Abstract
One of the most visible signs of hair ageing is greying of the hair, also known as canities. This hair disorder is mainly caused by oxidative stress. In preliminary work, we designed various models mimicking the impact of oxidative stress on hair pigmentation, showing an accumulation of reactive oxygen species (ROS) production and a decrease in the presence of melanocytes and melanoblasts, resulting in a decrease in hair pigmentation. A proteomic study on skin scalp explants was performed to identify the dysregulated biological pathways related to canities. We developed a smart active ingredient which has been tested on these biological pathways. We demonstrated that these negative effects were rectified in the presence of the ingredient, showing a reduction of ROS, protection of melanocyte reservoirs and reactivation of hair pigmentation. Finally, a clinical study was carried out on a panel of 44 male volunteers with grey hair. After 4 months, we evidenced a reduction in the proportion of grey hair and in the number of grey hairs/cm2 relative to Day 0. In conclusion, we clearly evidenced that oxidative stress is a key factor in triggering a cascade of events leading to a loss of hair pigmentation. We developed this active ingredient which is capable of restoring all the disrupted mechanisms and of providing hair repigmentation within only 4 months.
1. Introduction
1.1. Hair Pigmentation
As with the skin, hair pigmentation is instigated by melanocytes. The human hair follicle contains five to six different cell subpopulations of follicular melanocytes, distinguished by their degree of differentiation and ability to synthetise melanin [1]. We can observe the presence of melanocyte precursors, called melanoblasts, constituting the melanocyte reservoirs in the basal layer of the infundibulum and sebaceous gland and mostly in the hair bulge [2]. These melanoblasts differentiate into melanocyte stem cells (McSCs) [3]. These melanocyte precursors are undifferentiated amelanotic cells and are not melanogenically active but express the GP100 protein and so are positive for NKI/beteb staining [2,4]. They migrate downward along the outer root sheath (ORS) to populate the hair bulb around the dermal papilla (DP) [3,5]. In this compartment, under various signals from surrounding cells (mainly from the DP), the melanocyte precursors will differentiate into melanogenically active melanocytes and will proceed with melanogenesis [3,6]. The melanogenesis in the hair follicles is stringently coupled to the hair growth cycle and more precisely to the anagen phase [2,5]. During the anagen phase, the follicular active melanocytes are larger, with longer dendrites than epidermal melanocytes. They have a more extensive Golgi apparatus and rough endoplasmic reticulum and produce larger melanosomes which are transferred to the precortical keratinocytes of hair shafts [2]. At this stage of the hair cycle (anagen IV), the expression of anti-oxidant proteins is at its highest in order to neutralise the reactive oxygen species (ROS) produced during melanogenesis [2]. The production of free radicals during melanin synthesis is a normal metabolic process but it would seem that melanocytes from hair follicles are more sensitive to stress factors than epidermal melanocytes [1].
1.2. Canities
The progressive loss of hair pigmentation with age is called canities or senile “hair greying” and results in the emergence of grey hair fibres from the epidermis surface [7]. The “hair greying” is a consequence of melanogenesis reduction during the anagen phase of the hair cycle and seems to be universal. After the age of 50, 50% of the population have at least 50% “grey” hair [2,8]. This phenomenon could be variable according to ethnic origin [1,9]. The events leading to the onset of “hair greying” are not completely known but some works reveal that the accumulation of ROS could be the principal cause [10,11,12] These high concentrations of ROS are correlated to a reduction of catalase and methionine sulfoxide reductase activity [11,13]. Subsequently, it has been described that follicular melanocyte apoptosis was increased in ageing hair [2], leading to the exhaustion of melanocyte precursor reservoirs [9,11]. Moreover, it has been evidenced that in the case of canities, the melanocytes inside the pigmentary unit of the hair bulb lose their dendritic aspect to become more rounded and thus less differentiated and less melanogenically active [11].
Another hypothesis suggests that melanosome formation and their transfer could be defective [1,2]. The defective melanosomes seem to remain in the melanocytes within the autophagolysosomes, caused by a lack of recycling through autophagy [4,12]. The defective autophagy may lead to ROS leakage and finally to the death of melanocytes themselves [2]. All these events lead to a global decrease in melanogenesis [10].
Considering all previous work, we can hypothesise that melanocyte reservoirs can be protected through various mechanisms and mostly thanks to the maintenance of stem cell reservoirs and the neutralisation of oxidative stress [1,10]. In this work, we attempt to design ex vivo models to mimic the process of canities by inducing oxidative stress based on previous work [13,14,15]. We then designed a smart and innovative mixture to address this phenomenon. The final objective was to offer hair pigmentation recovery of grey hair.
2. Materials and Methods
2.1. Description and Preparation of the Active Ingredient
The Larix europea wood extract and Camellia sinensis leaf extract containing the active ingredient were stabilised using a proprietary enzyme-based process able to graft α-d-glucose residues to the pyrocatechol and 3,4,5-trihydroxylated rings of taxifolin (dihydroquercetin) and epigallocatechin gallate (EGCG), respectively. The α-d-glucosylating process was previously described by Auriol et al. in EP2027279B1 (Phenolic compounds with cosmetics and therapeutic applications). Briefly, the Leuconostoc mesenteroides ATCC 10830a dextransucrase (EC 2.4.1.5) is produced as described by Paul et al. [16]. The reaction media contain sucrose at 300 g L−1, with the extract previously dissolved in DMSO at a final concentration 12 g L−1 (final DMSO concentration 15%). The reaction takes place at pH 5.20 (sodium acetate buffer) at 30 °C. After 24 to 30 h of incubation under mild agitation, the enzyme is inactivated by decreasing the pH to 2.0 with concentrated sulfuric acid. The polyphenol-related substances are purified using an adsorbent resin (Diaion HP 20 from Mitsubishi Chemical Company, Tokyo, Japan). After adsorbing the polyphenol-related molecules onto the resin, the resin is extensively washed with water to remove all the water-soluble non-polyphenolic substances. Then, the extract is eluted with an alcohol, methanol. After complete alcohol removal by vacuum evaporation, the glucosylated substances contained in the extract are separated from the non-glucosylated ones by liquid–liquid extraction with ethyl acetate. Finally, the ethyl acetate present in the glucosylated extract-rich aqueous phase is removed by vacuum evaporation at 40 °C. The extract concentration is then adjusted by removing the excess water and filtered at 0.2 µm. The sterile aqueous solution containing the stabilised extract is used as such for the preparation of the active ingredient. Taxifolin and its glucosylated derivatives are adjusted to a final concentration of 0.17% (w/w) and EGCG and EGCG glucosides at 0.03% (w/w).
