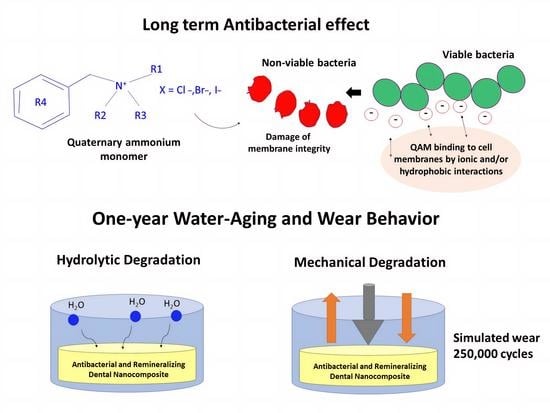
Sustained Antibacterial Effect and Wear Behavior of Quaternary Ammonium Contact-Killing Dental Polymers after One-Year of Hydrolytic Degradation
Abstract
1. Introduction
2. Materials and Methods
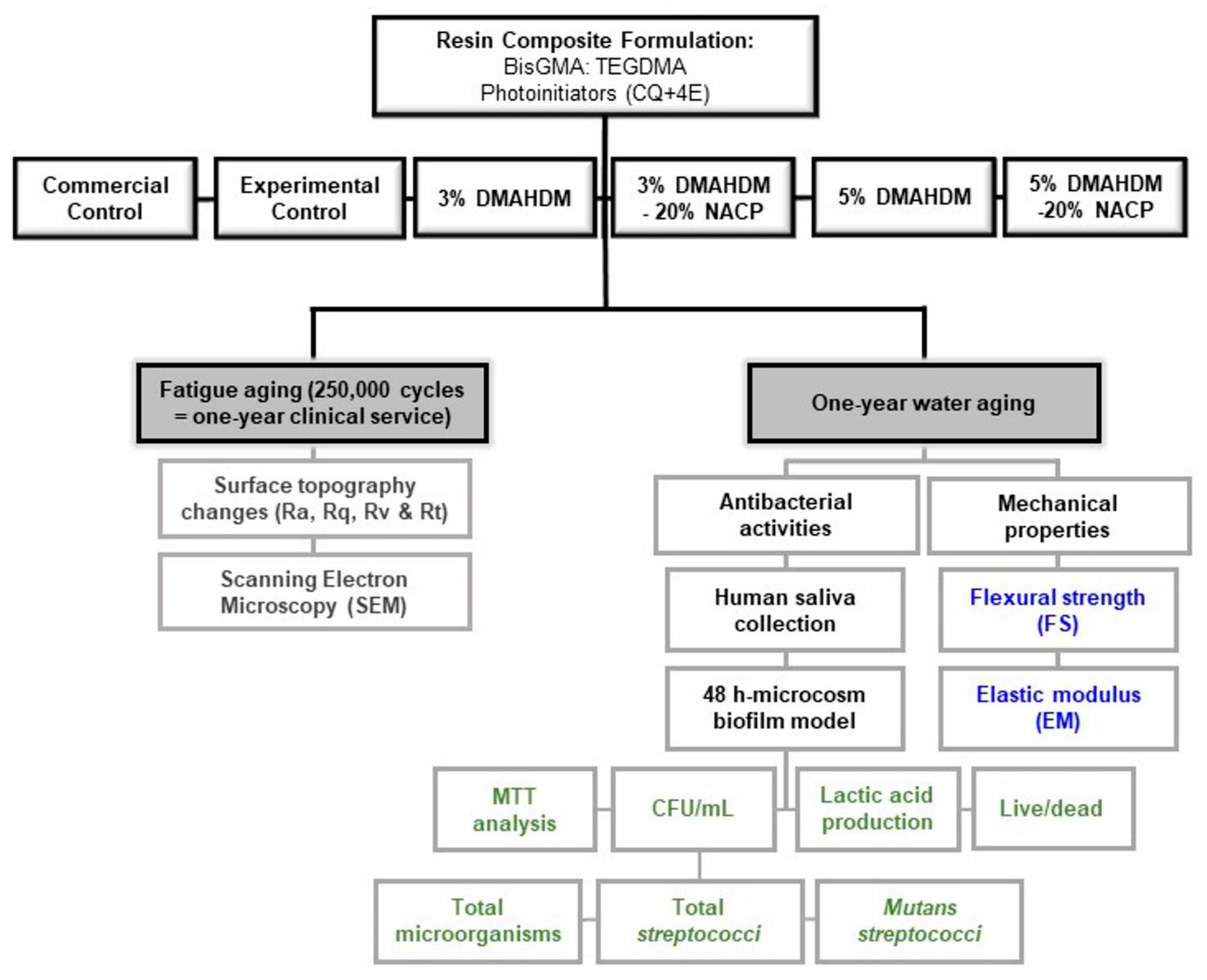
2.1. Experimental Design
- Commercial control (Heliomolar, Ivoclar Vivadent, Mississauga, ON, Canada): 22% BisGMA and Urethane dimethacrylate resin mix + 77% highly dispersed silicon dioxide, prepolymer, and ytterbium trifluoride as fillers + <1% stabilizers, catalysts, and pigments;
- Experimental Control: 35% BisGMA-TEGDMA resin mix 1:1 ratio + 65% glass particles;
- 3% DMAHDM: 32% BisGMA-TEGDMA resin mix 1:1 ratio + 3% DMAHDM + 65% glass particles;
- 5% DMAHDM: 30% BisGMA-TEGDMA resin mix 1:1 ratio + 5% DMAHDM + 65% glass particles;
- 3% DMAHDM+20% NACP: 32% BisGMA-TEGDMA resin mix 1:1 ratio + 3% DMAHDM + 20% NACP + 45% glass particles;
- 5% DMAHDM+20% NACP: 30% BisGMA-TEGDMA resin mix 1:1 ratio + 5% DMAHDM + 20% NACP + 45% glass particles.
