Specific Detection of PE-Included Vesicles Using Cyclic Voltammetry
Abstract
1. Introduction
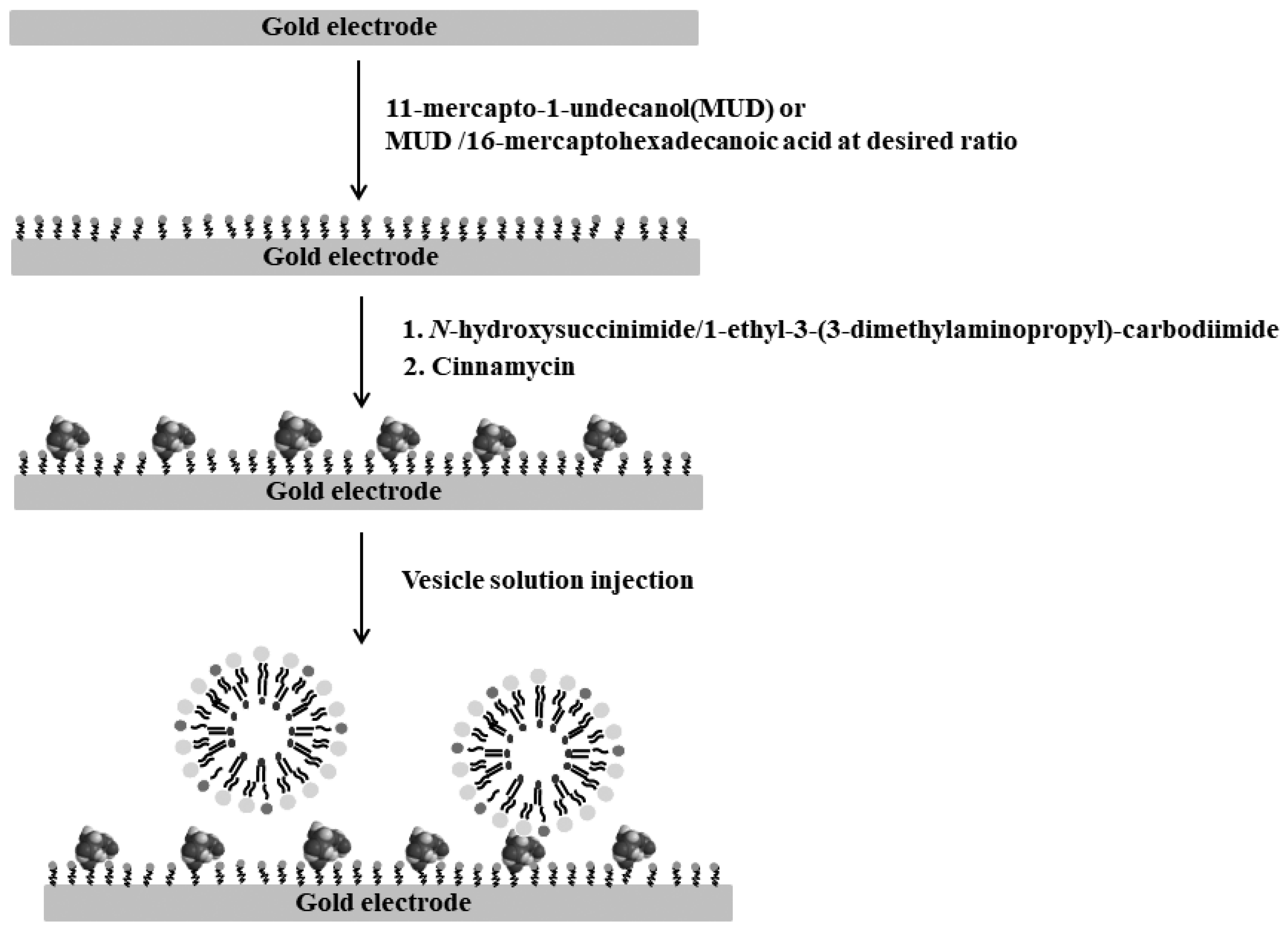
2. Materials and Methods
2.1. Vesicle Preparation
2.2. CV Experiments
3. Results and Discussion
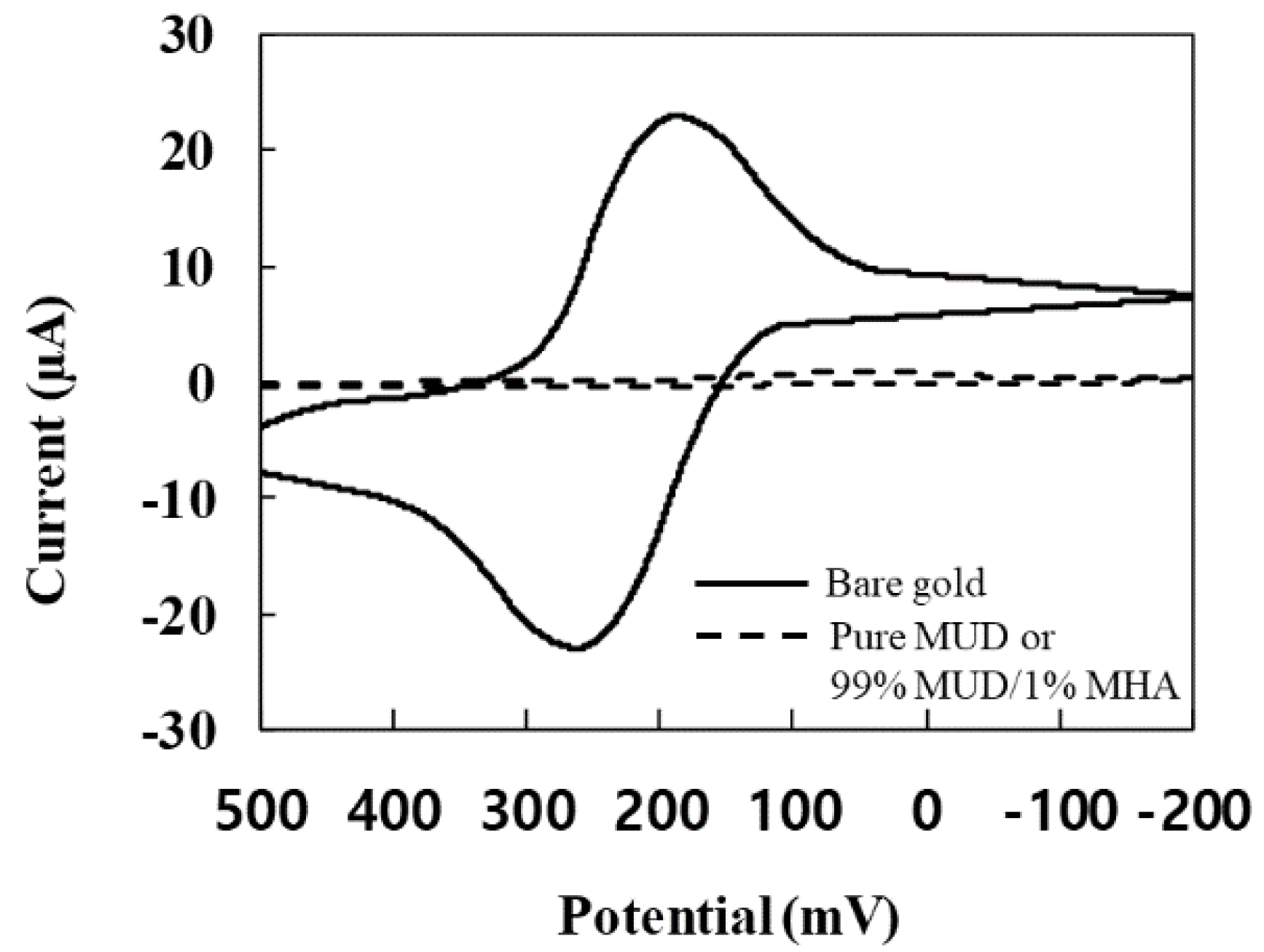
3.1. CV Response for the Monolayer Formation on the Gold Electrode
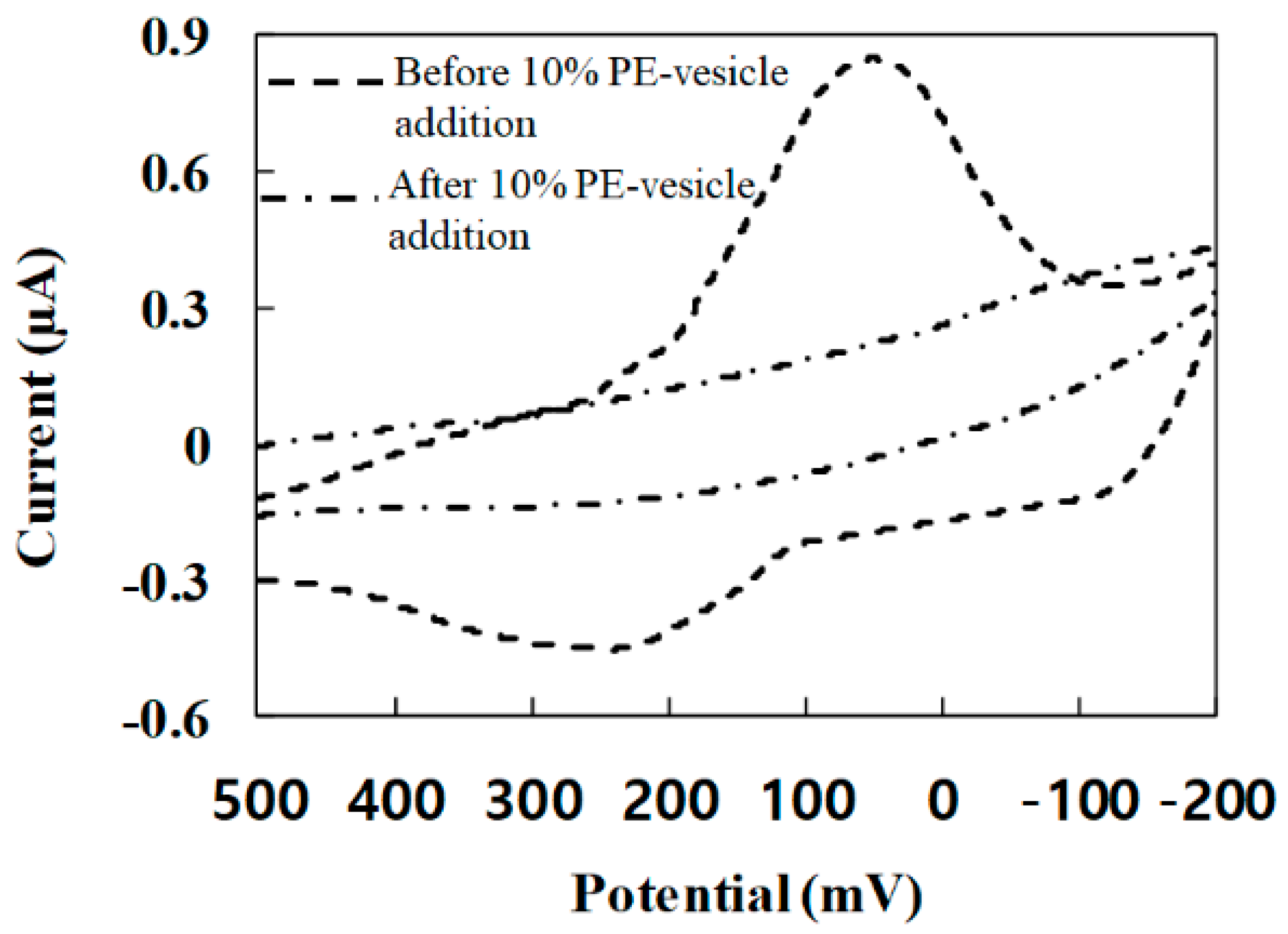
3.2. CV Response after PE-Vesicle Addition
3.3. CV Response Difference for Vesicle Composition
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wakamatsu, K.; Choung, S.Y.; Kobayashi, T.; Inoue, K.; Higashijima, T.; Miyazawa, T. Complex formation of peptide antibiotic Ro09-0198 with lysophosphatidylethanolamine: 1H NMR analyses in dimethyl sulfoxide solution. Biochemistry 1990, 29, 113–118. [Google Scholar] [CrossRef]
- Machaidze, G.; Ziegler, A.; Seelig, J. Specific binding of Ro 09-0198 (cinnamycin) to phosphatidylethanolamine: A thermodynamic analysis. Biochemistry 2002, 41, 1965–1971. [Google Scholar] [CrossRef] [PubMed]
- Choung, S.Y.; Kobayashi, T.; Takemoto, K.; Ishitsuka, H.; Inoue, K. Interaction of a cyclic peptide, Ro09-0198, with phosphatidylethanolamine in liposomal membranes. Biochim. Biophys. Acta 1988, 940, 180–187. [Google Scholar] [CrossRef]
- Emoto, K.; Kobayashi, T.; Yamaji, A.; Aizawa, H.; Yahara, I.; Inoue, K.; Umeda, M. Redistribution of phosphatidylethanolamine at the cleavage furrow of dividing cells during cytokinesis. Proc. Natl. Acad. Sci. USA 1996, 93, 12867–12872. [Google Scholar] [CrossRef] [PubMed]
