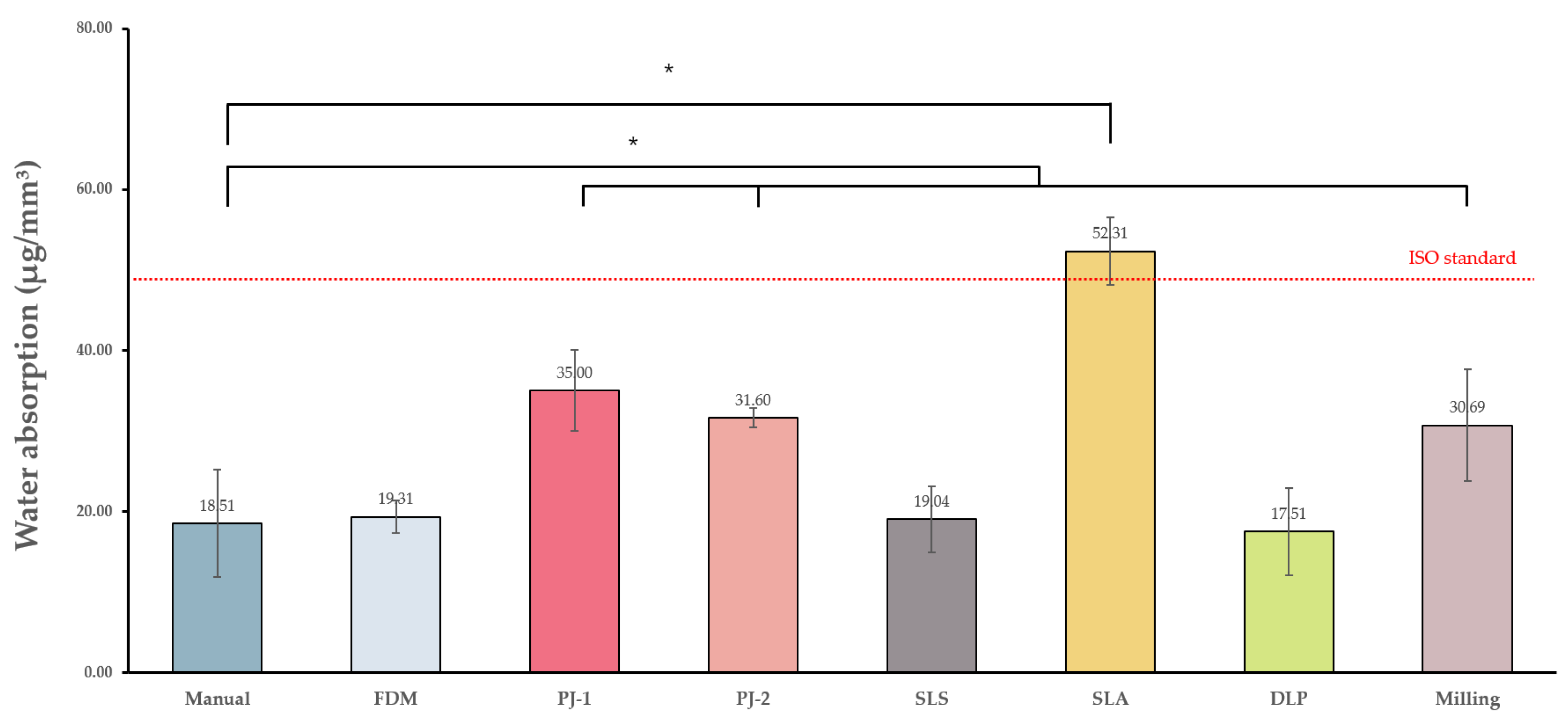
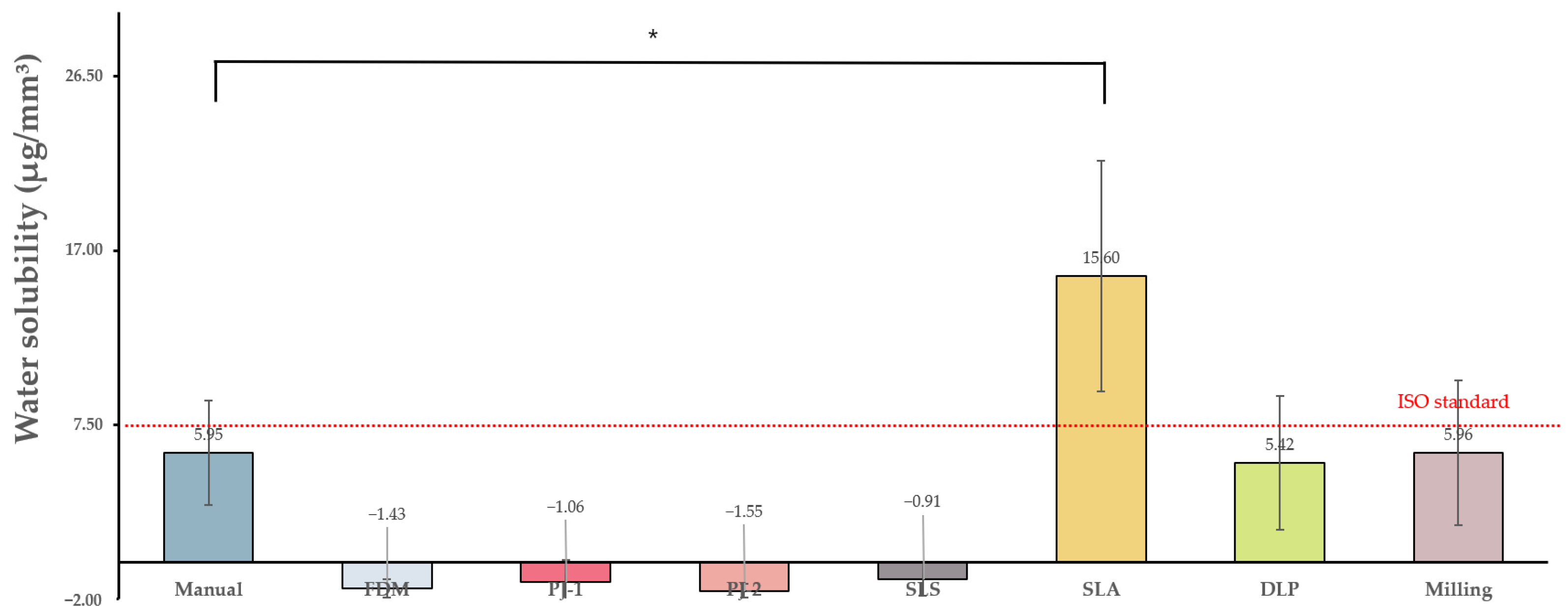
3.1.1. Water Absorption and Solubility
3.1.2. Color Stability
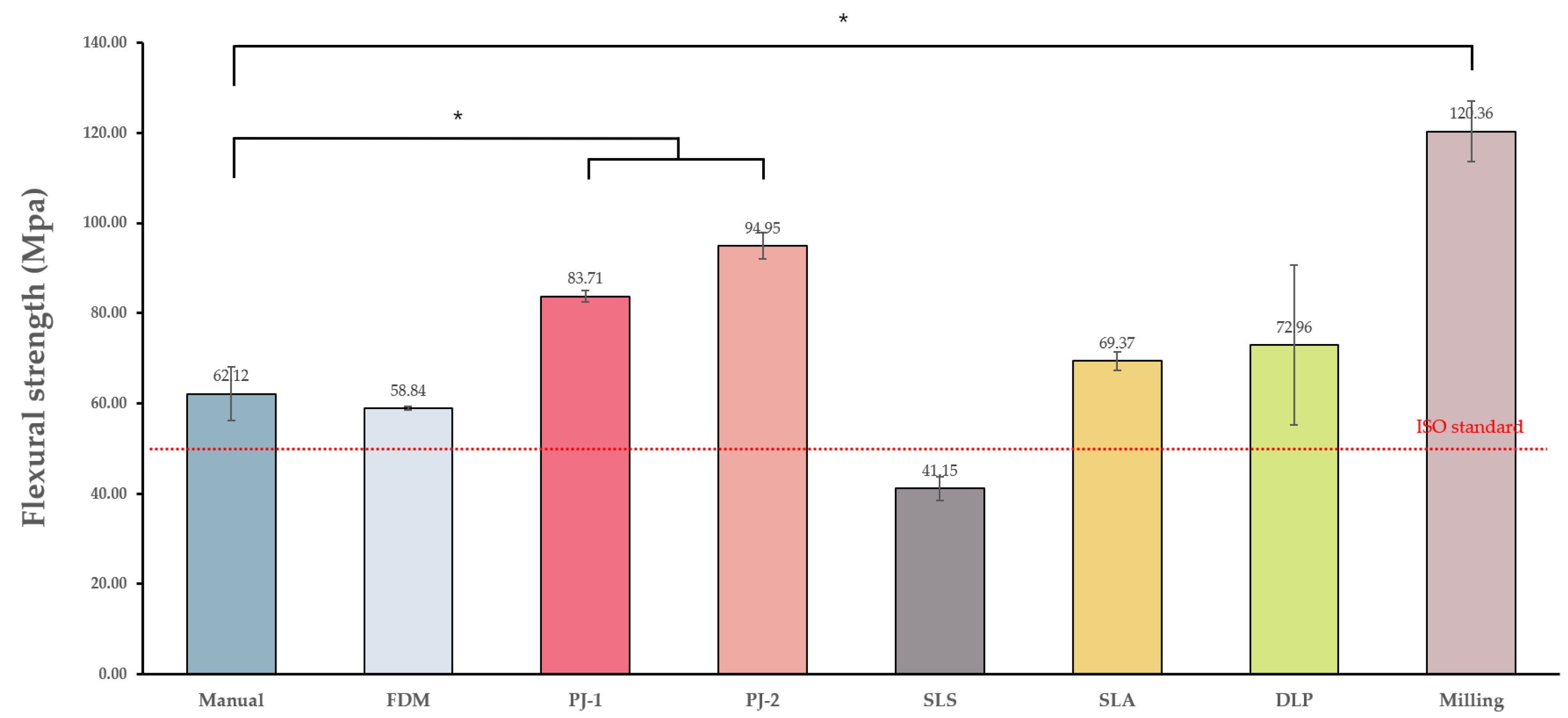
3.1.3. Flexural Strength
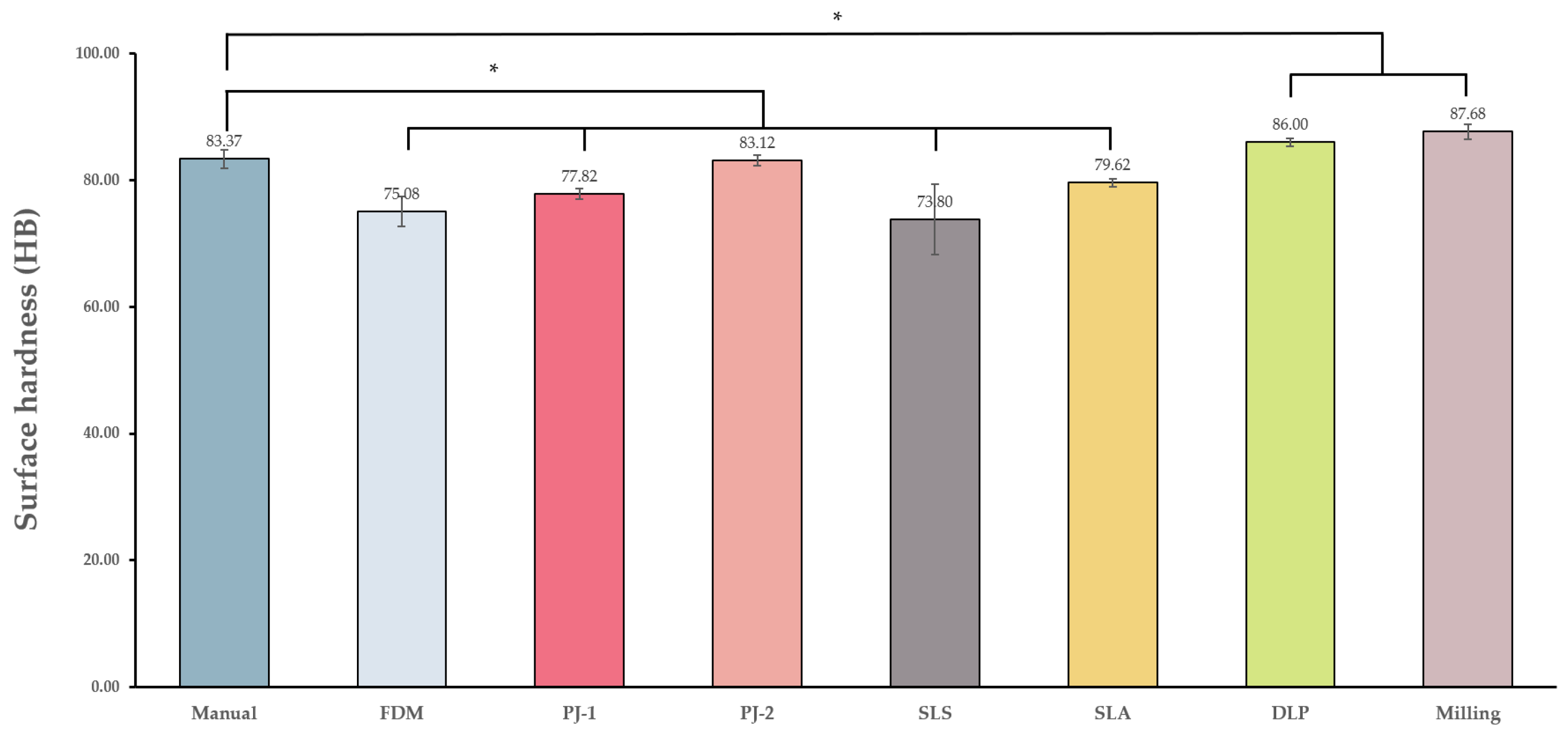
3.1.4. Surface Hardness
3.1.5. Fractured Surface Observation
4. Discussion
5. Conclusions
- The most suitable 3D-printing method for manufacturing oral appliances was the DLP method, since this exhibited comparable characteristics with the manual method and met the ISO standards in terms of water absorption and solubility, flexural strength, and surface hardness.
- Alternatively, the FDM method can be used for manufacturing confined oral appliances, since this method exhibited comparable characteristics to the manual method and met the ISO standards in water absorption and solubility and flexural strength, despite the low surface hardness.
- The color stability of 3D-printing materials should be improved to manufacture oral appliances, which require long-term esthetics.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Aakash, M.S.; Purvesh, M.S.; Romil, B.S. Clear aligners in orthodontics. Guident 2017, 10, 26–31. [Google Scholar]
- Wong, B.H. Invisalign A to Z. Am. J. Orthod. Dentofac. Orthop. 2002, 121, 540–541. [Google Scholar] [CrossRef] [PubMed]
- Shalish, M.; Cooper-Kazaz, R.; Ivgi, I.; Canetti, L.; Tsur, B.; Bachar, E.; Chaushu, S. Adult patients’ adjustability to orthodontic appliances. Part I: A comparison between Labial, Lingual, and Invisalign (TM). Eur. J. Orthod. 2012, 34, 724–730. [Google Scholar] [CrossRef] [Green Version]