2.2. Samples Aging
2.3. Mechanical Properties
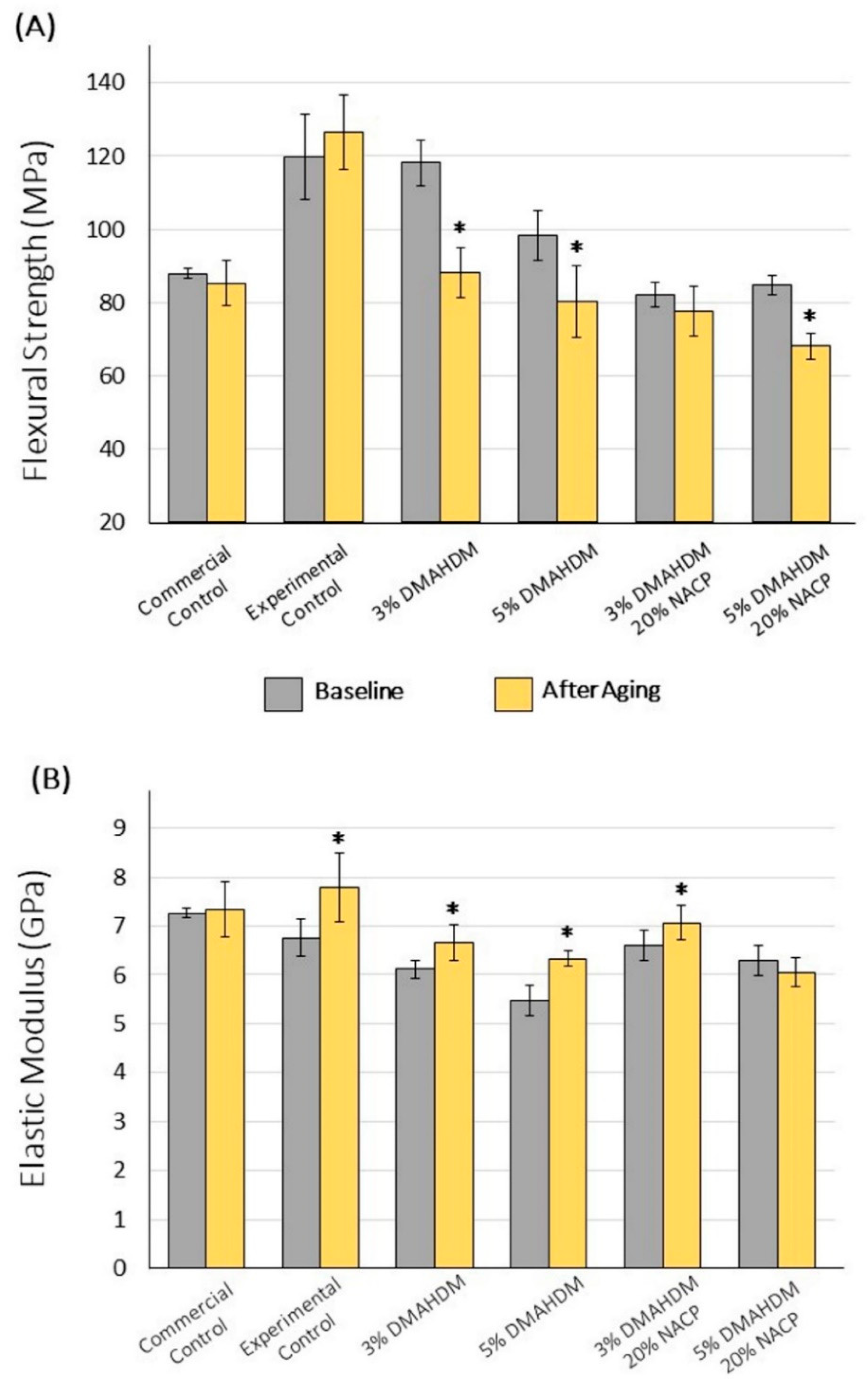
2.3.1. Flexural Strength and Elastic Modulus
2.3.2. Surface Roughness and Wear behavior
- Average surface roughness (Ra): Ra represents the average surface roughness from the roughness profile’s mean line.
- Maximum peak height (Rq): denoted as the highest peak produced by the chewing simulation. A high Rq value indicates a high amount of wear.
- Maximum valley depth (Rv): stated as the deepest valley produced by the chewing simulation. A high Rv value indicates a high amount of wear.
- Maximum high of the profile (Rt): Rt estimates the distance between the maximum peak height and the maximum valley depth.