- Kato, U.; Inadome, H.; Yamamoto, M.; Emoto, K.; Kobayashi, T.; Umeda, M. Role for Phospholipid Flippase Complex of ATP8A1 and CDC50A Proteins in Cell Migration. J. Biol. Chem. 2013, 288, 4922–4934. [Google Scholar] [CrossRef] [PubMed]
- Tafesse, F.G.; Vacaru, A.M.; Bosma, E.F.; Hermansson, M.; Jain, A.; Hilderink, A.; Somerharju, P.; Holthuis, J.C.M. Sphingomyelin synthase-related protein SMSr is a suppressor of ceramide-induced mitochondrial apoptosis. J. Cell Sci. 2014, 127, 445–454. [Google Scholar] [CrossRef] [PubMed]
- Stafford, J.H.; Thorpe, P.E. Increased exposure of phosphatidylethanolamine on the surface of tumor vascular endothelium. Neoplasia 2011, 13, 299–308. [Google Scholar] [CrossRef] [PubMed]
- Phoenix, D.A.; Harris, F.; Mura, M.; Dennison, S.R. The increasing role of phosphatidyl ethanolamine as a lipid receptor in the action of host defence peptides. Prog. Lipid Res. 2015, 59, 26–37. [Google Scholar] [CrossRef] [PubMed]
- Vestergaard, M.; Berglund, N.A.; Hsu, P.-C.; Song, C.; Koldsø, H.; Schiøtt, B.; Sansom, M.S.P. Structure and Dynamics of Cinnamycin−Lipid Complexes: Mechanisms of Selectivity for Phosphatidylethanolamine Lipids. ACS Omega 2019, 4, 18889–18899. [Google Scholar] [CrossRef] [PubMed]
- Okesli, A.; Cooper, L.E.; Fogle, E.J.; van der Donk, W.A. Nine Post-translational Modifications during the Biosynthesis of Cinnamycin. J. Am. Chem. Soc. 2011, 133, 13753–13760. [Google Scholar] [CrossRef]
