The Role of Cholinesterases in Post-Exercise HRV Recovery in University Volleyball Players
Abstract
1. Introduction
2. Materials and Methods
2.1. Headings
2.2. Subjects
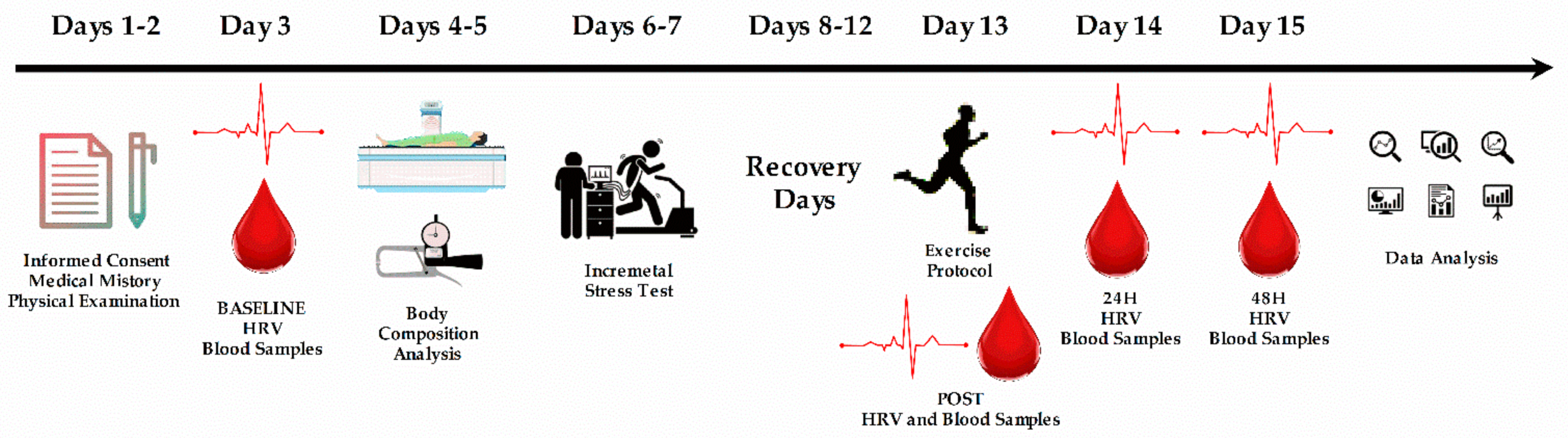
2.3. Procedure
2.4. Blood Samples
2.5. Exercise Protocol
2.6. Cholinesterases
2.7. Heart Rate Variability
2.8. Statistical Analysis
3. Results
4. Discussion
4.1. Limitations
4.2. Practical Applications
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bourdon, P.C.; Cardinale, M.; Murray, A.; Gastin, P.; Kellmann, M.; Varley, M.C.; Gabbett, T.J.; Coutts, A.J.; Burgess, D.J.; Gregson, W.; et al. Monitoring athlete training loads: Consensus statement. Int. J. Sports Physiol. Perform. 2017, 12, S2161–S2170. [Google Scholar] [CrossRef]
- Halson, S.L. Monitoring training load to understand fatigue in athletes. Sports Med. 2014, 44 (Suppl. 2), S139–S147. [Google Scholar] [CrossRef] [PubMed]
- Buchheit, M. Monitoring training status with HR measures: Do all roads lead to Rome? Front. Physiol. 2014, 5, 73. [Google Scholar] [CrossRef] [PubMed]
- Michael, S.; Graham, K.S.; Davis, G.M. Cardiac autonomic responses during exercise and post-exercise recovery using heart rate variability and systolic time intervals—A review. Front. Physiol. 2017, 8, 301. [Google Scholar] [CrossRef] [PubMed]
- Stanley, J.; Peake, J.M.; Buchheit, M. Cardiac parasympathetic reactivation following exercise: Implications for training prescription. Sports Med. 2013, 43, 1259–1277. [Google Scholar] [CrossRef]
- Camm, A.J.; Malik, M.; Bigger, J.T.; Breithardt, G.; Cerutti, S.; Cohen, R.J.; Coumel, P.; Fallen, E.L.; Kennedy, H.L.; Kleiger, R.E.; et al. Heart rate variability: Standards of measurement, physiological interpretation, and clinical use. Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology. Circulation 1996, 93, 1043–1065. [Google Scholar]
- Borresen, J.; Lambert, M.I. Autonomic control of heart rate during and after exercise—Measurements and implications for monitoring training status. Sports Med. 2008, 38, 633–646. [Google Scholar] [CrossRef]
- Daanen, H.A.M.; Lamberts, R.P.; Kallen, V.L.; Jin, A.; Van Meeteren, N.L.U. A systematic review on heart-rate recovery to monitor changes in training status in athletes. Int. J. Sports Physiol. Perform. 2012, 7, 251–260. [Google Scholar] [CrossRef]
- Wallace, L.K.; Slattery, K.M.; Coutts, A.J. A comparison of methods for quantifying training load: Relationships between modelled and actual training responses. Eur. J. Appl. Physiol. 2014, 114, 11–20. [Google Scholar] [CrossRef]