2.2. Evaluation of the Impact of Oxidative Stress on Microdissected Hair Follicles
2.2.1. Culture and Treatment of Hair Follicles
Human hair follicles (HFs) were dissected from a scalp explant from a 56-year-old donor after informed consent and ethics approval. After 18 h of pre-culture in modified William’s E medium (Gibco, Life Technologies, Carlsbad, CA, USA), only anagen VI HFs were selected for further experiments. Twelve hair follicles were used per treatment for each read out parameter. The culture medium was renewed every other day. After the selection, the HFs were incubated for 1 h 30 min for reactive oxygen species (ROS) production analysis and for 4 days for the NKI/Beteb immunostaining.
2.2.2. ROS Production Quantification Using DCFH-DA Probe
For ROS production analysis, the HFs were incubated for 1 h with the active ingredient dissolved at 1% (v/v) in culture medium or not for the untreated control. Afterwards, the HFs were then cultured for 30 min in the presence of dichlorofluoerescein diacetate (DCFH-DA, Life Technologies, Carlsbad, CA, USA), a probe that reacts with ROS, becoming fluorescent. Following the DCFH-DA incubation, the HFs were rinsed in phosphate-buffered solution (PBS) (Gibco, Life Technologies, Carlsbad, CA, USA) and incubated with cumene hydroperoxide (Sigma-Aldrich, St. Louis, MO, USA) at 50 µm, as an oxidative stimulus, for 1 h at 37 °C with 5% CO2. At the end of the experimental phase, the HFs were harvested, cryo-fixed in OCT (VWR, Avantor, Milan, Italy). The HFs were carefully orientated to obtain intact hair follicle sections and open dermal papillae. Section of 5 µm were obtained with a cryo-microtome. The ROS production was then analysed by fluorescence detection using epifluorescence microscopy at a magnification of 20× on cryo-sections. The intensity was quantified by image analysis using ImageJ software (NIH, New York, NY, USA). All data were expressed as mean ± standard error of the mean (SEM) for each experimental condition.
2.2.3. NKI/Beteb Immunostaining
Melanocyte and melanoblast quantification was obtained by NKI/Beteb immunofluorescence staining evaluation of the cryo-sections. Briefly, the 5 µm cryo-sections were fixed in acetone and washed in PBS (Gibco, Cleveland, TN, USA). Non-specific binding blocking was performed by incubating, for 1 h at room temperature, a solution of 10% normal goat serum (Sigma-Aldrich, St. Louis, MO, USA). Subsequently, the primary antibody against NKI/beteb (mouse monoclonal anti-melanoma-associated antigen, clone NKI/beteb, Cat. No. MON7006-1, MONOSAN, Uden, the Netherlands, diluted 1:50) was incubated for 1 h 30 min at room temperature. Samples were then incubated with rhodamine red conjugated to goat anti-mouse secondary antibody (Jackson ImmunoResearch Cat. No.115-295-062) and nuclei were counterstained with DAPI (Sigma-Aldrich, St. Louis, MO, USA). On the immunostained sections, pictures were taken using epifluorescence microscopy at a magnification of 10×. Image analysis was performed using ImageJ software in order to quantify the number of NKI/beteb-positive cells within each hair follicle. The obtained values were then normalised with the total number of cells of the considered area. All data were expressed as mean ± SEM for each experimental condition.
2.3. Evaluation of Melanin Production in Ex Vivo Grey Hair Follicles
Microdissected hair follicles (HFs) were obtained from occipital healthy human skin obtained from 2 donors of 35 years old and 53 years old undergoing hair transplantation surgery or from scalp biopsy after informed consent and ethics approval (University of Muenster, n.2015-602-f-S). HFs in human anagen VI were microdissected from scalp biopsies and they were cultured at 37 °C with 5% CO2 in a minimal media of William’s E media (Gibco, Life Technologies, Carlsbad, CA, USA) supplemented with 2 mM of L-glutamine (Gibco Life Technologies, Carlsbad, CA, USA), 10 ng·mL−1 hydrocortisone (Sigma-Aldrich, USA), 10 μg·mL−1 insulin (Sigma-Aldrich, St. Louis, MO, USA) and 1% penicillin/streptomycin mix (Gibco, Cleveland, TN, USA) to make Williams’ complete media (WCM) as previously described [17]. After 24 h, the medium was replaced with fresh medium containing either WCM (vehicle) or the active ingredient at 1%. Eleven grey (defined as having low pigment) anagen VI HFs were cultured per experimental group. HFs were cultured for a total of 3 days. The HFs were then frozen and sectioned with a cryostat (CM3050S, Leica Biosystems, Wetzlar, Germany) and 6 μm sections were collected. The HFs were carefully orientated to obtain intact hair follicle sections and open dermal papillae. Consecutive sections of hair follicles were collected and slides were stored at −80 °C. To evaluate melanin as a marker for HF pigmentation, Fontana-Masson (FM) staining was performed on frozen slides. Briefly, melanin was stained with silver nitrate (Caesar & Loretz, Hilden, Germany) ammonia-based solution and stopped with 5% aqueous sodium thiosulphate (Merck Millipore, Darmstadt, Germany). Pictures were taken with a Keyence Biozero Microscope 8100 and 9000 at a magnification of 200×. Three areas of 100 × 175 or 113 × 159 pixels were measured above the Auber’s line to assess pigmentation intensity for donor 1 or 2 in anagen HFs, respectively, using ImageJ software. All data were expressed as mean ± SEM.
2.4. Transcriptomic Analysis in Full Ex Vivo Scalp Skin
The assay was carried out on a NativeSkin® scalp skin explant, a full-thickness skin biopsy embedded in a solid and nourishing matrix, while its epidermal surface was left in contact with the air. The skin biopsy is firmly embedded in the matrix that prevents any lateral diffusion of topically applied formulations. The study was conducted on lift explants from 3 donors (mean age of 63 years) with sufficient hair follicles and an equivalent number between the samples from the same donor and after signed informed consent.
