The Use of the ROS Scavenger Cysteine as a Surface Ligand of Metal Nanoclusters and Its Bactericidal Elimination Effect
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Synthesis of the Cysteine-Conjugated Gold Nanoclusters
2.3. Synthesis of the Cysteine-Conjugated Silver Nanoclusters
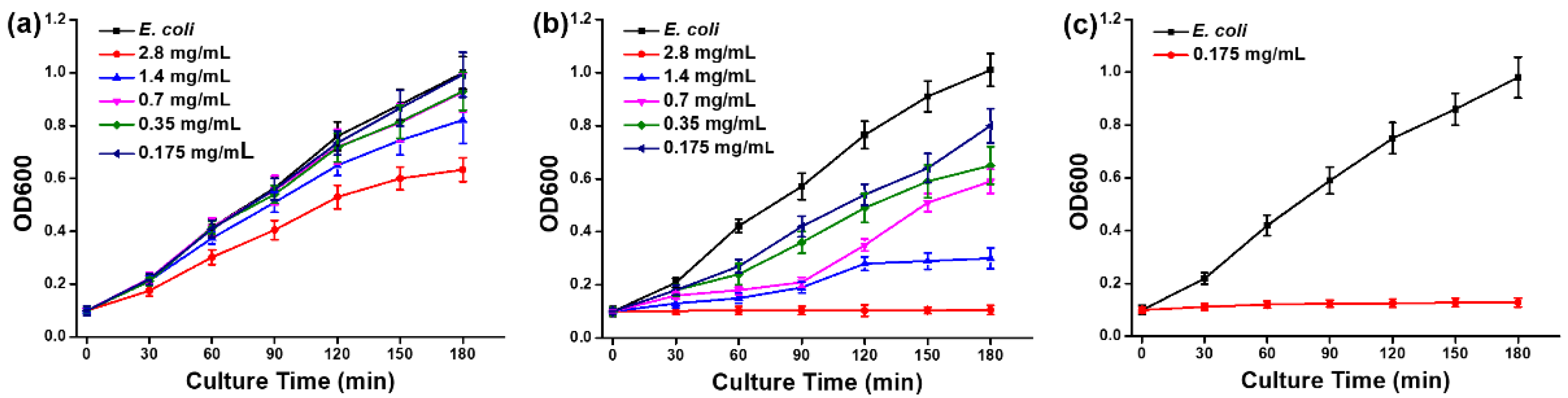
2.4. Bacterial Growth Curve Analysis
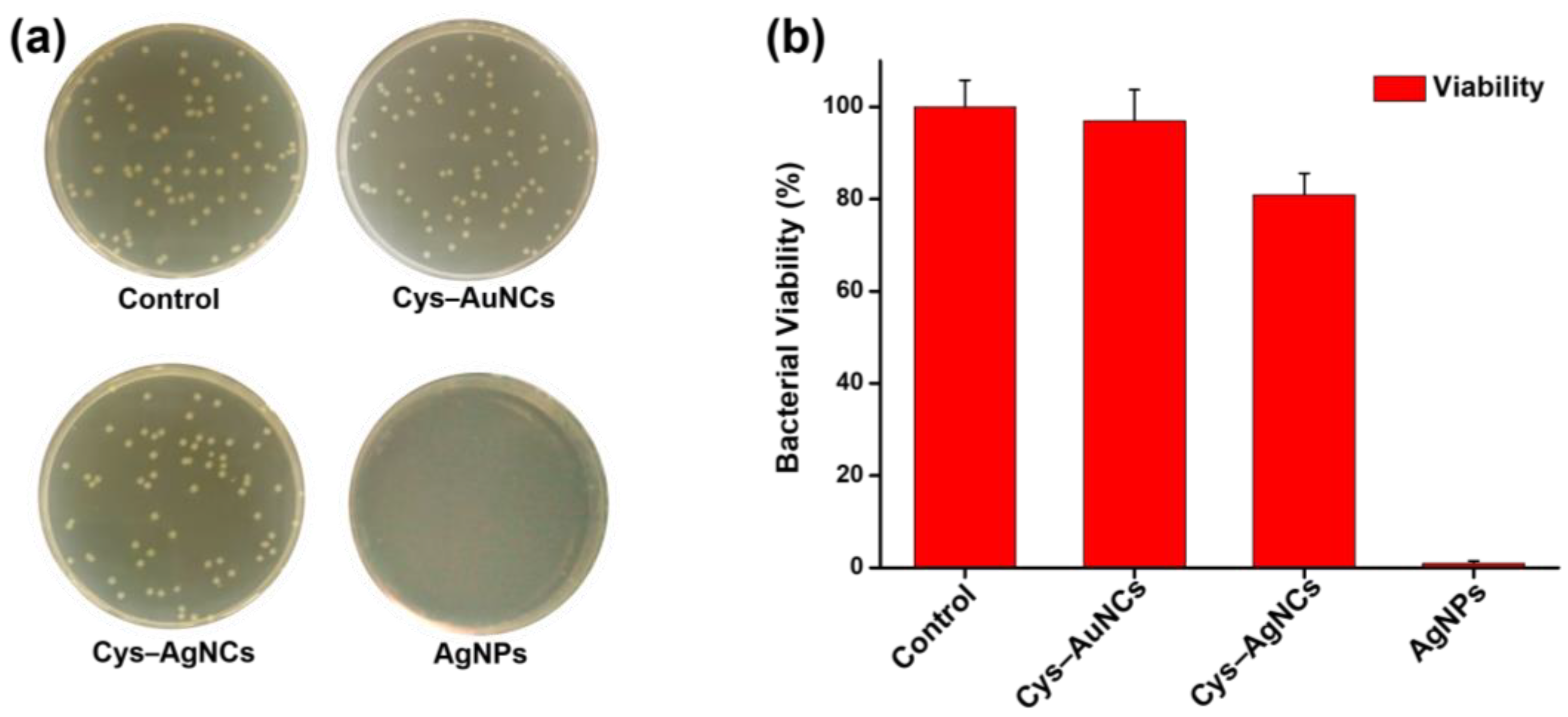
2.5. Assessment of the Total Number of Bacteria Using Plate Count Agar
2.6. Bacterial Imaging Using Confocal Fluorescence Microscopy
2.7. Measurement of the Absolute Quantum Yields Using an Integrating Sphere
3. Results and Discussion
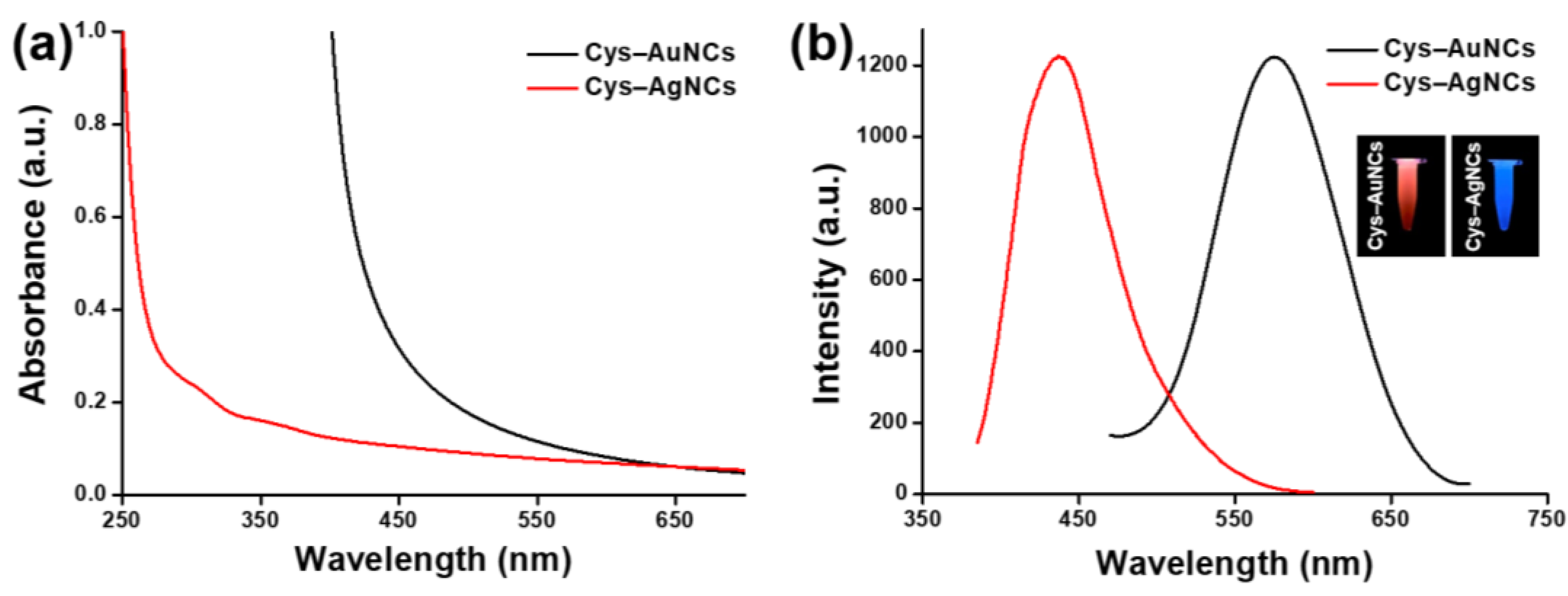
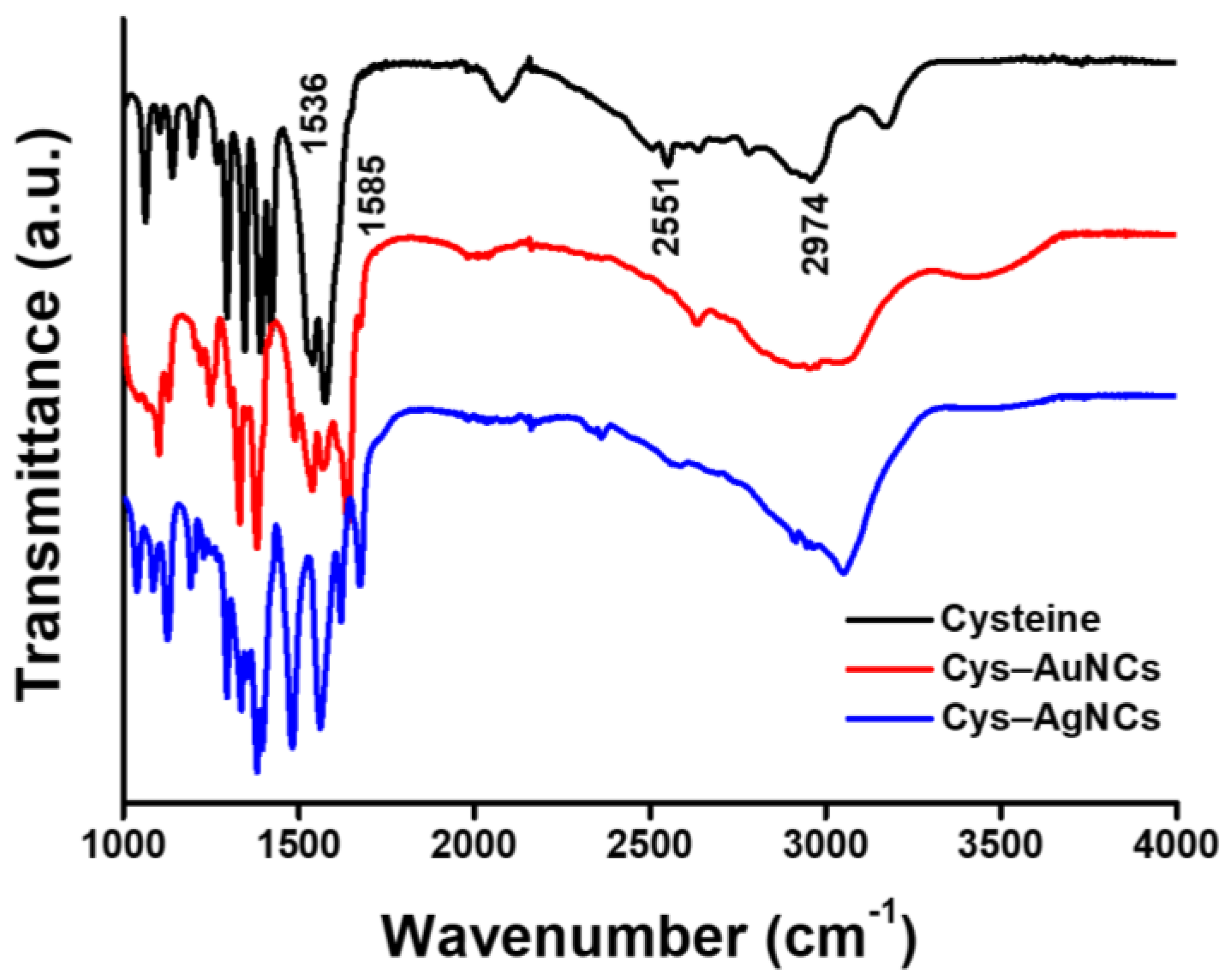
3.1. Structural and Optical Characterizations of the Cys–AuNCs and Cys–AgNCs
3.2. Bacterial Viability Assays of the Cys–AuNCs and Cys–AgNCs
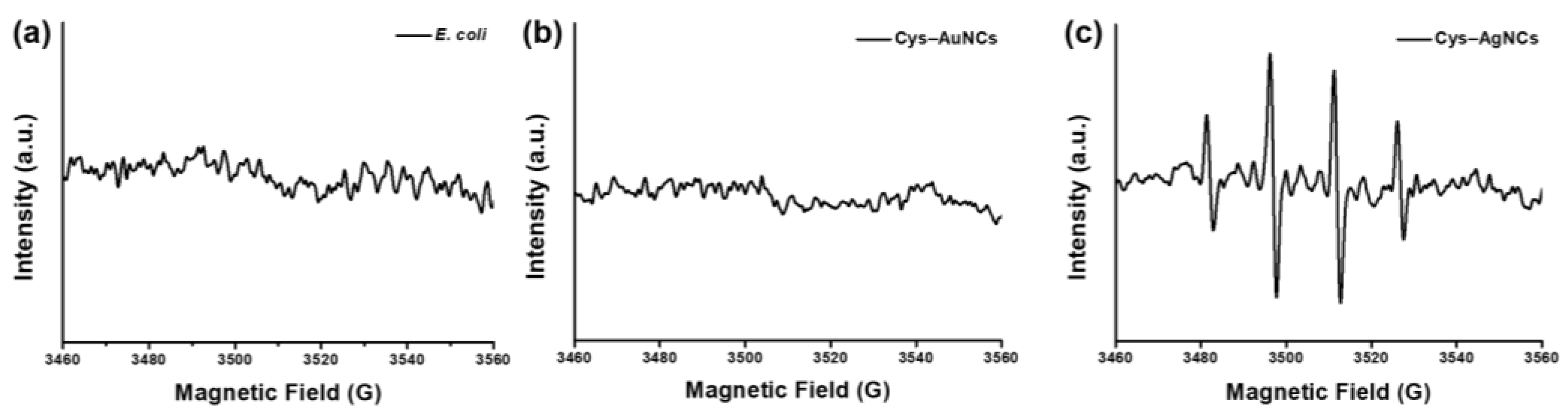
3.3. ROS Generation of the E. coli Incubated with the Cys–AuNCs and Cys–AgNCs
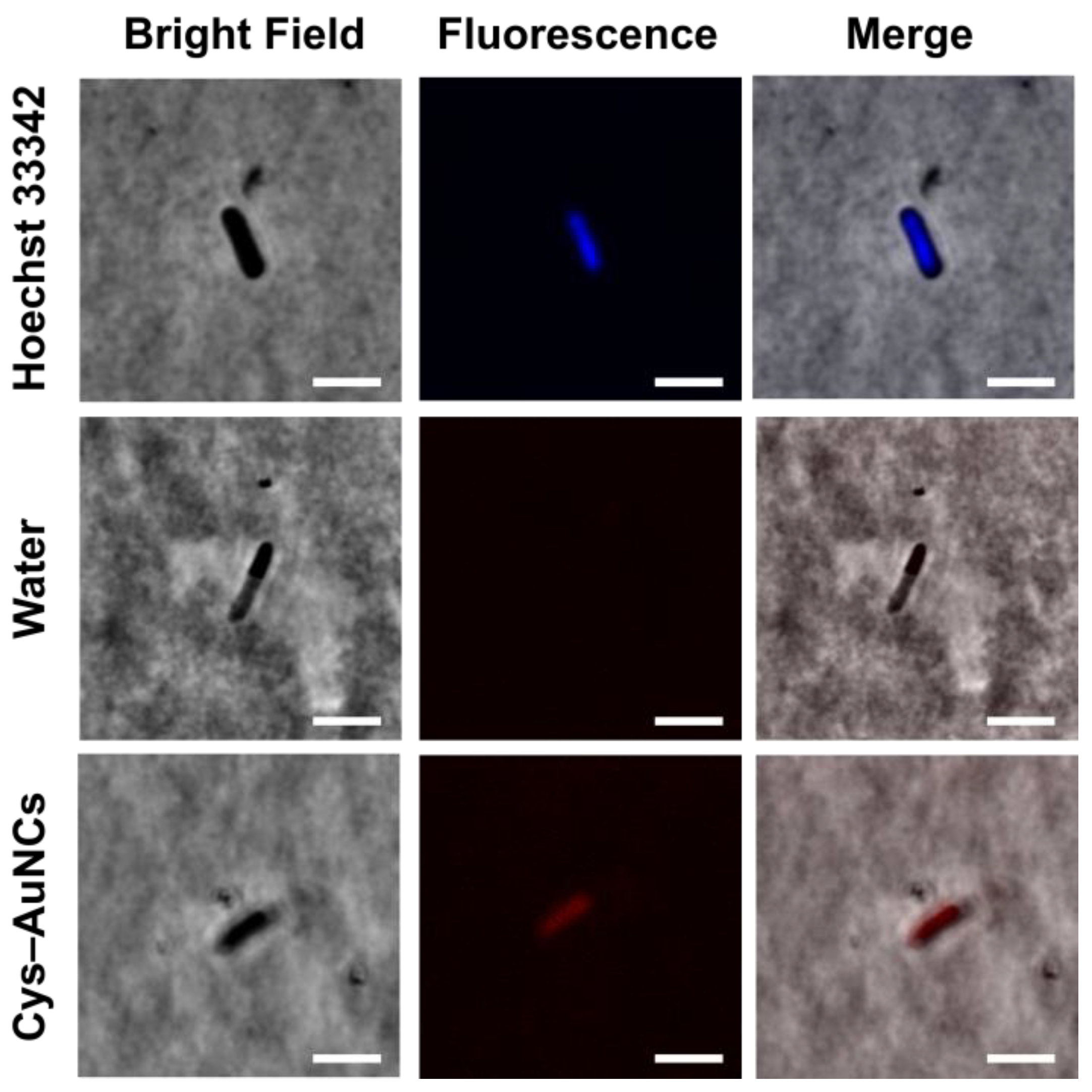
3.4. Fluorescence Imaging of the Cys–AuNCs in the E. coli
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kuo, T.R.; Chen, W.T.; Liao, H.J.; Yang, Y.H.; Yen, H.C.; Liao, T.W.; Wen, C.Y.; Lee, Y.C.; Chen, C.C.; Wang, D.Y. Improving hydrogen evolution activity of earth-abundant cobalt-doped iron pyrite catalysts by surface modification with phosphide. Small 2017, 13, 1603356. [Google Scholar] [CrossRef] [PubMed]
- Mutalik, C.; Hsiao, Y.-C.; Chang, Y.-H.; Krisnawati, D.I.; Alimansur, M.; Jazidie, A.; Nuh, M.; Chang, C.-C.; Wang, D.-Y.; Kuo, T.-R. High uv-vis-nir light-induced antibacterial activity by heterostructured TiO2-FeS2 nanocomposites. Int. J. Nanomed. 2020, 15, 8911. [Google Scholar] [CrossRef] [PubMed]
- Kuo, T.-R.; Liao, H.-J.; Chen, Y.-T.; Wei, C.-Y.; Chang, C.-C.; Chen, Y.-C.; Chang, Y.-H.; Lin, J.-C.; Lee, Y.-C.; Wen, C.-Y. Extended visible to near-infrared harvesting of earth-abundant FeS2-TiO2 heterostructures for highly active photocatalytic hydrogen evolution. Green Chem. 2018, 20, 1640–1647. [Google Scholar] [CrossRef]
