Abstract
Research on gold nanoparticles (AuNPs) has often focused on their physical, chemical, and crystalline characteristics. Commercial AuNPs have been applied in the diverse fields of biomedicine, catalysis, photovoltaics, and sensing. In this study, we explored the various activities of AuNPs to widen their applicability. This paper presents a simple and rapid synthesis process of AuNPs with bacteria isolated from a gold mining area. We also investigated the optimization of reaction parameters for AuNP synthesis. The study results revealed that among the isolated strains, Bifidobacterium lactis and Escherichia coli demonstrated the highest capabilities of AuNP synthesis. The optimal pH values for AuNP synthesis by B. lactis (BLAuNPs) and E. coli (ECAuNPs) were 5.0 for 72 h of incubation and 8.0 for 24 h of incubation. The average particle sizes of ECAuNPs and BLAuNPs were 4.2 and 5.6 nm, respectively. Furthermore, these biogenic AuNPs were found to be stable with no aggregation after 3 months of storage. BLAuNPs and ECAuNPs exhibited high levels of antimicrobial, antioxidant, photocatalytic, and antityrosinase activity. Moreover, they were noncytotoxic to skin cells even at 100% melanin inhibitory concentrations. Considering the demonstrated multifunctional activities of AuNPs, BLAuNPs and ECAuNPs have promising potential for commercialization.
1. Introduction
Nanotechnology is a major emerging research area for the manufacturing of new materials at the nanoscale level, which involves specialized structures, design, devices, and production at the nanometer scale [1]. The synthesis of nanoparticles (NPs) from gold has emerged as a vital application of nanotechnology [2]. Among metal NPs, AuNPs are very useful for efficient drug delivery owing to their simple, well-studied synthesis process and low toxicity [3]. AuNPs with a diameter of ≤5 nm can be used as a catalyst in biomedical reactions [4]. The major biomedical applications of AuNPs include targeted drug delivery, anticancer therapy, molecular imaging in living cells, antimicrobial activities, biosensors, and biocatalysts [4]. Furthermore, AuNPs have been used for cosmetic purposes, such as skin wound disinfection, anti-inflammation, and antiaging creams [5]. However, few studies have explored the antioxidant, antityrosinase, and antimelanogenesis properties of AuNPs.
In recent years, NPs have been synthesized using physical and chemical techniques. However, these synthesis techniques are expensive and involve the use of toxic chemicals [6]. NP synthesis using physical techniques requires high energy utilization, leading to high operational costs. Chemical synthesis techniques are low cost but involve the use of toxic solvents, which may result in contamination due to toxic byproducts. To overcome these challenges and to increase the range of application of AuNPs, a green and nontoxic synthesis method is urgently required. Biological synthesis methods are green alternatives for NP production. Many bacteria, fungi, yeast, plants, and algae can be used for high-yield, low-cost, and environment-friendly synthesis of NPs [7,8]. AuNPs synthesized by microorganisms are very useful in the production of effective antibacterial agents because of their nontoxic nature, polyvalent effects, and photothermal activity [9]. Among microorganisms, the use of prokaryotes for AuNP synthesis has received the most attention [10]. The surface energy of the NPs increases due to the surface reactive sites available on the NPs’ surfaces. This increases the contaminant removal efficiency of AuNPs even at low concentrations, making them suitable for use in water purification [11]. However, the exact mechanism by which microorganisms produce AuNPs remains unclear. Based on synthesis location, extracellular and intracellular synthesis mechanisms have been speculated on [8]. Different bacteria adopt different synthesis mechanisms and produce AuNPs with different sizes (1.9–100 nm) and shapes (cubic, spherical, triangular, hexagonal, and octahedral) [10]. Consequently, AuNPs produced by microorganisms exhibit different physiological activities or reactivities. However, studies have often focused more on determining the physical, chemical, and crystalline characteristics of AuNPs by using various precision instruments [9], and few studies have explored the multifunctional activities of AuNPs.
In this study, six different strains of bacteria—Pseudomonas aeruginosa, Bacillus cereus, Bacillus subtilis, Escherichia coli, Bifidobacterium lactis, and Lactobacillus acidophilus—were isolated from an abandoned gold mining area to evaluate their suitability for AuNPs production. Optimal strains were adopted to produce AuNPs and explore the antimicrobial, tyrosinase inhibitory, antioxidant, photocatalytic, and antimelanogenesis activities of the biosynthesized AuNPs. Furthermore, the cytotoxicity of the biosynthesized AuNPs to human epidermal melanocytes (HEMn) and the normal human skin fibroblast cell line (CCD-966SK) were examined. To our knowledge, this is the first study to evaluate multifunctional activities of biosynthesized AuNPs and their potential for use in food, environmental hygiene, medicine, and cosmetics.
2. Materials and Methods
2.1. Materials
All chemicals used in this experiment were of analytical grade. The AuNPs-producing bacteria (P. aeruginosa, B. cereus, B. subtilis, E. coli, B. lactis, and L. acidophilus) were isolated from an abandoned gold mine area (RuiFang District, New Taipei City, Taiwan). E. coli (ATCC 8739), Staphylococcus aureus (ATCC 6538), P. aeruginosa (ATCC 9027), Candida albicans (ATCC 10231), Cutibacterium acnes (ATCC 6919), and B. cereus (ATCC 10250) were purchased from the Bioresource Collection and Research Center (BCRC) (Hsinchu, Taiwan) and used in the antimicrobial experiment. Cultures of HEMn cells (Cascade cat. C-102–5C, Cascade Biologics, Inc., Portland, OR, USA) obtained from neonatal foreskin were propagated in medium 254 supplemented with human melanocyte growth supplement (Cascade Biologics, Inc., Portland, OR, USA), and the normal human skin fibroblast cell line CCD-966SK (ATCC CRL-1881) was purchased from BCRC. Mushroom tyrosinase was purchased from Sigma Chemical Co. (St. Louis, MO, USA). The 5-nm AuNPs (Chemicals-5 nm) were chemically synthesized using the classical method with sodium citrate [12] purchased from a local company (Li-hsin Chemical Company, Taiwan).
2.2. Identification of Isolates and Cultivation
The isolates were identified through PCR amplification and quantitated through 16S rRNA gene sequencing. The obtained sequences were compared with those of the reference strains in GenBank with >98.6% sequence similarity. P. aeruginosa, B. cereus, B. subtilis, and E. coli were aerobically cultured (pH 7.0) in a 1-L Erlenmeyer flask with 200 mL of nutrient broth and were cultivated for 24 h. B. lactis and L. acidophilus were anaerobically cultured (pH 6.0) in 200 mL Lactobacilli MRS broth with 0.05% cysteine in a 1-L Erlenmeyer flask; they were cultivated for 72 h.