- Plews, D.J.; Laursen, P.B.; Kilding, A.E.; Buchheit, M. Training adaptation and heart rate variability in elite endurance athletes—Opening the door to effective monitoring. Sports Med. 2013, 43, 773–781. [Google Scholar] [CrossRef]
- Naranjo Orellana, J.; de la Cruz Torres, B.; Sarabia Cachadiña, E.; de Hoyo, M.; Domínguez Cobo, S. Two new indexes for the assessment of autonomic balance in elite soccer players. Int. J. Sports Physiol. Perform. 2015, 10, 452–457. [Google Scholar] [CrossRef]
- Goldberger, J.J.; Le, F.K.; Lahiri, M.; Kannankeril, P.J.; Ng, J.; Kadish, A.H. Assessment of parasympathetic reactivation after exercise. Am. J. Physiol. Heart Circ. Physiol. 2006, 290, H2446–H2452. [Google Scholar] [CrossRef]
- Miranda-Mendoza, J.; Reynoso-Sánchez, L.F.; Hoyos-Flores, J.R.; Quezada-Chacón, J.T.; Naranjo, J.; Rangel-Colmenero, B.; Hernández-Cruz, G. Stress score and LnrMSSD as internal load parameters during competition. Rev. Int. Med. Cienc. Act. Fís. Deporte 2020, 20, 21–35. [Google Scholar]
- Saboul, D.; Balducci, P.; Millet, G.; Pialoux, V.; Hautier, C. A pilot study on quantification of training load: The use of HRV in training practice. Eur. J. Sport. Sci. 2015, 16, 172–181. [Google Scholar] [CrossRef]
- McLaren, S.J.; Macpherson, T.W.; Coutts, A.J.; Hurst, C.; Spears, I.R.; Weston, M. The relationships between internal and external measures of training load and intensity in team sports. A Meta-Analysis. Sports Med. 2018, 48, 641–658. [Google Scholar] [CrossRef]
- Ahmadian, M.; Roshan, V.D.; Hosseinzadeh, M. Parasympathetic reactivation in children: Influence of two various modes of exercise. Clin. Auton. Res. 2015, 25, 207–212. [Google Scholar] [CrossRef]
- Buchheit, M.; Laursen, P.B.; Ahmaidi, S. Parasympathetic reactivation after repeated sprint exercise. Am. J. Physiol. Heart Circ. Physiol. 2007, 293, H133–H141. [Google Scholar] [CrossRef]
- Noma, A.; Trautwein, W. Relaxation of the ACh-induced potassium current in the rabbit sinoatrial node cell. Pflug. Arch. 1978, 377, 193–200. [Google Scholar] [CrossRef]
- Osterrieder, W.; Noma, A.; Trautwein, W. On the kinetics of the potassium channel activated by acetylcholine in the S-A node of the rabbit heart. Pflug. Arch. 1980, 386, 101–109. [Google Scholar] [CrossRef]
- Dewland, T.A.; Androne, A.S.; Lee, F.A.; Lampert, R.J.; Katz, S.D. Effect of acetylcholinesterase inhibition with pyridostigmine on cardiac parasympathetic function in sedentary adults and trained athletes. Am. J. Physiol. Heart Circ. Physiol. 2007, 293, H86–H92. [Google Scholar] [CrossRef]