- Hsiao, Y.-C.; Jheng, P.-R.; Nguyen, H.T.; Chen, Y.-H.; Manga, Y.B.; Lu, L.-S.; Rethi, L.; Chen, C.-H.; Huang, T.-W.; Lin, J.-D.; et al. Photothermal-irradiated polyethyleneimine–polypyrrole nanopigment film-coated polyethylene fabrics for infrared-inspired with pathogenic evaluation. ACS Appl. Mater. Interfaces 2021, 13, 2483–2495. [Google Scholar] [CrossRef]
- Lu, K.-Y.; Jheng, P.-R.; Lu, L.-S.; Rethi, L.; Mi, F.-L.; Chuang, E.-Y. Enhanced anticancer effect of ROS-boosted photothermal therapy by using fucoidan-coated polypyrrole nanoparticles. Int. J. Biol. Macromol. 2021, 166, 98–107. [Google Scholar] [CrossRef]
- Burnouf, T.; Chen, C.-H.; Tan, S.-J.; Tseng, C.-L.; Lu, K.-Y.; Chang, L.-H.; Nyambat, B.; Huang, S.-C.; Jheng, P.-R.; Aditya, R.N. A bioinspired hyperthermic macrophage-based polypyrrole-polyethylenimine (ppy-pei) nanocomplex carrier to prevent and disrupt thrombotic fibrin clots. Acta Biomater. 2019, 96, 468–479. [Google Scholar] [CrossRef]
- Chen, Y.-P.; Xu, L.; Tang, T.-W.; Chen, C.-H.; Zheng, Q.-H.; Liu, T.-P.; Mou, C.-Y.; Wu, C.-H.; Wu, S.-H. Sting activator c-di-gmp-loaded mesoporous silica nanoparticles enhance immunotherapy against breast cancer. ACS Appl. Mater. Interfaces 2020, 12, 56741–56752. [Google Scholar] [CrossRef]
- Mutalik, C.; Wang, D.Y.; Krisnawati, D.I.; Jazidie, A.; Yougbare, S.; Kuo, T.R. Light-activated heterostructured nanomaterials for antibacterial applications. Nanomaterials 2020, 10, 643. [Google Scholar] [CrossRef]
- Yougbare, S.; Mutalik, C.; Krisnawati, D.I.; Kristanto, H.; Jazidie, A.; Nuh, M.; Cheng, T.M.; Kuo, T.R. Nanomaterials for the photothermal killing of bacteria. Nanomaterials 2020, 10, 1123. [Google Scholar] [CrossRef]