Oxidative stress was applied for 3 days, once a day, by adding 9 mg of hypoxanthin +10 units of xanthine oxidase for 1 h to produce O2− at the skin surface. The oxidative stress experimental condition, used here as a control, was only treated with Carbopol® ultrez 10 (Lubrizol, Wickliffe, OH, USA) (which was used as vehicle). The treatment with the active ingredient at 1% (v/v) was topically applied for 48 h. After treatment, the dermis of each explant was removed by microsurgery to focus gene quantification on epidermal cells. Skin explants were collected in specific lysis solution for mRNA extraction. Lysates were transferred onto plates in order to purify mRNA. Afterwards, a reverse transcription system was used. According to the Fluidigm® protocol, specific stages for a 48 × 48 chip preparation were starting following the manufacturer’s instructions. Briefly, a pre-amplification step was carried out with the primers used in the chip. A pre-amplified cDNA/PCR mix and primers were deposited on the chip. The mix blending was undertaken by an IFC Controller and then the chip was placed in a BioMark™ system in order to carry out real-time PCR. The specificity of each primer pair was checked for all cellular conditions by analysing the dissociation curves and the problematic points with double peaks were removed from the analysis. Each CT was then normalised by the reference genes GAPDH and YWHAZ and compared to the cell control condition to calculate ΔΔCT. Then the relative expression was calculated using the formula 2−ΔΔCT. The results are expressed as fold change. The targeted genes cover various functions, such as hair physiology, antioxidant activities and pigmentation pathways. To confirm an activating or inhibitory effect, the values were compared to the control treated with Carbopol® only.
2.5. Clinical Investigation
2.5.1. INCI Lotion Formula
Water, +/− 1% glycerin/acetyl tyrosine/sodium metabisulfite/Larix europae wood extract/glycine/zinc chloride/epigallocatechin gallatyl glucoside, sodium benzoate, citric acid, sodium citrate, PPG-26 buteth-26, PEG-40 hydrogenated castor oil, aqua/water, fragrance, butylphenyl, methylpropional, D-limonene, alpha-isomethylionone.
2.5.2. Panel Description
A double-blind, inter-individual and placebo-controlled clinical evaluation was performed on Caucasian male volunteers: 2 groups of 22 of subjects aged over 50 years with a proportion of 30% to 95% grey hair. The subjects agreed to keep a hair length greater than 2 cm throughout the study and to have a 1 cm2 zone on the scalp shaved. The study followed and was in compliance with the tenets of the Declaration of Helsinki.
One group of 22 volunteers tested the placebo product and a second group of 22 volunteers tested the hair lotion containing the active ingredient at 1% (v/v). The treatment was applied by massage on the scalp, once a day for 4 months.
Two clinical evaluation methods were carried out in order to demonstrate the in vivo efficacy of the active ingredient versus placebo.
2.5.3. Proportion of Grey Hair Assessment with the Scoring Method
The proportion of grey hair was assessed with the scoring method. Pictures of the scalp were taken using a Nikon D7100 in combination with the system Canfield Epiflash®, on the first day of the assay and after 4 months. A blind scoring was performed by experts to evaluate the proportion of grey hair in the picture. The experts used a pre-defined scale from 0 to 100% to estimate the proportion of grey hair. All data were expressed as mean ± standard deviation (SD).
2.5.4. Quantification of the Density of Grey Hair
The density of grey hair was assessed by counting the number of grey hairs in a specific and shaved area, giving a grey hair number per cm2 (hair cm−2) [18]. Briefly, a picture was taken two days after shaving a 1 cm2 area of the scalp at each appointment using a Nikon D7100 digital camera in combination with the system Canfield Epiflash® equipped with a contact lens (the contact lens allowed flattening of the hair on the scalp). A count of grey hairs was then done on images with a specific tool from Photoshop on a 0.7 cm2 test area (1 × 0.7 cm) defined in the image. All hairs with grey roots in the zone were counted. The size and the position of the studied area were the same for all evaluation times. In case of offset, the position of the test area was adjusted. All data were expressed as mean ± SD.
2.6. Statistical Analysis
All results are presented as mean ± standard error of the mean (SEM) of three independent triplicates. A Shapiro–Wilk test was used to verify whether the raw data followed the Gaussian law. In the case of normally distributed data, the mean values were compared using either an unpaired t-test (≤2 groups) or one-way ANOVA followed by a post hoc test (≥2 groups). In the case of non-normally distributed data, a Kruskal–Wallis test followed by a Mann–Whitney U test was used for unpaired data.
In all cases, results were considered as significant when p < 0.1 with #, p < 0.05 with *, p < 0.01 with ** and p < 0.001 with ***.
3. Results
3.1. Demonstration of Oxidative Stress Effect on Ex Vivo Models
A gene expression analysis was performed after repeated induction of oxidative stress on skin scalp explants. To explore the global effect of oxidative stress, we selected genes potentially responsible for canities development and involved in these three major functions: antioxidant activities, renewal, melanogenesis and related functions. In the category of renewal, we introduced hair cycle-related genes and genes involved in apoptosis and autophagy. The main objective of this study was to understand the impact of oxidative stress in the greying process that we hypothesised to be a key inducer. After the oxidative stress, the results in Figure 1 demonstrated a significant down-regulation of genes involved in autophagy (ATG7, MAP1LC3A). The significant down-regulation of KRT19, COX2, FST and BAX might describe a reduction of proliferation and the activation of stem cell quiescence. Moreover, the oxidative stress showed a clear effect on melanogenesis by down-regulating the expression of various genes directly linked to this synthesis (MITF, TYR, DKK1, EDN1) but also genes encoding for melanogenesis-related functions, such as melanosome formation and transfer (CTNS, HPS5, MLANA, MLPH, MYO5A, SLC24A5). The expression of the melanocyte precursor cell marker gene (DCT) was also significantly down-regulated. Finally, there was a significant negative impact on the expression of genes involved in antioxidant activities (FTL, GLRX, HMOX1, MGST1, PRDX1 and SOD2) even though the expression of GPX1 was activated. Overall, these results suggest that oxidative stress was able to induce a strong impact on renewal, pigmentation and antioxidant defence, which are the three main causes of canities development.
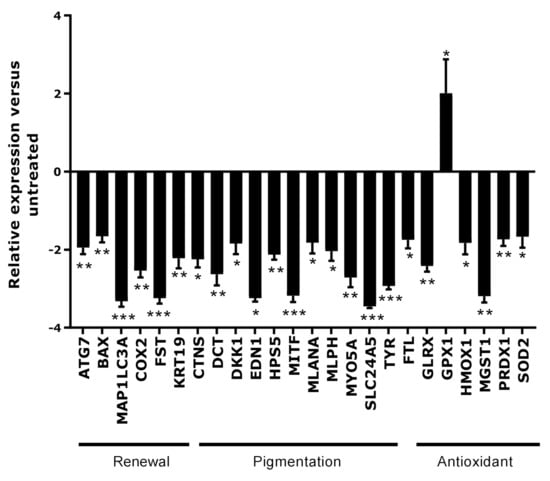
Figure 1.
