Antimicrobials from Medicinal Plants: An Emergent Strategy to Control Oral Biofilms
Abstract
1. Introduction
2. Oral Microbiome
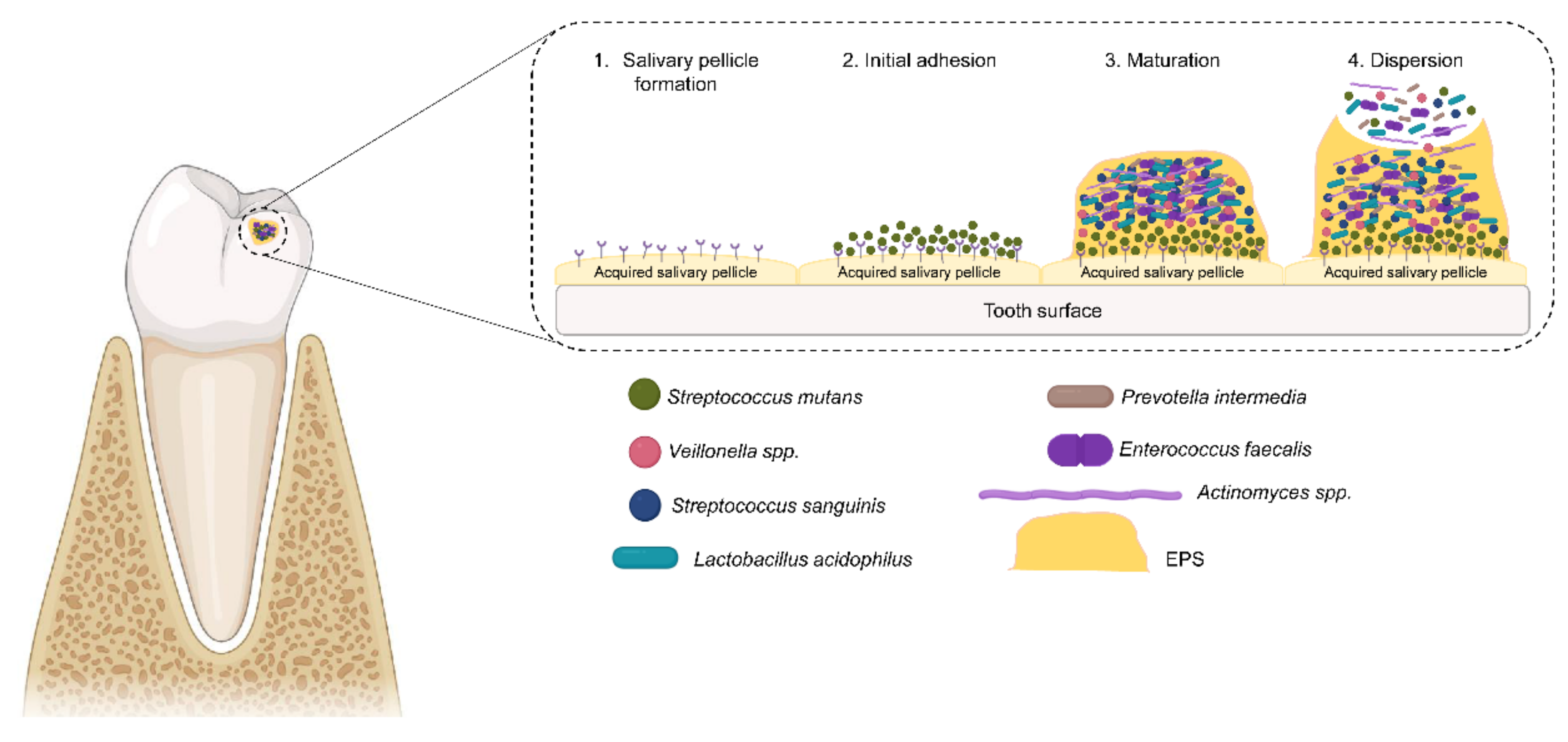
3. Oral Biofilm Formation
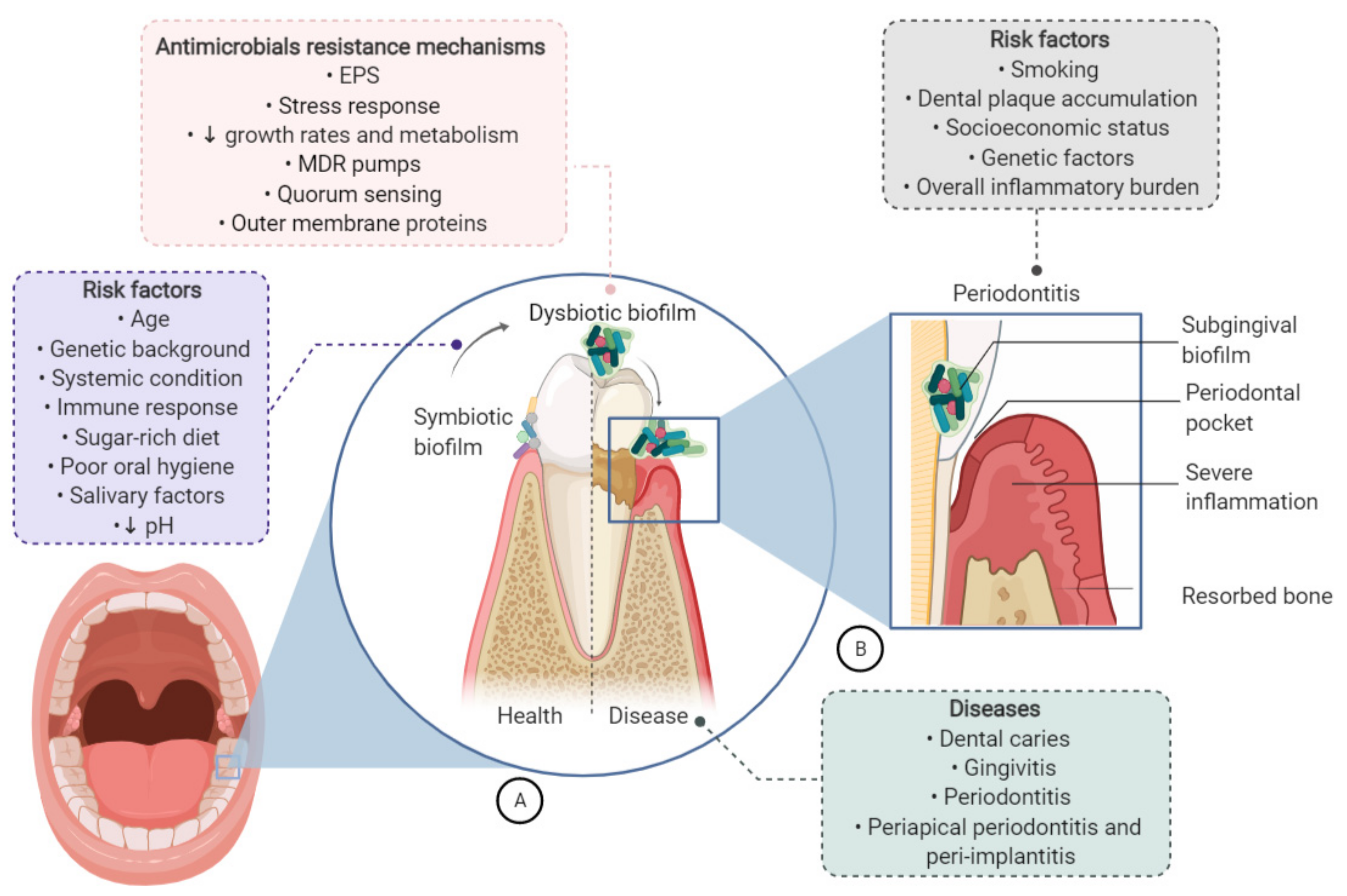
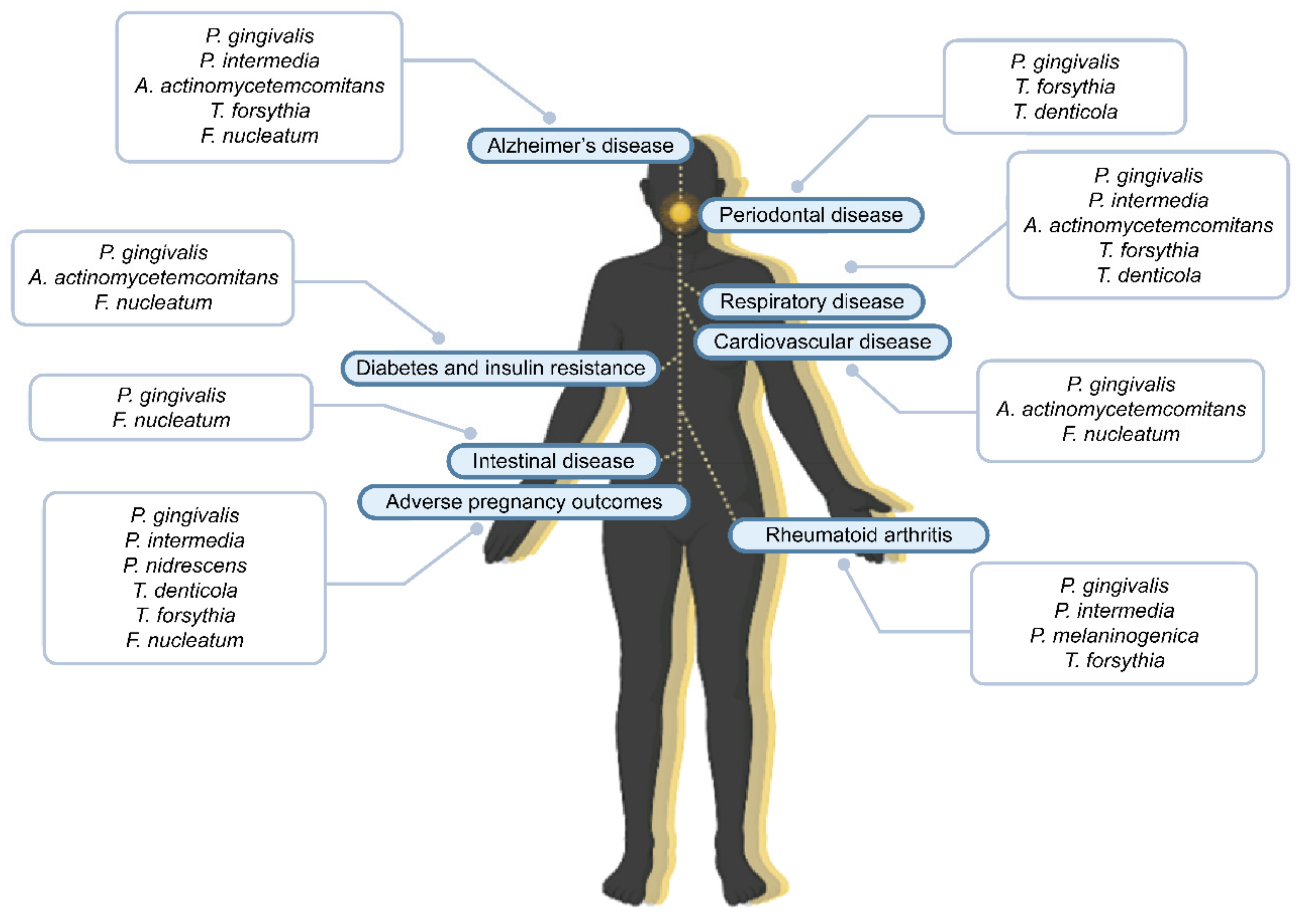
4. Oral Biofilms: From Dental Caries to Systemic Diseases
5. Biological Properties of Plants
6. The Most Promising Medicinal Plant Extracts in the Control of Oral Biofilms
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Marsh, P. Dental plaque as a microbial biofilm. Caries Res. 2004, 38, 204–211. [Google Scholar] [CrossRef]
- Chinsembu, K.C. Plants and other natural products used in the management of oral infections and improvement of oral health. Acta Trop. 2016, 154, 6–18. [Google Scholar] [CrossRef] [PubMed]
- Hwang, G.; Klein, M.I.; Koo, H. Analysis of the mechanical stability and surface detachment of mature Streptococcus mutans biofilms by applying a range of external shear forces. Biofouling 2014, 30, 1079–1091. [Google Scholar] [CrossRef]
- Nishikawara, F.; Nomura, Y.; Imai, S.; Senda, A.; Hanada, N. Evaluation of cariogenic bacteria. Eur. J. Dent. 2007, 1, 31–39. [Google Scholar] [CrossRef] [PubMed]
- Allaker, R.P.; Douglas, C.I. Novel anti-microbial therapies for dental plaque-related diseases. Int. J. Antimicrob. Agents 2009, 33, 8–13. [Google Scholar] [CrossRef] [PubMed]
- Maeda, H.; Hirai, K.; Mineshiba, J.; Yamamoto, T.; Kokeguchi, S.; Takashiba, S. Medical microbiological approach to Archaea in oral infectious diseases. Jpn. Dent. Sci. Rev. 2013, 49, 72–78. [Google Scholar] [CrossRef][Green Version]
- Loesche, W. Dental caries and periodontitis: Contrasting two infections that have medical implications. Infect. Dis. Clin. N. Am. 2007, 21, 471–502. [Google Scholar] [CrossRef] [PubMed]
- Alireza, R.G.; Afsaneh, R.; Hosein, M.S.; Siamak, Y.; Afshin, K.; Zeinab, K.; Mahvash, M.J.; Reza, R.A. Inhibitory activity of Salvadora persica extracts against oral bacterial strains associated with periodontitis: An in-vitro study. J. Oral Biol. Craniofac. Res. 2014, 4, 19–23. [Google Scholar] [CrossRef]
- Galvão, L.C.; Furletti, V.F.; Bersan, S.M.; da Cunha, M.G.; Ruiz, A.L.; Carvalho, J.E.; Sartoratto, A.; Rehder, V.L.; Figueira, G.M.; Teixeira Duarte, M.C.; et al. Antimicrobial activity of essential oils against Streptococcus mutans and their antiproliferative effects. Evid.-Based Complement. Altern. Med. 2012, 2012, 751435. [Google Scholar] [CrossRef]
- Teanpaisan, R.; Kawsud, P.; Pahumunto, N.; Puripattanavong, J. Screening for antibacterial and antibiofilm activity in Thai medicinal plant extracts against oral micro-organisms. J. Tradit. Complement. Med. 2017, 7, 172–177. [Google Scholar] [CrossRef]
- Vieira, D.R.; Amaral, F.M.; Maciel, M.C.; Nascimento, F.R.; Libério, S.A.; Rodrigues, V.P. Plant species used in dental diseases: Ethnopharmacology aspects and antimicrobial activity evaluation. J. Ethnopharmacol. 2014, 155, 1441–1449. [Google Scholar] [CrossRef]
- Brown, D. Antibiotic resistance breakers: Can repurposed drugs fill the antibiotic discovery void? Nat. Rev. Drug Discov. 2015, 14, 821–832. [Google Scholar] [CrossRef]
- Rana, R.; Sharma, R.; Kumar, A. Repurposing of existing statin drugs for treatment of microbial infections: How much promising? Infect. Disord. Drug Targets 2019, 19, 224–237. [Google Scholar] [CrossRef]
- Palombo, E.A. Traditional medicinal plant extracts and natural products with activity against oral bacteria: Potential applica-tion in the prevention and treatment of oral diseases. Evid.-Based Complement. Altern. Med. 2011, 2011, 680354. [Google Scholar] [CrossRef] [PubMed]
- Slobodníková, L.; Fialová, S.; Rendeková, K.; Kováč, J.; Mučaji, P. Antibiofilm activity of plant polyphenols. Molecules 2016, 21, 1717. [Google Scholar] [CrossRef] [PubMed]
- Daglia, M. Polyphenols as antimicrobial agents. Curr. Opin. Biotechnol. 2012, 23, 174–181. [Google Scholar] [CrossRef] [PubMed]
- Quideau, S.; Deffieux, D.; Douat-Casassus, C.; Pouységu, L. Plant polyphenols: Chemical properties, biological activities, and synthesis. Angew. Chem. Int. Ed. 2011, 50, 586–621. [Google Scholar] [CrossRef]
- Cushnie, T.T.; Lamb, A.J. Recent advances in understanding the antibacterial properties of flavonoids. Int. J. Antimicrob. Agents 2011, 38, 99–107. [Google Scholar] [CrossRef] [PubMed]
- Gyawali, R.; Ibrahim, S.A. Natural products as antimicrobial agents. Food Control 2014, 46, 412–429. [Google Scholar] [CrossRef]
- Silva, L.N.; Zimmer, K.R.; Macedo, A.J.; Trentin, D.S. Plant natural products targeting bacterial virulence factors. Chem. Rev. 2016, 116, 9162–9236. [Google Scholar] [CrossRef]
- Ren, Z.; Chen, L.; Li, J.; Li, Y. Inhibition of Streptococcus mutans polysaccharide synthesis by molecules targeting glycosyltransferase activity. J. Oral Microbiol. 2016, 8, 31095. [Google Scholar] [CrossRef] [PubMed]
- Koparde, A.A.; Doijad, R.C.; Magdum, C.S. Natural products in drug discovery. In Pharmacognosy—Medicinal Plants; IntechOpen: London, UK, 2019. [Google Scholar]
- Wade, W.G. Characterisation of the human oral microbiome. J. Oral Biosci. 2013, 55, 143–148. [Google Scholar] [CrossRef]
- Dewhirst, F.E.; Chen, T.; Izard, J.; Paster, B.J.; Tanner, A.C.R.; Yu, W.-H.; Lakshmanan, A.; Wade, W.G. The human oral microbiome. J. Bacteriol. 2010, 192, 5002–5017. [Google Scholar] [CrossRef] [PubMed]
- Lurie-Weinberger, M.N.; Gophna, U. Archaea in and on the human body: Health implications and future directions. PLoS Pathog. 2015, 11, e1004833. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, A.; Singh, A.; Verma, R.; Murari, A. Oral candidiasis: An overview. J. Oral Maxillofac. Pathol. 2014, 18, 81–85. [Google Scholar] [CrossRef]
- Ghannoum, M.A.; Jurevic, R.J.; Mukherjee, P.K.; Cui, F.; Sikaroodi, M.; Naqvi, A.; Gillevet, P.M. Characterization of the oral fungal microbiome (Mycobiome) in healthy individuals. PLoS Pathog. 2010, 6, e1000713. [Google Scholar] [CrossRef]
- Santosh, A.B.R.; Muddana, K. Viral infections of oral cavity. J. Fam. Med. Prim. Care 2020, 9, 36–42. [Google Scholar] [CrossRef]
- Sharma, N.; Bhatia, S.; Sodhi, A.S.; Batra, N. Oral microbiome and health. AIMS Microbiol. 2018, 4, 42–66. [Google Scholar] [CrossRef]
- Deo, P.N.; Deshmukh, R. Oral microbiome: Unveiling the fundamentals. J. Oral Maxillofac. Pathol. 2019, 23, 122–128. [Google Scholar] [CrossRef]
- Lamont, R.J.; Koo, H.; Hajishengallis, G. The oral microbiota: Dynamic communities and host interactions. Nat. Rev. Genet. 2018, 16, 745–759. [Google Scholar] [CrossRef]
- Loesche, W.J. Role of Streptococcus mutans in human dental decay. Microbiol. Rev. 1986, 50, 353. [Google Scholar] [CrossRef]
- Marsh, P.D. Microbiology of dental plaque biofilms and their role in oral health and caries. Dent. Clin. N. Am. 2010, 54, 441–454. [Google Scholar] [CrossRef] [PubMed]
- McNeill, K.; Hamilton, I. Acid tolerance response of biofilm cells of Streptococcus mutans. FEMS Microbiol. Lett. 2003, 221, 25–30. [Google Scholar] [CrossRef]
- Dahlen, G.; Basic, A.; Bylund, J. Importance of virulence factors for the persistence of oral bacteria in the inflamed gingival crevice and in the pathogenesis of periodontal disease. J. Clin. Med. 2019, 8, 1339. [Google Scholar] [CrossRef]
- Hannig, M.; Joiner, A. The structure, function and properties of the acquired pellicle. Monogr. Oral Sci. 2005, 19, 29–64. [Google Scholar] [CrossRef]
- Bos, R.; Van der Mei, H.C.; Busscher, H.J. Physico-chemistry of initial microbial adhesive interactions—Its mechanisms and methods for study. FEMS Microbiol. Rev. 1999, 23, 179–230. [Google Scholar] [CrossRef]
- Scannapieco, F.A.; Torres, G.; Levine, M.J. Salivary α-amylase: Role in dental plaque and caries formation. Crit. Rev. Oral Biol. Med. 1993, 4, 301–307. [Google Scholar] [CrossRef]
- Ihara, Y.; Takeshita, T.; Kageyama, S.; Matsumi, R.; Asakawa, M.; Shibata, Y.; Sugiura, Y.; Ishikawa, K.; Takahashi, I.; Yamashita, Y. Identification of initial colonizing bacteria in dental plaques from young adults using full-length 16S rRNA gene sequencing. mSystems 2019, 4, e00360-19. [Google Scholar] [CrossRef]
- Vitkov, L.; Krautgartner, W.D.; Hannig, M.; Fuchs, K. Fimbria-mediated bacterial adhesion to human oral epithelium. FEMS Microbiol. Lett. 2001, 202, 25–30. [Google Scholar] [CrossRef][Green Version]
- Okahashi, N.; Nakata, M.; Terao, Y.; Isoda, R.; Sakurai, A.; Sumitomo, T.; Yamaguchi, M.; Kimura, R.K.; Oiki, E.; Kawabata, S.; et al. Pili of oral Streptococcus sanguinis bind to salivary amylase and promote the biofilm formation. Microb. Pathog. 2011, 50, 148–154. [Google Scholar] [CrossRef]
- Karygianni, L.; Ren, Z.; Koo, H.; Thurnheer, T. Biofilm matrixome: Extracellular components in structured microbial communities. Trends Microbiol. 2020, 28, 668–681. [Google Scholar] [CrossRef] [PubMed]
- Costa, O.Y.A.; Raaijmakers, J.M.; Kuramae, E.E. Microbial extracellular polymeric substances: Ecological function and impact on soil aggregation. Front. Microbiol. 2018, 9, 1636. [Google Scholar] [CrossRef]
- Rickard, A.H.; Gilbert, P.; High, N.J.; E Kolenbrander, P.; Handley, P.S. Bacterial coaggregation: An integral process in the development of multi-species biofilms. Trends Microbiol. 2003, 11, 94–100. [Google Scholar] [CrossRef]
- Rabin, N.; Zheng, Y.; Opoku-Temeng, C.; Du, Y.; Bonsu, E.; O Sintim, H. Biofilm formation mechanisms and targets for developing antibiofilm agents. Future Med. Chem. 2015, 7, 493–512. [Google Scholar] [CrossRef] [PubMed]
- Kaplan, J. Biofilm dispersal: Mechanisms, clinical implications, and potential therapeutic uses. J. Dent. Res. 2010, 89, 205–218. [Google Scholar] [CrossRef] [PubMed]
- Bowen, W.H.; Burne, R.A.; Wu, H.; Koo, H. Oral biofilms: Pathogens, matrix, and polymicrobial interactions in microenvironments. Trends Microbiol. 2018, 26, 229–242. [Google Scholar] [CrossRef] [PubMed]
- Suppiger, S.; Astasov-Frauenhoffer, M.; Schweizer, I.; Waltimo, T.; Kulik, E.M. Tolerance and persister formation in oral streptococci. Antibiotics 2020, 9, 167. [Google Scholar] [CrossRef] [PubMed]
- Peres, M.A.; Macpherson, L.M.D.; Weyant, R.J.; Daly, B.; Venturelli, R.; Mathur, M.R.; Listl, S.; Celeste, R.K.; Guarnizo-Herreño, C.C.; Kearns, C.; et al. Oral diseases: A global public health challenge. Lancet 2019, 394, 249–260. [Google Scholar] [CrossRef]
- Maddi, A.; Scannapieco, F.A. Oral biofilms, oral and periodontal infections, and systemic disease. Am. J. Dent. 2013, 26, 249–254. [Google Scholar]
- Kouidhi, B.; Al Qurashi, Y.M.A.; Chaieb, K. Drug resistance of bacterial dental biofilm and the potential use of natural compounds as alternative for prevention and treatment. Microb. Pathog. 2015, 80, 39–49. [Google Scholar] [CrossRef]
- Matsumoto-Nakano, M. Role of Streptococcus mutans surface proteins for biofilm formation. Jpn. Dent. Sci. Rev. 2018, 54, 22–29. [Google Scholar] [CrossRef] [PubMed]