- Liu, C.H.; Wong, S.H.; Tai, C.J.; Tai, C.J.; Pan, Y.C.; Hsu, H.Y.; Richardson, C.D.; Lin, L.T. Ursolic acid and its nanoparticles are potentiators of oncolytic measles virotherapy against breast cancer cells. Cancers 2021, 13, 136. [Google Scholar] [CrossRef]
- Huang, T.W.; Ho, Y.C.; Tsai, T.N.; Tseng, C.L.; Lin, C.; Mi, F.L. Enhancement of the permeability and activities of epigallocatechin gallate by quaternary ammonium chitosan/fucoidan nanoparticles. Carbohydr. Polym. 2020, 242, 116312. [Google Scholar] [CrossRef]
- Yougbaré, S.; Chou, H.-L.; Yang, C.-H.; Krisnawati, D.I.; Jazidie, A.; Nuh, M.; Kuo, T.-R. Facet-dependent gold nanocrystals for effective photothermal killing of bacteria. J. Hazard. Mater. 2021, 407, 124617. [Google Scholar] [CrossRef]
- Weng, B.; Lu, K.-Q.; Tang, Z.; Chen, H.M.; Xu, Y.-J. Stabilizing ultrasmall Au clusters for enhanced photoredox catalysis. Nat. Commun. 2018, 9, 1543. [Google Scholar] [CrossRef]
- Li, C.-H.; Kuo, T.-R.; Su, H.-J.; Lai, W.-Y.; Yang, P.-C.; Chen, J.-S.; Wang, D.-Y.; Wu, Y.-C.; Chen, C.-C. Fluorescence-guided probes of aptamer-targeted gold nanoparticles with computed tomography imaging accesses for in vivo tumor resection. Sci. Rep. 2015, 5, 15675. [Google Scholar] [CrossRef]
- Fahmi, M.Z.; Ou, K.L.; Chen, J.K.; Ho, M.H.; Tzing, S.H.; Chang, J.Y. Development of bovine serum albumin-modified hybrid nanoclusters for magnetofluorescence imaging and drug delivery. RSC Adv. 2014, 4, 32762–32772. [Google Scholar] [CrossRef]
- Jin, R.C.; Zeng, C.J.; Zhou, M.; Chen, Y.X. Atomically precise colloidal metal nanoclusters and nanoparticles: Fundamentals and opportunities. Chem. Rev. 2016, 116, 10346–10413. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.C.; Corma, A. Metal catalysts for heterogeneous catalysis: From single atoms to nanoclusters and nanoparticles. Chem. Rev. 2018, 118, 4981–5079. [Google Scholar] [CrossRef] [PubMed]