- Martorelli, M.; Gerbino, S.; Giudice, M.; Ausiello, P. A comparison between customized clear and removable orthodontic appliances manufactured using RP and CNC techniques. Dent. Mater. 2013, 29, E1–E10. [Google Scholar] [CrossRef]
- Becker, C.M.; Kaiser, D.A. Surgical guide for dental implant placement. J. Prosthet. Dent. 2000, 83, 248–251. [Google Scholar] [CrossRef]
- Sutherland, K.; Vanderveken, O.M.; Tsuda, H.; Marklund, M.; Gagnadoux, F.; Kushida, C.A.; Cistulli, P.A. Oral Appliance Treatment for Obstructive Sleep Apnea: An Update. J. Clin. Sleep Med. 2014, 10, 215–227. [Google Scholar] [CrossRef] [Green Version]
- Dieltjens, M.; Vanderveken, O.M.; Hamans, E.; Verbraecken, J.A.; Wouters, K.; Willemen, M.; De Backer, W.A.; Van de Heyning, P.H.; Braem, M.J. Treatment of obstructive sleep apnea using a custom-made titratable duobloc oral appliance: A prospective clinical study. Sleep Breath. 2013, 17, 565–572. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ahrens, A.; McGrath, C.; Hagg, U. A systematic review of the efficacy of oral appliance design in the management of obstructive sleep apnoea. Eur. J. Orthod. 2011, 33, 318–324. [Google Scholar] [CrossRef] [Green Version]
- Srivastava, R.; Jyoti, B.; Devi, P. Oral splint for temporomandibular joint disorders with revolutionary fluid system. Dent. Res. J. 2013, 10, 307–313. [Google Scholar]