- Makino, A.; Baba, T.; Fujimoto, K.; Iwamoto, K.; Yano, Y.; Terada, N.; Ohno, S.; Sato, S.B.; Ohta, A.; Umeda, M.; et al. Cinnamycin (Ro 09-0198) promotes cell binding and toxicity by inducing transbilayer lipid movement. J. Biol. Chem. 2003, 278, 3204–3209. [Google Scholar] [CrossRef]
- Emoto, K.; Kuge, O.; Nishijima, M.; Umeda, M. Isolation of a Chinese hamster ovary cell mutant defective in intramitochondrial transport of phosphatidylserine. Proc. Natl. Acad. Sci. USA 1999, 96, 12400–12405. [Google Scholar] [CrossRef]
- Lee, S.-R.; Park, Y.; Park, J.-W. Kinetic and thermodynamic studies of cinnamycin specific-adsorption on PE-Included-Membranes using surface plasmon resonance. J. Biotechnol. 2020, 320, 77–79. [Google Scholar] [CrossRef] [PubMed]
- López-Cobo, S.; Campos-Silva, C.; Moyano, A.; Oliveira-Rodríguez, M.; Paschen, A.; Yáñez-Mó, M.; Blanco-López, M.C.; Valés-Gómez, M. Immunoassays for scarce tumour-antigens in exosomes: Detection of the human NKG2D-Ligand, MICA, in tetraspanin-containing nanovesicles from melanoma. J. Nanobiotechnol. 2018, 16, 47. [Google Scholar] [CrossRef]
- Carnell-Morris, P.; Tannetta, D.; Siupa, A.; Hole, P.; Dragovic, R. Analysis of Extracellular Vesicles Using Fluorescence Nanoparticle Tracking Analysis. Methods Mol. Biol. 2017, 1660, 153–173. [Google Scholar]