- Yang, B.B.; Xu, J.Y.; Bin, D.; Wang, J.; Zhao, J.Z.; Liu, Y.X.; Li, B.X.; Fang, X.N.; Liu, Y.; Qiao, L.; et al. Amorphous phosphatized ruthenium-iron bimetallic nanoclusters with Pt-like activity for hydrogen evolution reaction. Appl. Catal. B Environ. 2021, 283, 119583. [Google Scholar] [CrossRef]
- Wang, J.J.; Hu, Y.J.; Yu, X.X.; Zhuang, X.M.; Wang, Q.; Jiang, N.J.; Hu, J.Y. Recyclable and ultrasensitive sers sensing platform: Deposition of atomically precise Ag-152 nanoclusters on surface of plasmonic 3d ZnO-nc/aunp arrays. Appl. Surf. Sci. 2021, 540, 148324. [Google Scholar] [CrossRef]
- Guo, M.L.; Chi, J.T.; Zhang, C.; Wang, M.L.; Liang, H.; Hou, J.Y.; Ai, S.Y.; Li, X.Y. A simple and sensitive sensor for lactose based on cascade reactions in Au nanoclusters and enzymes co-encapsulated metal-organic frameworks. Food Chem. 2021, 339, 127863. [Google Scholar] [CrossRef]
- Cheng, T.M.; Chu, H.L.; Lee, Y.C.; Wang, D.Y.; Chang, C.C.; Chung, K.L.; Yen, H.C.; Hsiao, C.W.; Pan, X.Y.; Kuo, T.R.; et al. Quantitative analysis of glucose metabolic cleavage in glucose transporters overexpressed cancer cells by target-specific fluorescent gold nanoclusters. Anal. Chem. 2018, 90, 3974–3980. [Google Scholar] [CrossRef]
- Tan, S.-H.; Yougbaré, S.; Chu, H.-L.; Kuo, T.-R.; Cheng, T.-M. Hemoglobin-conjugated gold nanoclusters for qualitative analysis of haptoglobin phenotypes. Polymers 2020, 12, 2242. [Google Scholar] [CrossRef] [PubMed]
- Sheini, A. A point-of-care testing sensor based on fluorescent nanoclusters for rapid detection of septicemia in children. Sens. Actuators B Chem. 2021, 328, 129029. [Google Scholar] [CrossRef]
- Li, L.; Guo, X.P.; Peng, X.L.; Zhang, H.S.; Liu, Y.M.; Li, H.; He, X.J.; Shi, D.W.; Xiong, B.; Zhao, Y.B.; et al. Radiofrequency-responsive dual-valent gold nanoclusters for enhancing synergistic therapy of tumor ablation and artery embolization. Nano Today 2020, 35, 12. [Google Scholar] [CrossRef]