- Fournier, D.F. The conservative treatment of temporomandibular joint pain dysfunction syndrome. Ariz. Dent. J. 1977, 23, 30–31. [Google Scholar]
- Piehslinger, E. Treatment strategy in functional temporomandibular joint changes. Radiologe 2001, 41, 778–782. [Google Scholar] [CrossRef] [PubMed]
- Yakout, M.; Elbestawi, M.; Veldhuis, S.; Nangle-Smith, S. Influence of thermal properties on residual stresses in SLM of aerospace alloys. Rapid Prototyp. J. 2020, 26, 213–222. [Google Scholar] [CrossRef]
- Gibon, I.; Cheng, L.; Chow, S.; Cheung, W.; Beh, S.; Savalani, M.; Lee, S. The use of rapid prototyping to assist medical applications. Rapid Prototyp. J. 2006, 12, 53–58. [Google Scholar] [CrossRef] [Green Version]
- Khorasani, M.; Ghasemi, A.H.; Rolfe, B.; Gibson, I. Additive manufacturing a powerful tool for the aerospace industry. Rapid Prototyp. J. 2021, in press. [Google Scholar] [CrossRef]
- Nayar, S.; Bhuminathan, S.; Bhat, W.M. Rapid prototyping and stereolithography in dentistry. J. Pharm. Bioallied. Sci. 2015, 7, S216–S219. [Google Scholar] [CrossRef]
- Liu, Q.B.; Leu, M.C.; Schmitt, S.M. Rapid prototyping in dentistry: Technology and application. Int. J. Adv. Manuf. Technol. 2006, 29, 317–335. [Google Scholar] [CrossRef]
- Quadri, S.; Kapoor, B.; Singh, G.; Tewari, R.K. Rapid prototyping: An innovative technique in dentistry. J. Oral Res. Rev. 2017, 9, 96–102. [Google Scholar] [CrossRef]
- Dawood, A.; Marti Marti, B.; Sauret-Jackson, V.; Darwood, A. 3D printing in dentistry. Br. Dent. J. 2015, 219, 521–529. [Google Scholar] [CrossRef] [PubMed]