2.3. Biosynthesis and Purification of AuNPs
The extraction method of AuNPs from bacteria was modified from Roshmi et al.’s method [3]. After incubation, the bacterial biomass was collected by centrifugation at 6000 rpm for 10 min and washed thoroughly with sterile deionized water to remove any adhering media components. Approximately 2 g of bacterial wet biomass was resuspended in 100 mL of an aqueous solution of filter-sterilized 1 mM chloroauric acid (HAuCl4) in a 250 mL Erlenmeyer flask for AuNP synthesis. Bacterial biomass was incubated without HAuCl4 as control. After synthesis, the culture solution was centrifuged at 15,000× g for 15 min, and the collected pellets were washed and resuspended in 50 mM Tris buffer (pH 7). The bacterial cells were disrupted through ultrasonication (VCX750, Sonics, USA) over three 15s periods and with 45 s intervals between periods. The resulting solution was then filtered through a 0.22 μm filter (Merck KGaA, Burlington, Massachusetts, USA), and the filtrate containing AuNPs was lyophilized at −80 °C under vacuum. After lyophilization, the AuNPs were stored in screw cap bottles for further characterization.
2.4. Characterization of AuNPs
The formation and stability of the synthesized AuNPs were recorded at 300–700 nm by using a UV–visible (UV–vis) spectrophotometer (Thermo Scientific Evolution 201, USA). The morphology of AuNPs was characterized through transmission electron microscopy (Philips Tecnai F20, Netherlands). The particle size and particle-size distribution of AuNPs were obtained using a dynamic light scattering spectrometer (Malvern Nano ZS, UK). The stability of AuNPs was evaluated by storing AuNPs in deionized water, 5% bovine serum albumin, 5% NaCl or phosphate buffered saline (PBS) (pH 7.4) at 26 °C for 3 months. The solutions were monitored using a UV–vis spectrophotometer during the storage period.
2.5. Effect of pH on AuNP Synthesis
To evaluate the effect of pH on AuNP synthesis, E. coli pellets were collected after 24 h of culture and B. lactis pellets were collected after 72 h of culture. Then, 2 g of bacterial wet biomass was mixed with 1 mM HAuCl4 and cultured at different pH values (5.5–8.5 for E. coli and 4.0–7.0 for B. lactis) for 24, 48, and 72 h. The absorbance was measured using a UV–vis spectrophotometer, the change in solution color was observed, and the particle size of AuNPs was measured.
2.6. Minimum Inhibitory Concentration and Antibacterial Behavior of AuNPs
The antimicrobial activities of AuNPs were evaluated by analyzing the minimum inhibitory concentration (MIC). MIC was defined as the lowest concentration of antimicrobial agent required to inhibit the growth of microbes. The turbidity method was used to determine the MIC of AuNPs [13]. The 18 mL of sterilized broth was mixed with 1 mL of the tested strain and 1 mL of different concentrations of AuNPs in sterile test tubes. The final OD of inoculated strain and AuNPs final concentrations were 0.8 and 0.78–50 mg/L in the test tubes, respectively. The mixtures were shaken gently and then incubated. The tubes were incubated for 24 h (E. coli, Staphylococcus aureus, P. aeruginosa, and B. cereus), 48 h (Cutibacterium acnes), or 72 h (Candida albicans). The assay was repeated thrice, and the data are expressed as mean ± standard deviation. A negative control was prepared (without AuNPs, but with growth media).
To determine the release of cell constituents during the antibacterial process, a previously reported method was followed with slight modifications [14]. Briefly, the bacterial strains (E. coli and S. aureus) were grown in a nutrient broth at 37 °C for 24 h. The 100 mL bacterial suspension was centrifuged to obtain bacterial cells. The bacterial pellets were then rehomogenized in 50 mL of 0.1 M PBS (≈107 CFU/mL). The AuNPs synthesized by B. lactis (BLAuNPs) at MIC were mixed and incubated at 37 °C for 6 h with the bacterial cell suspension. After incubation, 3 mL of the solution was taken at hourly intervals and centrifuged at 10,000× g for 3 min. Then, the concentrations of constituents (i.e., nucleotides) released from the bacterial cells were measured and estimated at 260 nm by using a UV–vis spectrophotometer.
2.7. Analysis of Antioxidant Activity of AuNPs
The scavenging activity of the 2,2-diphenyl-1-picrylhydrazyl (DPPH) radical was used as an index for the antioxidant activity levels of the AuNPs, estimated according to the method of Wang et al. (2015) [15]. The DPPH solution (100 mM) was prepared in pure ethanol (97%). The reaction mixture comprised 1 mL of AuNPs and 4 mL of methanolic DPPH (0.1 mM). The absorbance of this mixture and a blank without the AuNPs was measured at 517 nm by using a UV–vis spectrophotometer after 1 h of incubation in the dark. The DPPH scavenging activity by AuNPs was calculated using the following equation:
where A0 is the absorbance of the blank and A is the absorbance of the test compound. The IC50 value of the DPPH radical by the AuNPs was evaluated at 50% scavenging activity.
2.8. AuNPs as Photocatalysts
The photocatalytic activities of the biosynthesized and chemically synthesized AuNPs were evaluated by monitoring the reduction of dyes, such as Victoria blue R (VBR), malachite green (MG), and methylene blue (MB). The solution containing 25 mg/L of AuNPs and 100 mg/L of dyes was stirred at 100 rpm for 20 min in the dark before it was exposed to visible light to reach adsorption equilibrium. Subsequently, the solution was irradiated with visible light for 12 min at 100 rpm. Then, 2 mL of the suspension was taken at constant intervals and centrifuged. The photocatalytic degradation of dyes was monitored using a UV–vis spectrometer at 610 nm for VBR, 660 nm for MG, and 619 nm for MB. The dye degradation percentage or removal efficiency was analyzed using the following formula.
where C0 and Ce correspond to the dye concentrations before and after photocatalysis, respectively.