- Bowen, W.; Koo, H. Biology of Streptococcus mutans-derived glucosyltransferases: Role in extracellular matrix formation of cariogenic biofilms. Caries Res. 2011, 45, 69–86. [Google Scholar] [CrossRef] [PubMed]
- Miller, M.B.; Bassler, B.L. Quorum sensing in bacteria. Annu. Rev. Microbiol. 2001, 55, 165–199. [Google Scholar] [CrossRef] [PubMed]
- Colombo, A.; Tanner, A. The role of bacterial biofilms in dental caries and periodontal and peri-implant diseases: A historical perspective. J. Dent. Res. 2019, 98, 373–385. [Google Scholar] [CrossRef] [PubMed]
- Mosaddad, S.A.; Tahmasebi, E.; Yazdanian, A.; Rezvani, M.B.; Seifalian, A.; Yazdanian, M.; Tebyanian, H. Oral microbial biofilms: An update. Eur. J. Clin. Microbiol. Infect. Dis. 2019, 38, 2005–2019. [Google Scholar] [CrossRef]
- Santos, A.L.; Siqueira, J.F., Jr.; Rôças, I.N.; Jesus, E.C.; Rosado, A.S.; Tiedje, J.M. Comparing the bacterial diversity of acute and chronic dental root canal infections. PLoS ONE 2011, 6, e28088. [Google Scholar] [CrossRef]
- Colombo, A.P.; do Souto, R.M.; da Silva-Boghossian, C.M.; Miranda, R.; Lourenço, T.G. Microbiology of oral biofilm-dependent diseases: Have we made significant progress to understand and treat these diseases? Curr. Oral Health Rep. 2015, 2, 37–47. [Google Scholar] [CrossRef]
- Ricucci, D.; Siqueira, J.F., Jr. Biofilms and apical periodontitis: Study of prevalence and association with clinical and histo-pathologic findings. J. Endod. 2010, 36, 1277–1288. [Google Scholar] [CrossRef]
- Socransky, S.S.; Haffajee, A.D.; Cugini, M.A.; Smith, C.; Kent, R.L., Jr. Microbial complexes in subgingival plaque. J. Clin. Periodontol. 1998, 25, 134–144. [Google Scholar] [CrossRef]
- Bui, F.Q.; Almeida-Da-Silva, C.L.C.; Huynh, B.; Trinh, A.; Liu, J.; Woodward, J.; Asadi, H.; Ojcius, D.M. Association between periodontal pathogens and systemic disease. Biomed. J. 2019, 42, 27–35. [Google Scholar] [CrossRef]
- Yao, Q.-W.; Zhou, D.-S.; Peng, H.-J.; Ji, P.; Liu, D.-S. Association of periodontal disease with oral cancer: A meta-analysis. Tumor Biol. 2014, 35, 7073–7077. [Google Scholar] [CrossRef] [PubMed]
- Michaud, D.S.; Fu, Z.; Shi, J.; Chung, M. Periodontal disease, tooth loss, and cancer risk. Epidemiol. Rev. 2017, 39, 49–58. [Google Scholar] [CrossRef]
- Inaba, H.; Sugita, H.; Kuboniwa, M.; Iwai, S.; Hamada, M.; Noda, T.; Morisaki, I.; Lamont, R.J.; Amano, A. Porphyromonas gingivalis promotes invasion of oral squamous cell carcinoma through induction of pro MMP 9 and its activation. Cell. Microbiol. 2014, 16, 131–145. [Google Scholar] [CrossRef] [PubMed]
- Fukugaiti, M.H.; Ignacio, A.; Fernandes, M.R.; Júnior, U.R.; Nakano, V.; AvilaCampos, M.J. High occurrence of Fusobacterium nucleatum and Clostridium difficile in the intestinal microbiota of colorectal carcinoma patients. Braz. J. Microbiol. 2015, 46, 1135–1140. [Google Scholar] [CrossRef] [PubMed]
- Castellarin, M.; Warren, R.L.; Freeman, J.D.; Dreolini, L.; Krzywinski, M.; Strauss, J.; Barnes, R.; Watson, P.; Allen-Vercoe, E.; Moore, R.A.; et al. Fusobacterium nucleatum infection is prevalent in human colorectal carcinoma. Genome Res. 2011, 22, 299–306. [Google Scholar] [CrossRef]
- Mealey, B.L.; Ocampo, G.L. Diabetes mellitus and periodontal disease. Periodontol. 2000 2007, 44, 127–153. [Google Scholar] [CrossRef] [PubMed]
- Teeuw, W.J.; Gerdes, V.E.A.; Loos, B.G. Effect of periodontal treatment on glycemic control of diabetic patients: A systematic review and meta-analysis. Diabetes Care 2010, 33, 421–427. [Google Scholar] [CrossRef] [PubMed]
- Nishimura, F.; Iwamoto, Y.; Mineshiba, J.; Shimizu, A.; Soga, Y.; Murayama, Y. Periodontal Disease and diabetes mellitus: The role of tumor necrosis factor-α in a 2-way relationship. J. Periodontol. 2003, 74, 97–102. [Google Scholar] [CrossRef]
- Kothari, M.; Spin-Neto, R.; Nielsen, J.F. Comprehensive oral-health assessment of individuals with acquired brain-injury in neuro-rehabilitation setting. Brain Inj. 2016, 30, 1103–1108. [Google Scholar] [CrossRef]
- Akiyama, H.; Barger, S.; Barnum, S.; Bradt, B.; Bauer, J.; Cole, G.M.; Cooper, N.R.; Eikelenboom, P.; Emmerling, M.; Fiebich, B.L.; et al. Inflammation and Alzheimer’s disease. Neurobiol. Aging 2000, 21, 383–421. [Google Scholar] [CrossRef]
- Kamer, A.R.; Craig, R.G.; Pirraglia, E.; Dasanayake, A.P.; Norman, R.G.; Boylan, R.J.; Nehorayoff, A.; Glodzik, L.; Brys, M.; de Leon, M.J. TNF-α and antibodies to periodontal bacteria discriminate between Alzheimer’s disease patients and normal subjects. J. Neuroimmunol. 2009, 216, 92–97. [Google Scholar] [CrossRef] [PubMed]
- Poole, S.; Singhrao, S.K.; Kesavalu, L.; Curtis, M.A.; Crean, S. Determining the presence of periodontopathic virulence factors in short-term postmortem Alzheimer’s disease brain tissue. J. Alzheimers Dis. 2013, 36, 665–677. [Google Scholar] [CrossRef] [PubMed]
- Bahekar, A.A.; Singh, S.; Saha, S.; Molnar, J.; Arora, R. The prevalence and incidence of coronary heart disease is significantly increased in periodontitis: A meta-analysis. Am. Heart J. 2007, 154, 830–837. [Google Scholar] [CrossRef]
- Figuero, E.; Sánchez-Beltrán, M.; Cuesta-Frechoso, S.; Tejerina, J.M.; Del Castro, J.A.; Gutiérrez, J.M.; Herrera, D.; Sanz, M. Detection of periodontal bacteria in atheromatous plaque by nested polymerase chain reaction. J. Periodontol. 2011, 82, 1469–1477. [Google Scholar] [CrossRef] [PubMed]
- Haraszthy, V.; Zambon, J.; Trevisan, M.; Zeid, M.; Genco, R. Identification of periodontal pathogens in atheromatous plaques. J. Periodontol. 2000, 71, 1554–1560. [Google Scholar] [CrossRef] [PubMed]
- Nakano, K.; Inaba, H.; Nomura, R.; Nemoto, H.; Takeda, M.; Yoshioka, H.; Matsue, H.; Takahashi, T.; Taniguchi, K.; Amano, A.; et al. Detection of cariogenic streptococcus mutans in extirpated heart valve and atheromatous plaque specimens. J. Clin. Microbiol. 2006, 44, 3313–3317. [Google Scholar] [CrossRef] [PubMed]
- Hajishengallis, G.; Wang, M.; Bagby, G.J.; Nelson, S. Importance of TLR2 in early innate immune response to acute pulmonary infection with Porphy-romonas gingivalis in mice. J. Immunol. 2008, 181, 4141–4149. [Google Scholar] [CrossRef]
- Sonti, R.; Fleury, C. Fusobacterium necrophorum presenting as isolated lung nodules. Respir. Med. Case Rep. 2015, 15, 80–82. [Google Scholar] [CrossRef][Green Version]
- Williams, D.M.; Kerber, C.A.; Tergin, H.F. Unusual presentation of Lemierre’s syndrome due to Fusobacterium nucleatum. J. Clin. Microbiol. 2003, 41, 3445–3448. [Google Scholar] [CrossRef]
- Gomes-Filho, I.S.; De Oliveira, T.F.L.; Da Cruz, S.S.; Passos-Soares, J.D.S.; Trindade, S.C.; Oliveira, M.T.; Souza-Machado, A.; Cruz, A.A.; Barreto, M.L.; Seymour, G.J. Influence of periodontitis in the development of nosocomial pneumonia: A case control study. J. Periodontol. 2014, 85, e82–e90. [Google Scholar] [CrossRef]
- Heo, S.M.; Sung, R.S.; Scannapieco, F.A.; Haase, E.M. Genetic relationships between Candida albicans strains isolated from dental plaque, trachea, and bron-choalveolar lavage fluid from mechanically ventilated intensive care unit patients. J. Oral Microbiol. 2011, 3, 6362. [Google Scholar] [CrossRef]
- Tonetto, M.R.; Rocatto, G.; Matos, F.Z.; Pedro, F.M.; Lima, S.L.; Aranha, A.F.; Porto, A.N.; Borges, A.H.; Borba, A.M.; Patil, S.; et al. Periodontal and microbiological profile of intensive care unit inpatients. J. Contemp. Dent. Pract. 2016, 17, 807–814. [Google Scholar] [CrossRef]
- Kaur, M.; Geisinger, M.L.; Geurs, N.C.; Griffin, R.; Vassilopoulos, P.J.; Vermeulen, L.; Haigh, S.; Reddy, M.S. Effect of intensive oral hygiene regimen during pregnancy on periodontal health, cytokine levels, and pregnancy outcomes: A pilot study. J. Periodontol. 2014, 85, 1684–1692. [Google Scholar] [CrossRef] [PubMed]
- Lin, D.; Moss, K.; Beck, J.D.; Hefti, A.; Offenbacher, S. Persistently high levels of periodontal pathogens associated with preterm pregnancy outcome. J. Periodontol. 2007, 78, 833–841. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Wang, X. Mobile microbiome: Oral bacteria in extra-oral infections and inflammation. J. Dent. Res. 2013, 92, 485–491. [Google Scholar] [CrossRef] [PubMed]
- Katz, J.; Chegini, N.; Shiverick, K.; Lamont, R. Localization of P. gingivalis in preterm delivery placenta. J. Dent. Res. 2009, 88, 575–578. [Google Scholar] [CrossRef] [PubMed]
- Reyes, L.; Phillips, P.; Wolfe, B.; Golos, T.G.; Walkenhorst, M.; Progulske-Fox, A.; Brown, M. Porphyromonas gingivalis and adverse pregnancy outcome. J. Oral Microbiol. 2017, 9, 1374153. [Google Scholar] [CrossRef]
- Potempa, J.; Mydel, P.; Koziel, J. The case for periodontitis in the pathogenesis of rheumatoid arthritis. Nat. Rev. Rheumatol. 2017, 13, 606–620. [Google Scholar] [CrossRef]
- Khan, R.; Adil, M.; Danishuddin, M.; Verma, P.K.; Khan, A.U. In vitro and in vivo inhibition of Streptococcus mutans biofilm by Trachyspermum ammi seeds: An approach of alternative medicine. Phytomedicine 2012, 19, 747–755. [Google Scholar] [CrossRef]
- Tiwari, R.; Rana, C. Plant secondary metabolites: A review. Int. J. Eng. Res. Gen. Sci. 2015, 3, 661–670. [Google Scholar]
- Gorlenko, C.L.; Kiselev, H.Y.; Budanova, E.V.; Zamyatnin, A.A.; Ikryannikova, L.N. Plant secondary metabolites in the battle of drugs and drug-resistant bacteria: New heroes or worse clones of antibiotics? Antibiotics 2020, 9, 170. [Google Scholar] [CrossRef] [PubMed]
- Hussein, R.A.; El-Anssary, A.A. Plants secondary metabolites: The key drivers of the pharmacological actions of medicinal plants. Herb. Med. 2019, 1, 13. [Google Scholar] [CrossRef]
- Anand, U.; Jacobo-Herrera, N.; Altemimi, A.; Lakhssassi, N. A comprehensive review on medicinal plants as antimicrobial therapeutics: Potential avenues of biocompatible drug discovery. Metabolites 2019, 9, 258. [Google Scholar] [CrossRef]
- Boudet, A.-M. Evolution and current status of research in phenolic compounds. Phytochemistry 2007, 68, 2722–2735. [Google Scholar] [CrossRef]
- Cohen, S.D.; Kennedy, J.A. Plant metabolism and the environment: Implications for managing phenolics. Crit. Rev. Food Sci. Nutr. 2010, 50, 620–643. [Google Scholar] [CrossRef]
- Zahin, M.; Aqil, F.; Khan, M.S.; Ahmad, I. Ethnomedicinal plants derived antibacterials and their prospects. In Ethnomedicine: A Source of Complementary Therapeutics; Research Signpost: Thiruvananthapuram, India, 2010; pp. 149–178. [Google Scholar]
- Carocho, M.; Barreiro, M.F.; Morales, P.; Ferreira, I.C. Adding molecules to food, pros and cons: A review on synthetic and natural food additives. Compr. Rev. Food Sci. Food Saf. 2014, 13, 377–399. [Google Scholar] [CrossRef]
- Silva, N.; Júnior, A.F. Biological properties of medicinal plants: A review of their antimicrobial activity. J. Venom. Anim. Toxins Incl. Trop. Dis. 2010, 16, 402–413. [Google Scholar] [CrossRef]
- Hassan, B.A.R. Medicinal plants (importance and uses). Pharm. Anal. Acta 2012, 3, 2153–2435. [Google Scholar] [CrossRef]
- Górniak, I.; Bartoszewski, R.; Króliczewski, J. Comprehensive review of antimicrobial activities of plant flavonoids. Phytochem. Rev. 2019, 18, 241–272. [Google Scholar] [CrossRef]
