Proteins as Hair Styling Agents
Abstract
1. Introduction
2. Materials and Methods
2.1. Expression and Purification of KP-UM and KP-Cryst Fusion Proteins
2.2. Keratin Extraction and Purification
2.3. Silk Fibroin (SF) Extraction and Purification
2.4. Hair Perming with Keratin, KP-UM, SF, BSA and KP-Cryst Proteins
2.5. Perming Efficiency
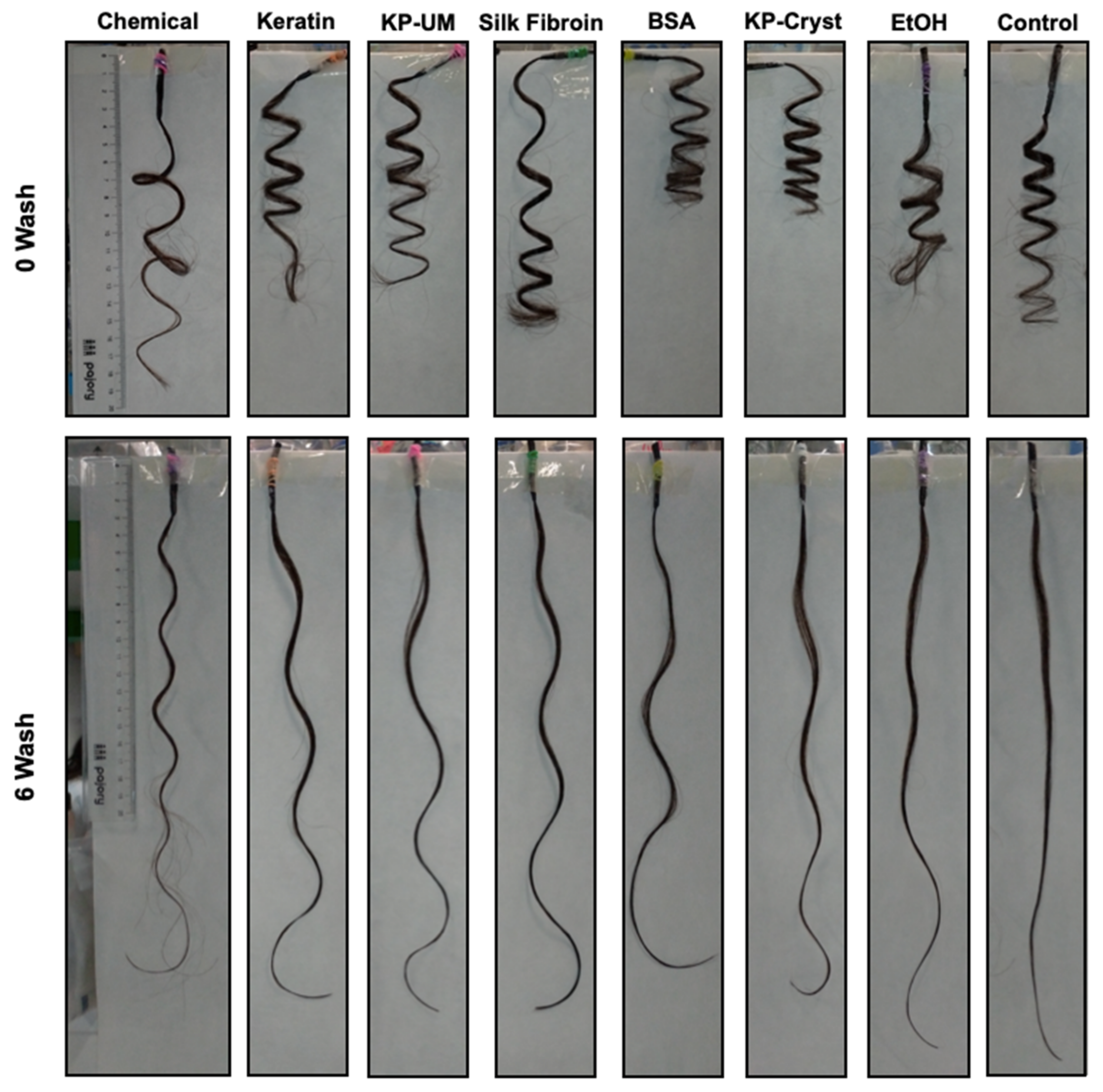
2.6. Perming Resistance to Shampoo
2.7. Hair Moisture Content
2.8. Statistical Analysis
3. Results and Discussion
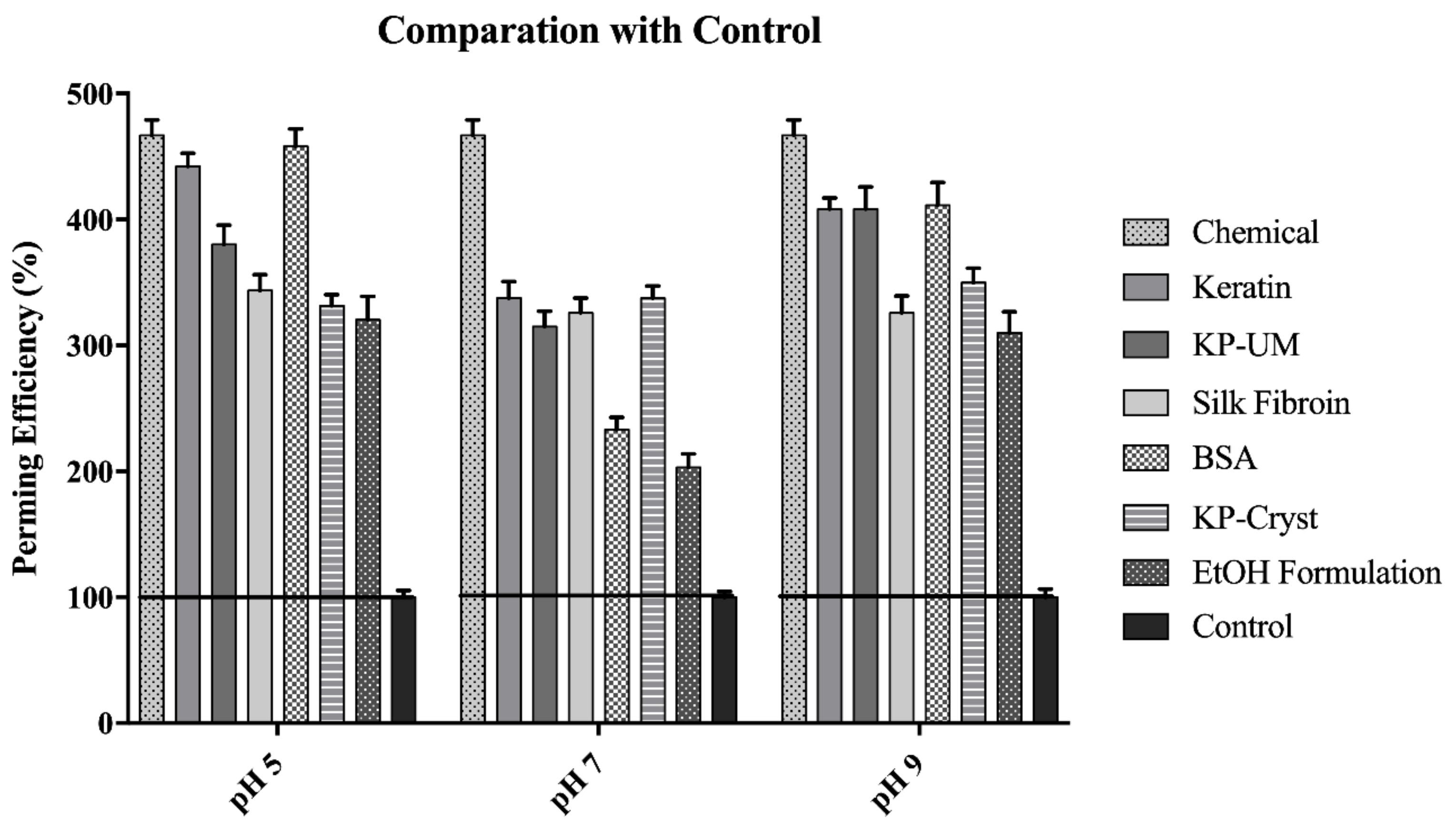
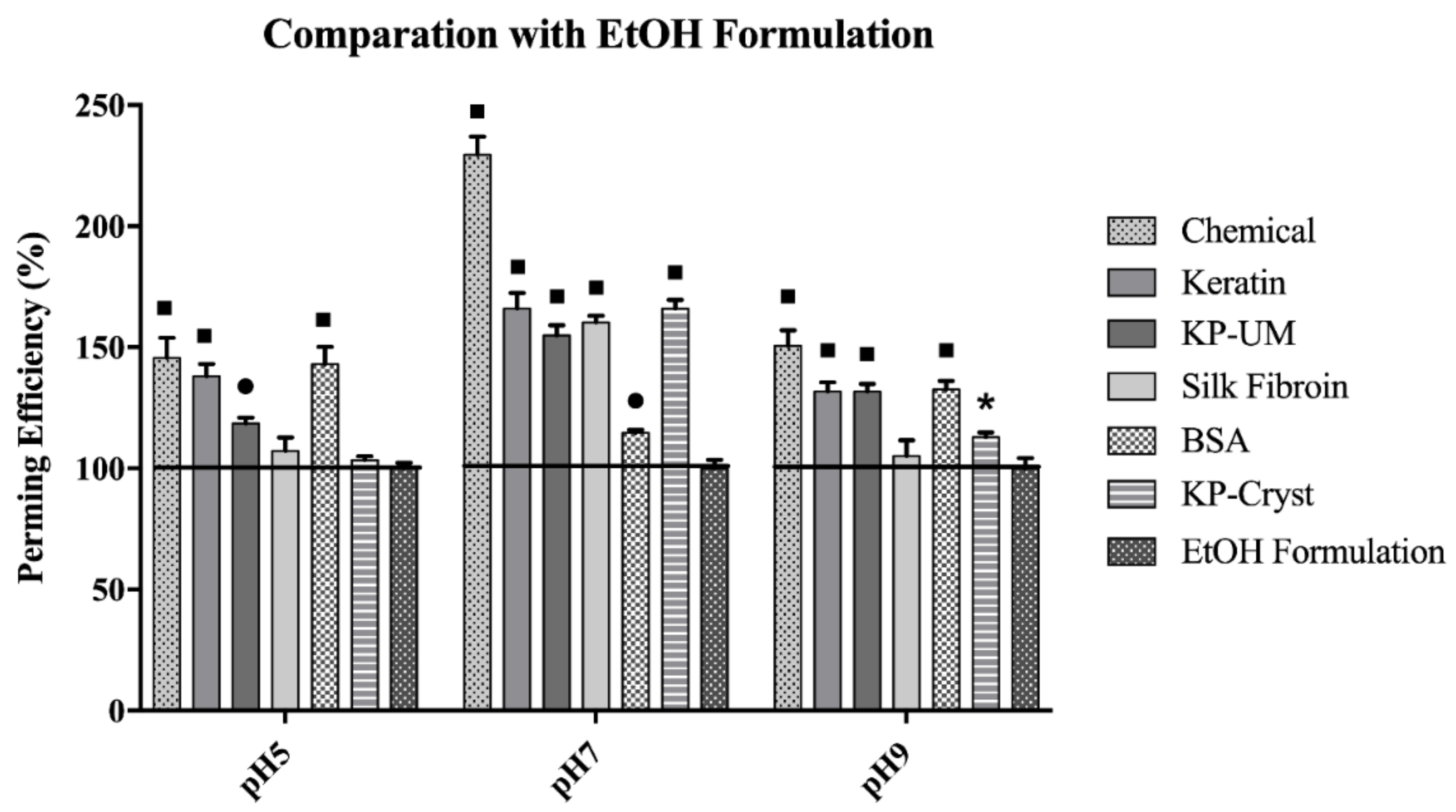
3.1. Perming efficiency
- pH 5EtOH formulation < KP-Cryst < Silk fibroin < KP-UM < Keratin < BSA
- pH 7EtOH formulation < BSA < KP-UM < Silk fibroin < KP-Cryst < Keratin
- pH 9EtOH formulation < KP-Cryst < Silk fibroin < KP-UM < Keratin < BSA
3.2. Perming Resistance to Shampoo
3.3. Effect of Protein Application on the Fibre Water Content
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Harrison, S.; Sinclair, R. Hair colouring, permanent styling and hair structure. J. Cosmet. Dermatol. 2004, 2, 180–185. [Google Scholar] [CrossRef] [PubMed]
- Bolduc, C.; Shapiro, J. Hair care products: Waving, straightening, conditioning, and coloring. Clin. Dermatol. 2001, 19, 431–436. [Google Scholar] [CrossRef]
- Cruz, C.F.; Martins, M.; Egipto, J.; Osório, H.; Ribeiro, A.; Cavaco-Paulo, A. Changing the shape of hair with keratin peptides. RSC Adv. 2017, 7, 51581–51592. [Google Scholar] [CrossRef]
- Mitsui, T. New Cosmetic Science; Elsevier: Amsterdam, The Netherlands, 1997; ISBN 9780080537498. [Google Scholar]
- Song, K.; Xu, H.; Yang, Y. Effects of chemical structures of polycarboxylic acids on molecular and performance manipulation of hair keratin Kaili. RSC Adv. 2016, 6, 58594–58603. [Google Scholar] [CrossRef]