- Van Noort, R. The future of dental devices is digital. Dent. Mater. 2012, 28, 3–12. [Google Scholar] [CrossRef] [PubMed]
- Indrani, D.J.; Cook, W.D.; Televantos, F.; Tyas, M.J.; Harcourt, J.K. Fracture-Toughness of Water-Aged Resin Composite Restorative Materials. Dent. Mater. 1995, 11, 201–207. [Google Scholar] [CrossRef]
- Kakami, C.; Nakano, H.; Hotta, Y.; Miyazaki, T.; Maki, K. A study of biocomposite resins for creating orthodontic appliances using a 3D printer. Orthod. Waves 2017, 76, 140–150. [Google Scholar] [CrossRef]
- Inokoshi, S.; Burrow, M.F.; Kataumi, M.; Yamada, T.; Takatsu, T. Opacity and color changes of tooth-colored restorative materials. Oper. Dent. 1996, 21, 73–80. [Google Scholar] [PubMed]
- Gruber, S.; Kamnoedboon, P.; Ozcan, M.; Srinivasan, M. CAD/CAM Complete Denture Resins: An In Vitro Evaluation of Color Stability. J. Prosthodont. 2021, 30, 430–439. [Google Scholar] [CrossRef] [PubMed]
- Garcia, C.R.; Rumpf, R.C.; Tsang, H.H.; Barton, J.H. Effects of extreme surface roughness on 3D printed horn antenna. Electron. Lett. 2013, 49, 734–735. [Google Scholar] [CrossRef]
- Ngo, T.D.; Kashani, A.; Imbalzano, G.; Nguyen, K.T.Q.; Hui, D. Additive manufacturing (3D printing): A review of materials, methods, applications and challenges. Compos. Part B-Eng. 2018, 143, 172–196. [Google Scholar] [CrossRef]
- Vaezi, M.; Seitz, H.; Yang, S.F. A review on 3D micro-additive manufacturing technologies. Int. J. Adv. Manuf. Technol. 2013, 67, 1721–1754. [Google Scholar] [CrossRef]