- Taylor, P. Acetylcholinesterase Agents. In Goodman and Gilman’s The Pharmacological Basis of Therapeutics, 13th ed.; Brunton, L., Hilal-Dandan, R., Knollmann, B.C., Eds.; McGraw-Hill Education: New York, NY, USA, 2017; pp. 163–175. [Google Scholar]
- Akselrod, S.; Gordon, D.; Madwed, J.B.; Snidman, N.C.; Shannon, D.C.; Cohen, R.J. Hemodynamic regulation: Investigation by spectral analysis. Am. J. Physiol. Heart Circ. Physiol. 1985, 249, H867–H875. [Google Scholar] [CrossRef]
- Levy, M.N. Brief Reviews: Sympathetic-parasympathetic Interactions in the heart. Circ. Res. 1971, 29, 437–445. [Google Scholar] [CrossRef]
- Nóbrega, A.C.L.; dos Reis, A.F.; Moraes, R.S.; Bastos, B.G.; Ferlin, E.L.; Ribeiro, J.P. Enhancement of heart rate variability by cholinergic stimulation with pyridostigmine in healthy subjects. Clin. Auton. Res. 2001, 11, 11–17. [Google Scholar] [CrossRef]
- Zarei, A.; Foroutan, S.; Foroutan, S.; Erfanian Omidvar, A. Enhancement of Frequency Domain Indices of Heart Rate Variability by Cholinergic Stimulation with Pyridostigmine Bromide. Iran. J. Pharm. Res. 2011, 10, 889–894. [Google Scholar]
- Soreq, H.; Seidman, S. Acetylcholinesterase—New roles for an old actor. Nat. Rev. Neurosci. 2001, 2, 294–302. [Google Scholar] [CrossRef]
- Ofek, K.; Krabbe, K.S.; Evron, T.; Debecco, M.; Nielsen, A.R.; Brunnsgaad, H.; Yirmiya, R.; Soreq, H.; Pedersen, B.K. Cholinergic status modulations in human volunteers under acute inflammation. J. Mol. Med. 2007, 85, 1239–1251. [Google Scholar] [CrossRef]
- Shenhar-Tsarfaty, S.; Berliner, S.; Bornstein, N.M.; Soreq, H. Cholinesterases as biomarkers for parasympathetic dysfunction and inflammation-related disease. J. Mol. Neurosci. 2014, 53, 298–305. [Google Scholar] [CrossRef]
- Zimmer, K.R.; Lencina, C.L.; Zimmer, A.R.; Thiesen, F.V. Influence of physical exercise and gender on acetylcholinesterase and butyrylcholinesterase activity in human blood samples. Int. J. Environ. Health Res. 2012, 22, 279–286. [Google Scholar] [CrossRef] [PubMed]
- Chamera, T.; Spieszny, M.; Klocek, T.; Kostrzewa-Nowak, D.; Nowak, R.; Lachowicz, M.; Buryta, R.; Ficek, K.; Eider, J.; Moska, W.; et al. Post-Effort Changes in Activity of Traditional Diagnostic Enzymatic Markers in Football Players’ Blood. J. Med. Biochem. 2015, 34, 179–190. [Google Scholar] [CrossRef] [PubMed]
- World Medical Association. World Medical Association Declaration of Helsinki: Ethical principles for medical research involving human subjects. JAMA 2013, 310, 2191–2194. [Google Scholar] [CrossRef] [PubMed]
- Stewart, A.; Marfell-Jones, M.; Olds, T.; De Ridder, H. International Standards for Anthropometric Assessment (ISAK); IAAS: Lower Hutt, New Zealand, 2011. [Google Scholar]