- Young, Y.-H.; Chuu, J.-J.; Liu, S.-H.; Lin-Shiau, S.-Y. Toxic Effects of Potassium Bromate and Thioglycolate on Vestibuloocular Reflex Systems of Guinea Pigs and Humans. Toxicol. Appl. Pharmacol. 2001, 177, 103–111. [Google Scholar] [CrossRef][Green Version]
- Nemer, M.; Kristensen, P.; Nijem, K.; Bjertness, E.; Skogstad, M. Respiratory function and chemical exposures among female hairdressers in Palestine. Occup. Med. 2013, 63, 73–76. [Google Scholar] [CrossRef][Green Version]
- Nemer, M.; Sikkeland, L.I.B.; Kasem, M.; Kristensen, P.; Nijem, K.; Bjertness, E.; Skare, Ø.; Bakke, B.; Kongerud, J. Airway inflammation and ammonia exposure among female Palestinian hairdressers: A cross-sectional study. Occup. Environ. Med. 2015, 72, 428–434. [Google Scholar] [CrossRef]
- Yang, S.; Wang, J.; Cong, W.; Cai, Z.; Ouyang, F. Effects of bisulfite and sulfite on the microalga Botryococcus braunii. Enzyme Microb. Technol. 2004, 35, 46–50. [Google Scholar] [CrossRef]
- Baker, M.D.; Mayfield, C.I.; Inniss, W.E.; Wong, P.T.S. Toxicity of pH, Heavy Metals and Bisulfite to a Freshwater Green Alga. Chemosphere 1983, 12, 35–44. [Google Scholar] [CrossRef]
- Medda, L.; Monduzzi, M.; Salis, A. The molecular motion of bovine serum albumin under physiological conditions is ion specific. Chem. Commun. 2015, 51, 6663–6666. [Google Scholar] [CrossRef]
- Qi, Y.; Wang, H.; Wei, K.; Yang, Y.; Zheng, R.-Y.; Kim, I.S.; Zhang, K.-Q. A Review of Structure Construction of Silk Fibroin Biomaterials from Single Structures to Multi-Level Structures. Int. J. Mol. Sci. 2017, 18, 237. [Google Scholar] [CrossRef]
- Hawley, T.G.; Johnson, T.B. The Isoelectric Point of Silk Fibroin. Ind. Eng. Chem. 1930, 22, 297–299. [Google Scholar] [CrossRef]