- Zhang, H.; Lyden, D. Asymmetric-flow field-flow fractionation technology for exomere and small extracellular vesicle separation and characterization. Nat. Protoc. 2019, 14, 1027–1053. [Google Scholar] [CrossRef]
- Marchisio, M.; Simeone, P.; Bologna, G.; Ercolino, E.; Pierdomenico, L.; Pieragostino, D.; Ventrella, A.; Antonini, F.; Zotto, G.D.; Vergara, D.; et al. Flow Cytometry Analysis of Circulating Extracellular Vesicle Subtypes from Fresh Peripheral Blood Samples. Int. J. Mol. Sci. 2021, 22, 48. [Google Scholar] [CrossRef]
- Yang, Y.; Zhai, C.; Zeng, Q.; Khan, A.L.; Yu, H. Multifunctional Detection of Extracellular Vesicles with Surface Plasmon Resonance Microscopy. Anal. Chem. 2020, 92, 4884–4890. [Google Scholar] [CrossRef] [PubMed]
- Huang-Doran, I.; Zhang, C.-Y.; Vidal-Puig, A. Extracellular Vesicles: Novel Mediators of Cell Communication In Metabolic Disease. Trends Endocrinol. Metab. 2017, 28, 3–18. [Google Scholar] [CrossRef] [PubMed]
- Pinazo, A.; Pons, R.; Marqués, A.; Farfan, M.; da Silva, A.; Perez, L. Biocompatible Catanionic Vesicles from Arginine-Based Surfactants: A New Strategy to Tune the Antimicrobial Activity and Cytotoxicity of Vesicular Systems. Pharmaceutics 2020, 12, 857. [Google Scholar] [CrossRef]
- Dang, X.T.T.; Kavishka, J.M.; Zhang, D.X.; Pirisinu, M.; Le, M.T.N. Extracellular Vesicles as an Efficient and Versatile System for Drug Delivery. Cells 2020, 9, 2191. [Google Scholar] [CrossRef] [PubMed]