- Zhang, L.; Kong, Y.; Wu, D.; Zhang, H.; Wu, J.; Chen, J.; Ding, J.; Hu, L.; Jiang, H.; Shen, X. Three flavonoids targeting the β-hydroxyacyl-acyl carrier protein dehydratase from Helicobacter pylori: Crystal structure characterization with enzymatic inhibition assay. Protein Sci. 2008, 17, 1971–1978. [Google Scholar] [CrossRef] [PubMed]
- Taylor, P.W.; Hamilton-Miller, J.M.T.; Stapleton, P.D. Antimicrobial properties of green tea catechins. Food Sci. Technol. Bull. Funct. Foods 2005, 2, 71–81. [Google Scholar] [CrossRef] [PubMed]
- Koo, H.; Hayacibara, M.F.; Schobel, B.D.; Cury, J.A.; Rosalen, P.L.; Park, Y.K.; Vacca-Smith, A.M.; Bowen, W.H. Inhibition of Streptococcus mutans biofilm accumulation and polysaccharide production by apigenin and tt-farnesol. J. Antimicrob. Chemother. 2003, 52, 782–789. [Google Scholar] [CrossRef] [PubMed]
- Hasan, S.; Singh, K.; Danisuddin, M.; Verma, P.K.; Khan, A.U. Inhibition of major virulence pathways of Streptococcus mutans by quercitrin and deoxynojirimycin: A synergistic approach of infection control. PLoS ONE 2014, 9, e91736. [Google Scholar] [CrossRef]
- Pagare, S.; Bhatia, M.; Tripathi, N.; Pagare, S.; Bansal, Y.K. Secondary metabolites of plants and their role: Overview. Curr. Trends Biotechnol. Pharm. 2015, 9, 293–304. [Google Scholar]
- Fokialakis, N.; Skaltsounis, A.L. Natural resins and bioactive natural products thereof as potential anitimicrobial agents. Curr. Pharm. Des. 2011, 17, 1267–1290. [Google Scholar] [CrossRef]
- Savoia, D. Plant-derived antimicrobial compounds: Alternatives to antibiotics. Futur. Microbiol. 2012, 7, 979–990. [Google Scholar] [CrossRef]
- Bazaka, K.; Jacob, M.V.; Chrzanowski, W.; Ostrikov, K. Anti-bacterial surfaces: Natural agents, mechanisms of action, and plasma surface modification. RSC Adv. 2015, 5, 48739–48759. [Google Scholar] [CrossRef]
- Sen, T.; Samanta, S.K. Medicinal plants, human health and biodiversity: A broad review. Adv. Biochem. Eng. Biotechnol. 2015, 147, 59–110. [Google Scholar]
- Patel, D.M.; Chauhan, J.B.; Ishnava, K.B. Studies on the anticariogenic potential of medicinal plant seed and fruit extracts. In Natural Oral Care in Dental Therapy; Wiley: Hoboken, NJ, USA, 2020; pp. 81–96. [Google Scholar]
- Cazella, L.N.; Glamoclija, J.; Soković, M.; Gonçalves, J.E.; Linde, G.A.; Colauto, N.B.; Gazim, Z.C. Antimicrobial activity of essential oil of Baccharis dracunculifolia DC (Asteraceae) aerial parts at flowering period. Front. Plant Sci. 2019, 10, 27. [Google Scholar] [CrossRef]
- Boeing, T.; Costa, P.; Venzon, L.; Meurer, M.; Mariano, L.N.B.; França, T.C.S.; Gouveia, L.; De Bassi, A.C.; Steimbach, V.; De Souza, P.; et al. Gastric healing effect of p-coumaric acid isolated from Baccharis dracunculifolia DC on animal model. Naunyn-Schmiedebergs Arch. Pharmacol. 2021, 394, 49–57. [Google Scholar] [CrossRef]
- Luchesi, L.A.; Paulus, D.; Busso, C.; Frata, M.T.; Oliveira, J.B. Chemical composition, antifungal and antioxidant activity of essential oils from Baccharis dracunculifolia and Pogostemon cablin against Fusarium graminearum. Nat. Prod. Res. 2020, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Lemos, M.; De Barros, M.P.; Sousa, J.P.B.; Filho, A.A.D.S.; Bastos, J.K.; De Andrade, S.F. Baccharis dracunculifolia, the main botanical source of Brazilian green propolis, displays antiulcer activity†. J. Pharm. Pharmacol. 2010, 59, 603–608. [Google Scholar] [CrossRef] [PubMed]
- Gilabert, M.; Ramos, A.N.; Schiavone, M.M.; Arena, M.E.; Bardoón, A. Bioactive sesqui- and diterpenoids from the Argentine Liverwort Porella chilensis. J. Nat. Prod. 2011, 74, 574–579. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.; Lee, J.-H.; Kim, S.-I.; Cho, M.H.; Lee, J. Anti-biofilm, anti-hemolysis, and anti-virulence activities of black pepper, cananga, myrrh oils, and nerolidol against Staphylococcus aureus. Appl. Microbiol. Biotechnol. 2014, 98, 9447–9457. [Google Scholar] [CrossRef]
- Piao, M.J.; Yoo, E.S.; Koh, Y.S.; Kang, H.K.; Kim, J.; Kim, Y.J.; Kang, H.H.; Hyun, J.W. Antioxidant effects of the ethanol extract from flower of Camellia japonica via scavenging of reactive oxygen species and induction of antioxidant enzymes. Int. J. Mol. Sci. 2011, 12, 2618–2630. [Google Scholar] [CrossRef]
- Kim, K.Y.; Davidson, P.M.; Chung, H.J. Antibacterial activity in extracts of Camellia japonica L. Petals and its application to a model food system. J. Food Prot. 2001, 64, 1255–1260. [Google Scholar] [CrossRef] [PubMed]
- Nam, H.H.; Nan, L.; Choo, B.K. Inhibitory effects of Camellia japonica on cell inflammation and acute rat reflux esopha-gitis. Chin. Med. 2021, 16, 1–12. [Google Scholar] [CrossRef]
- Zhang, Z.H.; Mi, C.; Wang, K.S.; Wang, Z.; Li, M.Y.; Zuo, H.X.; Xu, G.H.; Li, X.; Piao, L.X.; Ma, J.; et al. Chelidonine inhibits TNF-α-induced inflammation by suppressing the NF-κB pathways in HCT116 cells. Phytother. Res. 2018, 32, 65–75. [Google Scholar] [CrossRef]
- Dobrucka, R.; Dlugaszewska, J.; Kaczmarek, M. Cytotoxic and antimicrobial effects of biosynthesized ZnO nanoparticles using of Chelidonium majus extract. Biomed. Microdevices 2018, 20, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.-H.; Li, H.; Wu, Q.; Lee, H.J.; Ryu, J.-H. A new labdane diterpenoid with anti-inflammatory activity from Thuja orientalis. J. Ethnopharmacol. 2013, 146, 760–767. [Google Scholar] [CrossRef]
- Chakraborty, S.; Afaq, N.; Singh, N.; Majumdar, S. Antimicrobial activity of Cannabis sativa, Thuja orientalis and Psidium guajava leaf extracts against methicillin-resistant Staphylococcus aureus. J. Integr. Med. 2018, 16, 350–357. [Google Scholar] [CrossRef]
- Choi, H.-A.; Cheong, D.-E.; Lim, H.-D.; Kim, W.-H.; Ham, M.-H.; Oh, M.-H.; Wu, Y.; Shin, H.-J.; Kim, G.-J. Antimicrobial and anti-biofilm activities of the methanol extracts of medicinal plants against dental pathogens Streptococcus mutans and Candida albicans. J. Microbiol. Biotechnol. 2017, 27, 1242–1248. [Google Scholar] [CrossRef]
- Cushnie, T.T.; Lamb, A.J. Antimicrobial activity of flavonoids. Int. J. Antimicrob. Agents 2005, 26, 343–356. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Yang, W.; Tang, F.; Chen, X.; Ren, L. Antibacterial activities of flavonoids: Structure-activity relationship and mechanism. Curr. Med. Chem. 2014, 22, 132–149. [Google Scholar] [CrossRef] [PubMed]
- Dorri, M.; Hashemitabar, S.; Hosseinzadeh, H. Cinnamon (Cinnamomum zeylanicum) as an antidote or a protective agent against natural or chemical toxicities: A review. Drug Chem. Toxicol. 2018, 41, 338–351. [Google Scholar] [CrossRef]
- Kerekes, E.B.; Vidács, A.; Takó, M.; Petkovits, T.; Vágvölgyi, C.; Horváth, G.; Balázs, V.L.; Krisch, J. Anti-biofilm effect of selected essential oils and main components on mono- and polymicrobic bacterial cultures. Microorganisms 2019, 7, 345. [Google Scholar] [CrossRef] [PubMed]
- Bardají, D.; Reis, E.; Medeiros, T.; Lucarini, R.; Crotti, A.; Martins, C. Antibacterial activity of commercially available plant-derived essential oils against oral pathogenic bacteria. Nat. Prod. Res. 2015, 30, 1178–1181. [Google Scholar] [CrossRef]
- Taguchi, Y.; Takizawa, T.; Ishibashi, H.; Sagawa, T.; Arai, R.; Inoue, S.; Yamaguchi, H.; Abe, S. Therapeutic effects on murine oral candidiasis by oral administration of Cassia (Cinnamomum cassia) preparation. Nippon. Ishinkin Gakkai Zasshi 2010, 51, 13–21. [Google Scholar] [CrossRef]
- Ali, I.A.; Cheung, B.P.; Matinlinna, J.; Lévesque, C.M.; Neelakantan, P. Trans-cinnamaldehyde potently kills Enterococcus faecalis biofilm cells and prevents biofilm recovery. Microb. Pathog. 2020, 149, 104482. [Google Scholar] [CrossRef] [PubMed]
- Durgadevi, R.; Ravi, A.V.; Alexpandi, R.; Swetha, T.K.; Abirami, G.; Vishnu, S.; Pandian, S.K. Virulence targeted inhibitory effect of linalool against the exclusive uropathogen Proteus mirabilis. Biofouling 2019, 35, 508–525. [Google Scholar] [CrossRef]
- Kačániová, M.; Galovičová, L.; Ivanišová, E.; Vukovic, N.L.; Štefániková, J.; Valková, V.; Borotová, P.; Žiarovská, J.; Terentjeva, M.; Felšöciová, S.; et al. Antioxidant, antimicrobial and antibiofilm activity of coriander (Coriandrum sativum L.) essential oil for its application in foods. Foods 2020, 9, 282. [Google Scholar] [CrossRef]
- Can, E.; Kizak, V.; Can, Ş.S.; Özçiçek, E. Anesthetic efficiency of three medicinal plant oils for aquatic species: Coriander Coriandrum sativum, linaloe tree Bursera delpechiana, and lavender Lavandula hybrida. J. Aquat. Anim. Health 2019, 31, 266–273. [Google Scholar] [CrossRef] [PubMed]
- Bersan, S.M.F.; Galvão, L.C.C.; Goes, V.F.F.; Sartoratto, A.; Figueira, G.M.; Rehder, V.L.G.; Alencar, S.M.; Duarte, R.M.T.; Rosalen, P.L.; Duarte, M.C.T. Action of essential oils from Brazilian native and exotic medicinal species on oral biofilms. BMC Complement. Altern. Med. 2014, 14, 451. [Google Scholar] [CrossRef]
- Mukherjee, K.; Tribedi, P.; Mukhopadhyay, B.; Sil, A.K. Antibacterial activity of long-chain fatty alcohols against mycobacteria. FEMS Microbiol. Lett. 2013, 338, 177–183. [Google Scholar] [CrossRef] [PubMed]
- Ames-Sibin, A.P.; Barizão, C.L.; Castro-Ghizoni, C.V.; Silva, F.M.S.; Sá-Nakanishi, A.B.; Bracht, L.; Bersani-Amado, C.A.; Marçal-Natali, M.R.; Bracht, A.; Comar, J.F. β-Caryophyllene, the major constituent of copaiba oil, reduces systemic inflammation and oxidative stress in arthritic rats. J. Cell. Biochem. 2018, 119, 10262–10277. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, V.P.; Arruda, C.; Da Silva, J.J.M.; Mejia, J.A.A.; Furtado, N.A.J.C.; Bastos, J.K. Use of spinning band distillation equipment for fractionation of volatile compounds of Copaifera oleoresins for developing a validated gas chromatographic method and evaluating antimicrobial activity. Biomed. Chromatogr. 2019, 33, e4412. [Google Scholar] [CrossRef]
- Símaro, G.V.; Lemos, M.; da Silva, J.J.M.; Ribeiro, V.P.; Arruda, C.; Schneider, A.H.; Wanderley, C.W.D.S.; Carneiro, L.J.; Mariano, R.L.; Ambrósio, S.R.; et al. Antinociceptive and anti-inflammatory activities of Copaifera pubiflora Benth oleoresin and its major metabolite ent-hardwickiic acid. J. Ethnopharmacol. 2021, 271, 113883. [Google Scholar] [CrossRef]
- Moraes, T.D.S.; Leandro, L.F.; Santiago, M.B.; Silva, L.D.O.; Bianchi, T.C.; Veneziani, R.C.S.; Ambrósio, S.R.; Ramos, S.B.; Bastos, J.K.; Martins, C.H.G. Assessment of the antibacterial, antivirulence, and action mechanism of Copaifera pubiflora oleoresin and isolated compounds against oral bacteria. Biomed. Pharmacother. 2020, 129, 110467. [Google Scholar] [CrossRef]
- Carneiro, L.J.; Tasso, T.O.; Santos, M.F.; Goulart, M.O.; Santos, R.A.; Bastos, J.K.; da Silva, J.J.; Crotti, A.E.; Parreira, R.L.; Orenha, R.P.; et al. Copaifera multijuga, Copaifera pubiflora and Copaifera trapezifolia Oleoresins: Chemical characterization and in vitro cytotoxic potential against tumoral cell lines. J. Braz. Chem. Soc. 2020, 31, 1679–1689. [Google Scholar] [CrossRef]