- Moll, R.; Divo, M.; Langbein, L. The human keratins: Biology and pathology. Histochem. cell Biol. 2008, 129, 705–733. [Google Scholar] [CrossRef]
- Bragulla, H.H.; Homberger, D.G. Structure and functions of keratin proteins in simple, stratified, keratinized and cornified epithelia. J. Anat. 2009, 214, 516–559. [Google Scholar] [CrossRef]
- Majorek, K.A.; Porebski, P.J.; Dayal, A.; Zimmerman, M.D.; Jablonska, K.; Stewart, A.J.; Chruszcz, M.; Minor, W. Structural and immunologic characterization of bovine, horse, and rabbit serum albumins. Mol. Immunol. 2012, 52, 174–182. [Google Scholar] [CrossRef]
- Balkani, S.; Shamekhi, S.; Raoufinia, R.; Parvan, R.; Abdolalizadeh, J. Purification and characterization of bovine serum albumin using chromatographic method. Adv. Pharm. Bull. 2016, 6, 651–654. [Google Scholar] [CrossRef]
- Zhang, H.; Li, L.; Dai, F.; Zhang, H.; Ni, B.; Zhou, W.; Yang, X.; Wu, Y. Preparation and characterization of silk fibroin as a biomaterial with potential for drug delivery. J. Transl. Med. 2012, 10, 1–9. [Google Scholar] [CrossRef]
- Hakimi, O.; Knight, D.P.; Vollrath, F.; Vadgama, P. Spider and mulberry silkworm silks as compatible biomaterials. Compos. Part B Eng. 2007, 38, 324–337. [Google Scholar] [CrossRef]
- Cruz, C.F.; Fernandes, M.M.; Gomes, A.C.; Coderch, L.; Martí, M.; Méndez, S.; Gales, L.; Azoia, N.G.; Shimanovich, U.; Cavaco-Paulo, A. Keratins and lipids in ethnic hair. Int. J. Cosmet. Sci. 2013, 35, 244–249. [Google Scholar] [CrossRef]
- Deb-Choudhury, S.; Plowman, J.E.; Harland, D.P. Isolation and Analysis of Keratins and Keratin-Associated Proteins from Hair and Wool. In Methods in Enzymology; Elsevier Inc.: Amsterdam, The Netherlands, 2016; Volume 568, pp. 279–301. [Google Scholar]