- Anusavice, K.J.; Shen, C.; Rawls, H.R. Phillips’ Science of Dental Materials-12th Edition. Elsevier Health Sci. 2012, 15, 359–453. [Google Scholar]
- Prpic, V.; Slacanin, I.; Schauperl, Z.; Catic, A.; Dulcic, N.; Cimic, S. A study of the flexural strength and surface hardness of different materials and technologies for occlusal device fabrication. J. Prosthet. Dent. 2019, 121, 955–959. [Google Scholar] [CrossRef]
- Belter, J.T.; Dollar, A.M. Strengthening of 3D printed fused deposition manufactured parts using the fill compositing technique. PLoS ONE 2015, 10, e0122915. [Google Scholar] [CrossRef]
- Digholkar, S.; Madhav, V.N.; Palaskar, J. Evaluation of the flexural strength and microhardness of provisional crown and bridge materials fabricated by different methods. J. Indian Prosthodont. Soc. 2016, 16, 328–334. [Google Scholar]
- Hague, R.; Mansour, S.; Saleh, N.; Harris, R. Materials analysis of stereolithography resins for use in Rapid Manufacturing. J. Mater. Sci. 2004, 39, 2457–2464. [Google Scholar] [CrossRef] [Green Version]
- Craig, R.G.; Powers, J.M. Wear of Dental Tissues And Materials. Int. Dent. J. 1976, 26, 121–133. [Google Scholar] [PubMed]
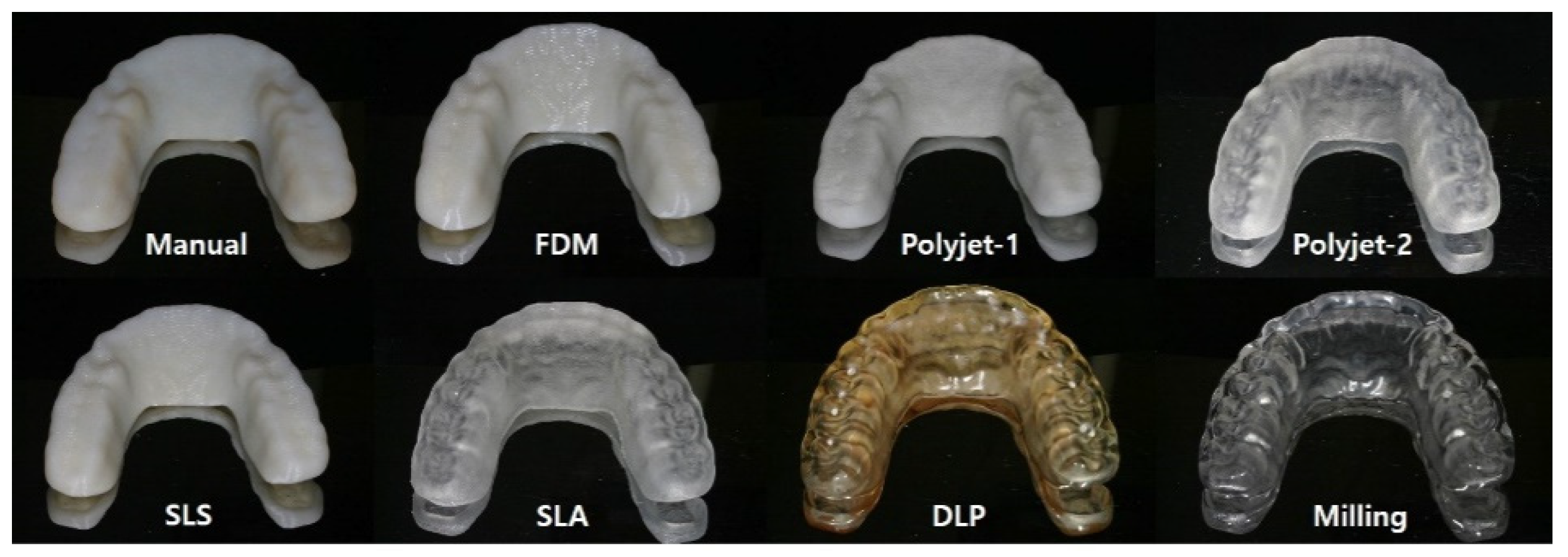

| Method | Material | Equipment | Layer Thickness (mm) |
|---|---|---|---|
| Manual (control) | Acrylic resin | Ortho jet (Lang Dental Manufacturing Company, Inc., Wheeling, IL, USA | - |
| FDM | ABS-M30 | FORTUS 450MC (Stratasys Inc., Hennepin County, MN, USA) | 0.1 |