2.9. Antityrosinase Activity
The antityrosinase activity of AuNPs was analyzed using the method of Wang et al. (2017) with modifications [15]. First, AuNPs were dissolved in a dimethyl sulfoxide (DMSO) solution (1 g/L) and further diluted to different concentrations with DMSO. Second, 30 μL of the solution containing AuNPs was mixed with 970 μL of sodium phosphate buffer (0.05 mM). The mixture was subsequently added to a solution containing 1 mL of L-tyrosine (100 mg/L) and 1 mL of mushroom tyrosinase solution (350 units/mL). The absorbance of the solution was measured at 490 nm by using a UV–vis spectrophotometer before and after 20 min of incubation. The IC50 value, at which half the original tyrosinase activity was inhibited, was analyzed through AuNPs treatment. The antityrosinase activity of AuNPs can be expressed as a percentage of tyrosinase inhibition by using the following formula:
where A is the absorbance at 490 nm without AuNPs (control), B is the absorbance at 490 nm without AuNPs and tyrosinase (blank), C is the absorbance at 490 nm with AuNPs and tyrosinase (experimental group), and D is the absorbance at 490 nm without tyrosinase (blank of C).
The antityrosinase activities of AuNPs in HEMn cells were analyzed as described previously [16]. Briefly, HEMn cells (106 cells/mL) were cultured in 24-well plates, exposed to 100 nM α-MSH for 48 h, and then treated with increasing doses of AuNPs for 24 h. Afterward, the cells were washed with PBS and lysed with 0.1 M PBS containing 1% Triton X-100 and protease inhibitors. The treated cells were then sonicated, and lysates were clarified through centrifugation at 9000× g for 15 min. The cellular tyrosinase content was determined using a BCA Protein Assay Kit (Thermo Scientific, Vantaa, Finland). The resulting solution was added to 96-well plates, with each well containing 20 μg of tyrosinase, 2.5 mM L-DOPA, and 0.1 M PBS. After incubation at 37 °C for 1 h, absorbance was measured at 475 nm by using an ELISA reader (Epoch 2 Bio-Tek Instruments, USA).
2.10. Cell Viability Assay
The AuNPs cytotoxicity on HEMn cells and CCD-966SK cells was assessed through the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) method [17]. Briefly, after a 24 h incubation, cells (3 × 106 cells/well) were washed, treated with the culture medium (control) or AuNPs for 24–72 h for cytotoxic analysis, and then subjected to MTT assay. Afterward, these cells were rewashed and incubated with 0.05% MTT solution at 37 °C for 4 h. After purple formazan crystals were formed, the media were removed, and 100 μL of DMSO was added to solubilize the crystals for 15 min. The absorbance was then measured at 595 nm by using an ELISA reader.
2.11. Cellular Melanin Content in HEMn Cells
Melanin contents were measured as described previously by Liao et al., 2012 [17]. Briefly, HEMn cells (3 × 106 cells/well) were incubated in a fresh medium containing various concentrations of AuNPs for 24 h. After the cells were washed with PBS, they were dissolved in 1 N NaOH containing 10% DMSO at 80 °C for 1 h, and the melanin released in cell suspensions was quantified. Relative melanin content was measured at 450 nm by using an ELISA reader. The melanin content was measured in comparison with the synthetic melanin standard (Sigma Chemical Co., St. Louis, MO, USA).
2.12. Statistical Analysis
Experimental results are reported as: mean ± standard deviation of three replicates. Data were subjected to one-way analysis of variance followed by Tukey’s post hoc test for multiple comparisons. Statistical analysis was conducted using SPSS version 24.0 (SPSS Inc. Chicago, IL, USA). Mean differences were considered significant when p < 0.05. The IC50 values were calculated using the Origin software package.
3. Results and Discussion
3.1. Effect of pH on AuNP Synthesis
The analysis of AuNPs biosynthesized by different bacterial strains indicated that at a pH of 7.0, P. aeruginosa, B. cereus, E. coli, and B. subtilis exhibited higher yields after 24 h of incubation than after incubation for 48 and 72 h. Furthermore, at a pH of 6.0, B. lactis and L. acidophilus exhibited higher yields after 72 h of incubation. The colloidal AuNPs biosynthesized by E. coli (called ECAuNPs) were bluish violet and exhibited a high optical density (1.45) at 530 nm. The colloidal AuNPs biosynthesized by B. lactis (called BLAuNPs) were wine red in color and exhibited a high optical density (1.63) at 540 nm. Among all tested strains, the optical densities of only AuNPs synthesized by E. coli and B. lactis were higher than 1.4, which indicated the potential of these two strains to synthesize AuNPs.
Regarding the effect of pH on AuNP synthesis, the results revealed that B. lactis produced the highest yield (OD: 2.49) of AuNPs at pH 5.0 after 72 h of incubation (Figure 1a), and E. coli produced the highest yield (OD: 1.95) at pH 8.0 after 24 h of incubation (Figure 1b). The optimal growth pH values of E. coli and B. lactis were 7.0 and 6.0, respectively, which indicates that the optimal growth pH might not be the optimal pH for AuNP synthesis. Piella et al. reported that the optimal pH was found to balance the reactivity of the gold precursor to promote rapid nucleation while ensuring a good electrostatic stabilization of the particles [18].
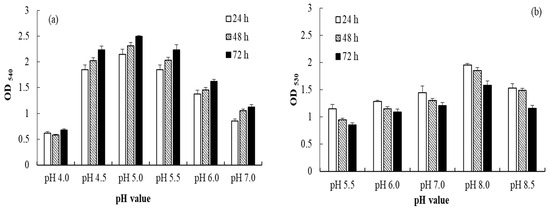
Figure 1.
Effects of pH on the AuNPs synthesized by (a) B. lactis and (b) E. coli after 24, 48, and 72 h of incubation.
3.2. Effect of pH on the Particle Size of AuNPs
The average particle sizes of AuNPs synthesized by B. lactis at pH 4.5, 5.0, 6.0, and 7.0 were 6.0 ± 0.8, 5.6 ± 0.3, 38.2 ± 1.5, and 32.6 ± 1.2 nm, respectively (Figure 2a). The average particle sizes of AuNPs synthesized by E. coli at pH 6.0, 7.0, and 8.0 were 58.3 ± 1.0, 6.4 ± 0.5, and 4.2 ± 0.5 nm, respectively (Figure 2b). A previous study reported that AuNPs with a diameter of ≤5 nm can be used as an efficient catalyst [4], which indicates that B. lactis and E. coli have strong potential to synthesize commercial AuNPs. Thus, the optimal pH was 5.0 for BLAuNP synthesis and 8.0 for ECAuNP synthesis. Hexagonal BLAuNPs were intracellularly synthesized because they could not be detected without breaking of the cell wall. Conversely, spherical ECAuNPs were produced through extracellular synthesis. Moreover, the stability of BLAuNPs and ECAuNPs was monitored through UV–vis spectroscopy. After storage for 3 months, no change was detected in the band wavelength and intensity, indicating that both AuNPs were highly stable. The particle sizes of BLAuNPs and ECAuNPs were 5.6 ± 0.3 nm (at pH 8.0) and 4.2 ± 0.5 nm (at pH 5.0), respectively—smaller than the particle size of AuNPs synthesized by most plants, such as Crescentia cujete L. (32.98 nm), Virola oleifera (15 nm), and Convolvulus fruticosus (35 nm) [12,19,20], and some bacteria, such as Bacillus sp. (12–32 nm) and Streptomyces sp. (10–30 nm) [3,21]. The relatively large size of AuNPs synthesized by plants may be due to the capped protein from plant extract adsorbed onto the AuNPs surface [12]. Additionally, the zeta potential of the BLAuNPs changed from +16.3 ± 1.6 mV at pH 4.0 to −28.2 ± 0.9 mV at pH 5.0 and that of the ECAuNPs changed from +23.6 ± 1.2 mV at pH 5.5 to −25.1 ± 1.6 mV at pH 8.0, leading to the stability of the nanoparticle. As B. lactis at pH 5.0 and E. coli at pH 8.0 synthesized AuNPs with high yields, small particle sizes, and high stability, BLAuNPs and ECAuNPs were used in the following evaluations.