2.3.3. Scanning Electron Microscopy
2.4. Microcosm Biofilm Model
2.4.1. Biofilm Plate Counting Method
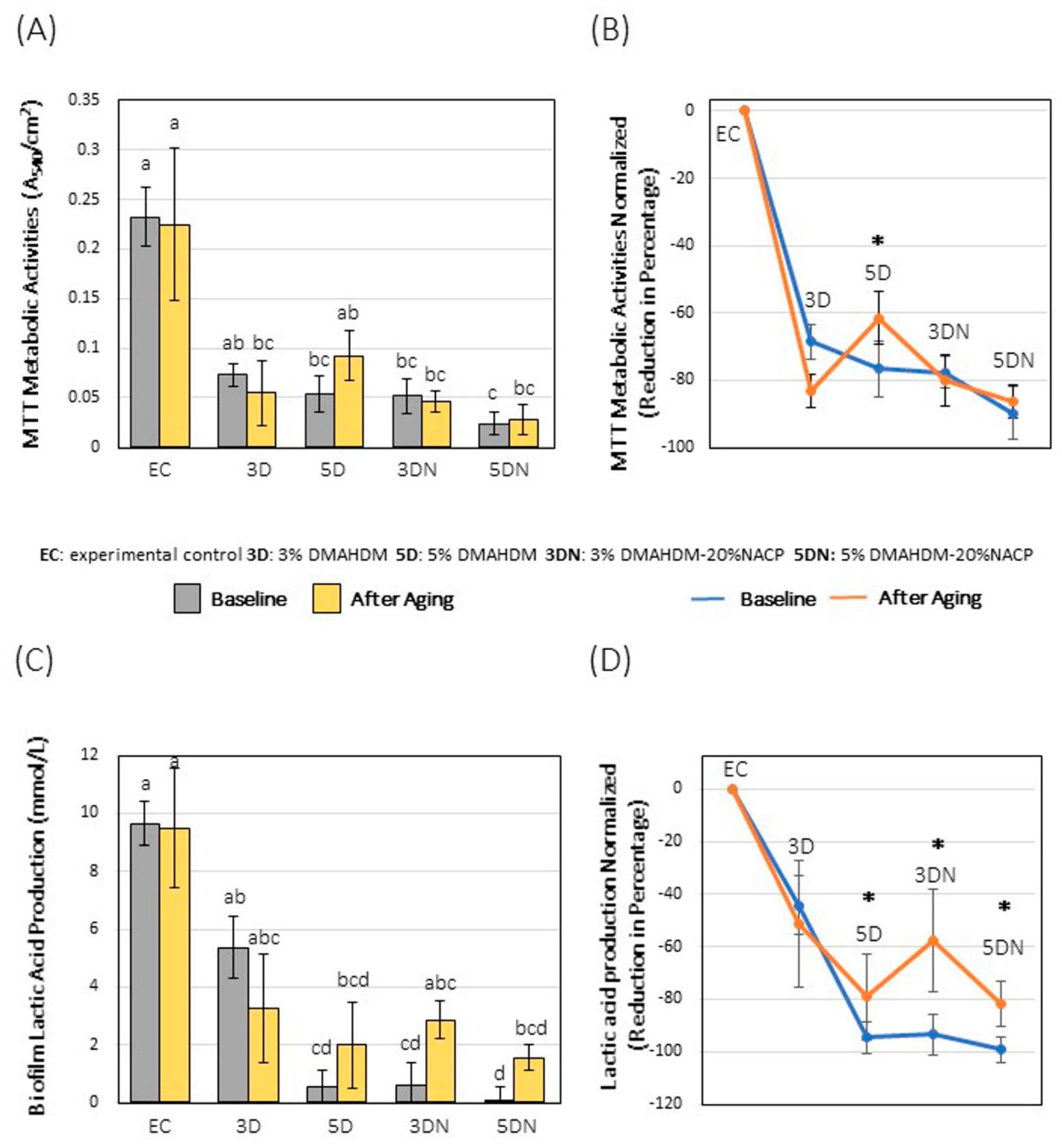
2.4.2. Metabolic Assay
2.4.3. Colorimetric Quantification of Lactic Acid
2.4.4. Baclight Bacterial Viability Assay
2.5. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhou, X.; Huang, X.; Li, M.; Peng, X.; Wang, S.; Zhou, X.; Cheng, L. Development and status of resin composite as dental restorative materials. J. Appl. Polym. Sci. 2019, 136, 48180. [Google Scholar] [CrossRef]
- Namgung, C.; Rho, Y.J.; Jin, B.H.; Lim, B.S.; Cho, B.-H. A Retrospective Clinical Study of Cervical Restorations: Longevity and Failure-Prognostic Variables. Oper. Dent. 2013, 38, 376–385. [Google Scholar] [CrossRef] [PubMed]
- Kubo, S. Longevity of resin composite restorations. Jpn. Dent. Sci. Rev. 2011, 47, 43–55. [Google Scholar] [CrossRef]
- Van Dijken, J.W.; Pallesen, U. Posterior bulk-filled resin composite restorations: A 5-year randomized controlled clinical study. J. Dent. 2016, 51, 29–35. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; Melo, M.A.; Weir, M.D.; Reynolds, M.A.; Bai, Y.; Xu, H.H. Do Dental Resin Composites Accumulate More Oral Biofilms and Plaque than Amalgam and Glass Ionomer Materials? Materials 2016, 9, 888. [Google Scholar] [CrossRef]
- Cocco, A.R.; Rosa, W.L.D.O.D.; da Silva, A.F.; Lund, R.G.; Piva, E. A systematic review about antibacterial monomers used in dental adhesive systems: Current status and further prospects. Dent. Mater. 2015, 31, 1345–1362. [Google Scholar] [CrossRef]
- Pirmoradian, M.; Hooshmand, T. Remineralization and antibacterial capabilities of resin-based dental nanocomposites. In Applications of Nanocomposite Materials in Dentistry; Asiri, A.M., Mohammad, A., Eds.; Woodhead Publishing: Cambridge, UK, 2019; pp. 237–269. [Google Scholar] [CrossRef]
- Imazato, S. Antibacterial properties of resin composites and dentin bonding systems. Dent. Mater. 2003, 19, 449–457. [Google Scholar] [CrossRef]
- Imazato, S. Bio-active restorative materials with antibacterial effects: New dimension of innovation in restorative dentistry. Dent. Mater. J. 2009, 28, 11–19. [Google Scholar] [CrossRef]
- Antonucci, J.M.; Zeiger, D.N.; Tang, K.; Lin-Gibson, S.; Fowler, B.O.; Lin, N.J. Synthesis and characterization of dimethacrylates containing quaternary ammonium functionalities for dental applications. Dent. Mater. 2012, 28, 219–228. [Google Scholar] [CrossRef]
- Alsahafi, R.; Balhaddad, A.A.; Mitwalli, H.; Ibrahim, M.S.; Melo, M.A.S.; Oates, T.W.; Xu, H.H.; Weir, M.D. Novel Crown Cement Containing Antibacterial Monomer and Calcium Phosphate Nanoparticles. Nanomaterials 2020, 10, 2001. [Google Scholar] [CrossRef]
- Mitwalli, H.; Balhaddad, A.A.; Alsahafi, R.; Oates, T.W.; Melo, M.A.S.; Xu, H.H.K.; Weir, M.D. Novel CaF2 Nanocomposites with Antibacterial Function and Fluoride and Calcium Ion Release to Inhibit Oral Biofilm and Protect Teeth. J. Funct. Biomater. 2020, 11, 56. [Google Scholar] [CrossRef]