- Cha, B.S.; Park, K.S.; Park, J.S. Signature mRNA markers in extracellular vesicles for the accurate diagnosis of colorectal cancer. J. Biol. Eng. 2020, 14, 4. [Google Scholar] [CrossRef]
- Ganesana, M.; Lee, S.T.; Wang, Y.; Venton, B.J. Analytical Techniques in Neuroscience: Recent Advances in Imaging, Separation, and Electrochemical Methods. Anal. Chem. 2017, 89, 314–334. [Google Scholar] [CrossRef]
- Lee, S.-R.; Park, J.-W. Trehalose-Induced Variation in Physical Properties of Fluidic Lipid Bilayer. J. Membr. Biol. 2018, 251, 705–709. [Google Scholar] [CrossRef] [PubMed]
- Raju, V.M.; Bhavana, V.; Gayathri, G.K.; Suryan, S.; Reddy, R.; Reddy, N.; Ravikumar, C.R.; Santosh, M.S. A novel disposable electrochemical DNA biosensor for the rapid detection of Bacillus thuringiensis. Microchem. J. 2020, 159, 105434. [Google Scholar] [CrossRef]
- Jozghorbani, M.; Fathi, M.; Kazemi, S.H.; Alinejadian, N. Determination of carcinoembryonic antigen as a tumor marker using a novel graphene-based label-free electrochemical immunosensor. Anal. Biochem. 2021, 613, 114017. [Google Scholar] [CrossRef] [PubMed]
- Lee, G.S.; Park, J.-W. Interactions of Cinnamycin-Immobilized Gold Nanorods with Biomimetic Membranes. J. Membr. Biol. 2020, 253, 37–42. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.-E.; Park, J.-W. Analysis of interactions between cinnamycin and biomimetic membranes. Colloids Surf. B Biointerfaces 2020, 185, 110595. [Google Scholar] [CrossRef] [PubMed]