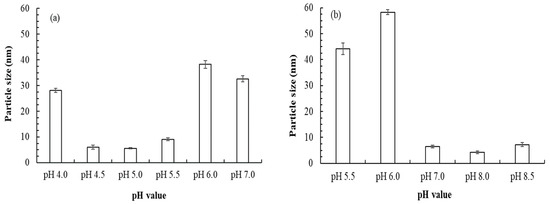
Figure 2.
Effect of pH on the particle size of AuNPs synthesized by (a) B. lactis after 72 h of incubation and (b) E. coli after 24 h of incubation.
3.3. Effect of AuNPs on MIC
The results in Table 1 indicated that the MICs of E. coli and S. aureus were 6.25 and 12.5 mg/L in BLAuNPs (Table 1). The results were similar to those of AuNPs biosynthesized by Olax nana, which had an MIC of 9.14 mg/L [22]. Zada et al. demonstrated that AuNPs caused bacterial membrane damage and cell disruption by inducing the production of intracellular reactive oxygen species (ROS). AuNPs can react with water vapor or oxygen present in the air, and generate ROS. Such active species can subsequently interact with other molecules present on the surface of a bacterial membrane, leading to further antimicrobial reactions. These cause oxidative damage to nucleic acids and bacteria [23]. Additionally, AuNPs can also play a role as an antioxidant (illustrated in Section 3.4). The inhibitory effect of BLAuNPs and chemically synthesized AuNPs on gram-negative bacteria E. coli and P. aeruginosa was stronger than that of BLAuNPs on gram-positive bacteria S. aureus, C. acnes, and B. cereus (Table 1). However, the inhibitory effects of ECAuNPs on all tested strains were equally strong. Due to their small size and positive charge, NPs can destroy the bacterial cell wall and interact with DNA, thereby resulting in cell death [23]. Based on the results of the release of the cell constituents, the leakage of nucleotides from E. coli and S. aureus treated with BLAuNPs increased with an increase in contact time (data not shown). Higher amounts of nucleotides were released from E. coli than from S. aureus, indicating that BLAuNPs had a stronger bactericidal effect on E. coli. Baruah et al. reported that the numerous peptidoglycan layers on the walls of gram-positive bacteria prevent the NPs from reaching the cytoplasmic membrane. Thus, gram-positive bacteria are less affected by the NPs than gram-negative bacteria are [24]. Both ECAuNPs and BLAuNPs exhibited optimal inhibitory effects on the opportunistic pathogenic yeast C. albicans (MIC: 3.125 mg/L). Furthermore, the antimicrobial activity of chemically synthesized AuNPs (Chemicals-5 nm) was significantly inferior to those of biogenic AuNPs (Table 1). Although the particle size of AuNPs was similar, the antimicrobial activity was obviously different. This result might be attributed to the shape of AuNPs, the uniform dispersion of AuNPs, or the surface capped molecules on AuNPs [25]. AuNPs synthesized by Sargassum incisifolium and Prunus persica were ineffective against bacteria [26,27]. The MIC values of AuNPs synthesized by Alpinia nigra against E. coli, synthesized by Convolvulus fruticosus against E. coli and S. aureus, and synthesized by Trichoderma harzianum against E. coli were 300, 75, and 20 mg/L, respectively [20,24,28]. Clearly, ECAuNPs and BLAuNPs were effective broad-spectrum antibiotics. As E. coli, S. aureus, and B. cereus are foodborne bacteria [29] and E. coli, S. aureus, P. aeruginosa, C. albicans, and C. acnes are frequently found in cosmetics or associated with common skin disorders [30], ECAuNPs and BLAuNPs have the potential applications in the food, environmental hygiene, and cosmetics.

Table 1.
Minimum inhibitory concentrations (mg/L) of BLAuNPs, ECAuNPs, and chemically synthesized AuNPs (5 nm) against tested strains.
3.4. Antioxidant Activity
Free radicals cause damage and mutation of human cells, and antioxidants play a crucial role in fighting them. The antioxidant activity of chemicals is often evaluated by measuring the DPPH radical scavenging activity [31]. Figure 3 indicates that the DPPH radical scavenging activity increases as the concentration of AuNPs increases. A similar dose-dependent behavior was observed among AuNPs biosynthesized using thyme extract [32]. Scavenging activities of 92.5%, 100%, and 35% were achieved for BLAuNPs, ECAuNPs, and Chemicals–5 nm, respectively, at 26 mg/L. The IC50 values of BLAuNPs, ECAuNPs, and Chemicals–5 nm were calculated as 18.2, 14.8, 32.4 mg/L, respectively. The results showed that biosynthesized AuNPs exhibited higher antioxidant activity than chemically synthesized AuNPs. IC50 values for DPPH radical scavenging activity of AuNPs synthesized by Albizia amara [33], Prunus persica [27], Alpinia nigra [24], and P. ginseng [5] were 25.25, 92.6, 52.16, and 141.3 mg/L, respectively. Compared with these biogenic AuNPs, ECAuNPs exhibited higher antioxidant activity. This may be because AuNPs exists in two oxidation states, Au+1 and Au+3; thus, they have a high tendency (especially AuNPs of a small particle size or coated with bioactive compounds) to accept or donate electrons or hydrogen to create a strong bond based on the existing oxidation state [34].