- Tinoco, A.; Gonçalves, F.; Costa, A.F.; Cavaco-Paulo, A.; Ribeiro, A. Keratin:Zein particles as vehicles for fragrance release on hair. Ind. Crops Prod. 2021, 159, 113067. [Google Scholar] [CrossRef]
- Tinoco, A.; Antunes, E.; Martins, M.; Gonçalves, F.; Gomes, A.C.; Silva, C.; Cavaco-paulo, A.; Ribeiro, A. Fusion proteins with chromogenic and keratin binding modules. Sci. Rep. 2019, 9, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Tinoco, A.; Gonçalves, J.; Silva, C.; Cavaco-Paulo, A.; Ribeiro, A. Crystallin Fusion Proteins Improve the Thermal Properties of Hair. Front. Bioeng. Biotechnol. 2019, 7, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Ayutthaya, S.I.N.; Tanpichai, S.; Wootthikanokkhan, J. Keratin Extracted from Chicken Feather Waste: Extraction, Preparation, and Structural Characterization of the Keratin and Keratin/Biopolymer Films and Electrospuns. J. Polym. Environ. 2015, 23, 506–516. [Google Scholar] [CrossRef]
- Tinoco, A.; Gonçalves, J.; Silva, C.; Loureiro, A.; Gomes, A.C.; Cavaco-Paulo, A.; Ribeiro, A. Keratin-based particles for protection and restoration of hair properties. Int. J. Cosmet. Sci. 2018, 40, 408–419. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Cavaco-Paulo, A.; Xu, B.; Martins, M. Polymeric Hydrogel Coating for Modulating the Shape of Keratin Fiber. Front. Chem. 2019, 7. [Google Scholar] [CrossRef] [PubMed]
- Barba, C.; Scott, S.; Roddick-Lanzilotta, A.; Kelly, R.; Manich, A.M.; Parra, J.L.; Coderch, L. Restoring Important Hair Properties with Wool Keratin Proteins and Peptides. Fibers Polym. 2010, 11, 1055–1061. [Google Scholar] [CrossRef]
- Azoia, N.G.; Fernandes, M.M.; Micaêlo, N.M.; Soares, C.M.; Cavaco-Paulo, A. Molecular modeling of hair keratin/peptide complex: Using MM-PBSA calculations to describe experimental binding results. Proteins Struct. Funct. Bioinform. 2012, 80, 1409–1417. [Google Scholar] [CrossRef] [PubMed]