Gene expression analysis in full scalp skin explants after oxidative stress. The results (mean ± SEM) are expressed as relative expression versus the untreated condition. Statistical analysis was performed using one-way ANOVA followed by Fisher’s t-test in comparison with the untreated condition, with * p < 0.05, ** p < 0.01 and *** p < 0.001.
Pursuing our investigation further, we attempted to induce oxidative stress in microdissected hair follicles in order to confirm the hypothesis proposed following the gene expression results. After the stress, we observed a significant production of reactive oxygen species (ROS) inside the hair follicles, 264% in comparison with the untreated condition (Figure 2). Moreover, we evaluated the number of NKI/beteb-positive cells to observe the effect of oxidative stress on the melanocyte reservoirs. The counting of the red fluorescent cells showed a significant decrease by 78% of the number of NKI/beteb-positive cells compared with the untreated condition (Figure 3).
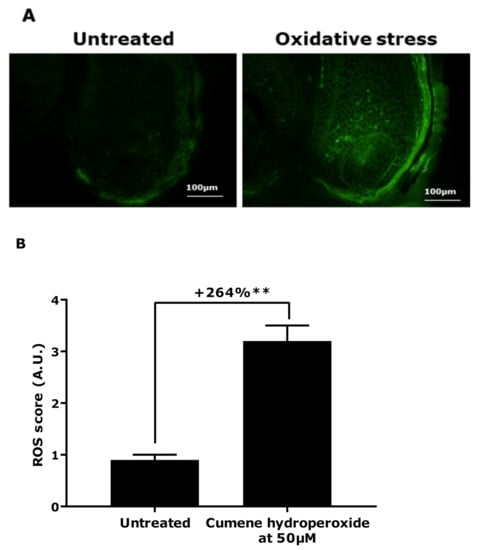
Figure 2.
Reactive oxygen species (ROS) production assessment following oxidative stress (cumene hydroperoxide at 50 µm) in hair follicles. (A) Representative images. Magnification: 20×. (B) Quantification of the mean fluorescence intensity (± SEM) of dichlorofluoerescein diacetate (DCFH-DA) probe. Statistical analysis was performed using one-way ANOVA with a permutation test followed by Tukey’s test, with ** p < 0.01.
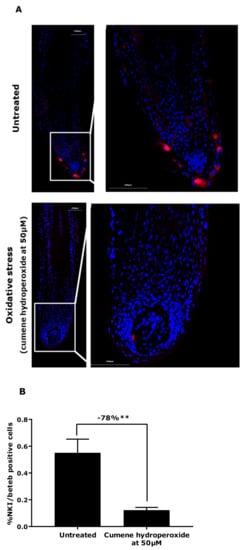
Figure 3.
Melanocyte and melanoblast quantification by NKI/beteb immunofluorescence staining following oxidative stress (cumene hydroperoxide at 50 µm) in hair follicles. (A) Representative images. Magnification: 10×. (B) Data are presented as mean ± SEM. Statistical analysis was performed using one-way ANOVA with permutation test followed by a t-test with permutation, with ** p < 0.01.
These results confirmed that oxidative stress triggers the redox balance, as observed with the reduction of gene expressions coding for antioxidant enzymes but also by the ROS release. As a consequence, it induced stem cell quiescence and the depletion of the melanocyte reservoirs, as demonstrated at the transcriptomic level and by the strong reduction of melanocyte detection. Finally, it also reduced the ability to synthesise melanin.
In this first part of this work, we evidenced that oxidative stress triggered biological dysregulations which are the main causes of canities and, therefore, demonstrated the key role of oxidative stress in its development. We used this canities representative model for further investigations.
3.2. Effect of Active Ingredient on Canities
The second step of our investigation was to identify whether our active ingredient was able to act globally on all pathways impacted by the process of canities. We evaluated the gene expression after incubation for 48 h with the active ingredient at 1% (v/v) on scalp skin explants under oxidative stress exposure mimicking the canities model. In Figure 4, the results demonstrated that after the treatment with the active ingredient, the genes encoding for melanogenesis were up-regulated (EDN1, MC1R, MITF, POMC). The formation of melanosomes and their transfer were reactivated, as a result of the up-regulation of AP3B1, CTNS, HPS5, KRT5 and MYO5A expressions. The expressions of genes involved in renewal (FST, KRT19) and autophagy (MAP1LC3A) were also up-regulated. Finally, the treatment with the active ingredient showed a significant stimulation of genes involved in antioxidant activities (HMOX1, GLRX, GSS, MGST1, NRF2 and TXN). These results suggested oxidation level control and boosting of the melanin synthesis pathway.
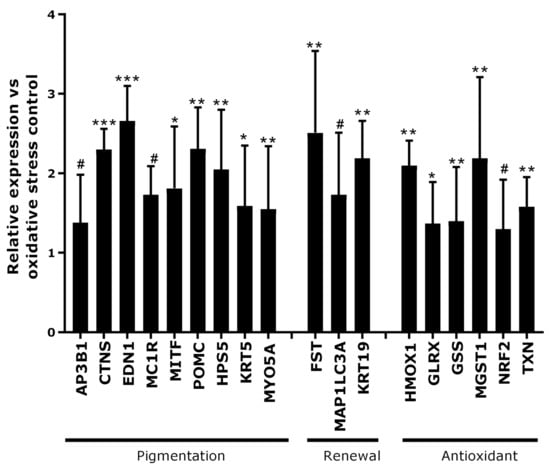
Figure 4.
Gene expression analysis in scalp skin explants in a canities-induced condition following treatment with the active ingredient at 1%. The results (mean ± SEM) are expressed as relative expression versus the oxidative stress control condition. Statistical analysis was performed using one-way ANOVA followed by Fisher’s t-test in comparison with oxidative stress control condition, with # p < 0.1, * p < 0.05, ** p < 0.01 and *** p < 0.001.