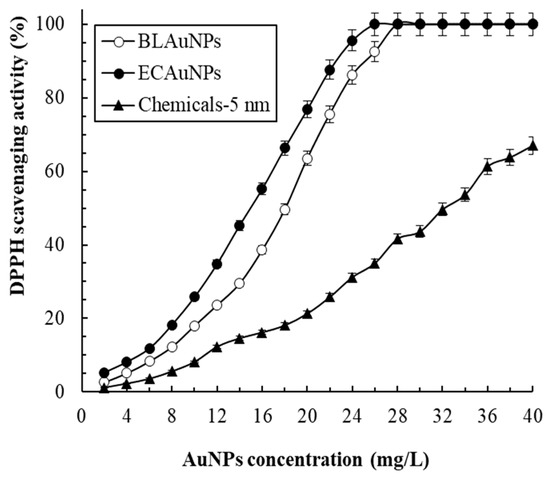
Figure 3.
DPPH radical scavenging activity of BLAuNPs, ECAuNPs, and Chemicals-5 nm at different concentrations.
3.5. Photocatalytic Activity of AuNPs
Studies that have investigated the photodegradation of dyes or other pollutants by AuNPs have reported some disadvantages of AuNPs use. First, high AuNPs concentration was required to achieve satisfactory efficiency; for example, 60–2000 mg/L biogenic AuNPs was required to degrade dye [20,25,35]. Second, only treatment with dilute dye concentration did not fulfil the actual requirements of dye treatment; for example, treatment with 5–22.4 mg/L MB was insufficient [20,25,36]. Finally, only in the presence of NaBH4, strong photocatalytic activity was achieved for efficient degradation of dyes [24]. To solve these problems, we evaluated the efficiency of 25 mg/L AuNPs in degrading 100 mg/L dye without the addition of NaBH4.
Dye degradation of AuNPs can be ranked from the easiest to the hardest as follows (Figure 4): VBR, MG, and MB. This ranking might be attributed to differences in the chemical structures of the dyes. Furthermore, ECAuNPs exhibited the highest photocatalytic efficiency and Chemicals-5 nm exhibited the lowest removal efficiency. The reaction was of pseudo-first order, and the respective rate constants (k) for photocatalytic reactions by ECAuNPs for VBR, MG, and MB were 0.702, 0.452, and 0.357 min−1 (r2 > 0.97). The photodegradation of MB by ECAuNPs was more efficient than the photodegradation of MB by AuNPs synthesized by Longan fruit (k = 0.29 min−1) [25] and Lagerstroemia speciosa (k = 0.198 min−1) [37]. Moreover, 100% removal of 40 mg/L MG by 60 mg/L AuNPs synthesized by Enterococcus species required 48 h [35], but ECAuNPs and BLAuNPs could photodegrade 100 mg/L MG in only 8 and 12 min in this study, respectively. The high photocatalytic activity of the ECAuNPs can be attributed to their small size and spherical morphology. These results indicated that AuNPs synthesized in this study were competitive. Baruah et al. reported that a photocatalytic reaction is triggered by the excitation of electrons in AuNPs from the low energy valence band to the high energy conduction band in the presence of sunlight [24]. The electrons from the valence band on the AuNPs surface are transferred to these dyes, thereby degrading them. Briefly, biogenic AuNPs synthesized in this study exhibit high potential to be used in the removal of dyes or other environmental pollutants.
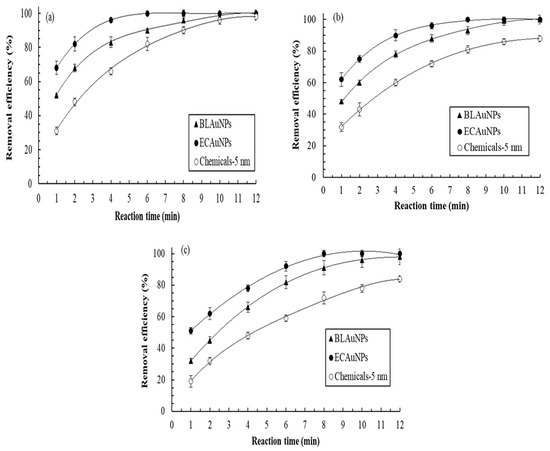
Figure 4.
Removal efficiencies of (a) VBR (b) MG and (c) MB by BLAuNPs, ECAuNPs, and Chemicals-5 nm during the 12 min treatment periods (dye concentration: 100 mg/L; AuNPs concentration: 25 mg/L).
3.6. Cell Viability Assessment
To evaluate user safety, the cytotoxic effects of BLAuNPs, ECAuNPs, and Chemicals-5 nm on viable HEMn cells and CCD966SK cells were assessed after a 72 h treatment by using the MTT method. Based on previous results (Figure 3 and Table 1), the maximum required concentrations for BLAuNPs, ECAuNPs, and Chemicals-5 nm to achieve optimal antioxidant and antimicrobial activity were 18.2, 14.8, and 50 mg/L, respectively. Therefore, the effects of 0–100 mg/L AuNPs on cell viability were evaluated.
Figure 5 shows that the cytotoxicity of AuNPs to the tested cells increases as the AuNPs concentration increases. Compared with BLAuNPs, ECAuNPs were more toxic. At up to 2 × IC50 for BLAuNPs and ECAuNPs (36.4 and 29.6 mg/L, respectively), the cell viability of HEMn and CCD966SK cells still exceeded 98.0%. When the tested concentration of Chemicals-5 nm was up to 2 × MIC (100 mg/L), the cell viability exceeded 92.1%. No obvious signs of cytotoxicity were observed compared with control. In addition, the differences in the inhibition of cell viability by BLAuNPs and ECAuNPs were nonsignificant (p > 0.05) between CCD966SK and HEMn cells when the tested concentration was ≤60 mg/L. However, previous reports have indicated that AuNPs biosynthesized by Albizia amara exerted cytotoxic effects on HeLa and Vero cells, with IC50 values of 47.77 and 72.28 mg/L, respectively [33]. The IC50 of AuNPs biosynthesized by Olax nana on HepG2 cells was 2.97 mg/L [22]. Previous studies have revealed that AuNPs could be uptaken by cells through the clathrin-mediated endocytosis pathway [12,38]. Thus, the cytotoxicity of BLAuNPs and ECAuNPs to CCD966SK and HEMn cells can be prevented in a similar manner. These results clearly indicated that BLAuNPs and ECAuNPs can be safely applied in the medicine and cosmetics.