- Pelikh, O.; Keck, C.M. Hair follicle targeting and dermal drug delivery with curcumin drug nanocrystals—Essential influence of excipients. Nanomaterials 2020, 10, 2323. [Google Scholar] [CrossRef]
- Cheng, Y.; Koh, L.; Li, D.; Ji, B.; Han, M.; Zhang, Y. On the strength of β-sheet crystallites of Bombyx mori silk fibroin. J. R. Soc. Interface 2014, 11, 20140305. [Google Scholar] [CrossRef]
- Kundu, S.; Pandit, S.; Abbas, S.; Aswal, V.K.; Kohlbrecher, J. Structures and interactions among globular proteins above the isoelectric point in the presence of divalent ions: A small angle neutron scattering and dynamic light scattering study. Chem. Phys. Lett. 2018, 693, 176–182. [Google Scholar] [CrossRef]
- Malinauskyte, E.; Cornwell, P.A.; Reay, L.; Shaw, N.; Petkov, J. Effect of equilibrium pH on the structure and properties of bleach-damaged human hair fibers. Biopolymers 2020, e23401. [Google Scholar] [CrossRef]
- Yamasaki, M.; Yano, H.; Aoki, K. Differential scanning calorimetric studies on bovine serum albumin: I. Effects of pH and ionic strength. Int. J. Biol. Macromol. 1990, 12, 263–268. [Google Scholar] [CrossRef]
- Basit, A.; Asghar, F.; Sadaf, S.; Akhtar, M.W. Health improvement of human hair and their reshaping using recombinant keratin K31. Biotechnol. Rep. 2018, 20, e00288. [Google Scholar] [CrossRef]
- Skopp, G.; Pötsch, L.; Moeller, M.R. On cosmetically treated hair—Aspects and pitfalls of interpretation. Forensic Sci. Int. 1997, 84, 43–52. [Google Scholar] [CrossRef]
- Lu, R.; Li, W.-W.; Katzir, A.; Raichlin, Y.; Yu, H.-Q.; Mizaikof, B. Probing the secondary structure of bovine serum albumin during heat-induced denaturation using mid-infrared fiberoptic sensors. Analyst 2015, 140, 765–770. [Google Scholar] [CrossRef] [PubMed]