- Ekpenyong, C.E.; Akpan, E.E. Use of Cymbopogon citratus essential oil in food preservation: Recent advances and future perspectives. Crit. Rev. Food Sci. Nutr. 2017, 57, 2541–2559. [Google Scholar] [CrossRef]
- Hacke, A.C.M.; Miyoshi, E.; Marques, J.A.; Pereira, R.P. Anxiolytic properties of Cymbopogon citratus (DC.) stapf extract, essential oil and its constituents in zebrafish (Danio rerio). J. Ethnopharmacol. 2020, 260, 113036. [Google Scholar] [CrossRef]
- Oliveira, J.B.; Teixeira, M.A.; Paiva, L.F.; Oliveira, R.F.; Mendonça, A.R.; Brito, M.J. In vitro and in vivo antimicrobial activity of Cymbopogon citratus (DC.) Stapf. against Staphylococcus spp. isolated from newborn babies in an intensive care unit. Microb. Drug Resist. 2019, 25, 1490–1496. [Google Scholar] [CrossRef]
- Ortega-Ramirez, L.A.; Gutiérrez-Pacheco, M.M.; Vargas-Arispuro, I.; González-Aguilar, G.A.; Martínez-Téllez, M.A.; Ayala-Zavala, J.F. Inhibition of glucosyltransferase activity and glucan production as an antibiofilm mechanism of lemongrass essential oil against Escherichia coli O157:H7. Antibiotics 2020, 9, 102. [Google Scholar] [CrossRef]
- Ortega-Cuadros, M.; Tofiño-Rivera, A.P.; Merini, L.J.; Martínez-Pabón, M.C. Antimicrobial activity of Cymbopogon citratus (Poaceae) on Streptococcus mutans biofilm and its cytotoxic effects. Rev. Biol. Trop. 2018, 66, 1519–1529. [Google Scholar] [CrossRef]
- Tofiño-Rivera, A.; Ortega-Cuadros, M.; Galvis-Pareja, D.; Jiménez-Rios, H.; Merini, L.; Martínez-Pabón, M. Effect of Lippia alba and Cymbopogon citratus essential oils on biofilms of Streptococcus mutans and cytotoxicity in CHO cells. J. Ethnopharmacol. 2016, 194, 749–754. [Google Scholar] [CrossRef] [PubMed]
- Chaves-Quirós, C.; Usuga-Usuga, J.-S.; Morales-Uchima, S.-M.; Tofiño-Rivera, A.-P.; Tobón-Arroyave, S.-I.; Martínez-Pabón, M.-C.; Rivera, A.T. Assessment of cytotoxic and antimicrobial activities of two components of Cymbopogon citratus essential oil. J. Clin. Exp. Dent. 2020, 12, e749–e754. [Google Scholar] [CrossRef]
- González-Burgos, E.; Liaudanskas, M.; Viškelis, J.; Žvikas, V.; Janulis, V.; Gómez-Serranillos, M.P. Antioxidant activity, neuroprotective properties and bioactive constituents analysis of varying polarity extracts from Eucalyptus globulus leaves. J. Food Drug Anal. 2018, 26, 1293–1302. [Google Scholar] [CrossRef] [PubMed]
- Tsukatani, T.; Sakata, F.; Kuroda, R.; Akao, T. Biofilm eradication activity of herb and spice extracts alone and in combination against oral and food-borne pathogenic bacteria. Curr. Microbiol. 2020, 77, 2486–2495. [Google Scholar] [CrossRef]
- Nagata, H.; Inagaki, Y.; Yamamoto, Y.; Maeda, K.; Kataoka, K.; Osawa, K.; Shizukuishi, S. Inhibitory effects of macrocarpals on the biological activity of Porphyromonas gingivalis and other periodontopathic bacteria. Oral Microbiol. Immunol. 2006, 21, 159–163. [Google Scholar] [CrossRef]
- Sebei, K.; Sakouhi, F.; Herchi, W.; Khouja, M.L.; Boukhchina, S. Chemical composition and antibacterial activities of seven Eucalyptus species essential oils leaves. Biol. Res. 2015, 48, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Issarachot, P.; Sangkaew, W.; Sianglum, W.; Saeloh, D.; Limsuwan, S.; Voravuthikunchai, S.P.; Joycharat, N. α-glucosidase inhibitory, antibacterial, and antioxidant activities of natural substances from the wood of Derris reticulata Craib. Nat. Prod. Res. 2019, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Pulbutr, P.; Rattanakiat, S.; Phetsaardeiam, N.; Modtaku, P.; Denchai, R.; Jaruchotikamol, A.; Khunawattanakul, W. Anticariogenic activities of Derris reticulata ethanolic stem extract against Streptococcus mutans. Pak. J. Biol. Sci. 2018, 21, 300–306. [Google Scholar] [CrossRef] [PubMed]
- Getie, M.; Gebre-Mariam, T.; Rietz, R.; Höhne, C.; Huschka, C.; Schmidtke, M.; Abate, A.; Neubert, R. Evaluation of the anti-microbial and anti-inflammatory activities of the medicinal plants Dodonaea viscosa, Rumex nervosus and Rumex abyssinicus. Fitoterapia 2003, 74, 139–143. [Google Scholar] [CrossRef]
- Khalil, N.; Sperotto, J.; Manfron, M. Antiinflammatory activity and acute toxicity of Dodonaea viscosa. Fitoterapia 2006, 77, 478–480. [Google Scholar] [CrossRef] [PubMed]
- Qureshi, S.; Khan, M.; Ahmad, M. A survey of useful medicinal plants of Abbottabad in northern Pakistan. Trakia J. Sci. 2008, 6, 39–51. [Google Scholar]
- Naidoo, R.; Patel, M.; Gulube, Z.; Fenyvesi, I. Inhibitory activity of Dodonaea viscosa var. angustifolia extract against Streptococcus mutans and its biofilm. J. Ethnopharmacol. 2012, 144, 171–174. [Google Scholar] [CrossRef] [PubMed]
- Karkanis, A.; Martins, N.; Petropoulos, S.; Ferreira, I. Phytochemical composition, health effects, and crop management of liquorice (Glycyrrhiza glabra L.): A medicinal plant. Food Rev. Int. 2016, 34, 182–203. [Google Scholar] [CrossRef]
- Chakotiya, A.S.; Tanwar, A.; Narula, A.; Sharma, R.K. Alternative to antibiotics against Pseudomonas aeruginosa: Effects of Glycyrrhiza glabra on membrane permeability and inhibition of efflux activity and biofilm formation in Pseudomonas aeruginosa and its in vitro time-kill activity. Microb. Pathog. 2016, 98, 98–105. [Google Scholar] [CrossRef]
- Suwannakul, S.; Chaibenjawong, P. Antibacterial activities of Glycyrrhiza gabra Linn. (licorice) root extract against Porphyromonas gingivalis rand its inhibitory effects on cysteine proteases and biofilms. J. Dent. Indones. 2017, 24, 85–92. [Google Scholar] [CrossRef]
- Kim, S.-R.; Jeon, H.-J.; Park, H.-J.; Kim, M.-K.; Choi, W.-S.; Jang, H.-O.; Bae, S.-K.; Jeong, C.-H.; Bae, M.-K. Glycyrrhetinic acid inhibits Porphyromonas gingivalis lipopolysaccharide-induced vascular permeability via the suppression of interleukin-8. Inflamm. Res. 2012, 62, 145–154. [Google Scholar] [CrossRef]
- Hennebelle, T.; Sahpaz, S.; Joseph, H.; Bailleul, F. Ethnopharmacology of Lippia alba. J. Ethnopharmacol. 2008, 116, 211–222. [Google Scholar] [CrossRef]
- Mączka, W.; Wińska, K.; Grabarczyk, M. One hundred faces of geraniol. Molecules 2020, 25, 3303. [Google Scholar] [CrossRef]
- Nikavar, B.; Ali, N.A.; Kamalnezhad, M. Evaluation of the antioxidant properties of five Mentha species. Iran J. Pharm. Res. 2008. [Google Scholar] [CrossRef]
- Shafiei, Z.; Rahim, Z.H.; Philip, K.; Thurairajah, N.; Yaacob, H. Potential effects of Psidium sp., Mangifera sp., Mentha sp. and its mixture (PEM) in reducing bacterial populations in biofilms, adherence and acid production of S. sanguinis and S. mutans. Arch. Oral Biol. 2020, 109, 104554. [Google Scholar] [CrossRef] [PubMed]
- Wi, W.N.; Fathilah, A.; Rahim, Z. Plant extracts of Psidium guajava, Mangifera and Mentha sp. inhibit the growth of the population of single-species oral biofilm. Altern. Integr. Med. 2013, 31, 1–6. [Google Scholar]
- Rahim, Z.H.A.; Shaikh, S.; Ismail, W.N.H.W.; Harun, W.H.-A.W.; Razak, F.A. The effect of selected plant extracts on the development of single-species dental biofilms. J. Coll. Physicians Surg. Pak. 2014, 24, 796–801. [Google Scholar] [PubMed]
- Shafiei, Z.; Rahim, Z.H.; Philip, K.; Thurairajah, N. Antibacterial and anti-adherence effects of a plant extract mixture (PEM) and its individual constituent ex-tracts (Psidium sp., Mangifera sp., and Mentha sp.) on single- and dual-species biofilms. PeerJ 2016, 4, e2519. [Google Scholar] [CrossRef]
- Aleksic, V.; Knezevic, P. Antimicrobial and antioxidative activity of extracts and essential oils of Myrtus communis L. Microbiol. Res. 2014, 169, 240–254. [Google Scholar] [CrossRef]
- Kaya, D.A.; Ghica, M.V.; Dănilă, E.; Öztürk, Ş.; Türkmen, M.; Kaya, M.G.A.; Dinu-Pîrvu, C.-E. Selection of optimal operating conditions for extraction of Myrtus Communis L. essential oil by the steam distillation method. Molecules 2020, 25, 2399. [Google Scholar] [CrossRef]
- Sateriale, D.; Imperatore, R.; Colicchio, R.; Pagliuca, C.; Varricchio, E.; Volpe, M.G.; Salvatore, P.; Paolucci, M.; Pagliarulo, C. Phytocompounds vs. dental plaque bacteria: In vitro effects of myrtle and pomegranate polyphenolic extracts against single-species and multispecies oral biofilms. Front. Microbiol. 2020, 11, 11. [Google Scholar] [CrossRef]
- Sateriale, D.; Facchiano, S.; Colicchio, R.; Pagliuca, C.; Varricchio, E.; Paolucci, M.; Volpe, M.G.; Salvatore, P.; Pagliarulo, C. In vitro synergy of polyphenolic extracts from honey, myrtle and pomegranate against oral pathogens, S. mutans and R. dentocariosa. Front. Microbiol. 2020, 11, 1465. [Google Scholar] [CrossRef] [PubMed]
- Panossian, A.; Wikman, G.; Sarris, J. Rosenroot (Rhodiola rosea): Traditional use, chemical composition, pharmacology and clinical efficacy. Phytomedicine 2010, 17, 481–493. [Google Scholar] [CrossRef] [PubMed]
- Chiang, H.-M.; Chen, H.-C.; Wu, C.-S.; Wu, P.-Y.; Wen, K.-C. Rhodiola plants: Chemistry and biological activity. J. Food Drug Anal. 2015, 23, 359–369. [Google Scholar] [CrossRef] [PubMed]
- Elameen, A.; Kosman, V.M.; Thomsen, M.; Pozharitskaya, O.N.; Shikov, A.N. Variability of major phenyletanes and phenylpropanoids in 16-year-old Rhodiola rosea L. clones in Norway. Molecules 2020, 25, 3463. [Google Scholar] [CrossRef]
- Zhang, Z.; Liu, Y.; Lu, M.; Lyu, X.; Gong, T.; Tang, B.; Wang, L.; Zeng, J.; Li, Y. Rhodiola rosea extract inhibits the biofilm formation and the expression of virulence genes of cariogenic oral pathogen Streptococcus mutans. Arch. Oral Biol. 2020, 116, 104762. [Google Scholar] [CrossRef]
- De Oliveira, J.R.; Camargo, S.E.A.; De Oliveira, L.D. Rosmarinus officinalis L. (rosemary) as therapeutic and prophylactic agent. J. Biomed. Sci. 2019, 26, 1–22. [Google Scholar] [CrossRef]
- Birtić, S.; Dussort, P.; Pierre, F.-X.; Bily, A.C.; Roller, M. Carnosic acid. Phytochemistry 2015, 115, 9–19. [Google Scholar] [CrossRef]
- Mirpour, M.; Siahmazgi, Z.G.; Kiasaraie, M.S. Antibacterial activity of clove, gall nut methanolic and ethanolic extracts on Streptococcus mutans PTCC 1683 and Streptococcus salivarius PTCC 1448. J. Oral Biol. Craniofacial Res. 2015, 5, 7–10. [Google Scholar] [CrossRef]
- Philander, L.A. An ethnobotany of Western Cape Rasta bush medicine. J. Ethnopharmacol. 2011, 138, 578–594. [Google Scholar] [CrossRef]
- Akhalwaya, S.; van Vuuren, S.; Patel, M. An in vitro investigation of indigenous South African medicinal plants used to treat oral infections. J. Ethnopharmacol. 2018, 210, 359–371. [Google Scholar] [CrossRef] [PubMed]
- Nikolić, M.; Glamočlija, J.; Ferreira, I.C.; Calhelha, R.C.; Fernandes, Â.; Marković, T.; Marković, D.; Giweli, A.; Soković, M. Chemical composition, antimicrobial, antioxidant and antitumor activity of Thymus serpyllum L., Thymus algeriensis Boiss. and Reut and Thymus vulgaris L. essential oils. Ind. Crops Prod. 2014, 52, 183–190. [Google Scholar] [CrossRef]
- De Oliveira Carvalho, I.; Purgato, G.A.; Píccolo, M.S.; Pizziolo, V.R.; Coelho, R.R.; Diaz-Muñoz, G.; Diaz, M.A.N. In vitro anticariogenic and antibiofilm activities of toothpastes formulated with essential oils. Arch. Oral Biol. 2020, 117, 104834. [Google Scholar] [CrossRef] [PubMed]