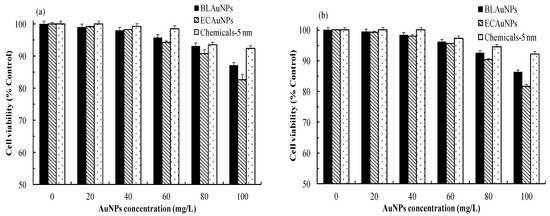
Figure 5.
Cell viability of (a) HEMn cells and (b) CCD966SK cells treated by BLAuNPs, ECAuNPs, and Chemicals-5 nm at various concentrations after 72 h treatments.
3.7. In Vitro and In Vivo Tyrosinase Activity Inhibition
Figure 6 shows that the antityrosinase activities in mushroom and HEMn cells increase with an increase in AuNPs concentration. Regarding the in vitro antityrosinase activity against mushroom tyrosinase, the IC50 values of BLAuNPs, ECAuNPs, and Chemicals-5 nm were 14.2, 10.2, and 24.1 mg/L, respectively (Figure 6a). Moreover, the antityrosinase activity of biogenic AuNPs was superior to that of Chemicals-5 nm. It was suggested that some bioactive compounds produced by bacteria are coated on the AuNPs surface without capping agents, thereby enhancing the tyrosinase inhibition activity [38]. Furthermore, our results were more favorable than those for the control arbutin (IC50: 18.2 mg/L) and AuNPs synthesized by Panax ginseng leaves (IC50: 16.06 mg/L) [38], but inferior to that for P. ginseng berries (IC50: 6.6 mg/L) [5].
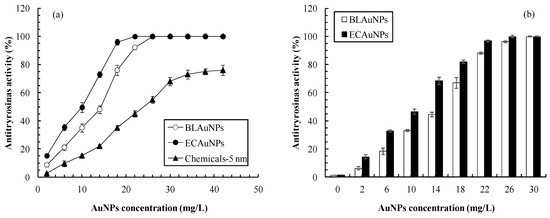
Figure 6.
Effects of AuNP concentrations on antityrosinase activities in (a) mushroom tyrosinase and (b) HEMn cells.
The results of antityrosinase activity in human cells better reflected the actual effect compared with the results of the mushroom tyrosinase inhibition. The IC50 values of BLAuNPs and ECAuNPs for inhibition of tyrosinase activity in HEMn cells were 15.4 and 11.8 mg/L, respectively (Figure 6b). The tyrosinase inhibition in human cells (in vivo) was slightly weaker than the mushroom tyrosinase inhibition (in vitro). This finding might be attributed to the difference in the chemical structure of tyrosinase between mushrooms and human cells [39].
The inhibition of melanin production in HEMn cells decreased in a dose-dependent manner (Figure 7). Melanin was completely inhibited by ECAuNPs and BLAuNPs at 26 and 30 mg/L, respectively. The trend of melanin content inhibition shown in Figure 7 was similar to that of tyrosinase inhibition in shown in Figure 6b. As ECAuNPs and BLAuNPs were noncytotoxic at such concentrations (Figure 5), the inhibition of melanogenesis may be attributed to the inhibition of tyrosinase or related enzymes, rather than melanocyte death. The underlying mechanism must be confirmed through Western blotting. ECAuNPs and BLAuNPs at a concentration of 10 mg/L reduced the melanin contents in HEMn to 46.5% and 33.5%, respectively (Figure 7)—higher than the inhibitions observed when HEMn were treated with the same concentration of P. ginseng leaf-mediated AuNPs (25%) and P. ginseng berry-mediated AuNPs (16%) [5,38]. These results suggested that biogenic AuNPs synthesized in this study have the potential to be used in skin whitening agents.
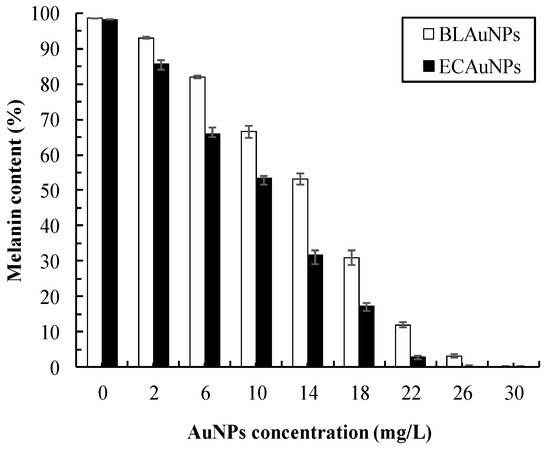
Figure 7.
Effects of AuNP concentrations on the melanin content of HEMn cells. Cells were treated with different concentrations of BLAuNPs and ECAuNPs for 24 h.
4. Conclusions
In this study, the antimicrobial, antioxidant, photocatalytic, and antityrosinase activities of biosynthesized and chemically synthesized AuNPs were studied. The cytotoxicity of biogenic AuNPs to CCD966SK and HEMn cells was also evaluated. Through strain screening and controlling of pH conditions, approximately 5-nm AuNPs with high reactivity were synthesized. Results indicate that the biogenic AuNPs are highly antimicrobial against strains often found in contaminated food or cosmetics. The photocatalytic, antioxidant, and antityrosinase activities of ECAuNPs and BLAuNPs were considerably higher than those of chemically synthesized AuNPs. Furthermore, biogenic AuNPs demonstrated high photocatalytic activity by efficiently degrading various dyes without the addition of NaBH4. The high antityrosinase activity of ECAuNPs with a small IC50 significantly reduced the melanin production in HEMn cells, and there was no cytotoxicity toward the human cells tested. Thus, the multifunctional activities and safety of AuNPs synthesized by E. coli and B. lactis make these AuNPs suitable for applications in food, environment, medicine, and cosmetics.