- Malinauskyte, E.; Shrestha, R.; Cornwell, P.A.; Gourion-Arsiquaud, S.; Hindley, M. Penetration of different molecular weight hydrolysed keratins into hair fibres and their effects on the physical properties of textured hair. Int. J. Cosmet. Sci. 2021, 43, 26–37. [Google Scholar] [CrossRef]
- Snyder, M.J.; Bjurman, S. Water-Vapor Hair Treatment Apparatus. U.S. Patent 5,010,905, 30 April 1991. [Google Scholar]
- Piérard-Franchimont, C.; Piérard, G.E. Hair weathering and hair capacitance mapping: A pilot study. J. Cosmet. Dermatol. 2012, 11, 179–182. [Google Scholar] [CrossRef]
- Barba, C.; Méndez, S.; Martí, M.; Parra, J.L.; Coderch, L. Water content of hair and nails. Thermochim. Acta 2009, 494, 136–140. [Google Scholar] [CrossRef]
- Barba, C.; Martí, M.; Manich, A.M.; Carilla, J.; Parra, J.L.; Coderch, L. Water absorption / desorption of human hair and nails. Thermochim. Acta 2010, 503–504, 33–39. [Google Scholar] [CrossRef]
- Benzarti, M.; Jamart, J.; Zahouani, H. The Effect of Hydration on the Mechanical Behaviour of Hair. Exp. Mech. 2014, 54, 1411–1419. [Google Scholar] [CrossRef]
- Zayas, J.F. Water Holding Capacity of Proteins. In Functionality of Proteins in Food; Springer: Berlin/Heidelberg, Germany, 1997; pp. 76–133. [Google Scholar]
- Peters, J.P.C.M.; Vergeldt, F.J.; Boom, R.M.; van der Goot, A.J. Water-binding capacity of protein-rich particles and their pellets. Food Hydrocoll. 2017, 65, 144–156. [Google Scholar] [CrossRef]
- Hill, V.; Loni, E.; Cairns, T.; Sommer, J.; Schaffer, M. Identification and analysis of damaged or porous hair. Drug Test. Anal. 2014, 6, 42–54. [Google Scholar] [CrossRef] [PubMed]



| Protein | Molecular Weight (kDa) | Isoelectric Point | Structure |
|---|---|---|---|