- Dong, J.; Zhang, L.; Liu, Y.; Xu, N.; Zhou, S.; Yang, Q.; Yang, Y.; Ai, X. Thymol protects channel catfish from Aeromonas hydrophila infection by inhibiting aerolysin expression and biofilm formation. Microorganisms 2020, 8, 636. [Google Scholar] [CrossRef] [PubMed]
- Hajiaghapour, M.; Rezaeipour, V. Comparison of two herbal essential oils, probiotic, and mannan-oligosaccharides on egg production, hatchability, serum metabolites, intestinal morphology, and microbiota activity of quail breeders. Livest. Sci. 2018, 210, 93–98. [Google Scholar] [CrossRef]
- Vitali, L.A.; Beghelli, D.; Nya, P.C.B.; Bistoni, O.; Cappellacci, L.; Damiano, S.; Lupidi, G.; Maggi, F.; Orsomando, G.; Papa, F.; et al. Diverse biological effects of the essential oil from Iranian Trachyspermum ammi. Arab. J. Chem. 2016, 9, 775–786. [Google Scholar] [CrossRef]
- Dahake, P.; Dadpe, M.; Dhore, S.; Kale, Y.; Kendre, S.; Siddiqui, A. Evaluation of antimicrobial efficacy of Trachyspermum ammi (Ajwain) oil and chlorhexidine against oral bacteria: An in vitro study. J. Indian Soc. Pedod. Prev. Dent. 2018, 36, 357–363. [Google Scholar] [CrossRef]
- Murugan, K.; Sekar, K.; Sangeetha, S.; Ranjitha, S.; Sohaibani, S.A. Antibiofilm and quorum sensing inhibitory activity of Achyranthes aspera on cariogenic Streptococcus mutans: An in vitro and in silico study. Pharm. Biol. 2013, 51, 728–736. [Google Scholar] [CrossRef]
- Yang, Y.; Hwang, E.-H.; Park, B.-I.; Choi, N.-Y.; Kim, K.-J.; You, Y.-O. Artemisia princeps inhibits growth, biofilm formation, and virulence factor expression of Streptococcus mutans. J. Med. Food 2019, 22, 623–630. [Google Scholar] [CrossRef]
- Teanpaisan, R.; Senapong, S.; Puripattanavong, J. In vitro antimicrobial and antibiofilm activity of Artocarpus Lakoocha (Moraceae) extract against some oral pathogens. Trop. J. Pharm. Res. 2014, 13, 1149. [Google Scholar] [CrossRef]
- Geethashri, A.; Manikandan, R.; Ravishankar, B.; Shetty, A.V. Comparative evaluation of biofilm suppression by plant extracts on oral pathogenic bacteria. J. Appl. Pharm. Sci. 2014, 4, 20. [Google Scholar]
- Pereira, C.A.; Costa, A.C.B.P.; Liporoni, P.C.S.; Rego, M.A.; Jorge, A.O.C. Antibacterial activity of Baccharis dracunculifolia in planktonic cultures and biofilms of Streptococcus mutans. J. Infect. Public Health 2016, 9, 324–330. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.-H. Antimicrobial effects of herbal extracts on Streptococcus mutans and normal oral streptococci. J. Microbiol. 2013, 51, 484–489. [Google Scholar] [CrossRef] [PubMed]
- Alshahrani, A.M.; Gregory, R.L. In vitro Cariostatic effects of cinnamon water extract on nicotine-induced Streptococcus mutans biofilm. BMC Complement. Med. Ther. 2020, 20, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Wiwattanarattanabut, K.; Choonharuangdej, S.; Srithavaj, T. In vitro anti-cariogenic plaque effects of essential oils extracted from culinary herbs. J. Clin. Diagn. Res. 2017, 11, DC30–DC35. [Google Scholar] [CrossRef]
- Azizan, N.; Mohd-Said, S.; Mazlan, M.K.; Chelvan, K.T.; Hanafiah, R.M.; Zainal-Abidin, Z. In-vitro inhibitory effect of Cinnamomum zeylanicum and Eugenia caryophyllata oils on multispecies anaerobic oral biofilm. J. Int. Dent. Med. Res. 2019, 12, 411–417. [Google Scholar]
- Wang, Y.; Zhang, Y.; Shi, Y.-Q.; Pan, X.-H.; Lu, Y.-H.; Cao, P. Antibacterial effects of cinnamon (Cinnamomum zeylanicum) bark essential oil on Porphyromonas gingivalis. Microb. Pathog. 2018, 116, 26–32. [Google Scholar] [CrossRef]
- Hickl, J.; Argyropoulou, A.; Sakavitsi, M.E.; Halabalaki, M.; Al-Ahmad, A.; Hellwig, E.; Aligiannis, N.; Skaltsounis, A.L.; Wittmer, A.; Vach, K.; et al. Mediterranean herb extracts inhibit microbial growth of representative oral microorganisms and biofilm formation of Streptococcus mutans. PLoS ONE 2018, 13, e0207574. [Google Scholar] [CrossRef]
- Barbieri, D.S.; Tonial, F.; Lopez, P.V.; Maia, B.H.; Santos, G.D.; Ribas, M.O.; Glienke, C.; Vicente, V.A. Antiadherent activity of Schinus terebinthifolius and Croton urucurana extracts on in vitro biofilm formation of Candida albicans and Streptococcus mutans. Arch. Oral Biol. 2014, 59, 887–896. [Google Scholar] [CrossRef]
- Oliveira, M.A.; Borges, A.C.; Brighenti, F.L.; Salvador, M.J.; Gontijo, A.V.; Koga-Ito, C.Y. Cymbopogon citratus essential oil: Effect on polymicrobial caries-related biofilm with low cytotoxicity. Braz. Oral Res. 2017, 31, e89. [Google Scholar] [CrossRef] [PubMed]
- Marinković, J.; Ćulafić, D.M.; Nikolić, B.; Đukanović, S.; Marković, T.; Tasić, G.; Ćirić, A.; Marković, D. Antimicrobial potential of irrigants based on essential oils of Cymbopogon martinii and Thymus zygis towards in vitro multispecies biofilm cultured in ex vivo root canals. Arch. Oral Biol. 2020, 117, 104842. [Google Scholar] [CrossRef] [PubMed]
- Martos, J.; Luque, C.M.; González-Rodríguez, M.P.; Arias-Moliz, M.T.; Baca, P. Antimicrobial activity of essential oils and chloroform alone and combinated with cetrimide against Enterococcus faecalis biofilm. Eur. J. Microbiol. Immunol. 2013, 3, 44–48. [Google Scholar] [CrossRef] [PubMed]
- Raoof, M.; Khaleghi, M.; Siasar, N.; Mohannadalizadeh, S.; Haghani, J.; Amanpour, S. Antimicrobial activity of methanolic extracts of Myrtus Communis L. and Eucalyptus Galbie and their combination with calcium hydroxide powder against Enterococcus Faecalis. J. Dent. Shiraz 2019, 20, 195–202. [Google Scholar]
- Goldbeck, J.C.; Nascimento, J.E.D.; Jacob, R.G.; Fiorentini, Â.M.; da Silva, W.P. Bioactivity of essential oils from Eucalyptus globulus and Eucalyptus urograndis against planktonic cells and biofilms of Streptococcus mutans. Ind. Crops Prod. 2014, 60, 304–309. [Google Scholar] [CrossRef]
- Sulistyani, H.; Fujita, M.; Miyakawa, H.; Nakazawa, F. Effect of roselle calyx extract on in vitro viability and biofilm formation ability of oral pathogenic bacteria. Asian Pac. J. Trop. Med. 2016, 9, 119–124. [Google Scholar] [CrossRef] [PubMed]
- Sekita, Y.; Murakami, K.; Yumoto, H.; Amoh, T.; Fujiwara, N.; Ogata, S.; Matsuo, T.; Miyake, Y.; Kashiwada, Y. Preventive effects of Houttuynia cordata extract for oral infectious diseases. BioMed Res. Int. 2016, 2016, 2581876. [Google Scholar] [CrossRef]
- Süntar, I.; Oyardı, O.; Akkol, E.K.; Ozçelik, B. Antimicrobial effect of the extracts from Hypericum perforatum against oral bacteria and biofilm formation. Pharm. Biol. 2016, 54, 1065–1070. [Google Scholar] [CrossRef]
- Merghni, A.; Marzouki, H.; Hentati, H.; Aouni, M.; Mastouri, M. Antibacterial and antibiofilm activities of Laurus nobilis L. essential oil against Staphylococcus aureus strains associated with oral infections. Curr. Res. Transl. Med. 2016, 64, 29–34. [Google Scholar] [CrossRef]
- Zhang, Z.; Zeng, J.; Zhou, X.; Xu, Q.; Li, C.; Liu, Y.; Zhang, C.; Wang, L.; Zeng, W.; Li, Y. Activity of Ligustrum robustum (Roxb.) Blume extract against the biofilm formation and exopolysaccharide synthesis of Streptococcus mutans. Mol. Oral Microbiol. 2021, 36, 67–79. [Google Scholar] [CrossRef]
- Ahmad, I.; Wahab, S.; Nisar, N.; Dera, A.A.; Alshahrani, M.Y.; Abullias, S.S.; Irfan, S.; Alam, M.M.; Srivastava, S. Evaluation of antibacterial properties of Matricaria aurea on clinical isolates of periodontitis patients with special reference to red complex bacteria. Saudi Pharm. J. 2020, 28, 1203–1209. [Google Scholar] [CrossRef]
- Nurrahman, H.F.; Widyarman, A.S. Effectiveness of Matricaria chamomilla essential oil on Aggregatibacter actinomy-cetemcomitans and Treponema denticola biofilms. J. Indones. Dent. Assoc. 2020, 3, 77–82. [Google Scholar]
- Song, Y.-M.; Zhou, H.-Y.; Wu, Y.; Wang, J.; Liu, Q.; Mei, Y.-F. In vitro evaluation of the antibacterial properties of tea tree oil on planktonic and biofilm-forming Streptococcus mutans. AAPS PharmSciTech 2020, 21, 1–12. [Google Scholar] [CrossRef]
- Ben Lagha, A.; Vaillancourt, K.; Huacho, P.M.; Grenier, D. Effects of labrador tea, peppermint, and winter savory essential oils on Fusobacterium nucleatum. Antibiotics 2020, 9, 794. [Google Scholar] [CrossRef]
- Pires, J.G.; Zabini, S.S.; Braga, A.S.; de Cássia Fabris, R.; de Andrade, F.B.; de Oliveira, R.C.; Magalhães, A.C. Hydroalcoholic extracts of Myracrodruon urundeuva All. and Qualea grandiflora Mart. leaves on Streptococcus mutans biofilm and tooth demineralization. Arch. Oral Biol. 2018, 91, 17–22. [Google Scholar] [CrossRef] [PubMed]
- Tsujii, T.; Kawada-Matsuo, M.; Migita, H.; Ohta, K.; Oogai, Y.; Yamasaki, Y.; Komatsuzawa, H. Antibacterial activity of phellodendron bark against Streptococcus mutans. Microbiol. Immunol. 2020, 64, 424–434. [Google Scholar] [CrossRef] [PubMed]
- Orrù, G.; Demontis, C.; Mameli, A.; Tuveri, E.; Coni, P.; Pichiri, G.; Coghe, F.; Rosa, A.; Rossi, P.; D’Hallewin, G. The selective interaction of Pistacia lentiscus oil vs. human Streptococci, an old functional food revisited with new tools. Front. Microbiol. 2017, 8, 2067. [Google Scholar] [CrossRef] [PubMed]
- Magi, G.; Marini, E.; Brenciani, A.; Di Lodovico, S.; Gentile, D.; Ruberto, G.; Cellini, L.; Nostro, A.; Facinelli, B.; Napoli, E. Chemical composition of Pistacia vera L. oleoresin and its antibacterial, anti-virulence and anti-biofilm activities against oral streptococci, including Streptococcus mutans. Arch. Oral Biol. 2018, 96, 208–215. [Google Scholar] [CrossRef] [PubMed]
- Massunari, L.; Novais, R.Z.; Oliveira, M.T.; Valentim, D.; Junior, E.D.; Duque, C. Antimicrobial activity and biocompatibility of the Psidium cattleianum extracts for endodontic purposes. Braz. Dent. J. 2017, 28, 372–379. [Google Scholar] [CrossRef]
- Dastjerdi, E.V.; Abdolazimi, Z.; Ghazanfarian, M.; Amdjadi, P.; Kamalinejad, M.; Mahboubi, A. Effect of Punica granatum L. flower water extract on five common oral bacteria and bacterial biofilm formation on orthodontic wire. Iran. J. Public Health 2014, 43, 1688–1694. [Google Scholar]
- De Sousa, M.B.; Júnior, J.O.; Barbosa, W.L.; da Silva Valério, E.; da Mata Lima, A.; de Araújo, M.H.; Muzitano, M.F.; Nakamura, C.V.; de Mello, J.C.; Teixeira, F.M. Pyrostegia venusta (Ker Gawl.) Miers crude extract and fractions: Prevention of dental biofilm formation and immunomodulatory capacity. Pharmacogn. Mag. 2016, 12, S218–S222. [Google Scholar]
- De Oliveira, J.R.; de Jesus, D.; Figueira, L.W.; de Oliveira, F.E.; Pacheco Soares, C.; Camargo, S.E.; Jorge, A.O.; de Oliveira, L.D. Biological activities of Rosmarinus officinalis L.(rosemary) extract as analyzed in microorganisms and cells. Exp. Biol. Med. 2017, 242, 625–634. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, J.R.; Santana-Melo, G.D.; Camargo, S.E.; Vasconcellos, L.M.; Oliveira, L.D. Total protein level reduction of odontopathogens biofilms by Rosmarinus officinalis L. (rosemary) extract: An analysis on Candida albicans and Streptococcus mutans. Braz. Dent. Sci. 2019, 22, 260–266. [Google Scholar] [CrossRef]