- Balhaddad, A.A.; Kansara, A.A.; Hidan, D.; Weir, M.D.; Xu, H.H.; Melo, M.A.S. Toward dental caries: Exploring nanoparticle-based platforms and calcium phosphate compounds for dental restorative materials. Bioact. Mater. 2019, 4, 43–55. [Google Scholar] [CrossRef]
- Bhadila, G.; Filemban, H.; Wang, X.; Melo, M.A.S.; Arola, D.D.; Tay, F.R.; Oates, T.W.; Weir, M.D.; Sun, J.; Xu, H.H. Bioactive low-shrinkage-stress nanocomposite suppresses S. mutans biofilm and preserves tooth dentin hardness. Acta Biomater. 2020, 114, 146–157. [Google Scholar] [CrossRef]
- Balhaddad, A.A.; Ibrahim, M.S.; Weir, M.D.; Xu, H.H.; Melo, M.A.S. Concentration dependence of quaternary ammonium monomer on the design of high-performance bioactive composite for root caries restorations. Dent. Mater. 2020, 36, e266–e278. [Google Scholar] [CrossRef]
- Melo, M.A.; Guedes, S.F.; Xu, H.H.; Rodrigues, L.K. Nanotechnology-based restorative materials for dental caries management. Trends Biotechnol. 2013, 31, 459–467. [Google Scholar] [CrossRef] [PubMed]
- Balhaddad, A.A.; Ibrahim, M.S.; Garcia, I.M.; Collares, F.M.; Weir, M.D.; Xu, H.H.; Melo, M.A.S. Pronounced Effect of Antibacterial Bioactive Dental Composite on Microcosm Biofilms Derived From Patients With Root Carious Lesions. Front. Mater. 2020, 7. [Google Scholar] [CrossRef]
- Szczesio-Wlodarczyk, A.; Sokolowski, J.; Kleczewska, J.; Bociong, K. Ageing of Dental Composites Based on Methacrylate Resins—A Critical Review of the Causes and Method of Assessment. Polymers 2020, 12, 882. [Google Scholar] [CrossRef]
- Krüger, J.; Maletz, R.; Ottl, P.; Warkentin, M. In vitro aging behavior of dental composites considering the influence of filler content, storage media and incubation time. PLoS ONE 2018, 13, e0195160. [Google Scholar] [CrossRef] [PubMed]
- Ferracane, J.L. Resin-based composite performance: Are there some things we can’t predict? Dent. Mater. 2013, 29, 51–58. [Google Scholar] [CrossRef] [PubMed]
- Hahnel, S.; Henrich, A.; Bürgers, R.; Handel, G.; Rosentritt, M. Investigation of Mechanical Properties of Modern Dental Composites after Artificial Aging for One Year. Oper. Dent. 2010, 35, 412–419. [Google Scholar] [CrossRef] [PubMed]
- Tsujimoto, A.; Barkmeier, W.W.; Fischer, N.G.; Nojiri, K.; Nagura, Y.; Takamizawa, T.; Latta, M.A.; Miazaki, M. Wear of resin composites: Current insights into underlying mechanisms, evaluation methods and influential factors. Jpn. Dent. Sci. Rev. 2018, 54, 76–87. [Google Scholar] [CrossRef]
- Bhadila, G.; Baras, B.H.; Weir, M.D.; Wang, H.; Melo, M.A.S.; Hack, G.D.; Bai, Y.; Xu, H.H.K. Novel antibacterial calcium phosphate nanocomposite with long-term ion recharge and re-release to inhibit caries. Dent. Mater. J. 2020, 39, 678–689. [Google Scholar] [CrossRef]
- Zhang, N.; Zhang, K.; Melo, M.A.S.; Weir, M.D.; Xu, D.J.; Bai, Y.; Xu, H.H.K. Effects of Long-Term Water-Aging on Novel Anti-Biofilm and Protein-Repellent Dental Composite. Int. J. Mol. Sci. 2017, 18, 186. [Google Scholar] [CrossRef] [PubMed]