- Li, C.; Yang, W.T.; Yuan, R.; Xu, W.J. Antibody-responsive signal-off fluorescence of DNA-harbored silver nanoclusters for direct, rapid and sensitive immunoassay. Sens. Actuators B Chem. 2019, 301, 127148. [Google Scholar] [CrossRef]
- Kaur, N.; Aditya, R.N.; Singh, A.; Kuo, T.-R. Biomedical applications for gold nanoclusters: Recent developments and future perspectives. Nanoscale Res. Lett. 2018, 13, 1–12. [Google Scholar] [CrossRef]
- Sangubotla, R.; Lakshmi, B.A.; Kim, S.; Kim, J. Bio-inspired green fluorescent gold-naringin nanoclusters as a dual-functional optical probe for bio-imaging and intracellular sensing applications. Appl. Surf. Sci. 2020, 510, 145417. [Google Scholar] [CrossRef]
- Niu, Q.Q.; Gao, P.F.; Yuan, M.J.; Zhang, G.M.; Zhou, Y.; Dong, C.; Shuang, S.M.; Zhang, Y. Development of sensing method for mercury ions and cell imaging based on highly fluorescent gold nanoclusters. Microchem. J. 2019, 146, 1140–1149. [Google Scholar] [CrossRef]
- Raju, S.; Joseph, M.M.; Kuttanpillai, R.P.; Padinjarathil, H.; Usha, P.G.N.; Nair, S.T.T. Polysaccharide enabled biogenic fabrication of ph sensing fluorescent gold nanoclusters as a biocompatible tumor imaging probe. Microchim. Acta 2020, 187, 246. [Google Scholar] [CrossRef]
- Mutas, M.; Strelow, C.; Kipp, T.; Mews, A. Specific binding and internalization: An investigation of fluorescent aptamer-gold nanoclusters and cells with fluorescence lifetime imaging microscopy. Nanoscale 2018, 10, 20453–20461. [Google Scholar] [CrossRef] [PubMed]
- Yougbare, S.; Chang, T.-K.; Tan, S.-H.; Kuo, J.-C.; Hsu, P.-H.; Su, C.-Y.; Kuo, T.-R. Antimicrobial gold nanoclusters: Recent developments and future perspectives. Int. J. Mol. Sci. 2019, 20, 2924. [Google Scholar] [CrossRef]
- Chang, T.-K.; Cheng, T.-M.; Chu, H.-L.; Tan, S.-H.; Kuo, J.-C.; Hsu, P.-H.; Su, C.-Y.; Chen, H.-M.; Lee, C.-M.; Kuo, T.-R. Metabolic mechanism investigation of antibacterial active cysteine-conjugated gold nanoclusters in escherichia coli. ACS Sustain. Chem. Eng. 2019, 7, 15479–15486. [Google Scholar] [CrossRef]