- Al-Sohaibani, S.; Murugan, K. Anti-biofilm activity of Salvadora persica on cariogenic isolates of Streptococcus mutans: In vitro and molecular docking studies. Biofouling 2012, 28, 29–38. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.; Duhan, J.; Tewari, S.; Sangwan, P.; Yadav, A.; Singh, G.; Juneja, R.; Saini, H. Comparative evaluation of antimicrobial efficacy of Syzygium aromaticum, Ocimum sanctum and Cinnamomum zeylanicum plant extracts against Enterococcus faecalis: A preliminary study. Int. Endod. J. 2013, 46, 775–783. [Google Scholar] [CrossRef]
- De Oliveira, J.R.; de Jesus Viegas, D.; Martins, A.P.; Carvalho, C.A.; Soares, C.P.; Camargo, S.E.; Jorge, A.O.; de Oliveira, L.D. Thymus vulgaris L. extract has antimicrobial and anti-inflammatory effects in the absence of cytotoxicity and genotoxicity. Arch. Oral Biol. 2017, 82, 271–279. [Google Scholar] [CrossRef] [PubMed]
| Plant Name | Plant Extract | Compound | Microorganism | Results | References | |||
|---|---|---|---|---|---|---|---|---|
| Antimicrobial Activity | Antibiofilm Activity | |||||||
| Acacia karroo Hayne | Dichloromethane: methanol (leaves) | - | S. mutans ATCC 25175 | MIC | 0.50 mg·mL−1 | 88.8% inhibition | 0.25 mg·mL−1 | [183] |
| Achyranthes aspera L. | Methanol | Betulin; 3,12-oleandione | S. mutans (CI) | MIC | 125 µg·mL−1 | 94.9% inhibition | 125 µg·mL−1 | [190] |
| IZD | 23.0 mm (250 mg·mL−1) | |||||||
| Aloysia gratissima (Aff & Hook) Tr | Essential oil (leaves) (Ag4 fraction) | - | S. mutans UA159 | MIC | 31.2–62.5 µg·mL−1 | >90% inhibition | 62.5 µg·mL−1 | [9] |
| MBC | 62.5–125 µg·mL−1 | |||||||
| Essential oil (leaves) | (E)-pinocamphone; β-pinene; guaiol | F. nucleatum ATCC 25586 | MIC | 0.125 mg·mL−1 | 55.83% inhibition | 1.0 mg·mL−1 | [136] | |
| MBC | 0.250 mg·mL−1 | |||||||
| P. gingivalis ATCC 33277 | MIC | 0.125 mg·mL−1 | 39.12% inhibition | |||||
| MBC | ||||||||
| S. sanguis ATCC 10556 | MIC | 0.500 mg·mL−1 | 60.83% inhibition | |||||
| MBC | 1.0 mg·mL−1 | |||||||
| S. mitis ATCC 903 | MICMBC | 0.25 mg·mL−1 | 9.00% inhibition | |||||
| Artemisia princeps Pamp. | Ethanol (leaves) | - | S. mutans ATCC 25175 | MIC | 0.40 mg·mL−1 | ≈80.0% inhibition | 0.40 mg·mL−1 | [191] |
| MBC | 3.2 mg·mL−1 | |||||||
| Artocarpus lakoocha Roxb. | Aqueous | Oxyresveratrol | S. mutans ATCC 25175 | MIC | 0.10 mg·mL−1 | ≥90.0% inhibition ≥90.0% reduction | 3.12 mg·mL−1 6.25 mg·mL−1 | [192] |
| MBC | 0.20 mg·mL−1 | |||||||
| IZD | 30.5 mm (10% (w/v)) | |||||||
| A. actinomycetemcomitans ATCC 33384 | MIC | 0.100 mg·mL−1 | ≥90.0% inhibition ≥90.0% reduction | 0.39 mg·mL−1 3.12 mg·mL−1 | ||||
| MBC | 0.200 mg·mL−1 | |||||||
| IZD | 29.5 mm (10% (w/v)) | |||||||
| Azadirachta indica A.Juss. | Aqueous (leaves) | - | E. faecalis ATCC 29212 | - | 53.6% reduction | 30 mg·mL−1 | [193] | |
| S. aureus ATCC 25923 | 48.2% reduction | |||||||
| Baccharis dracunculifolia DC. | Essential oil (leaves) | - | S. mutans ATCC 35688 | MIC | 6.0% (v/v) | 39.3% reduction | 6.0% (v/v) | [194] |
| S. mutans 22 (CI) | 78.9% reduction | |||||||
| S. mutans 24 (CI) | 90.9% reduction | |||||||
| S. mutans 28 (CI) | 91.1% reduction | |||||||
| Essential oil (leaves) | Trans-nerolidol; spathulenol | S. mutans UA159 | MIC | 15.6–31.2 µg·mL−1 | 95.0% inhibition | 31.2 µg·mL−1 | [9] | |
| MBC | 125–250 µg·mL−1 | |||||||
| Berula erecta (Huds.) Coville | Dichloromethane: methanol (rhizome) | - | S. mutans ATCC 25175 | MIC | 0.50 mg·mL−1 | 37.7% inhibition | 0.25 mg·mL−1 | [183] |
| Betula schmidtii Regel. | Methanol | - | S mutans ATCC 25175 | MIC | 31.3 mg·mL−1 | ≈46.0% reduction | 125 mg·mL−1 | [195] |
| Camellia japonica L. | Methanol (leaves) | - | S. mutans ATCC 25175 | MIC | 0.5 mg·disk−1 | 99.0% GTFs inhibition | 1.0 mg·mL−1 | [125] |
| IZD | 12 mm (2 mg·disk−1) | |||||||
| Chelidonium majus subsp. asiaticum H. Hara | Methanol (whole plant) | - | S. mutans ATCC 25175 | MIC | 1.0 mg·disk−1 | 99.0% GTFs inhibition | 1.0 mg·mL−1 | [125] |
| IZD | 8 mm (1.0 mg·disk−1) | |||||||
| Chrysosplenium flagelliferum F.Schmidt | Methanol (whole plant) | - | S. mutans ATCC 25175 | MIC | 1.0 mg·disk−1 | 99.0% GTFs inhibition | 1.0 mg·mL−1 | [125] |
| IZD | 12 mm (2 mg·disk−1) | |||||||
| Cinnamomum burmannii (Nees & T.Nees) Blume | Aqueous | - | S. mutans UA159 | MIC | 2.5 mg·mL−1 | MBIC | 2.5 mg·mL−1 | [196] |
| MBBC | 10 mg·mL−1 | |||||||
| >99% inhibition | 10 mg·mL−1 | |||||||
| Cinnamomum zeylanicum Blume | Essential oil | β-linalool; (E)-cinnamaldehyde; cinnamyl acetate | S. mutans ATCC 25175 | MIC | 0.056 mg·mL−1 | ≈99.0% inhibition | 0.224 mg·mL−1 | [185] |
| IZD | 10 mm (50 mg·mL−1) | |||||||
| E. faecalis ATCC 19433 | MIC | 0.315 mg·mL−1 | ≈47.0% inhibition | 1.26 mg·mL−1 | ||||
| IZD | 10 mm (50 mg·mL−1) | |||||||
| S. aureus ATCC 29213 | MIC | 0.315 mg·mL−1 | ≈93.0% inhibition | 0.315 mg·mL−1 | ||||
| IZD | 11 mm (50 mg·mL−1) | |||||||
| Essential oil (bark) | - | S. mutans KPSK2 | MIC | 0.08% (v/v) | 88.2% inhibition 81.2% reduction | 0.32% (v/v) 0.32% (v/v) | [197] | |
| MBC | 0.08% (v/v) | |||||||
| IZD | 32.2 mm (20% (v/v)) | |||||||
| Essential oil (bark) | - | P. gingivalis ATCC 5397 | - | 85.7% inhibition | 5.0 mg·mL−1 | [198] | ||
| F. nucleatum ATCC 25586 | 75.3% reduction | |||||||
| Essential oil (bark) | Cinnamaldehyde | P. gingivalis ATCC 33177 | MIC | 6.25 μg·mL−1 | 74.5% inhibition | 4.17 μg·mL−1 | [199]) | |
| 33.5% reduction | 25 μg·mL−1 | |||||||
| Cistus creticus L. | Methanol (aerial plant parts) | Quercetin; 3-O-β-D-glucopyranoside | S. mutans DSM 20523 | MIC | 5 mg·mL−1 | ≈80% inhibition | 0.600 mg·mL−1 | [200] |
| MBC | 10 mg·mL−1 | |||||||
| Cistus monspeliensis L. | Methanol (aerial plant parts) | Cistodioic acid | S. mutans DSM 20523 | MIC | 2.5 mg·mL−1 | ≈60% inhibition | 0.600 mg·mL−1 | [200] |
| MBC | NA | |||||||
| Copaifera pubiflora Benth. | Oleoresin | Ent-hardwickiic acid; schistochilic acid B; ent-7α-acetoxy hardwickiic acid; (13E)-ent-labda-7,13-dien-1-5-oic acid | S. sanguinis ATCC 10556 | MIC | 12.5 µg·mL−1 | MBIC50 MBEC | 6.25 µg·mL−1 50.0 µg·mL−1 | [141] |
| MBC | 25.0 µg·mL−1 | |||||||
| S. sanguinis (CI) | MIC | 25.0 µg·mL−1 | MBIC50 | 6.25 µg·mL−1 | ||||
| MBC | ||||||||
| S. mutans ATCC 25175 | MIC | 12.5 µg·mL−1 | MBIC50 | 12.5 µg·mL−1 | ||||
| MBC | ||||||||
| L. paracasei (CI) | MIC | 12.5 µg·mL−1 | MBIC50 | 12.5 µg·mL−1 | ||||
| MBC | ||||||||
| P. gingivalis ATCC 33277 | MIC | 12.5 µg·mL−1 50.0 µg·mL−1 | MBIC50 | 12.5 µg·mL−1 | ||||
| MBC | ||||||||
| P. gingivalis (CI) | MIC MBC | 50.0 µg·mL−1 | MBIC50 | 100 µg·mL−1 | ||||
| F. nucleatum (CI) | MIC | 25.0 µg·mL−1 | MBIC50 | 400 µg·mL−1 | ||||
| MBC | 50.0 µg·mL−1 | |||||||
| P. micra (CI) | MIC | 12.5 µg·mL−1 | MBIC50MBEC | 25.0 µg·mL−1 | ||||
| MBC | 25.0 µg·mL−1 | 50.0 µg·mL−1 | ||||||
| Coriandrum sativum L. | Essential oil (leaves) | 1-decanol, E-2-decen-1-ol; 2-dodecen-1-ol | S. mutans UA159 | MIC | 15.6–31.2 µg·mL−1 | >95% inhibition | 31.2 μg·mL−1 | [9] |
| MBC | 31.2–62.5 µg·mL−1 | |||||||
| Essential oil (leaves) | 1-decanol, E-2-decen-1-ol; 2-dodecen-1-ol | F. nucleatum ATCC 25586 | MIC | 0.015 mg·mL−1 | 55.8% inhibition | 1.0 mg·mL−1 | [136] | |
| MBC | 0.125 mg·mL−1 | |||||||
| P. gingivalis ATCC 33277 | MIC | 0.125 mg·mL−1 | 39.7% inhibition | |||||
| MBC | ||||||||
| S. sanguis ATCC 10556 | MIC | 0.250 mg·mL−1 | 58.3% inhibition | |||||
| MBC | 0.500 mg·mL−1 | |||||||
| S. mitis ATCC 903 | MIC | 0.062 mg·mL−1 | 1.5% inhibition | |||||
| MBC | 0.125 mg·mL−1 | |||||||
| Croton urucurana Baill. | Ethyl acetate-ethanol (stem bark) | - | S. mutans UA159 | - | 34% inhibition | 0.007 mg·mL−1 | [201] | |
| Curcuma longa L. | Ethanol | - | P. gingivalis JCM12257 | - | 99.7% reduction | 5.0% (v/v) | [151] | |
| S. mutans NBRC13955 | 99.1% reduction | |||||||
| Cymbopogon citratus (DC.) Stapf | Essential oil (leaves) | Citral; myrcene | S. mutans ATCC UA159 | - | 93% inhibition | 1.0 µg·mL−1 | [147] | |
| Essential oil (leaves) | Geranial; neral; myrcene | S. mutans ATCC 35688 | MIC | 2.61 mg·mL−1 | 95% inhibition | 26.1 mg·mL−1 | [202] | |
| MBC | 10.54 mg·mL−1 | |||||||
| IZD | 11 mm (100% (v/v)) | |||||||
| L. acidophilus ATCC 4356 | MIC | 1.32 mg·mL−1 | 99.6% inhibition | 13.2 mg·mL−1 | ||||
| MBC | 2.61 mg·mL−1 | |||||||
| IZD | 8 mm (100% (v/v)) | |||||||
| Essential oil | Geranial; neral; myrcene | S. mutans ATCC 35668 | - | 95.4% reduction | 0.10 µg·mL−1 | [148] | ||
| Cymbopogon martinii (Roxb.) W. Watson | Essential oil | Geraniol; geranyl acetate | S. mitis (CI) | MIC | 0.25 mg·mL−1 | 28% reduction | 0.25 mg·mL−1 | [203] |
| MBC | >2.0 mg·mL−1 | |||||||
| E. faecalis (CI) | MIC | 0.25 mg·mL−1 | 36% reduction | 1.0 mg·mL−1 | ||||
| MBC | 1.0 mg·mL−1 | |||||||
| S. mitis + S. sanguinis + E. faecalis | - | 20% reduction | ||||||
| Cyperus articulatus L. | Essential oil (bulbs) | α-pinene; mustakone; α-bulnesene | F. nucleatum ATCC 25586 | MIC | 0.250 mg·mL−1 | 61.67% inhibition | 1.0 mg·mL−1 | [136] |
| MBC | ||||||||
| P. gingivalis ATCC 33277 | MIC | 0.250 mg·mL−1 | 43.53% inhibition | |||||
| MBC | ||||||||
| S. sanguis ATCC 10556 | MIC | 0.250 mg·mL−1 0.500 mg·mL−1 | 63.96% inhibition | |||||
| MBC | ||||||||
| S. mitis ATCC 903 | MIC | 0.250 mg·mL−1 | 5.00% inhibition | |||||
| MBC | 0.500 mg·mL−1 | |||||||
| Derris reticulata Craib | Ethanol (stem) | - | S. mutans DMST 1877 | MIC | 0.875 mg·mL−1 | 102.8% inhibition | 750 µg·mL−1 | [155] |
| MBC | 1.75 mg·mL−1 | |||||||
| Dodonaea viscosa var. angustifólia (L.f.) Benth | Methanol (leaves) | Xylopyranoside; 2,2′-methylenebis[6-(1,1-dimethyl)]-4-methyl); 2-(3-Hydroxy-4-methoxyphenyl)-3,7-dimethoxy-4H-chromen-4-one; trans-3′,4′,5′-Trimethoxy-4-(methylthio)chalcone; stigmasterol | S. mutans NCTC 1091 | MIC MBC | 0.78 mg·mL−1 3.125 mg·mL−1 | 99% inhibition | 0.78 mg·mL−1 | [159] |
| Englerophytum magalismontanum(Sond.) T.D.Penn. | Dichloromethane: methanol (stems) | - | S. mutans ATCC 25175 | MIC | 0.83 mg·mL−1 | 49.28% inhibition | 0.25 mg·mL−1 | [183] |
| Erythrina lysistemon Hutch. | Dichloromethane: methanol (stems) | - | S. mutans ATCC 25175 | MIC | 0.50 mg·mL−1 | 72.54% inhibition | 0.25 mg·mL−1 | [183] |
| Eucalyptus sp. | Essential oil | - | E. faecalis ATCC 29212 | - | 71.6% reduction | 100% (v/v) | [204] | |
| 78.5% reduction | ||||||||
| Eucalyptus galbie | Methanol | - | E. faecalis PTCC 1237 | MIC | 12.5 mg·mL−1 | 77.7% adherence reduction | 6.25 mg·mL−1 | [205] |
| IZD | 9.63 mm (100 mg·mL−1) | |||||||
| Eucalyptus globulus Labill. | Ethanol | Macrocarpal A; macrocarpal B; macrocarpal C; eucalyptin; 1,8-cineole | P. gingivalis JCM12257 S. mutans NBRC13955 | - | MBEC MBEC | 49.1 µg·mL−1 393 µg·mL−1 | [151] | |
| Essential oil (leaves) | 1,8-cineole; α-pinene | S. mutans ATCC 700610 | MIC | 0.013 mg·mL−1 | 81.1% reduction | 2.0 mg·mL−1 | [206] | |
| IZD | 34.7 mm (100% v/v) | |||||||
| Eucalyptus x urograndis | Essential oil (leaves) | 1,8-cineole; α-pinene | S. mutans ATCC 700610 | MIC | 0.025 mg·mL−1 | 35.1% reduction | 2.0 mg·mL−1 | [206] |
| IZD | 23.0 mm (100% (v/v)) | |||||||