- Christ, K.; Rüttubger, H.-H.; Höpfner, M.; Rothe, U.; Bendas, G. The detection of UV-induced membrane damages by a combination of two biosensor techniques. Photochem. Photobiol. 2005, 81, 1417–1423. [Google Scholar] [CrossRef]
- Park, J.W.; Ahn, D.J. Temperature effect on nanometer-scale physical properties of mixed phospholipid monolayers. Colloids Surf. B Biointerfaces 2008, 62, 157–161. [Google Scholar] [CrossRef] [PubMed]

| Step | Charge (μC) | Charge Permeability (%) | |||
|---|---|---|---|---|---|
| MUD | 90% MUD/10% MHA | MUD | 90% MUD/10% MHA | ||
| Monolayer | 1.0 | 1.0 | 5 | 5 | |
| Cinnamycin/Monolayer | 1.0 | 1.0 | 5 | 5 | |
| Vesicles/Cinnamycin/Monolayer | DPPC | 1.0 | 1.0 | 5 | 5 |
| 90% DPPC/10% DPPE | 1.0 | 0.6 | 5 | 3 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, Y.; Park, J.-W. Specific Detection of PE-Included Vesicles Using Cyclic Voltammetry. Appl. Sci. 2021, 11, 3660. https://doi.org/10.3390/app11083660
Park Y, Park J-W. Specific Detection of PE-Included Vesicles Using Cyclic Voltammetry. Applied Sciences. 2021; 11(8):3660. https://doi.org/10.3390/app11083660
Chicago/Turabian StylePark, Yeseul, and Jin-Won Park. 2021. "Specific Detection of PE-Included Vesicles Using Cyclic Voltammetry" Applied Sciences 11, no. 8: 3660. https://doi.org/10.3390/app11083660
APA StylePark, Y., & Park, J.-W. (2021). Specific Detection of PE-Included Vesicles Using Cyclic Voltammetry. Applied Sciences, 11(8), 3660. https://doi.org/10.3390/app11083660