- Garcia, I.M.; Balhaddad, A.A.; Aljuboori, N.; Ibrahim, M.S.; Mokeem, L.; Ogubunka, A.; Collares, F.M.; de Melo, M.A.S. Wear Behavior and Surface Quality of Dental Bioactive Ions-Releasing Resins Under Simulated Chewing Conditions. Front. Oral Health 2021, 2. [Google Scholar] [CrossRef]
- Passos, V.F.; Melo, M.A.; Vasconcellos, A.A.; Rodrigues, L.K.A.; Santiago, S.L. Comparison of methods for quantifying dental wear caused by erosion and abrasion. Microsc. Res. Tech. 2012, 76, 178–183. [Google Scholar] [CrossRef]
- ISO E. 4049:2009. Dentistry-Polymer-Based Restorative Materials; ISO International Organization for Standardization: Geneva, Switzerland, 2009. [Google Scholar]
- Maktabi, H.; Ibrahim, M.; Alkhubaizi, Q.; Weir, M.; Xu, H.; Strassler, H.; Fugolin, A.P.P.; Pfeifer, C.S.; Melo, M.A.S. Underperforming light curing procedures trigger detrimental irradiance-dependent biofilm response on incrementally placed dental composites. J. Dent. 2019, 88, 103110. [Google Scholar] [CrossRef] [PubMed]
- Bhadila, G.; Wang, X.; Zhou, W.; Menon, D.; Melo, M.A.S.; Montaner, S.; Oates, T.W.; Weir, M.D.; Sun, J.; Xu, H.H.K. Novel low-shrinkage-stress nanocomposite with remineralization and antibacterial abilities to protect marginal enamel under biofilm. J. Dent. 2020, 99, 103406. [Google Scholar] [CrossRef]
- Baras, B.H.; Wang, S.; Melo, M.A.S.; Tay, F.; Fouad, A.F.; Arola, D.D.; Weir, M.D.; Xu, H.H. Novel bioactive root canal sealer with antibiofilm and remineralization properties. J. Dent. 2019, 83, 67–76. [Google Scholar] [CrossRef]
- Al-Dulaijan, Y.A.; Cheng, L.; Weir, M.D.; Melo, M.A.S.; Liu, H.; Oates, T.W.; Wang, L.; Xu, H.H. Novel rechargeable calcium phosphate nanocomposite with antibacterial activity to suppress biofilm acids and dental caries. J. Dent. 2018, 72, 44–52. [Google Scholar] [CrossRef] [PubMed]
- Al-Dulaijan, Y.A.; Weir, M.D.; Melo, M.A.S.; Sun, J.; Oates, T.W.; Zhang, K.; Xu, H.H. Protein-repellent nanocomposite with rechargeable calcium and phosphate for long-term ion release. Dent. Mater. 2018, 34, 1735–1747. [Google Scholar] [CrossRef]
- Al-Qarni, F.D.; Tay, F.; Weir, M.D.; Melo, M.A.; Sun, J.; Oates, T.W.; Xie, X.; Xu, H.H. Protein-repelling adhesive resin containing calcium phosphate nanoparticles with repeated ion-recharge and re-releases. J. Dent. 2018, 78, 91–99. [Google Scholar] [CrossRef]
- McBain, A.J. Chapter 4 In Vitro Biofilm Models: An Overview. In Advances in Applied Microbiology; Academic Press: Cambridge, MA, USA, 2009; pp. 99–132. [Google Scholar] [CrossRef]
- Barker, S. Preparation and colorimetric determination of lactic acid. In Methods in Enzymology; Academic Press: Cambridge, MA, USA, 1957; pp. 241–246. [Google Scholar] [CrossRef]
- LIVE/DEAD® BacLightTM Bacterial Viability Kit Protocol-US, (n.d.). Available online: //www.thermofisher.com/us/en/home/references/protocols/cell-and-tissue-analysis/protocols/live-dead-baclight-bacterial-viability-protocol.html (accessed on 23 March 2021).
- ISO 4049:2009-Dentistry-Polymer-Based Restorative Materials, (n.d.). Available online: https://webstore.ansi.org/Standards/ISO/ISO40492009?gclid=EAIaIQobChMI49fVzfqo7wIVGOXICh3o-QogEAAYASAAEgIIpfD_BwE (accessed on 11 March 2021).