- Skinner, J.S.; McLellan, T.H. The transition from aerobic to anaerobic metabolism. Res. Q. Exerc. Sport 1980, 51, 234–248. [Google Scholar] [CrossRef]
- Nicholas, C.W.; Nuttall, F.E.; Williams, C. The Loughborough Intermittent Shuttle Test: A field test that simulates the activity pattern of soccer. J. Sports Sci. 2000, 18, 97–104. [Google Scholar] [CrossRef]
- Hopkins, W.G.; Marshall, S.W.; Batterham, A.M.; Hanin, J. Progressive statistics for studies in sports medicine and exercise science. Med. Sci. Sports Exerc. 2009, 41, 3–13. [Google Scholar] [CrossRef]
- Kannankeril, P.J.; Goldberger, J.J. Parasympathetic effects on cardiac electrophysiology during exercise and recovery. Am. J. Physiol. Heart Circ. Physiol. 2002, 282, H2091–H2098. [Google Scholar] [CrossRef]
- Kannankeril, P.J.; Le, F.K.; Kadish, A.H.; Goldberger, J.J. Parasympathetic effects on heart rate recovery after exercise. J. Investig. Med. 2004, 52, 394–401. [Google Scholar] [CrossRef]
- Das, U.N. Acetylcholinesterase and butyrylcholinesterase as possible markers of low-grade systemic inflammation. Med. Sci. Monit. 2007, 13, RA214–RA221. [Google Scholar]
- Reale, M.; Costantini, E.; Di Nicola, M.; D’Angelo, C.; Franchi, S.; D’Aurora, M.; Di Bari, M.; Orlando, V.; Galizia, S.; Ruggieri, S.; et al. Butyrylcholinesterase and acetylcholinesterase polymorphisms in multiple sclerosis patients: Implication in peripheral inflammation. Sci. Rep. 2018, 8, 1319. [Google Scholar] [CrossRef]
- Masuda, Y.; Kawamura, A. Acetylcholinesterase inhibitor (donepezil hydrochloride) reduces heart rate variability. J. Cardiovasc. Pharmacol. 2003, 41, S67–S71. [Google Scholar]
- Jalife, J.; Slenter, V.A.; Salata, J.J.; Michaels, D.C. Dynamic vagal control of pacemaker activity in the mammalian sinoatrial node. Circ. Res. 1983, 52, 642–656. [Google Scholar] [CrossRef]
- Seiler, S.; Haugen, O.; Kuffel, E. Autonomic recovery after exercise in trained athletes: Intensity and duration effects. Med. Sci. Sports Exerc. 2007, 39, 1366–1373. [Google Scholar] [CrossRef]
- Casonatto, J.; Tinucci, T.; Dourado, A.C.; Polito, M. Cardiovascular and autonomic responses after exercise sessions with different intensities and durations. Clinics 2011, 66, 453–458. [Google Scholar] [CrossRef] [PubMed]
- Naranjo, J.; La Cruz, B.; de Sarabia, E.; Hoyo, M.; de Domínguez-Cobo, S. Heart rate variability. A follow-up in elite soccer players throughout the season. Int. J. Sports Med. 2015, 36, 881–886. [Google Scholar] [CrossRef] [PubMed]