To further our work, we used the microdissected hair follicle canities-induced model to evaluate the effect of the active ingredient on redox balance control and melanocyte reservoir protection using ROS quantification and NKI/beteb immunostaining. In Figure 5, we observed that, in the presence of the active ingredient at 1%, the ROS production was significantly reduced by 54%. In Figure 6, the presence of the active ingredient demonstrated a significant protection of melanocyte reservoirs against oxidative stress, as observed with the increase in the NKI/beteb-positive cell number by 190%. These results confirmed that the active ingredient was able to neutralise the high level of oxidative stress and thus protected the melanocyte reservoirs in hair follicles.
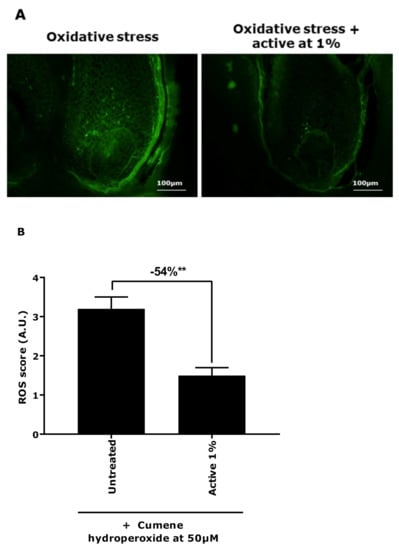
Figure 5.
ROS production assessment in hair follicles in a canities-induced condition (cumene hydroperoxide at 50 µm) in the presence of active ingredient at 1%. (A) Representative images. Magnification: 20×. (B) Quantification of the mean fluorescence intensity (± SEM) of DCFH-DA probe. Statistical analysis was performed using one-way ANOVA with permutation test followed by Tukey’s test, with ** p < 0.01.
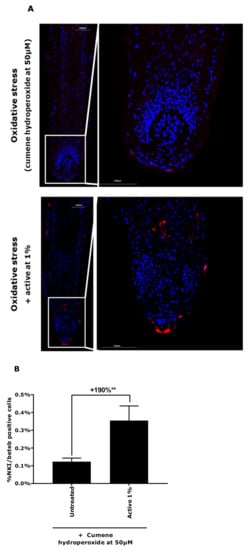
Figure 6.
Melanocyte and melanoblast quantification by NKI/beteb immunostaining in a canities-induced condition (cumene hydroperoxide at 50 µm) in the presence of active ingredient at 1% in hair follicles. (A) Representative images. Magnification: 10×. (B) Data are presented as mean ± SEM. Statistical analysis was performed using one-way ANOVA with permutation test followed by a t-test with permutation, with ** p < 0.01.
The final expectation of the active ingredient was for it to reactivate the pigmentation in a natural canities condition. The melanin content of grey hair follicles was evaluated by Fontana-Masson staining. In Figure 7, the results of melanin content quantification showed a significant increase in production of melanin by 15% after 3 days of treatment in presence of the active ingredient at 1%.
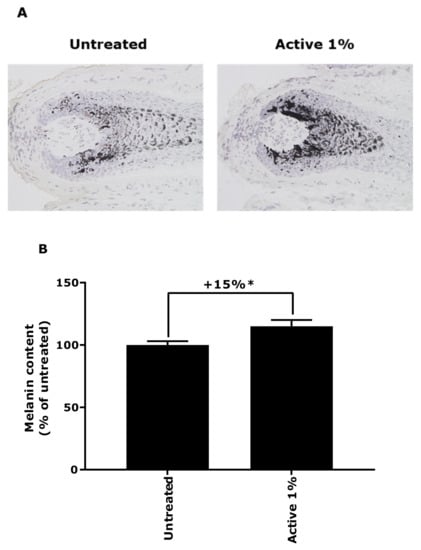
Figure 7.
Evaluation of melanin content by Fontana-Masson staining of grey hair follicles treated with active ingredient at 1%. (A) Representative images. Magnification: 200×. (B) Results expressed as percentage of untreated condition (data are presented as mean ± SEM). Statistical analysis was performed using a Student’s t-test, with * p < 0.05.
Thanks to these models developed to mimic canities, we demonstrated that our active ingredient was able to protect hair follicles against oxidative stress, preserve stem cells and melanocytes and reactivate melanogenesis. These first steps led to a total control of the main causes of canities development and promoted restoration of hair follicle pigmentation.
We then carried out a clinical study with double-blind and placebo-controlled conditions. A panel of 44 male volunteers presenting 30% to 95% of grey hair, randomly distributed in two groups, was used. In this study, the canities was firstly analysed by scoring the proportion of grey hair using a specific scale before and after product application. We can observe in Figure 8 that after 4 months of daily application, the proportion of grey hair was significantly reduced by the active product, showing −17%, while the placebo evidenced a lower efficacy, with only 8%. Moreover, we demonstrated that the active product was significantly more efficient than placebo, as observed by a significant 2.1-fold reduction of the proportion of grey hair after 4 months of application. The representative images in Figure 8 show this clear effect, observed with the net reduction of the proportion of grey hair after 4 months of application of active product.
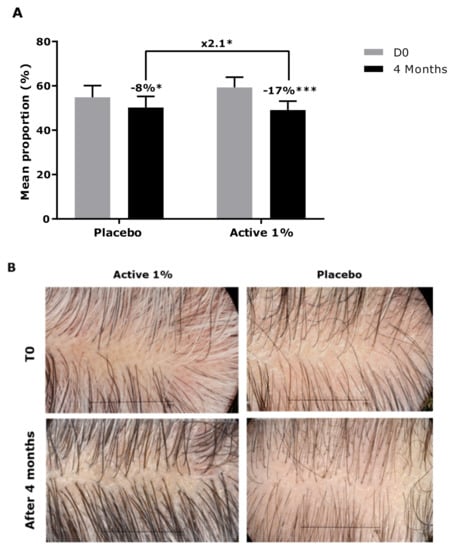
Figure 8.
(A) Scoring of the proportion of grey hair (%) after 4 months of application. Data are presented as mean ± SD (B) Representative images of a volunteer who applied the active ingredient at 1% (Volunteer 7) and a volunteer who applied placebo (Volunteer 2). Statistical analysis was performed using a mixed ANOVA, with * p < 0.05, *** p < 0.001.
The measure of grey hair density, which is a more quantitative method represented by the number of grey hairs per cm2, was performed on the volunteers. In Figure 9, the results showed that after 4 months of active product application, the number of grey hairs per cm2 was significantly reduced by 14%, while the placebo application evidenced only a slight and not significant reduction of 5%. Interestingly, the active product showed a better and significant reduction of grey hair density than the placebo, with an efficacy that was 2.8 times higher (Figure 9).