- Drummond, J.L. Degradation, Fatigue, and Failure of Resin Dental Composite Materials. J. Dent. Res. 2008, 87, 710–719. [Google Scholar] [CrossRef] [PubMed]
- Takeshige, F.; Kawakami, Y.; Hayashi, M.; Ebisu, S. Fatigue behavior of resin composites in aqueous environments. Dent. Mater. 2007, 23, 893–899. [Google Scholar] [CrossRef]
- Mitwalli, H.; Alsahafi, R.; Balhaddad, A.A.; Weir, M.D.; Xu, H.H.K.; Melo, M.A.S. Emerging Contact-Killing Antibacterial Strategies for Developing Anti-Biofilm Dental Polymeric Restorative Materials. Bioengineering 2020, 7, 83. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, M.S.; Garcia, I.M.; Vila, T.; Balhaddad, A.A.; Collares, F.M.; Weir, M.D.; Xu, H.H.K.; Melo, M.A.S. Multifunctional antibacterial dental sealants suppress biofilms derived from children at high risk of caries. Biomater. Sci. 2020, 8, 3472–3484. [Google Scholar] [CrossRef]
- Makvandi, P.; Jamaledin, R.; Jabbari, M.; Nikfarjam, N.; Borzacchiello, A. Antibacterial quaternary ammonium compounds in dental materials: A systematic review. Dent. Mater. 2018, 34, 851–867. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, Y.; Hu, Y.; Huang, F.; Xiao, Y. Quaternary ammonium compounds in dental restorative materials. Dent. Mater. J. 2018, 37, 183–191. [Google Scholar] [CrossRef]
- Marsh, P.D. Dental diseases—Are these examples of ecological catastrophes? Int. J. Dent. Hyg. 2006, 4 (Suppl. 1), 3–10, discussion 50–52. [Google Scholar] [CrossRef]
- Rego, G.F.; Vidal, M.L.; Viana, G.M.; Cabral, L.M.; Schneider, L.F.J.; Portela, M.B.; Cavalcante, L.M. Antibiofilm properties of model composites containing quaternary ammonium methacrylates after surface texture modification. Dent. Mater. 2017, 33, 1149–1156. [Google Scholar] [CrossRef] [PubMed]
- Vidal, M.L.; Rego, G.F.; Viana, G.M.; Cabral, L.M.; Souza, J.P.B.; Silikas, N.; Schneider, L.F.; Cavalcante, L.M. Physical and chemical properties of model composites containing quaternary ammonium methacrylates. Dent. Mater. 2018, 34, 143–151. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Wang, H.; Ren, B.; Li, H.; Weir, M.D.; Zhou, X.; Oates, T.W.; Cheng, L.; Xu, H.H. Do quaternary ammonium monomers induce drug resistance in cariogenic, endodontic and periodontal bacterial species? Dent. Mater. 2017, 33, 1127–1138. [Google Scholar] [CrossRef]