Author Contributions
All authors collaborated to carry out the work presented here. C.-Y.C. and Y.-C.C. (Ying-Chien Chung) conceived and designed the experiments; Y.-C.C. (Yung-Chu Chang), M.-H.L. and T.-H.T. performed the experiments; C.-Y.C. and Y.-C.C. (Ying-Chien Chung) wrote the paper; C.-Y.C., T.-H.T. and Y.-C.C. (Ying-Chien Chung) reviewed and edited the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by the Ministry of Science and Technology of Taiwan, grant numbers MOST 109-2313-B-157-001 and MOST 109-2622-E-157-001.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Acknowledgments
We thank Huang, C.L., for helping with some of the paperwork and data processing.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Rao, P.V.; Nallappan, D.; Madhavi, K.; Rahman, S.; Wei, L.J.; Gan, S.H. Phytochemicals and biogenic metallic nanoparticles as anticancer agents. Oxid. Med. Cell. Longev. 2016, 2016, 3685671. [Google Scholar] [CrossRef]
- Manivasagan, P.; Venkatesan, J.; Sivakumar, K.; Kim, S.K. Actinobacteria mediated synthesis of nanoparticles and their biological properties: A review. Crit. Rev. Microbiol. 2016, 42, 209–221. [Google Scholar] [CrossRef]
- Roshmi, T.; Soumya, K.R.; Jyothis, M.; Radhakrishnan, E.K. Effect of biofabricated gold nanoparticle-based antibiotic conjugates on minimum inhibitory concentration of bacterial isolates of clinical origin. Gold Bull. 2015, 48, 63–71. [Google Scholar] [CrossRef]
- Shivaramakrishnan, B.; Gurumurthy, B.; Balasubramanian, A. Potential biomedical applications of metallic nanobiomaterials: A review. Int. J. Pharm. Sci. Res. 2017, 8, 985–1000. [Google Scholar]
- Jiménez Pérez, Z.E.; Mathiyalagan, R.; Markus, J.; Kim, Y.J.; Kang, H.M.; Abbai, R.; Seo, K.H.; Wang, D.; Soshnikova, V.; Yang, D.C. Ginseng-berry-mediated gold and silver nanoparticle synthesis and evaluation of their in vitro antioxidant, antimicrobial, and cytotoxicity effects on human dermal fibroblast and murine melanoma skin cell lines. Int. J. Nanomed. 2017, 12, 709–723. [Google Scholar] [CrossRef]
- Thakkar, K.N.; Mhatre, S.S.; Parikh, R.Y. Biological synthesis of metallic nanoparticles. Nanomed. Nanotechnol. Biol. Med. 2010, 6, 257–262. [Google Scholar] [CrossRef]
- Pantidos, N.; Horsfall, L.E. Biological synthesis of metallic nanoparticles by bacteria, fungi and plants. J. Nanomed. Nanotechnol. 2014, 5, 1–10. [Google Scholar] [CrossRef]
- Das, R.K.; Pachapur, V.L.; Lonappan, L.; Naghdi, M.; Pulicharla, R.; Maiti, S.; Celdon, M.; Dalila, L.M.A.; Sarma, S.J.; Brar, S.K. Biological synthesis of metallic nanoparticles: Plants, animals and microbial aspects. Nanotechnol. Environ. Eng. 2017, 2, 18–21. [Google Scholar] [CrossRef]
- Singh, J.; Dutta, T.; Kim, K.H.; Rawat, M.; Samddar, P.; Kumar, P. Green synthesis of metals and their oxide nanoparticles: Applications for environmental remediation. J. Nanobiotechnol. 2018, 16, 84–107. [Google Scholar] [CrossRef]
- Shedbalkar, U.; Singh, R.; Wadhwani, S.; Gaidhani, S.; Chopade, B.A. Microbial synthesis of gold nanoparticles: Current status and future prospects. Adv. Colloid Interface Sci. 2014, 209, 40–48. [Google Scholar] [CrossRef]
- Pradeep, T. Noble metal nanoparticles for water purification: A critical review. Thin Solid Films 2009, 517, 6441–6478. [Google Scholar] [CrossRef]
- Dos Santos Corrêa, A.; Contreras, L.A.; Keijok, W.J.; Barcelos, D.H.F.; Pereira, A.C.H.; Kitagawa, R.R.; Scherer, R.; Gomes, D.C.D.O.; Silva, A.R.D.; Endringer, D.C.; et al. Virola oleifera-capped gold nanoparticles showing radical-scavenging activity and low cytotoxicity. Mater. Sci. Eng. C 2018, 91, 853–858. [Google Scholar] [CrossRef] [PubMed]
- Kuhn, D.M.; George, T.; Chandra, J.; Mukherjee, P.K.; Ghannoum, M.A. Antifungal susceptibility of Candida biofilms: Unique efficacy of amphotericin B lipid formulations and echinocandins. Antimicrob. Agents Chemother. 2002, 46, 1773–1780. [Google Scholar] [CrossRef] [PubMed]
- Chung, Y.C.; Chen, C.Y. Antibacterial characteristics and activity of acid-soluble chitosan. Bioresour. Technol. 2008, 99, 2806–2814. [Google Scholar] [CrossRef]
- Wang, G.H.; Chen, C.Y.; Tsai, T.H.; Chen, C.K.; Cheng, C.Y.; Huang, Y.H.; Hsieh, M.C.; Chung, Y.C. Evaluation of tyrosinase inhibitory and antioxidant activities of Angelica dahurica root extracts for four different probiotic bacteria fermentations. J. Biosci. Bioeng. 2017, 123, 679–684. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.H.; Lin, Y.M.; Kuo, J.T.; Lin, C.P.; Chang, C.F.; Hsieh, M.C.; Cheng, C.Y.; Chung, Y.C. Comparison of biofunctional activity of Asparagus cochinchinensis (Lour.) Merr. extract before and after fermentation with Aspergillus oryzae. J. Biosci. Bioeng. 2019, 127, 59–65. [Google Scholar] [CrossRef] [PubMed]
- Liao, W.T.; Huang, T.S.; Chiu, C.C.; Pan, J.L.; Liang, S.S.; Chen, B.H.; Chen, S.H.; Liu, P.L.; Wang, H.C.; Wen, Z.H.; et al. Biological properties of acidic cosmetic water from seawater. Int. J. Mol. Sci. 2012, 13, 5952–5971. [Google Scholar] [CrossRef]
- Piella, J.; Bastús, N.G.; Puntes, V. Size-controlled synthesis of sub-10-nanometer citrate-stabilized gold nanoparticles and related optical properties. Chem. Mater. 2016, 28, 1066–1075. [Google Scholar] [CrossRef]
- Seetharaman, P.; Chandrasekaran, R.; Gnanasekar, S.; Mani, I.; Sivaperumal, S. Biogenic gold nanoparticles synthesized using Crescentia cujete L. and evaluation of their different biological activities. Biocatal. Agric. Biotechnol. 2017, 11, 75–82. [Google Scholar] [CrossRef]