| Firmiana simplex (L.) W.Wight | Methanol (bark) | - | S. mutans ATCC 25175 | MIC IZD | 1.0 mg·disk−1 9 mm (1.0 mg·disk−1) | 35.7% GTFs inhibition | 1.0 mg·mL−1 | [125] |
| Foeniculum vulgare Mill. | Essential oil (seeds) | - | S. mutans KPSK2 | MIC MBC IZD | 1.25% (v/v) 2.50% (v/v) 9.17 mm [20% (v/v)] | 84.4% inhibition 69.7 % reduction | 5.0% (v/v) | [197] |
| Ethanol | - | P. gingivalis JCM12257 | - | 99.9% reduction | 5.0% (v/v) | [151] | ||
| S. mutans NBRC13955 | 71.4% reduction | |||||||
| Geranium sibiricum L. | Methanol (whole plant) | - | S. mutans ATCC 25175 | MIC | 0.5 mg·disk−1 | 69.3 % GTFs inhibition | 1.0 mg·mL−1 | [125] |
| IZD | 15 mm (2.0 mg·disk−1) | |||||||
| Ginkgo biloba L. | Methanol | - | S mutans ATCC 25175 | MIC | 62.5 mg·mL−1 | ≈38.5% reduction | 125 mg·mL−1 | [195] |
| Glycyrrhiza glabra L. | Ethanol (roots) | - | P. gingivalis ATCC 33277 | MIC | 62.5 µg·mL−1 | 92.3% inhibition MBEC | 500 µg·mL−1 62.5 µg·mL−1 | [162] |
| MBC | 125 µg·mL−1 | |||||||
| Hibiscu sabdariffa L. | Ethanol (calices) | - | S. mutans Ingbritt | MIC | 7.2 mg·mL−1 | 99.0% inhibition | 3.60 mg·mL−1 | [207] |
| MBC | 57.6 mg·mL−1 | |||||||
| S. sanguinis ATCC 10556T | MIC | 28.8 mg·mL−1 | 97.0% inhibition | 14.4 mg·mL−1 | ||||
| MBC | 57.6 mg·mL−1 | |||||||
| L. casei ATCC 4646 | MIC | 28.8 mg·mL−1 | 92.0% inhibition | 28.8 mg·mL−1 | ||||
| MBC | >57.6 mg·mL−1 | |||||||
| A. naeslundii ATCC 12104T | MIC | 14.4 mg·mL−1 | 97.0% inhibition | 14.4 mg·mL−1 | ||||
| MBC | >57.6 mg·mL−1 | |||||||
| A. actinomycetemcomitans ATCC 29522 | MIC | 28.8 mg·mL−1 | 97.0% inhibition | 28.8 mg·mL−1 | ||||
| MBC | 57.6 mg·mL−1 | |||||||
| F. nucleatum JCM 6328 | MIC | 7.2 mg·mL−1 | 83.0% inhibition | 1.8 mg·mL−1 | ||||
| MBC | 14.4 mg·mL−1 | |||||||
| P. gingivalis ATCC 33277T | MIC | 7.2 mg·mL−1 | 98.0% inhibition | 14.4 mg·mL−1 | ||||
| MBC | 28.8 mg·mL−1 | |||||||
| P. intermedia ATCC 25611T | MIC | 14.4 mg·mL−1 | 89.0% inhibition | 7.2 mg·mL−1 | ||||
| MBC | 28.8 mg·mL−1 | |||||||
| Houttuynia cordata Thunb. | Ethanol (leaves) | - | S. mutans MT8148 | MIC | 1.09 µg·mL−1 | ≈80.0% reduction | 10% (v/v) | [208] |
| F. nucleatum JCM8532 | MIC | 0.543 µg·mL−1 | ≈90.0% reduction | |||||
| Hypericum perforatum L. | Ethanol | - | S. mutans ATCC 21752 | MIC | 64.0 µg·mL−1 | MBIC50 | 18.0 µg·mL−1 | [209] |
| S. sobrinus ATCC 6715 | MIC | 16.0 µg·mL−1 | 7.23 µg·mL−1 | |||||
| L. plantarum ATCC 80141 | MIC | 32.0 µg·mL−1 | 29.9 µg·mL−1 | |||||
| E. faecalis ATCC 29912 | MIC | 32.0 µg·mL−1 | 18.1 µg·mL−1 | |||||
| Hexane | S. mutans ATCC 21752 | MIC | 64.0 µg·mL−1 | 16.3 µg·mL−1 | ||||
| S. sobrinus ATCC 6715 | MIC | 16.0 µg·mL−1 | 7.29 µg·mL−1 | |||||
| L. plantarum ATCC 80141 | MIC | 32.0 µg·mL−1 | 27.6 µg·mL−1 | |||||
| E. faecalis ATCC 29912 | MIC | 32.0 µg·mL−1 | 16.1 µg·mL−1 | |||||
| Chloroform | S. mutans ATCC 21752 | MIC | 32.0 µg·mL−1 | 17.2 µg·mL−1 | ||||
| S. sobrinus ATCC 6715 | MIC | 16.0 µg·mL−1 | 8.03 µg·mL−1 | |||||
| L. plantarum ATCC 80141 | MIC | 32.0 µg·mL−1 | 27.5 µg·mL−1 | |||||
| E. faecalis ATCC 29912 | MIC | 32.0 µg·mL−1 | 17.2 µg·mL−1 | |||||
| Acetic acid | S. mutans ATCC 21752 | MIC | 32.0 µg·mL−1 | 17.7 µg·mL−1 | ||||
| S. sobrinus ATCC 6715 | MIC | 16.0 µg·mL−1 | 7.52 µg·mL−1 | |||||
| L. plantarum ATCC 80141 | MIC | 16.0 µg·mL−1 | 25.1 µg·mL−1 | |||||
| E. faecalis ATCC 29912 | MIC | 16.0 µg·mL−1 | 17.7 µg·mL−1 | |||||
| Butanol | S. mutans ATCC 21752 | MIC | 32.0 µg·mL−1 | 17.5 µg·mL−1 | ||||
| S. sobrinus ATCC 6715 | MIC | 16.0 µg·mL−1 | 7.25 µg·mL−1 | |||||
| L. plantarum ATCC 80141 | MIC | 16.0 µg·mL−1 | 27.3 µg·mL−1 | |||||
| E. faecalis ATCC 29912 | MIC | 32.0 µg·mL−1 | 17.5 µg·mL−1 | |||||
| Aqueous | S. mutans ATCC 21752 | MIC | 32.0 µg·mL−1 | 20.1 µg·mL−1 | ||||
| S. sobrinus ATCC 6715 | MIC | 8.00 µg·mL−1 | 7.60 µg·mL−1 | |||||
| L. plantarum ATCC 80141 | MIC | 8.00 µg·mL−1 | 24.9 µg·mL−1 | |||||
| E. faecalis ATCC 29912 | MIC | 16.0 µg·mL−1 | 20.1 µg·mL−1 | |||||
| Laurus nobilis L. | Essential oil (from Gafsa) | 1,8-cineole; methyl eugenol; α-terpinyl acetate; linalool | S. aureus ATCC 6538 | MIC | 31.25 mg·mL−1 | 95.35% inhibition 78.4 % eradication | 31.25 mg·mL−1 100 mg mL−1 | [210] |
| MBC | 62.5 mg·mL−1 | |||||||
| IZD | 7.75 mm (100% (v/v)) | |||||||
| S. aureus L36 (CI) | MIC | 15.625 mg·mL−1 | 78.92% inhibition 27.2% eradication | 15.625 mg·mL−1 100 mg·mL−1 | ||||
| MBC | 125 mg·mL−1 | |||||||
| IZD | 8.0 mm (100% (v/v)) | |||||||
| S. aureus L37 (CI) | MIC | 15.625 mg·mL−1 | 86.07% inhibition 30.0% eradication | 15.625 mg·mL−1 100 mg·mL−1 | ||||
| MBC | 125 mg·mL−1 | |||||||
| IZD | 9.5 mm (100% (v/v)) | |||||||
| Essential oil (from Sousse) | 1,8-cineole; methyl eugenol; α-terpinyl acetate; linalool | S. aureus ATCC 6538 | MIC | 31.25 mg·mL−1 | 96.61% inhibition 78.0 % eradication | 15.625 mg·mL−1 100 mg·mL−1 | ||
| MBC | 62.5 mg·mL−1 | |||||||
| IZD | 10.5 mm (100% (v/v)) | |||||||
| S. aureus L36 (CI) | MIC | 3.91 mg·mL−1 | 88.22% inhibition 45.0% eradication | 3.91 mg·mL−1 100 mg·mL−1 | ||||
| MBC | 31.25 mg·mL−1 | |||||||
| IZD | 11.5 mm (100% (v/v)) | |||||||
| S. aureus L37 (CI) | MIC | 3.91 mg·mL−1 | 93.00% inhibition 31.0% eradication | 3.91 mg·mL−1 100 mg·mL−1 | ||||
| MBC | 62.5 mg·mL−1 | |||||||
| IZD | 10.75 mm (100% (v/v)) | |||||||
| Ligustrum robustum (Roxb.) | Methanol (roots) | Ligurobustoside B, N, J and C | S. mutans UA159 | MIC | 0.40% (w/v) | ≈35.0% inhibition MBIC-0.8% (w/v) | 0.10% (w/v) | [211] |
| MBC | 0.80% (w/v) | |||||||
| S. mutans C1 (CI) | MIC | 0.25% (w/v) | ≈61.0% inhibition MBIC-0.5% (w/v) | |||||
| MBC | 0.50% (w/v) | |||||||
| S. mutans C2 (CI) | MIC | 0.25% (w/v) | ≈62.0% inhibition MBIC-0.5% (w/v) | |||||
| MBC | 0.50% (w/v) | |||||||
| S. mutans C3 (CI) | MIC | 0.35% (w/v) | ≈41.0% inhibition MBIC-0.7% (w/v) | |||||
| MBC | 0.70% (w/v) | |||||||
| S. mutans C4 (CI) | MIC | 0.25% (w/v) | ≈54.0% inhibition MBIC-0.5% (w/v) | |||||
| MBC | 0.50% (w/v) | |||||||
| S. mutans C5 (CI) | MIC | 0.25% (w/v) | ≈57.0% inhibition MBIC-0.25% (w/v) | |||||
| MBC | 0.50% (w/v) | |||||||
| S. mutans C6 (CI) | MIC | 0.25% (w/v) | ≈47.0% inhibition MBIC-0.5% (w/v) | |||||
| MBC | 0.50% (w/v) | |||||||
| S. mutans C7 (CI) | MIC | 0.30% (w/v) | ≈42.0% inhibition MBIC-0.6% (w/v) | |||||
| MBC | 0.60% (w/v) | |||||||
| S. mutans C8 (CI) | MIC | 0.35% (w/v) | ≈40.0% inhibition MBIC-0.7% (w/v) | |||||
| MBC | 0.70% (w/v) | |||||||
| Lindera glauca (Siebold & Zucc.) Blume | Methanol (leaves) | - | S. mutans ATCC 25175 | MIC | 1.0 mg·disk−1 | 85.9% GTFs inhibition | 1.0 mg·mL−1 | [125] |
| IZD | 8 mm (1.0 mg·disk−1) | |||||||
| Lippia alba (Mill.) N.E.Br. ex Britton & P.Wilson | Essential oil | Geraniol; citral | S. mutans ATCC 35668 | - | 95.8% reduction | 0.10 µg·mL−1 | [148] | |
| Lippia sidoides Cham. | Essential oil (leaves) | Thymol | S. mutans UA159 | MIC | 62.5–125 µg·mL−1 | >90% inhibition | 125 µg·mL−1 | [9] |
| MBC | 125–250 µg·mL−1 | |||||||
| Essential oil (leaves) | Thymol; p-cymene; α-caryophyllene | F. nucleatum ATCC 25586 | MIC | 0.125 mg·mL−1 | 58.33% inhibition | 1.0 mg·mL−1 | [136] | |
| MBC | ||||||||
| P. gingivalis ATCC 33277 | MIC | 0.250 mg·mL−1 | 12.94% inhibition | |||||
| MBC | ||||||||
| S. sanguis ATCC 10556 | MIC | 0.125 mg·mL−1 0.500 mg·mL−1 | 58.13% inhibition | |||||
| MBC | ||||||||
| S. mitis ATCC 903 | MIC | 0.250 mg·mL−1 >1.0 mg·mL−1 | 5.50% inhibition | |||||
| MBC | ||||||||
| Mangifera sp. | Aqueous (leaves) | Quinic acid; benzophenone C-glycoside isomer; benzophenone C-glycoside; quercetin-3-O-glucoside | S. mutans ATCC 25175 | - | 99.4% reduction | 0.50 mg·mL−1 | [167] | |
| S. sanguinis ATCC BAA-1455 | 61.4% reduction | |||||||
| Mangifera indica L. | Aqueous (leaves) | - | E. faecalis ATCC 29212 | - | 37.1% reduction | 30 mg·mL−1 | [193] | |
| S. aureus ATCC 25923 | 30.3% reduction | |||||||
| Matricaria aurea (Loefl.) Sch.Bip. | Ethanol (flowers) | - | P. gingivalis (CI) | MIC | 0.78 mg·mL−1 | 78% inhibition | 1.56 mg·mL−1 | [212] |
| MBC | 3.12 mg·mL−1 | |||||||
| IZD | 20 mm (0.2 g·mL−1) | |||||||
| T. denticola (CI) | MIC | 1.56 mg·mL−1 | 74% inhibition | 3.12 mg·mL−1 | ||||
| MBC | 3.12 mg·mL−1 | |||||||
| IZD | 15 mm (0.2 g·mL−1) | |||||||
| T. forsythia (CI) | MIC | 0.39 mg·mL−1 | 86% inhibition | 0.78 mg·mL−1 | ||||
| MBC | 1.56 mg·mL−1 | |||||||
| IZD | 23 mm (0.2 g·mL−1) | |||||||
| A. actinomycetemcomitans (CI) | MIC | 1.56 mg·mL−1 | 62% inhibition | 3.12 mg·mL−1 | ||||
| MBC | 6.25 mg·mL−1 | |||||||
| IZD | 13 mm (0.2 g·mL−1) | |||||||
| Matricaria recutita L. | Essential oil (leaves) | Alkaloid; saponin; tannin, phenolic; flavonoid; triterpenoid and glycoside compounds | A. actinomycetemcomitans ATCC 29522 | - | 87.6% reduction | 100% (v/v) | [213] | |
| T. denticola ATCC 35405 | 99.1% reduction | 50% (v/v) | ||||||
| Melaleuca alternifolia (Maiden & Betche) Cheel Myrtaceae | Essential oil | - | S. mutans ATCC 25175 | MIC | 0.125% (v/v) | ≈94% reduction (metabolic activity) | 1.0% (v/v) | [214] |
| MBC | 0.25% (v/v) | ≈85% reduction (biomass) | 0.5% (v/v) | |||||
| IZD | 20.3 mm [20% (v/v)] | |||||||
| Mentha sp. | Aqueous (leaves) | Methyl-2-[cyclohex-2-en-1-yl(hydroxy)methyl]-3-hydroxy-4-(2-hydroxyethyl)-3-methyl-5-oxoprolinate | S. mutans ATCC 25175 | - | 98.5% reduction | 0.50 mg·mL−1 | [167] | |
| S. sanguinis ATCC BAA-1455 | 85.5% reduction | |||||||
| Mentha × piperita L. | Essential oil (leaves) | - | S. mutans KPSK2 | MIC | 1.25% (v/v) | 83.42% inhibition 71.01% reduction | 5% (v/v) | [197] |
| MBC | 1.25% (v/v) | |||||||
| IZD | 11.33 mm (20% (v/v)) | |||||||
| Essential oil (flowers) | Menthol; menthone | F. nucleatum ATCC 25586 | MIC | 0.25% (v/v) | >90.0% inhibition | 0.25% (v/v) | [215] | |
| MBC | 0.5% (v/v) | |||||||
| Mentha spicata L. | Methanol (leaves) | - | S. mutans KPSK2 | MIC | 1.25% (v/v) | 82.7% inhibition 71.5% reduction | 5.0% (v/v) | [197] |
| MBC | 1.25% (v/v) | |||||||
| IZD | 9.50 mm (20% (v/v)) | |||||||
| Mikania glomerata Spreng | Essential oil (leaves) | Germacrene D; α-caryophyllene; bicyclogermacrene | F. nucleatum ATCC 25586 | MIC | 0.25 mg·mL−1 | 58.96% inhibition | 1.0 mg·mL−1 | [136] |
| MBC | 0.50 mg·mL−1 | |||||||
| P. gingivalis ATCC 33277 | MIC | 0.50 mg·mL−1 | 40.00% inhibition | |||||
| MBC | >1.00 mg·mL−1 | |||||||
| S. sanguis ATCC 10556 | MIC | 0.062 mg·mL−1 | 54.79% inhibition | |||||
| MBC | 0.125 mg·mL−1 | |||||||
| S. mitis ATCC 903 | MIC | 0.125 mg·mL−1 | 1.00% inhibition | |||||
| MBC | ||||||||
| Myracrodruon urundeuva All. | Hydroalcoholic (leaves) | - | S. mutans ATCC 25175 | MIC | 2.5 mg·mL−1 | MBIC | 1.25 mg·mL−1 | [216] |
| MBC | MBEC | 2.5 mg·mL−1 | ||||||
| Myrtus communis L. | Hydroethanolic (leaves) | - | S. mutans ATCC 25175 | - | MBIC MBEC | 40 µg.µL−1 120 µg.µL−1 | [173] | |
| S. oralis (CI) | MBIC MBEC | 20 µg.µL−1 40 µg.µL−1 | ||||||
| S. mitis (CI) | MBIC MBEC | 20 µg.µL−1 40 µg.µL−1 | ||||||
| R. dentocariosa (CI) | MBIC MBEC | 40 µg.µL−1 120 µg.µL−1 | ||||||