- Sideridou, I.D.; Karabela, M.M.; Vouvoudi, E.C. Physical properties of current dental nanohybrid and nanofill light-cured resin composites. Dent. Mater. 2011, 27, 598–607. [Google Scholar] [CrossRef]
- Rüttermann, S.; Alberts, I.; Raab, W.H.M.; Janda, R.R. Physical properties of self-, dual-, and light-cured direct core materials. Clin. Oral Investig. 2010, 15, 597–603. [Google Scholar] [CrossRef]
- Alzraikat, H.; Burrow, M.F.; Maghaireh, G.A.; Taha, N.A. Nanofilled Resin Composite Properties and Clinical Performance: A Review. Oper. Dent. 2018, 43, E173–E190. [Google Scholar] [CrossRef]
- Fúcio, S.B.; Carvalho, F.G.; Sobrinho, L.C.; Sinhoreti, M.A.; Puppin-Rontani, R.M. The influence of 30-day-old Streptococcus mutans biofilm on the surface of esthetic restorative materials—An in vitro study. J. Dent. 2008, 36, 833–839. [Google Scholar] [CrossRef] [PubMed]
- Ionta, F.Q.; Mendonça, F.L.; de Oliveira, G.C.; de Alencar, C.R.B.; Honório, H.M.; Magalhães, A.C.; Rios, D. In vitro assessment of artificial saliva formulations on initial enamel erosion remineralization. J. Dent. 2014, 42, 175–179. [Google Scholar] [CrossRef]
- Sagsoz, O.; Sagsoz, N.P. Chemical degradation of dental CAD/CAM materials. Bio-Med. Mater. Eng. 2019, 30, 419–426. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, M.S.; Garcia, I.M.; Kensara, A.; Balhaddad, A.A.; Collares, F.M.; Williams, M.A.; Ibrahim, A.S.; Lin, N.J.; Weir, M.D.; Xu, H.H.; et al. How we are assessing the developing antibacterial resin-based dental materials? A scoping review. J. Dent. 2020, 99, 103369. [Google Scholar] [CrossRef]
- Melo, M.A.S.; Weir, M.D.; Passos, V.F.; Rolim, J.P.M.; Lynch, C.D.; Rodrigues, L.K.A.; Xu, H.H.K. Human In Situ Study of the effect of Bis(2-Methacryloyloxyethyl) Dimethylammonium Bromide Immobilized in Dental Composite on Controlling Mature Cariogenic Biofilm. Int. J. Mol. Sci. 2018, 19, 3443. [Google Scholar] [CrossRef] [PubMed]