- Ebrahimzadeh, M.A.; Naghizadeh, A.; Mohammadi-Aghdam, S.; Khojasteh, H.; Ghoreishi, S.M.; Mortazavi-Derazkola, S. Enhanced catalytic and antibacterial efficiency of biosynthesized Convolvulus fruticosus extract capped gold nanoparticles. J. Photochem. Photobiol. B Biol. 2020, 209, 111949. [Google Scholar] [CrossRef]
- Zonooz, N.F.; Salouti, M.; Shapouri, R.; Nasseryan, J. Biosynthesis of gold nanoparticles by Streptomyces sp. ERI-3 supernatant and process optimization for enhanced production. J. Cluster Sci. 2012, 23, 375–382. [Google Scholar] [CrossRef]
- Ovais, M.; Khalil, A.T.; Raza, A.; Islam, N.U.; Ayaz, M.; Saravanan, M.; Ali, M.; Ahmad, I.; Shahid, M.; Shinwari, Z.K. Multifunctional theranostic applications of biocompatible green-synthesized colloidal nanoparticles. Appl. Microbiol. Biotechnol. 2018, 102, 4393–4408. [Google Scholar] [CrossRef] [PubMed]
- Zada, S.; Ahmad, A.; Khan, S.; Iqbal, A.; Ahmad, S.; Ali, H.; Fu, P. Biofabrication of gold nanoparticles by Lyptolyngbya JSC-1 extract as super reducing and stabilizing agents: Synthesis, characterization and antibacterial activity. Microb. Pathogenes. 2018, 114, 116–123. [Google Scholar] [CrossRef] [PubMed]
- Baruah, D.; Goswami, M.; Yadav, R.N.S.; Yadav, A.; Das, A.M. Biogenic synthesis of gold nanoparticles and their application in photocatalytic degradation of toxic dyes. J. Photochem. Photobiol. B Biol. 2018, 186, 51–58. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.U.; Yuan, Q.; Wei, Y.; Khan, G.M.; Khan, Z.U.H.; Khan, S.; Ali, F.; Tahir, K.; Ahmad, A.; Khan, F.U. Photocatalytic and antibacterial response of biosynthesized gold nanoparticles. J. Photochem. Photobiol. B Biol. 2016, 162, 273–277. [Google Scholar] [CrossRef]
- Mmola, M.; Roes-Hill, M.L.; Durrell, K.; Bolton, J.J.; Sibuyi, N.; Meyer, M.E.; Beukes, D.R.; Antunes, E. Enhanced antimicrobial and anticancer activity of silver and gold nanoparticles synthesised using Sargassum incisifolium aqueous extracts. Molecules 2016, 21, 1633. [Google Scholar] [CrossRef]
- Patra, J.K.; Baek, K.H. Comparative study of proteasome inhibitory, synergistic antibacterial, synergistic anticandidal, and antioxidant activities of gold nanoparticles biosynthesized using fruit waste materials. Int. J. Nanomed. 2016, 11, 4691–4705. [Google Scholar]
- Tripathi, R.M.; Shrivastav, B.R.; Shrivastav, A. Antibacterial and catalytic activity of biogenic gold nanoparticles synthesised by Trichoderma harzianum. IET Nanobiotechnol. 2018, 12, 509–513. [Google Scholar] [CrossRef]
- Nedorostova, L.; Kloucek, P.; Kokoska, L.; Stolcova, M.; Pulkrabek, J. Antimicrobial properties of selected essential oils in vapour phase against foodborne bacteria. Food Control 2009, 20, 157–160. [Google Scholar] [CrossRef]
- DeCarlo, A.; Zeng, T.; Dosoky, N.S.; Satyal, P.; Setzer, W.N. The essential oil composition and antimicrobial activity of Liquidambar formosana oleoresin. Plants 2020, 9, 822. [Google Scholar] [CrossRef]
- Gulcin, İ. Antioxidants and antioxidant methods: An updated overview. Arch. Toxicol. 2020, 94, 651–715. [Google Scholar] [CrossRef]
- Hamelian, M.; Varmira, K.; Veisi, H. Green synthesis and characterizations of gold nanoparticles using Thyme and survey cytotoxic effect, antibacterial and antioxidant potential. J. Photochem. Photobiol. B Biol. 2018, 184, 71–79. [Google Scholar] [CrossRef] [PubMed]
- Balasubramani, G.; Ramkumar, R.; Raja, R.K.; Aiswarya, D.; Rajthilak, C.; Perumal, P. Albizia amara Roxb. mediated gold nanoparticles and evaluation of their antioxidant, antibacterial and cytotoxic properties. J. Clust. Sci. 2017, 28, 259–275. [Google Scholar] [CrossRef]
- Soshnikova, V.; Kim, Y.J.; Singh, P.; Huo, Y.; Markus, J.; Ahn, S.; Castro-Aceituno, V.; Kang, J.; Chokkalingam, M.; Mathiyalagan, R.; et al. Cardamom fruits as a green resource for facile synthesis of gold and silver nanoparticles and their biological applications. Artif. Cells Nanomed. Biotechnol. 2018, 46, 108–117. [Google Scholar] [CrossRef] [PubMed]
- Oladipo, I.C.; Lateef, A.; Elegbede, J.A.; Azeez, M.A.; Asafa, T.B.; Yekeen, T.A.; Akinboro, A.; Gueguim-Kana, E.B.; Beukes, L.S.; Oluyide, T.O.; et al. Enterococcus species for the one-pot biofabrication of gold nanoparticles: Characterization and nanobiotechnological applications. J. Photochem. Photobiol. B Biol. 2017, 173, 250–257. [Google Scholar] [CrossRef] [PubMed]
- López-Miranda, J.L.; Esparza, R.; Rosas, G.; Pérez, R.; Estévez-González, M. Catalytic and antibacterial properties of gold nanoparticles synthesized by a green approach for bioremediation applications. 3 Biotech 2019, 9, 135. [Google Scholar] [CrossRef]
- Choudhary, B.C.; Paul, D.; Gupta, T.; Tetgure, S.R.; Garole, V.J.; Borse, A.U.; Garole, D.J. Photocatalytic reduction of organic pollutant under visible light by green route synthesized gold nanoparticles. J. Environ. Sci. 2017, 55, 236–246. [Google Scholar] [CrossRef] [PubMed]
- Jiménez-Pérez, Z.E.; Singh, P.; Kim, Y.J.; Mathiyalagan, R.; Kim, D.H.; Lee, M.H.; Yang, D.C. Applications of Panax ginseng leaves-mediated gold nanoparticles in cosmetics relation to antioxidant, moisture retention, and whitening effect on B16BL6 cells. J. Ginseng Res. 2018, 42, 327–333. [Google Scholar] [CrossRef]
- Wu, L.; Chen, C.; Cheng, C.; Dai, H.; Ai, Y.; Lin, C.; Chung, Y. Evaluation of tyrosinase inhibitory, antioxidant, antimicrobial, and antiaging activities of Magnolia officinalis extracts after Aspergillus niger fermentation. BioMed Res. Int. 2018, 2018, 5201786. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).