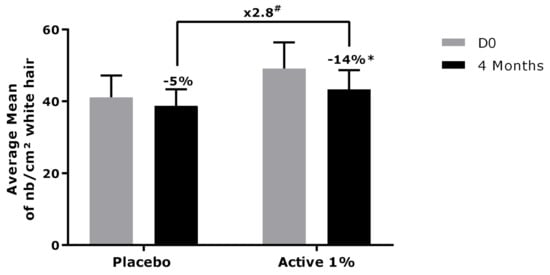
Figure 9.
Measurement of density of grey hair (number/cm2). Data are presented as mean ± SD. Statistical analysis was performed using a mixed ANOVA, with # p < 0.1, * p < 0.05 and ** p < 0.01.
Overall, these clinical data demonstrated that our active ingredient was able to reactivate the hair pigmentation process by significantly reducing the canities on volunteers after 4 months. The control of oxidative stress, the preservation of melanocyte reservoirs and the ability to reactivate melanogenesis triggered by our active ingredient were responsible for these clinical benefits, clearly proving the efficiency of this active ingredient against canities.
4. Discussion
Chronological ageing is well described today and the main cause is oxidative stress [19]. This stress is triggered by external and internal factors, such as UV, pollution, chemicals, cigarette smoke and psycho-emotional stress [9,20]. Recently, research on hair ageing has also pointed to oxidative stress as a major cause of “hair greying” [10,11,21]. In an attempt to understand the phenomenon of canities and offer a smart solution to prevent it, we designed ex vivo models and exposed them to oxidative stress in order to mimic this phenomenon. First, we repeatedly exposed scalp skin explants to free radicals to induce premature canities. After this exposure, we analysed the gene expressions and demonstrated that oxidative stress had clearly reduced the antioxidant defences of the cells by down-regulating the expression of numerous genes. The expressions of genes involved in autophagy and stemness, which are also known to play a role in perturbation of skin pigmentation, were also disturbed [22,23,24]. The expression of major actors (DKK1, TYR, EDN1) of melanogenesis was significantly down-regulated, especially the expression of DCT and MITF, which are important during the differentiation of melanogenically active melanocytes [2,25]. Interestingly, it has also been evidenced that MITF has a link with the maintenance of stemness in melanocyte precursors [3,26]. This result confirmed the observations made for COX2, FST and KRT19 in our own work but also the work of Shi and colleagues, showing a down-regulation of KRT19 in unpigmented hair [13]. We also confirmed the results obtained by Tang and colleagues, by showing that the genes encoding for melanosome formation and transfer (CTNS, HPS5, MLPH, SLC24A5) were disturbed with oxidative stress, especially MYO5A [1,14]. Unfortunately, we did not find any significant modulation of KIT or Bcl-2 or Pmel17/gp-100, which are widely described as essential in human hair pigmentation [1,2,11,19,25].
The fine regulation of redox balance is crucial in melanocytes and the slightest deviation could disturb melanogenesis and trigger apoptosis [10,14]. Exposition of hair follicles to oxidative stress allowed us to work on a robust model of premature canities. The exposure to oxidative stress triggered a higher oxidation level, evidenced by the induction of ROS production. This stress had a major impact on hair follicles and the consequence was the depletion of melanocyte and melanoblast reservoirs, as observed with the NKI/beteb staining, which is already evidenced in the literature [4].
On the basis of these results, we designed a smart combination of molecules to act on the key players of hair pigmentation during canities. The glucosylation of taxifolin (dihydroquercetin, DHQ) and epigallocatechin gallate (EGCG) provided better solubility to aglycones. In this work, we evaluated the efficacy of this active ingredient on the main causes of canities development. Indeed, these compounds are well known for their ROS-scavenging property. We hypothesised that their antioxidant property could play a major role in canities development since oxidative stress plays a central role in its induction. Interestingly, it is described in the literature that antioxidants can preserve stemness by controlling redox homeostasis and maintaining a low level of ROS. Moreover, we have already demonstrated that antioxidant compounds can preserve the pool of stem cells by reducing cell apoptosis and stress induced by the oxygen level [27]. We proposed the hypothesis that our active ingredient could also protect melanocyte stem cells against oxidative stress and thereby control hair pigmentation [21,27,28,29,30]. In addition, a precursor for melanin synthesis was added to boost and potentialise the reactivation of melanogenesis.
In this work, we demonstrated that this smart active ingredient can reactivate the hair pigmentation mechanism and fight against canities by acting at various levels. We showed, thanks to mRNA analysis, that the active ingredient was able to stimulate the expression of the main regulators of melanogenesis (MC1R, MITF, POMC) and related genes (AP3B1, CTNS, HPS5, MYO5A) in scalp skin explants exposed to oxidative stress, mimicking the canities model. Melanosome formation and autophagy were also reactivated. The induction of FST and KRT19 expressions, both involved in hair morphogenesis [31], suggests a renewal and protection of the stem cell reservoirs [13,17]. Moreover, the expressions of antioxidant enzymes were up-regulated. In order to confirm these first observations, the active ingredient was then assessed in the premature canities-induced hair follicle model. Hair follicles are considered as an ex vivo model [17,21], and a major route for substance penetration [32,33]. Indeed, it is described that the hair follicle plays a subordinate role in percutaneous penetration as it allows a major proportion of global skin penetration [34]. Consequently, the active ingredient was tested at 1%, the same concentration as those for an in vivo application since it was clearly the most optimal concentration to use. As expected, the active ingredient demonstrated ROS scavenging activity by significantly reducing the ROS production. This clear reduction can also be correlated to the results observed with the mRNA analysis that showed an increase in gene expressions encoding for antioxidant enzymes. Moreover, the number of positive cells stained by NKI/beteb, specific marker of active melanocytes and melanoblasts, was significantly increased in the presence of the active ingredient. This result suggests that the melanocyte reservoirs were thus protected, which is also important for hair follicle renewal [17]. Therefore, we can confirm that, thanks to the scavenging property of the active ingredient, the pool of melanocyte precursors was preserved. In this way, one of main causes of canities development was controlled.