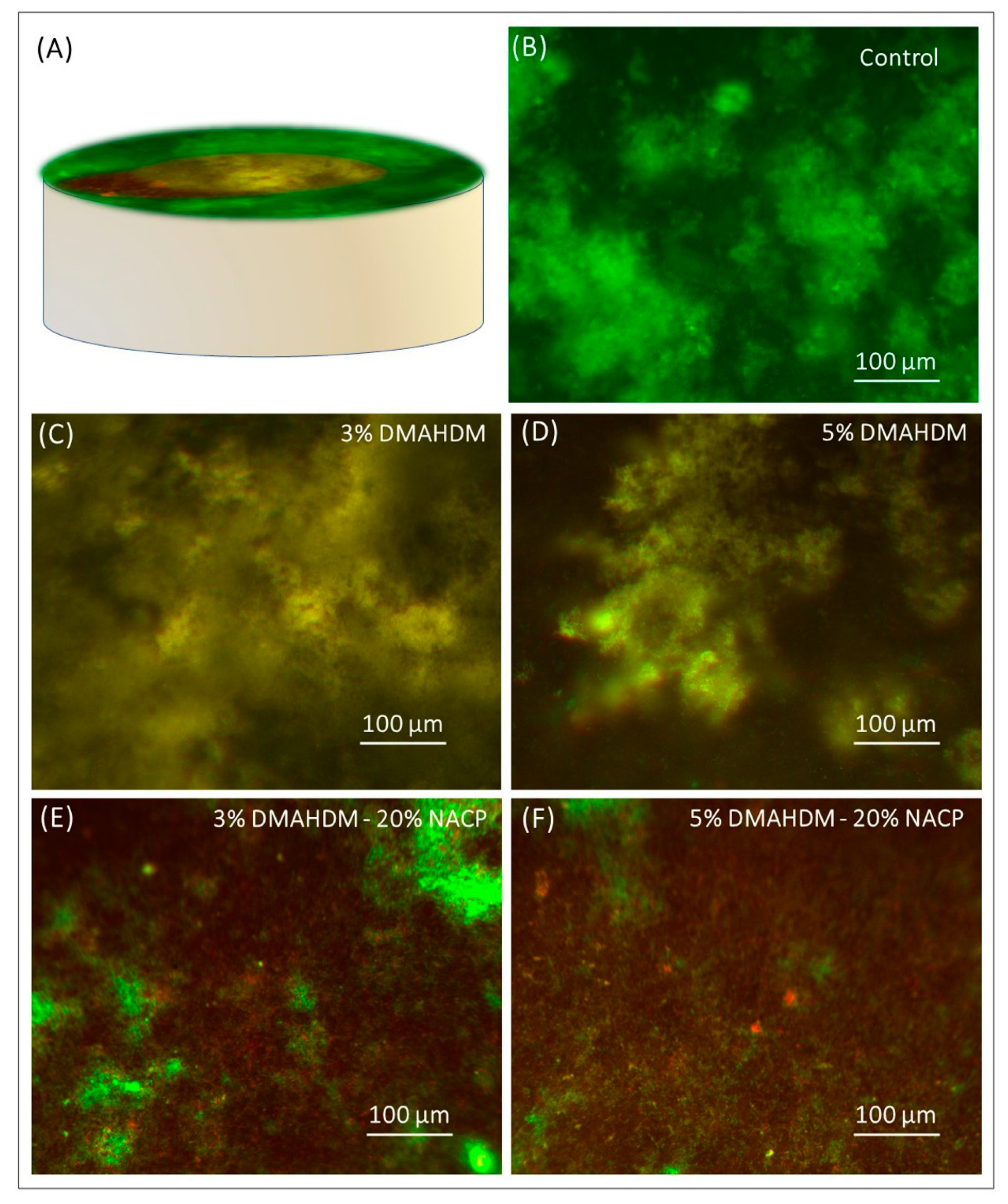
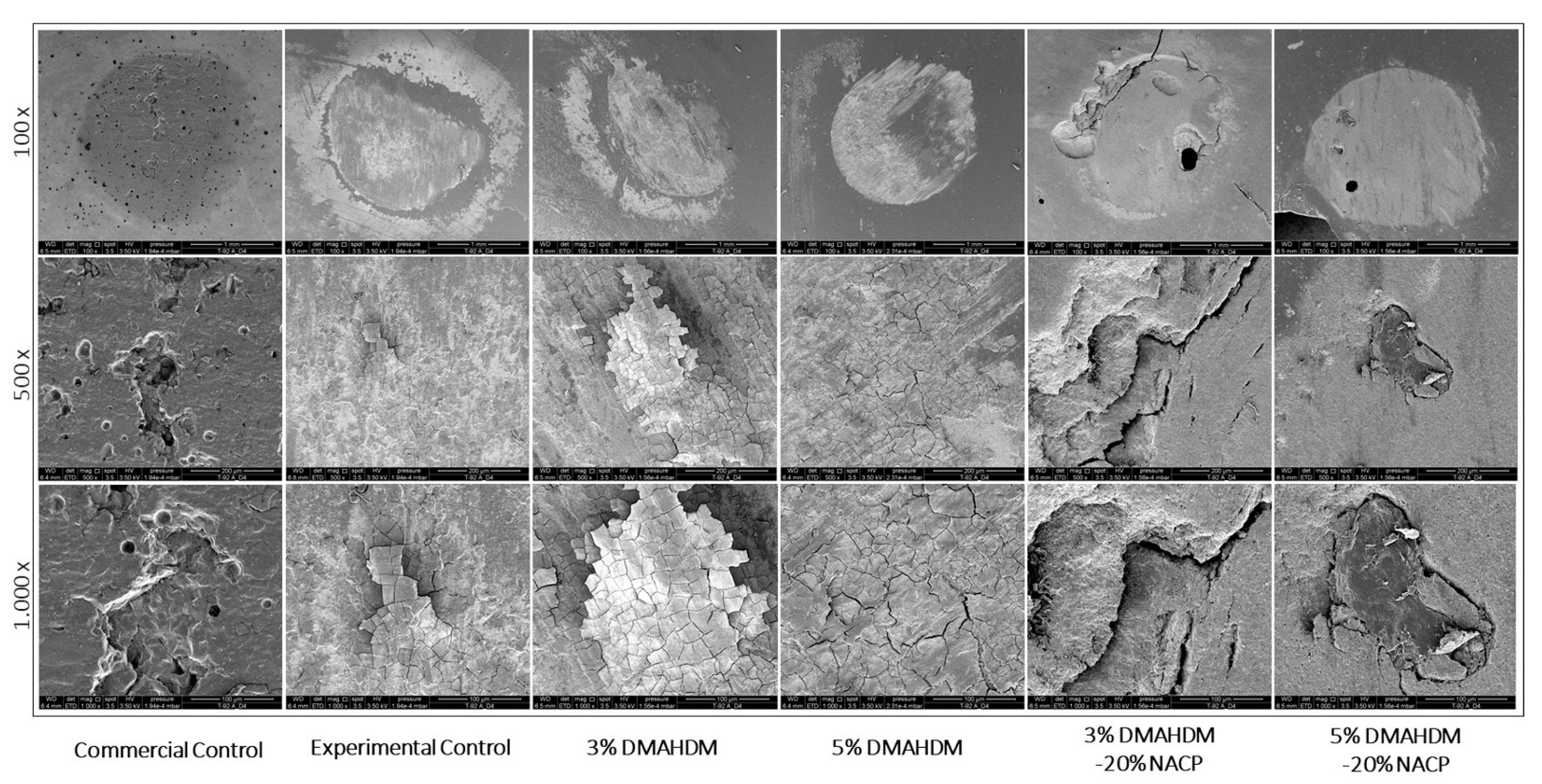
| Resin Composite Formulation | Ra (μm) | Rq (μm) | Rv (μm) | Rt (μm) |
|---|---|---|---|---|
| Commercial Control | 0.292 ± 0.098 a | 0.189 ± 0.109 a | 0.590 ± 0.178 a | 1.583 ± 0.429 ab |
| Experimental Control | 0.104 ± 0.067 a | 0.250 ± 0.107 a | 0.221 ± 0.092 a | 0.325 ± 0.048 a |
| 3% DMAHDM | 0.223 ± 0.145 a | 0.307 ± 0.158 a | 0.439 ± 0.363 a | 1.212 ± 0.627 ab |
| 5% DMAHDM | 0.310 ± 0.227 a | 0.304 ± 0.164 a | 0.591 ± 0.549 a | 1.674 ± 1.032 ab |
| 3% DMAHDM-20% NACP | 0.282 ± 0.176 a | 0.323 ± 0.180 a | 0.604 ± 0.438 a | 1.250 ± 1.036 ab |
| 5% DMAHDM-20% NACP | 0.313 ± 0.166 a | 0.388 ± 0.249 a | 0.790 ± 0.422 a | 2.120 ± 1.537 b |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Balhaddad, A.A.; Mokeem, L.S.; Weir, M.D.; Xu, H.; Melo, M.A.S. Sustained Antibacterial Effect and Wear Behavior of Quaternary Ammonium Contact-Killing Dental Polymers after One-Year of Hydrolytic Degradation. Appl. Sci. 2021, 11, 3718. https://doi.org/10.3390/app11083718
Balhaddad AA, Mokeem LS, Weir MD, Xu H, Melo MAS. Sustained Antibacterial Effect and Wear Behavior of Quaternary Ammonium Contact-Killing Dental Polymers after One-Year of Hydrolytic Degradation. Applied Sciences. 2021; 11(8):3718. https://doi.org/10.3390/app11083718
Chicago/Turabian StyleBalhaddad, Abdulrahman A., Lamia S. Mokeem, Michael D. Weir, Huakun Xu, and Mary Anne S. Melo. 2021. "Sustained Antibacterial Effect and Wear Behavior of Quaternary Ammonium Contact-Killing Dental Polymers after One-Year of Hydrolytic Degradation" Applied Sciences 11, no. 8: 3718. https://doi.org/10.3390/app11083718
APA StyleBalhaddad, A. A., Mokeem, L. S., Weir, M. D., Xu, H., & Melo, M. A. S. (2021). Sustained Antibacterial Effect and Wear Behavior of Quaternary Ammonium Contact-Killing Dental Polymers after One-Year of Hydrolytic Degradation. Applied Sciences, 11(8), 3718. https://doi.org/10.3390/app11083718