| PJ-1 | Vero-clear | J750 (Stratasys Inc., Hennepin County, MN, USA) | 0.03 |
| PJ-2 | Med-610 | OBJET260 CONNEX 2 (Stratasys Inc., Hennepin County, MN, USA) | 0.05 |
| SLS | Polyamide | sPRO 140 (3Dsystems, Wilsonville, OR, USA) | 0.06 |
| SLA | Exclusive Form2 clear resin | Form2 (Formlabs, Middlesex County, MA, USA) | 0.05 |
| DLP | NextDent ortho Rigid | NextDent 5100 (3Dsystems, Wilsonville, OR, USA) | 0.025 |
| Milling | Clear PMMA block | ARUM 5X-200 (Arum dentistry Co., Ltd., Daejeon, Korea) | - |
| Absorption (μg/mm3) | Solubility (μg/mm3) | Color Change (ΔE) | Flexural Strength (Mpa) | Surface Hardness (HB) | ||
|---|---|---|---|---|---|---|
| Classification | Group | Mean ± SD | Mean ± SD | Mean ± SD | Mean ± SD | Mean ± SD |
| Control group | Manual | 18.51 ± 6.69 a | 5.95 ± 2.84 ab | 13.34 ± 16.12 a | 62.12 ± 5.99 a | 83.37 ± 1.5 a |
| Experimental groups | FDM | 19.31 ± 2.02 a | −1.43 ± 0.49 a | 32.58 ± 0.94 b | 58.84 ± 0.39 a | 75.08 ± 2.33 b |
| PJ-1 | 35 ± 5.05 b | −1.06 ± 1.19 a | 31.82 ± 1.67 b | 83.71 ± 1.24 b | 77.82 ± 0.86 b | |
| PJ-2 | 31.6 ± 1.2 b | −1.55 ± 0.38 a | 24.67 ± 2.96 b | 94.95 ± 2.94 b | 83.12 ± 0.82 a | |
| SLS | 19.04 ± 4.12 a | −0.63 ± 0.87 a | 60.95 ± 4.94 c | 41.15 ± 2.58 a | 73.8 ± 5.58 b | |
| SLA | 52.31 ± 4.17 c | 15.6 ± 6.29 c | 12.76 ± 1.97 a | 69.37 ± 2.1 a | 79.62 ± 0.63 b | |
| DLP | 17.51 ± 5.39 a | 5.42 ± 3.64 b | 14.26 ± 4.61 a | 72.96 ± 17.63 a | 86 ± 0.58 c | |