Finally, we conducted experiments to evaluate the effect of our active ingredient on grey hair follicles, which is the final proof of the control of canities development. We demonstrated with Fontana-Masson staining that the melanin content was significantly increased in natural grey hair follicles, proving the global reactivation of canities-impaired mechanisms, including oxidative stress control, stem cell protection and melanogenesis. After this validation, we carried out a clinical study to finally evaluate the active ingredient and confirmed its activity on male volunteers presenting 30 to 95% grey hair. We used two complementary studies: the evaluation of grey hair proportion as a semi-qualitative method and the grey hair density per cm2 measurement as a quantitative one. The results presented a clear and significant reduction of the proportion of grey hair and the effect was significantly higher than the placebo. A second method of measurement demonstrated that the density of grey hair per cm2 was significantly reduced. Overall, these clinical data confirmed the efficacy of our active ingredient against canities by reactivating hair pigmentation.
5. Conclusions
Oxidative stress is the major inducer of the cascade of events leading to canities. We designed ex vivo models to reproduce this phenomenon and better understand the pathways involved in its emergence, thus allowing the confirmation of various work. We designed an active complex to counteract the main causes of canities induction. The complex demonstrated the stimulation of melanin synthesis by controlling the redox balance and protecting the melanocyte reservoirs in hair follicles. At the clinical level, it allowed hair repigmentation after only 4 months of twice daily application. Our work demonstrated that the control of oxidative stress, which leads to an efficient preservation of melanocytes stem cell reservoirs, combined with melanogenesis stimulation are two key essential factors in reactivating hair pigmentation, leading to a drastic reduction of canities.
6. Patents
EP2027279B1, Daniel Auriol, Renaud Nalin, Patrick Robe and Fabrice Lefevre 2006.
Author Contributions
Conceptualisation, R.R. and A.S.; methodology, D.A. and M.D.T.; validation, A.S. and M.D.T.; investigation, P.A. and E.C.; writing—original draft preparation, M.D.T.; writing—review and editing, M.D.T. and A.S.; project administration, R.R. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted according to the ethical guidelines of the Declaration of Helsinki. This study, performed on cosmetic products coming within the definition of article L. 5131-1 of the French Public Health Code is in accordance with Decree n° 2017-884 of May 9, 2017 modifying some regulatory requirements concerning researches involving human subjects.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study. Written informed consent has been obtained from the patient(s) to publish this paper.
Data Availability Statement
Data available on request due to restrictions e.g., privacy or ethical. The data presented in this study are available on request from the corresponding author. The data are not publicly available due to GDPR compliance.
Acknowledgments
The authors would like to thank, in particular, Claire Leduc and her team from Syntivia (Toulouse, France) for their involvement and support in this project. They would also like to thank Marta Bertolini and her team from Monasterium Laboratory (Münster, Germany) for their help in conducting these studies and for meeting short deadlines.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Tobin, D.J. The cell biology of human hair follicle pigmentation. Pigment Cell Melanoma Res. 2010, 24, 75–88. [Google Scholar] [CrossRef] [PubMed]
- Tobin, D.J. Human hair pigmentation biological aspects. Int. J. Cosmet. Sci. 2008, 30, 233–257. [Google Scholar] [CrossRef]
- Qiu, W.; Chuong, C.-M.; Lei, M. Regulation of melanocyte stem cells in the pigmentation of skin and its appendages: Biological patterning and therapeutic potentials. Exp. Dermatol. 2019, 28, 395–405. [Google Scholar] [CrossRef]
- Tobin, D.; Paus, R. Graying: Gerontobiology of the hair follicle pigmentary unit. Exp. Gerontol. 2001, 36, 29–54. [Google Scholar] [CrossRef]
- Slominski, A.; Paus, R. Melanogenesis Is Coupled to Murine Anagen: Towar B New Concepts for the Role of Melanocytes and the Regulation of Melanogenesis in Hair Growth. J. Investig. Dermatol. 1993, 101, 8. [Google Scholar] [CrossRef]
- Li, H.; Hou, L. Regulation of melanocyte stem cell behavior by the niche microenvironment. Pigment Cell Melanoma Res. 2018, 31, 556–569. [Google Scholar] [CrossRef]
- Commo, S.; Gaillard, O.; Bernard, B.A. Human hair greying is linked to a specific depletion of hair follicle melanocytes affecting both the bulb and the outer root sheath. Br. J. Dermatol. 2004, 150, 435–443. [Google Scholar] [CrossRef] [PubMed]
- Singal, A.; Daulatabad, D.; Grover, C.; Sharma, S.B.; Chhillar, N. Assessment of oxidative stress in patients with premature canities. Int. J. Trichology 2015, 7, 91. [Google Scholar] [CrossRef]
- Pandhi, D.; Khanna, D. Premature graying of hair. Indian J. Dermatol. Venereol. Leprol. 2013, 79, 641. [Google Scholar] [CrossRef] [PubMed]
- Wood, J.M.; Decker, H.; Hartmann, H.; Chavan, B.; Rokos, H.; Spencer, J.D.; Hasse, S.; Thornton, M.J.; Shalbaf, M.; Paus, R.; et al. Senile hair graying: H2O2-mediated oxidative stress affects human hair color by blunting methionine sulfoxide repair. FASEB J. 2009, 23, 2065–2075. [Google Scholar] [CrossRef]
- Arck, P.C.; Overall, R.; Spatz, K.; Liezman, C.; Handjiski, B.; Klapp, B.F.; Birch-Machin, M.A.; Peters, E.M.J. Towards a “free radical theory of graying”: Melanocyte apoptosis in the aging human hair follicle is an indicator of oxidative stress induced tissue damage. FASEB J. 2006, 20, 1567–1569. [Google Scholar] [CrossRef] [PubMed]