| BSA | 66.5 | 4.7 [11] | Globular |
| Silk Fibroin | Heavy chain 390 [12] Light chain 26 | 2.1 [13] | Anti-parallel β-sheet |
| Keratin | 44 to 66 [14] | (4.9–5.4) * (6.5–8.5) ** [15] | α -helix |
| KP-UM | 26.3 | 8.39 | β-barrel |
| KP-Cryst | 24.8 | 7.13 | Greek Key |
| Perming Efficiency Ratio (Sample/Chemical) | |||
|---|---|---|---|
| Sample | pH 5 | pH 7 | pH 9 |
| Keratin | 0.947 | 0.723 | 0.875 |
| KP-UM | 0.815 | 0.675 | 0.872 |
| Silk Fibroin | 0.736 | 0.698 | 0.698 |
| BSA | 0.982 | 0.500 | 0.882 |
| KP-Cryst | 0.711 | 0.723 | 0.750 |
| EtOH Formulation | 0.686 | 0.435 | 0.664 |
| Perming Resistance (%) | |||
|---|---|---|---|
| pH 5 | pH 7 | pH 9 | |
| Chemical | 70.37 | 70.37 | 70.37 |
| Keratin | 48.65 | 29.66 | 47.64 |
| KP-UM | 28.97 | 24.00 | 37.07 |
| Silk Fibroin | 41.45 | 30.00 | 36.08 |
| BSA | 93.58 | 11.85 | 35.26 |
| KP-Cryst | 36.49 | 17.36 | 40.83 |
| EtOH Formulation | 33.90 | 15.32 | 29.29 |
| Control | 7.62 | 7.62 | 7.62 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tinoco, A.; Costa, A.F.; Luís, S.; Martins, M.; Cavaco-Paulo, A.; Ribeiro, A. Proteins as Hair Styling Agents. Appl. Sci. 2021, 11, 4245. https://doi.org/10.3390/app11094245
Tinoco A, Costa AF, Luís S, Martins M, Cavaco-Paulo A, Ribeiro A. Proteins as Hair Styling Agents. Applied Sciences. 2021; 11(9):4245. https://doi.org/10.3390/app11094245
Chicago/Turabian StyleTinoco, Ana, André F. Costa, Salomé Luís, Madalena Martins, Artur Cavaco-Paulo, and Artur Ribeiro. 2021. "Proteins as Hair Styling Agents" Applied Sciences 11, no. 9: 4245. https://doi.org/10.3390/app11094245
APA StyleTinoco, A., Costa, A. F., Luís, S., Martins, M., Cavaco-Paulo, A., & Ribeiro, A. (2021). Proteins as Hair Styling Agents. Applied Sciences, 11(9), 4245. https://doi.org/10.3390/app11094245