- Hui, S.H.; Liu, Q.Q.; Huang, Z.Z.; Yang, J.; Liu, Y.M.; Jiang, S. Gold nanoclusters-decorated zeolitic imidazolate frameworks with reactive oxygen species generation for photoenhanced antibacterial study. Bioconjugate Chem. 2020, 31, 2439–2445. [Google Scholar] [CrossRef] [PubMed]
- Nakal-Chidiac, A.; Garcia, O.; Garcia-Fernandez, L.; Martin-Saavedra, F.M.; Sanchez-Casanova, S.; Escudero-Duch, C.; San Roman, J.; Vilaboa, N.; Aguilar, M.R. Chitosan-stabilized silver nanoclusters with luminescent, photothermal and antibacterial properties. Carbohydr. Polym. 2020, 250, 116973. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.J.; Ren, L.T.; Sun, L.X.; Bai, X.; Zhuang, G.Q.; Cao, B.; Hu, G.Q.; Zheng, N.F.; Liu, S.J. Amphiphilic silver nanoclusters show active nano-bio interaction with compelling antibacterial activity against multidrug-resistant bacteria. NPG Asia Mater. 2020, 12, 56. [Google Scholar] [CrossRef]
- Zheng, K.; Setyawati, M.I.; Leong, D.T.; Xie, J. Antimicrobial gold nanoclusters. ACS Nano 2017, 11, 6904–6910. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Liu, Y.; Yang, J.; Liu, Y.; Hu, F.; Zhu, K.; Jiang, X. Gold nanoclusters for targeting methicillin-resistant staphylococcus aureus in vivo. Angew. Chem. Int. Ed. 2018, 57, 3958–3962. [Google Scholar] [CrossRef]
- Eun, H.; Kwon, W.Y.; Kalimuthu, K.; Kim, Y.; Lee, M.; Ahn, J.O.; Lee, H.; Lee, S.H.; Kim, H.J.; Park, H.G.; et al. Melamine-promoted formation of bright and stable DNA-silver nanoclusters and their antimicrobial properties. J. Mater. Chem. B 2019, 7, 2512–2517. [Google Scholar] [CrossRef]
- Tang, Q.; Hu, G.; Fung, V.; Jiang, D.-e. Insights into interfaces, stability, electronic properties, and catalytic activities of atomically precise metal nanoclusters from first principles. Acc. Chem. Res. 2018, 51, 2793–2802. [Google Scholar] [CrossRef] [PubMed]