| Ocimum basilicum L. | Essential oil (leave) | - | S. mutans KPSK2 | MIC | 0.31% (v/v) | 86.8% inhibition 73.3% reduction | 1.25% (v/v) | [197] |
| MBC | 0.31% (v/v) | |||||||
| IZD | 14.7 mm (20% (v/v)) | |||||||
| Ethanol (seeds) | - | P. gingivalis JCM12257 | - | 99.9% reduction | 5.0% (v/v) | [151] | ||
| S. mutans NBRC13955 | 90.9% reduction | |||||||
| Origanum vulgare L. | Methanol (aerial plant parts) | Rosmarinic acid | S. mutans DSM 20523 | MIC | 2.5 mg·mL−1 | ≈60.0% inhibition | 5.0 mg·mL−1 | [200] |
| MBC | 5.0 mg·mL−1 | |||||||
| Essential oil | O-cymene; carvacrol | S. mutans ATCC 25175 | MIC | 0.315 mg·mL−1 | ≈97.0% inhibition | 1.26 mg·mL−1 | [185] | |
| IZD | 10.0 mm (50 mg·mL−1) | |||||||
| E. faecalis ATCC 19433 | MIC | 0.625 mg·mL−1 | ≈60.0% inhibition | 2.5 mg·mL−1 | ||||
| IZD | 7.0 mm (50 mg·mL−1) | |||||||
| S. aureus ATCC 29213 | MIC | 0.625 mg·mL−1 | ≈94% inhibition | 0.625 mg·mL−1 | ||||
| IZD | 8.0 mm (50 mg·mL−1) | |||||||
| Phellodendron amurense Rupr. | Ethanol (bark) | - | S. mutans UA159 | MIC | 0.313 mg·mL−1 | 100% inhibition | 1.25 mg·mL−1 | [217] |
| Piper betle L. | Aqueous (leaves) | - | E. faecalis ATCC 29212 | - | 53.6% reduction | 30.0 mg·mL−1 | [193] | |
| S. aureus ATCC 25923 | 32.7% reduction | |||||||
| Ethanol (leaves) | 4-chromanol | S. mutans ATCC 25175 | MIC | 1.56 mg·mL−1 | 90% inhibition | 1.56 mg·mL−1 | [10] | |
| MBC | 3.17 mg·mL−1 | 90% reduction | 6.25 mg·mL−1 | |||||
| A. actinomycetemcomitans ATCC 33384 | MIC | 1.04 mg·mL−1 | 90% inhibition | 0.39 mg·mL−1 | ||||
| MBC | 2.08 mg·mL−1 | 90 reduction | 3.13 mg·mL−1 | |||||
| Piper nigrum L. | Methanol (seed) | - | S. mutans KPSK2 | MIC | 1.25% (v/v) | 82.7% inhibition 67.8% reduction | 5.0% (v/v) | [197] |
| MBC | 2.50% (v/v) | |||||||
| IZD | 14.0 mm (20% (v/v)) | |||||||
| Aqueous (leaves) | - | E. faecalis ATCC 29212 | - | 49.1% reduction | 30.0 mg·mL−1 | [193] | ||
| S. aureus ATCC 25923 | 44.2% reduction | |||||||
| Pistacia lentiscus L. | Essential oil (berry) | Phenols; free unsaturated-saturated fatty acids | S. salivarius K12 | MIC MBC | >50.0% (v/v) | MBIC | > 50% (v/v) | [218] |
| S. salivarius M18 | MIC MBC | >50.0% (v/v) | MBIC | >50% (v/v) | ||||
| S. pyogenes (CI) | MIC MBC | 50.0% (v/v) | MBIC | 25.0–3.0% (v/v) | ||||
| S. agalactiae (CI) | MIC MBC | 50.0% (v/v) | MBIC | 25.0–3.0% (v/v) | ||||
| S. mutans CIP103220 | MIC MBC | 50.0% (v/v) | MBIC | 25.0–3.0% (v/v) | ||||
| S. intermedius DSM 20573 | MIC | 6.0–3.0% (v/v) | MBIC | <4.0% (v/v) | ||||
| MBC | 50.0% (v/v) | |||||||
| S. mitis (CI) | MIC | 6.0–3.0% (v/v) | MBIC | <4.0% (v/v) | ||||
| MBC | 50.0% (v/v) | |||||||
| Pistacia vera L. | Purified oleoresin | α-pinene; β-pinene | S. mutans ATCC 25175 | MBC | >2048 µg·mL−1 | 49.4% inhibition | 256 µg·mL−1 | [219] |
| S. sanguinis ATCC 10556 | MBC | 1024 µg·mL−1 | 71.2% inhibition | |||||
| Psidium sp. | Aqueous (leaves) | Methyl quercetin sulfate; 2,6-dihydroxy-3-methyl-4-O-(6″-O-galloyl-β-D-glucopyranosyl)-benzophenone | S. mutans ATCC 25175 | - | 93.8% reduction | 0.5 mg·mL−1 | [167] | |
| S. sanguinis ATCC BAA-1455 | 48.6% reduction | |||||||
| Psidium cattleianum Sabine | Aqueous (leaves) | - | E. faecalis ATCC 51299 | MIC MBC | 0.5 mg·mL−1 | >99.9% reduction | 5.0 mg·mL−1 | [220] |
| P. aeruginosa ATCC 15442 | MIC MBC | 4.0 mg·mL−1 | - | - | ||||
| Hydroethanol (leaves) | E. faecalis ATCC 51299 | MICMBC | 0.5 mg·mL−1 | >99.9% reduction | 2.5 mg·mL−1 | |||
| P. aeruginosa ATCC 15442 | MIC MBC | 4.0 mg·mL−1 | >99.9% reduction | 40 mg·mL−1 | ||||
| Punica granatum L. | Flowers infusion | - | S. mutans ATCC 35608 | MIC MBC | 50.0 mg·mL−1 | 84.4% reduction | 25 mg·mL−1 | [221] |
| S. sanguinis ATCC 10556 | MIC | 6.25 mg·mL−1 | 100% reduction | 1.56 mg·mL−1 | ||||
| MBC | 25.0 mg·mL−1 | |||||||
| S. sobrinus ATCC 27607 | MIC MBC | 25.0 mg·mL−1 | 99.9% reduction | 12.5 mg·mL−1 | ||||
| S. salivarius ATCC 9222 | MIC | 25.0 mg·mL−1 | 86.5% reduction | 12.5 mg·mL−1 | ||||
| MBC | 100 mg·mL−1 | |||||||
| E. faecalis CIP 55142 | MIC MBC | 50.0 mg·mL−1 | 56.3% reduction | 50 mg·mL−1 | ||||
| Pyrostegia venusta (Ker Gawl.) Miers | Hydroalcoholic (flowers) | - | S. mutans ATCC 25175 | MIC | 500 mg·mL−1 | 68.9% inhibition | 500 mg·mL−1 | [222] |
| MBC | 1000 mg·mL−1 | |||||||
| Qualea grandiflora Mart. | Hydroalcoholic (leaves) | - | S. mutans ATCC 25175 | MIC | 5.0 mg·mL−1 | MBIC | 5.0 mg·mL−1 | [216] |
| MBEC | 10.0 mg·mL−1 | |||||||
| Rhodiola rosea L. | Ethanol (root) | - | S. mutans UA159 | - | ≈95.0% inhibition | 0.50 µg.µL−1 | [178] | |
| ≈48.6% reduction | ||||||||
| Rosa rugosa Thunb. | Methanol (leaves) | - | S. mutans ATCC 25175 | MIC | 1.0 mg·disk−1 | 64.9% GTFs inhibition | 1.0 mg·mL−1 | [125] |
| IZD | 12 mm (2.0 mg·disk−1) | |||||||
| Rosmarinus officinalis L. | Ethanol | Carnosic acid; carnosol; rosmarinic acid | S. mutans NBRC13955 | - | MBEC | 97.8 µg·mL−1 | [151] | |
| P. gingivalis JCM12257 | MBEC | 195.5 µg·mL−1 | ||||||
| Extract (leaves) | - | S. aureus ATCC 6538 | MIC | 25 mg·mL−1 | 99% reduction | 200 mg·mL−1 | [223] | |
| MMC | >50 mg·mL−1 | |||||||
| E. faecalis ATCC 4083 | MIC | 50 mg·mL−1 | 80% reduction | |||||
| MMC | >50 mg·mL−1 | |||||||
| S. mutans ATCC 35688 | MIC | 25 mg·mL−1 | 80% reduction | |||||
| MMC | >50 mg·mL−1 | |||||||
| P. aeruginosa ATCC 15442 | MIC | 6.25 mg·mL−1 | 70% reduction | |||||
| MMC | ||||||||
| Methanol (aerial plant parts) | 7-methoxyrosmanol, rosmanol isomers; rosmarinic acid | S. mutans DSM 20523 | MIC | 0.60 mg·mL−1 | >90% inhibition | 1.25 mg·mL−1 | [200] | |
| MBC | 2.50 mg·mL−1 | |||||||
| Extract (leaves) | S. mutans ATCC 35688 | - | 32% reduction of total biofilm protein | 200 mg·mL−1 | [224] | |||
| Salvadora persica L. | Methanol | Benzyl (6Z,9Z,12Z)-6,9,12-octadecatrienoate; 3-benzyloxy-1-nitro-butan-2-ol; 1,3-cyclohexane dicarbohydrazide | S. mutans CI | MIC IZD | 2.60 mg·mL−1 24.0 mm (2.5 g. mL−1) | 87.9% inhibition | 2.6 mg·mL−1 | [225] |
| Salvia sclarea L. | Methanol (aerial plant parts) | Rosmarinic acid | S. mutans DSM 20523 | MIC | 1.25 mg·mL−1 | 60% inhibition | 2.5 mg·mL−1 | [200] |
| MBC | 5.0 mg·mL−1 | |||||||
| Schinus terebinthifolia Raddi. | Methanol (leaves) | p-anisaldehyde | S. mutans UA159 | - | 41% inhibition | 0.0035 mg·mL−1 | [201] | |
| Sophora flavescens Aiton. | Methyl-alcohol | - | S mutans ATCC 25175 | MIC | 62.5 mg·mL−1 | ≈88.5% reduction | 125 mg·mL−1 | [195] |
| Spirostachys africana Sond. | Dichloromethane: methanol (leaves) | - | S. mutans ATCC 25175 | MIC | 0.50 mg·mL−1 | 97.56% inhibition | 0.25 mg·mL−1 | [183] |
| Syzygium aromaticum (L.) Merr. & L.M.Perry | Essential oil (flower buds) | - | P. gingivalis ATCC 53978 | - | 78.6% inhibition | 5.0 mg·mL−1 | [198] | |
| F. nucleatum ATCC 25586 | 76.2% reduction | 1.25 mg·mL−1 | ||||||
| Ethanol | Eugenol; eugenol acetate; β-caryophyllene | P. gingivalis JCM12257 | - | MBEC | 435.5 μg·mL−1 | [151] | ||
| S. mutans NBRC13955 | MBEC | 871 μg·mL−1 | ||||||
| Essential oil | Eugenol; β-caryophyllene | S. mutans ATCC 25175 | MIC | 0.315 mg·mL−1 | 96% reduction | 1.26 mg·mL−1 | [185] | |
| IZD | 5 mm (50 mg·mL−1) | |||||||
| S. aureus ATCC 29213 | MIC | 0.625 mg·mL−1 | 94% reduction | 2.5 mg·mL−1 | ||||
| IZD | 6 mm (50 mg·mL−1) | |||||||
| E. faecalis ATCC 19433 | MIC | 1.25 mg·mL−1 | 45% reduction | 5.0 mg·mL−1 | ||||
| IZD | 1 mm (50 mg·mL−1) | |||||||
| Hydroethanol (buds) | Eugenol; β-caryophyllene; eugenol acetate | E. faecalis ATCC 29212 | MBC | 10% (w/v) | 100% reduction | 10% (w/v) | [226] | |
| IZD | 5.467 mm (20% (w/v)) | |||||||
| Tarchonanthus camphoratus L. | Dichloromethane: methanol (bark) | - | S. mutans ATCC 25175 | MIC | 0.67 mg·mL−1 | 86.37% inhibition | 0.08 mg·mL−1 | [183] |
| Tecoma capensis (Thunb.) Lindl. | Dichloromethane: methanol (leaves) | - | S. mutans ATCC 25175 | MIC | 0.67 mg·mL−1 | 53.10% inhibition | 0.25 mg·mL−1 | [183] |
| Thuja orientalis L. | Methanol (leaves and stems) | - | S. mutans ATCC 25175 | MIC | 0.1 mg·disk−1 | 99.0% GTFs inhibition | 1.0 mg·mL−1 | [125] |
| IZD | 13 mm (2.0 mg·disk−1) | |||||||
| Thymus longicaulis C.Presl. | Methanol (aerial parts) | Rosmarinic acids; flavonoids; triterpenic acids | S. mutans DSM 20523 | MIC | 0.60 mg·mL−1 | > 95% inhibition | 5.0 mg·mL−1 | [200] |
| MBC | 1.25 mg·mL−1 | |||||||
| Thymus vulgaris L. | Extract (leaves) | - | S. aureus ATCC 6538 | MIC MMC | >50.0 mg·mL−1 | 66% reduction | 200 mg·mL−1 | [227] |
| E. faecalis ATCC 4083 | MIC MMC | >50.0 mg·mL−1 | 82% reduction | |||||
| S. mutans ATCC 35688 | MIC MMC | >50.0 mg·mL−1 | 64% reduction | |||||
| P. aeruginosa ATCC 15442 | MIC MMC | >50.0 mg·mL−1 | 88% reduction | |||||
| Essential oil | O-cymene; thymol | S. mutans ATCC 25175 | MIC | 0.625 mg·mL−1 | 96% inhibition | 2.5 mg·mL−1 | [185] | |
| IZD | 10 mm (50 mg·mL−1) | |||||||
| S. aureus ATCC 29213 | MIC | 0.625 mg·mL−1 | 96% inhibition | 0.156 mg·mL−1 | ||||
| IZD | 8 mm (50 mg·mL−1) | |||||||
| E. faecalis ATCC 19433 | MIC | 0.625 mg·mL−1 | 55% inhibition | 2.5 mg·mL−1 | ||||
| IZD | 9 mm (50 mg·mL−1) | |||||||
| Thymus zygis L. | Essential oil | Thymol; p-cymene; terpinene; linalool; carvacrol | S. mitis (CI) | MIC MBC | 1 mg·mL−1 2 mg·mL−1 | 50% inhibition | 0.5 mg·mL−1 | [203] |
| S. sanguinis (CI) | 16% inhibition | 4 mg·mL−1 | ||||||
| E. faecalis (CI) | 22% inhibition | 4 mg·mL−1 | ||||||
| S. mitis + S. sanguinis + E. faecalis | - | 16% inhibition | 0.5 mg·mL−1 | |||||
| Trachyspermum ammi L. | Ethanol (seeds) | 2-isopropyl-5-methyl- phenol; oleic acid; octadecanoic acid | S. mutans ATCC 700610 | MIC | 320 µg·mL−1 | 61% inhibition | 160 µg·mL−1 | [90] |
| 89% reduction | ||||||||
| Petroleum ether fraction (seeds) | 2-isopropyl-5-methyl-phenol; oleic acid | S. mutans ATCC 700610 | MIC | 40 µg·mL−1 | 100% inhibition | 40 µg·mL−1 | ||
| 100% reduction | 80 µg·mL−1 | |||||||
| Zingiber officinale Roscoe. | Ethanol | - | P. gingivalis JCM12257 S. mutans NBRC13955 | - | 99.9% reduction 97.6% reduction | 5.0% (v/v) | [151] | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Milho, C.; Silva, J.; Guimarães, R.; Ferreira, I.C.F.R.; Barros, L.; Alves, M.J. Antimicrobials from Medicinal Plants: An Emergent Strategy to Control Oral Biofilms. Appl. Sci. 2021, 11, 4020. https://doi.org/10.3390/app11094020
Milho C, Silva J, Guimarães R, Ferreira ICFR, Barros L, Alves MJ. Antimicrobials from Medicinal Plants: An Emergent Strategy to Control Oral Biofilms. Applied Sciences. 2021; 11(9):4020. https://doi.org/10.3390/app11094020
Chicago/Turabian StyleMilho, Catarina, Jani Silva, Rafaela Guimarães, Isabel C. F. R. Ferreira, Lillian Barros, and Maria José Alves. 2021. "Antimicrobials from Medicinal Plants: An Emergent Strategy to Control Oral Biofilms" Applied Sciences 11, no. 9: 4020. https://doi.org/10.3390/app11094020
APA StyleMilho, C., Silva, J., Guimarães, R., Ferreira, I. C. F. R., Barros, L., & Alves, M. J. (2021). Antimicrobials from Medicinal Plants: An Emergent Strategy to Control Oral Biofilms. Applied Sciences, 11(9), 4020. https://doi.org/10.3390/app11094020