- Nieto-Jiménez, C.; Pardos-Mainer, E.; Ruso-Álvarez, J.F.; Naranjo-Orellana, J. Training Load and HRV in a Female Athlete: A Case Study. Rev. Int. Med. Cienc. Act. Fís. Deporte 2020, 20, 321–333. [Google Scholar]
- Proietti, R.; Di Fronso, S.; Pereira, L.A.; Bortoli, L.; Robazza, C.; Nakamura, F.Y.; Bertollo, M. Heart rate variability discriminates competitive levels in professional soccer players. J. Strength Cond. Res. 2017, 31, 1719–1725. [Google Scholar] [CrossRef]
- Corrales, M.M.; de la Cruz Torres, B.; Garrido Esquivel, A.; Garrido Salazar, M.A.; Naranjo Orellana, J. Normal values of heart rate variability at rest in a young, healthy and active mexican population. Health 2012, 4, 377–385. [Google Scholar] [CrossRef]
- Al Haddad, H.; Laursen, P.B.; Ahmaidi, S.; Buchheit, M. Nocturnal heart rate variability following supramaximal intermittent exercise. Int. J. Sport Physiol. Perform. 2009, 4, 435–447. [Google Scholar]
- Schmitt, L.; Regnard, J.; Millet, G.P. Monitoring fatigue status with HRV measures in elite athletes: An avenue beyond RMSSD? Front. Physiol. 2015, 6, 343. [Google Scholar] [CrossRef]
- Naranjo, J. Variabilidad de la Frecuencia Cardíaca: Fundamentos y Aplicaciones a la Actividad Física y el Deporte; Fénix Editora: Sevilla, Spain, 2018. [Google Scholar]
- Cruz, G.H.; Orellana, J.N.; Taraco, A.R.; Colmenero, B.R. Leukocyte populations are associated with heart rate variability after a triathlon. J. Hum. Kinet. 2016, 54, 55–63. [Google Scholar] [CrossRef]
- DeBlauw, J.A.; Crawford, D.A.; Kurtz, B.K.; Drake, N.B.; Heinrich, K.M. Evaluating the Clinical Utility of Daily Heart Rate Variability Assessment for Classifying Meaningful Change in Testosterone-to-Cortisol Ratio: A Preliminary Study. Int. J. Exerc. Sci. 2021, 14, 260–273. [Google Scholar]
- Weippert, M.; Behrens, M.; Mau-Moeller, A.; Bruhn, S.; Behrens, K. Relationship between morning heart rate variability and creatine kinase response during intensified training in recreational endurance athletes. Front. Physiol. 2018, 9, 1267. [Google Scholar] [CrossRef]
- Campbell, G.D.; Edwards, F.R.; Hirst, G.D.; O’shea, J.E. Effects of vagal stimulation and applied acetylcholine on pacemaker potentials in the guinea-pig heart. J. Physiol. 1989, 415, 57–68. [Google Scholar] [CrossRef]
- Dong, J.G. The role of heart rate variability in sports physiology. Exp. Ther. Med. 2016, 11, 1531–1536. [Google Scholar] [CrossRef]
- Coote, J.H. Recovery of heart rate following intense dynamic exercise. Exp. Physiol. 2010, 95, 431–440. [Google Scholar] [CrossRef]
- Pierpont, G.L.; Voth, E.J. Assessing autonomic function by analysis of heart rate recovery from exercise in healthy subjects. Am. J. Cardiol. 2004, 94, 64–68. [Google Scholar] [CrossRef]