- Muhammed, M.A.H.; Verma, P.K.; Pal, S.K.; Kumar, R.A.; Paul, S.; Omkumar, R.V.; Pradeep, T. Bright, NIR-Emitting Au23 from Au25: Characterization and applications including biolabeling. Chem. Eur. J. 2009, 15, 10110–10120. [Google Scholar] [CrossRef]
- Luo, Z.; Yuan, X.; Yu, Y.; Zhang, Q.; Leong, D.T.; Lee, J.Y.; Xie, J. From aggregation-induced emission of Au (i)–thiolate complexes to ultrabright Au (0)@ Au (i)–thiolate core–shell nanoclusters. J. Am. Chem. Soc. 2012, 134, 16662–16670. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.; Zhou, C.; Yu, M.; Liu, J. Different sized luminescent gold nanoparticles. Nanoscale 2012, 4, 4073–4083. [Google Scholar] [CrossRef] [PubMed]
- Hsu, P.-H.; Yougbaré, S.; Kuo, J.-C.; Krisnawati, D.I.; Jazidie, A.; Nuh, M.; Chou, P.-T.; Hsiao, Y.-C.; Kuo, T.-R. One-pot synthesis of thiol-modified liquid crystals conjugated fluorescent gold nanoclusters. Nanomaterials 2020, 10, 1755. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Guo, Q.; Xie, Y.; Ma, H. Green chemistry for the preparation of l-cysteine functionalized silver nanoflowers. Chem. Phys. Lett. 2016, 652, 148–151. [Google Scholar] [CrossRef]
- Nieto-Ortega, B.; Bürgi, T. Vibrational properties of thiolate-protected gold nanoclusters. Acc. Chem. Res. 2018, 51, 2811–2819. [Google Scholar] [CrossRef]
- Xie, J.; Zheng, Y.; Ying, J.Y. Protein-directed synthesis of highly fluorescent gold nanoclusters. J. Am. Chem. Soc. 2009, 131, 888–889. [Google Scholar] [CrossRef]
- Zheng, K.; Setyawati, M.I.; Leong, D.T.; Xie, J. Surface ligand chemistry of gold nanoclusters determines their antimicrobial ability. Chem. Mater. 2018, 30, 2800–2808. [Google Scholar] [CrossRef]
- Wang, S.; Wang, Y.; Peng, Y.; Yang, X. Exploring the antibacteria performance of multicolor Ag, Au, and Cu nanoclusters. ACS Appl. Mater. Interfaces 2019, 11, 8461–8469. [Google Scholar] [CrossRef]
- Sakimoto, K.K.; Wong, A.B.; Yang, P. Self-photosensitization of nonphotosynthetic bacteria for solar-to-chemical production. Science 2016, 351, 74–77. [Google Scholar] [CrossRef]