- Trüeb, R.M. The impact of oxidative stress on hair. Int. J. Cosmet. Sci. 2015, 37, 25–30. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Luo, L.-F.; Liu, X.-M.; Zhou, Q.; Xu, S.-Z.; Lei, T.-C. Premature Graying as a Consequence of Compromised Antioxidant Activity in Hair Bulb Melanocytes and Their Precursors. PLoS ONE 2014, 9, e93589. [Google Scholar] [CrossRef][Green Version]
- Tang, L.; Li, J.; Lin, X.; Wu, W.; Kang, K.; Fu, W. Oxidation Levels Differentially Impact Melanocytes: Low versus High Concentration of Hydrogen Peroxide Promotes Melanin Synthesis and Melanosome Transfer. Dermatology 2012, 224, 145–153. [Google Scholar] [CrossRef]
- Jiménez-Cervantes, C.; Martinez-Esparza, M.; Pérez, C.; Daum, N.; Solano, F.; Garcia-Borron, J.C. Melanogenesis inhibition by oxidative stress: Transient downregulation of melanocyte differentiation markers and possible involvement of microphthalmia transcription factor. J. Cell Sci. 2001, 114, 2335–2344. [Google Scholar]
- Paul, F.; Auriol, D.; Oriol, E.; Monsan, P. Production and Purification of Dextransucrase from Leuconostoc mesenteroides, NRRL B 512 (F). Ann. N. Y. Acad. Sci. 1984, 434, 267–270. [Google Scholar] [CrossRef]
- Langan, E.A.; Philpott, M.P.; Kloepper, J.E.; Paus, R. Human hair follicle organ culture: Theory, application and perspectives. Exp. Dermatol. 2015, 24, 903–911. [Google Scholar] [CrossRef] [PubMed]
- Singal, A.; Daulatabad, D.; Grover, C. Graying severity score: A useful tool for evaluation of premature canities. Indian Dermatol. Online J. 2016, 7, 164. [Google Scholar] [CrossRef]
- Triwongwaranat, D.; Thuangtong, R.; Arunkajohnsak, S. A review of the etiologies, clinical characteristics, and treatment of canities. Int. J. Dermatol. 2019, 58, 659–666. [Google Scholar] [CrossRef]
- Krutmann, J.; Bouloc, A.; Sore, G.; Bernard, B.A.; Passeron, T. The skin aging exposome. J. Dermatol. Sci. 2017, 85, 152–161. [Google Scholar] [CrossRef]
- O’Sullivan, J.D.B.; Nicu, C.; Picard, M.; Chéret, J.; Bedogni, B.; Tobin, D.J.; Paus, R. The biology of human hair greying. Biol. Rev. 2021, 96, 107–128. [Google Scholar] [CrossRef] [PubMed]
- Qiao, Z.; Wang, X.; Xiang, L.; Zhang, C. Dysfunction of Autophagy: A Possible Mechanism Involved in the Pathogenesis of Vitiligo by Breaking the Redox Balance of Melanocytes. Oxidative Med. Cell. Longev. 2016, 2016, 1–7. [Google Scholar] [CrossRef]
- Setaluri, V. Autophagy as a Melanocytic Self-Defense Mechanism. J. Investig. Dermatol. 2015, 135, 1215–1217. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.-F.; Gruber, F.; Ni, C.; Mildner, M.; Koenig, U.; Karner, S.; Barresi, C.; Rossiter, H.; Narzt, M.-S.; Nagelreiter, I.M.; et al. Suppression of Autophagy Dysregulates the Antioxidant Response and Causes Premature Senescence of Melanocytes. J. Investig. Dermatol. 2015, 135, 1348–1357. [Google Scholar] [CrossRef]
- Samuelov, L.; Sprecher, E.; Sugawara, K.; Singh, S.K.; Tobin, D.J.; Tsuruta, D.; Bíró, T.; Kloepper, J.E.; Paus, R. Topobiology of Human Pigmentation: P-Cadherin Selectively Stimulates Hair Follicle Melanogenesis. J. Investig. Dermatol. 2013, 133, 1591–1600. [Google Scholar] [CrossRef]
- Adhikari, K.; Fontanil, T.; Cal, S.; Mendoza-Revilla, J.; Fuentes-Guajardo, M.; Chacón-Duque, J.-C.; Al-Saadi, F.; Johansson, J.A.; Quinto-Sanchez, M.; Acuña-Alonzo, V.; et al. A genome-wide association scan in admixed Latin Americans identifies loci influencing facial and scalp hair features. Nat. Commun. 2016, 7. [Google Scholar] [CrossRef]
- Meunier, M.; Scandolera, A.; Chapuis, E.; Lambert, C.; Jarrin, C.; Robe, P.; Chajra, H.; Auriol, D.; Reynaud, R. From stem cells protection to skin microbiota balance: Orobanche rapum extract, a new natural strategy. J. Cosmet. Dermatol. 2019, 18, 1140–1154. [Google Scholar] [CrossRef]
- Liao, N.; Shi, Y.; Zhang, C.; Zheng, Y.; Wang, Y.; Zhao, B.; Zeng, Y.; Liu, X.; Liu, J. Antioxidants inhibit cell senescence and preserve stemness of adipose tissue-derived stem cells by reducing ROS generation during long-term in vitro expansion. Stem Cell Res. Ther. 2019, 10. [Google Scholar] [CrossRef] [PubMed]
- Xican, L.; Xie, H.; Jiang, Q.; Wei, G.; Lin, L.; Li, C.; Ou, X.; Yang, L.; Xie, Y.; Fu, Z.; et al. The mechanism of (+) taxifolin’s protective antioxidant effect for •OH-treated bone marrow-derived mesenchymal stem cells. Cell. Mol. Biol. Lett. 2017, 22. [Google Scholar] [CrossRef]
- Petruk, G.; Del Giudice, R.; Rigano, M.M.; Monti, D.M. Antioxidants from Plants Protect against Skin Photoaging. Oxidative Med. Cell. Longev. 2018, 2018, 1–11. [Google Scholar] [CrossRef]
- Ma, G.-W.; Chu, Y.-K.; Zhang, W.-J.; Qin, F.-Y.; Xu, S.-S.; Yang, H.; Rong, E.-G.; Du, Z.-Q.; Wang, S.-Z.; Li, H.; et al. Polymorphisms of FST gene and their association with wool quality traits in Chinese Meri sheep. PLoS ONE 2017, 12, e0174868. [Google Scholar] [CrossRef]
- Chandrasekaran, N.C.; Sanchez, W.Y.; Mohammed, Y.H.; Grice, J.E.; Roberts, M.S.; Barnard, R.T. Permeation of topically applied Magnesium ions through human skin is facilitated by hair follicles. Magnes. Res. 2016, 29, 35–42. [Google Scholar] [CrossRef] [PubMed]
- Illel, B.; Schaefer, H.; Wepierre, J.; Doucet, O. Follicles Play an Important Role in Percutaneous Absorption. J. Pharm. Sci. 1991, 80, 424–427. [Google Scholar] [CrossRef] [PubMed]
- Otberg, N.; Patzelt, A.; Rasulev, U.; Hagemeister, T.; Linscheid, M.; Sinkgraven, R.; Sterry, W.; Lademann, J. The role of hair follicles in the percutaneous absorption of caffeine. Br. J. Clin. Pharmacol. 2008, 65, 488–492. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).