| Variable | M | SD | CV (%) | |
|---|---|---|---|---|
| Blocks 1–5 | Total distance (m) | 7500 | 0 | 0 |
| Mean speed (km·h−1) | 8.7 | 0.8 | 9.1 | |
| VT2 speed (km·h−1) | 14.9 | 0.9 | 6.3 | |
| VT2 (%) | 58.6 | 6.4 | 10.9 | |
| Block 6 | Distance (m) | 546 | 322.2 | 58.9 |
| Mean speed (km·h−1) | 11 | 0.7 | 6.6 | |
| VT2 (%) | 74 | 2.3 | 3.1 | |
| Total exercise protocol | Total time (min) | 52.2 | 5 | 9.6 |
| Total distance (m) | 8046.7 | 322.2 | 4 |
| Variable | BASELINE | AFTER | 24H | 48H | |
|---|---|---|---|---|---|
| ChE (pg/mL) | M | 1818.41 | 2218.78 | 1608.81 | 1454.54 |
| SD | 588.75 | 1101.58 | 546.88 | 580.45 | |
| RMSSD (ms) | M | 42.64 | 17.72 | 43.83 | 46.18 |
| SD | 12.86 | 12.55 | 24.50 | 33.22 | |
| SS (AU) | M | 8.76 | 21.93 | 10.93 | 11.86 |
| SD | 1.93 | 10.05 | 5.16 | 4.32 | |
| S:PS ratio (AU) | M | 0.32 | 3.26 | 0.46 | 0.50 |
| SD | 0.14 | 3.28 | 0.32 | 0.28 |
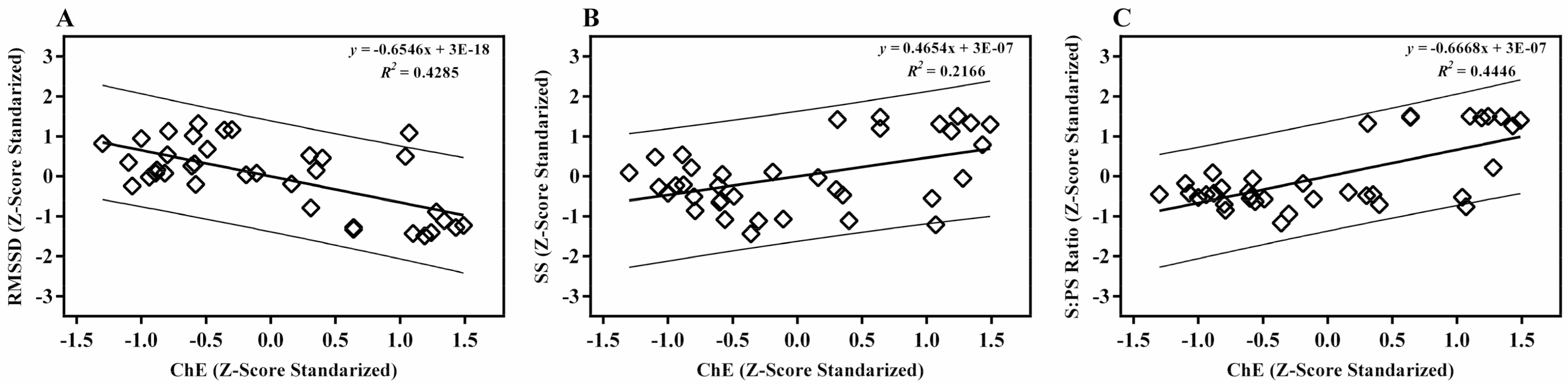
| ChE | RMSSD | SS | ||
|---|---|---|---|---|
| RMSSD | Pearson’s correlation | −0.654 ** | ||
| SS | Pearson’s correlation | 0.465 ** | −0.879 ** | |
| S:PS ratio | Pearson’s correlation | 0.666 ** | −0.926 ** | 0.942 ** |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hoyos-Flores, J.R.; Rangel-Colmenero, B.R.; Alonso-Ramos, Z.N.; García-Dávila, M.Z.; Cruz-Castruita, R.M.; Naranjo-Orellana, J.; Hernández-Cruz, G. The Role of Cholinesterases in Post-Exercise HRV Recovery in University Volleyball Players. Appl. Sci. 2021, 11, 4188. https://doi.org/10.3390/app11094188
Hoyos-Flores JR, Rangel-Colmenero BR, Alonso-Ramos ZN, García-Dávila MZ, Cruz-Castruita RM, Naranjo-Orellana J, Hernández-Cruz G. The Role of Cholinesterases in Post-Exercise HRV Recovery in University Volleyball Players. Applied Sciences. 2021; 11(9):4188. https://doi.org/10.3390/app11094188
Chicago/Turabian StyleHoyos-Flores, José Raúl, Blanca R. Rangel-Colmenero, Zeltzin N. Alonso-Ramos, Myriam Z. García-Dávila, Rosa M. Cruz-Castruita, José Naranjo-Orellana, and Germán Hernández-Cruz. 2021. "The Role of Cholinesterases in Post-Exercise HRV Recovery in University Volleyball Players" Applied Sciences 11, no. 9: 4188. https://doi.org/10.3390/app11094188
APA StyleHoyos-Flores, J. R., Rangel-Colmenero, B. R., Alonso-Ramos, Z. N., García-Dávila, M. Z., Cruz-Castruita, R. M., Naranjo-Orellana, J., & Hernández-Cruz, G. (2021). The Role of Cholinesterases in Post-Exercise HRV Recovery in University Volleyball Players. Applied Sciences, 11(9), 4188. https://doi.org/10.3390/app11094188