- Zhang, H.; Liu, H.; Tian, Z.; Lu, D.; Yu, Y.; Cestellos-Blanco, S.; Sakimoto, K.K.; Yang, P. Bacteria photosensitized by intracellular gold nanoclusters for solar fuel production. Nat. Nanotech. 2018, 13, 900–905. [Google Scholar] [CrossRef]
- Jackson, S.K.; Thomas, M.P.; Smith, S.; Madhani, M.; Rogers, S.C.; James, P.E. In vivo epr spectroscopy: Biomedical and potential diagnostic applications. Faraday Discuss. 2004, 126, 103–117. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Krisnawati, D.I.; Hsu, P.-H.; Lin, Y.-H.; Alimansur, M.; Atmojo, D.S.; Rahmawati, E.Q.; Rahayu, D.; Khafid, M.; Lu, S.-C.; Kuo, T.-R. The Use of the ROS Scavenger Cysteine as a Surface Ligand of Metal Nanoclusters and Its Bactericidal Elimination Effect. Appl. Sci. 2021, 11, 4095. https://doi.org/10.3390/app11094095
Krisnawati DI, Hsu P-H, Lin Y-H, Alimansur M, Atmojo DS, Rahmawati EQ, Rahayu D, Khafid M, Lu S-C, Kuo T-R. The Use of the ROS Scavenger Cysteine as a Surface Ligand of Metal Nanoclusters and Its Bactericidal Elimination Effect. Applied Sciences. 2021; 11(9):4095. https://doi.org/10.3390/app11094095
Chicago/Turabian StyleKrisnawati, Dyah Ika, Po-Hsuan Hsu, Yu-Hsiang Lin, Moh Alimansur, Didik Susetiyanto Atmojo, Elfi Quyumi Rahmawati, Dwi Rahayu, Muhamad Khafid, Ssu-Chiao Lu, and Tsung-Rong Kuo. 2021. "The Use of the ROS Scavenger Cysteine as a Surface Ligand of Metal Nanoclusters and Its Bactericidal Elimination Effect" Applied Sciences 11, no. 9: 4095. https://doi.org/10.3390/app11094095
APA StyleKrisnawati, D. I., Hsu, P.-H., Lin, Y.-H., Alimansur, M., Atmojo, D. S., Rahmawati, E. Q., Rahayu, D., Khafid, M., Lu, S.-C., & Kuo, T.-R. (2021). The Use of the ROS Scavenger Cysteine as a Surface Ligand of Metal Nanoclusters and Its Bactericidal Elimination Effect. Applied Sciences, 11(9), 4095. https://doi.org/10.3390/app11094095