| Milling | 30.69 ± 6.9 b | 5.11 ± 4.25 b | 2.82 ± 0.29 a | 120.36 ± 6.63 c | 87.68 ± 1.21 c |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, H.-J.; Lim, S.-W.; Lee, M.-K.; Ju, S.W.; Park, S.-H.; Ahn, J.-S.; Hwang, K.-G. Which Three-Dimensional Printing Technology Can Replace Conventional Manual Method of Manufacturing Oral Appliance? A Preliminary Comparative Study of Physical and Mechanical Properties. Appl. Sci. 2022, 12, 130. https://doi.org/10.3390/app12010130
Kim H-J, Lim S-W, Lee M-K, Ju SW, Park S-H, Ahn J-S, Hwang K-G. Which Three-Dimensional Printing Technology Can Replace Conventional Manual Method of Manufacturing Oral Appliance? A Preliminary Comparative Study of Physical and Mechanical Properties. Applied Sciences. 2022; 12(1):130. https://doi.org/10.3390/app12010130
Chicago/Turabian StyleKim, Hyo-Jin, Seung-Weon Lim, Mi-Kyung Lee, Sung Won Ju, Suk-Hee Park, Jin-Soo Ahn, and Kyung-Gyun Hwang. 2022. "Which Three-Dimensional Printing Technology Can Replace Conventional Manual Method of Manufacturing Oral Appliance? A Preliminary Comparative Study of Physical and Mechanical Properties" Applied Sciences 12, no. 1: 130. https://doi.org/10.3390/app12010130
APA StyleKim, H.-J., Lim, S.-W., Lee, M.-K., Ju, S. W., Park, S.-H., Ahn, J.-S., & Hwang, K.-G. (2022). Which Three-Dimensional Printing Technology Can Replace Conventional Manual Method of Manufacturing Oral Appliance? A Preliminary Comparative Study of Physical and Mechanical Properties. Applied Sciences, 12(1), 130. https://doi.org/10.3390/app12010130






