Abstract
Pulsed electromagnetic fields (PEMFs) are used as non-invasive tools to enhance microcirculation and tissue oxygenation, with a modulatory influence on the microvasculature. This study aimed to measure the acute effect of PEMF on muscle oxygenation and its influence on pulmonary oxygen kinetics during exercise. Eighteen male cyclists performed, on different days, a constant-load exercise in both active (ON) and inactive (OFF) PEMF stimulations while deoxyhemoglobin and pulmonary oxygen kinetics, total oxygenation index, and blood lactate were collected. PEMF enhanced muscle oxygenation, with higher values of deoxyhemoglobin both at the primary component and at the steady-state level. Moreover, PEMF accelerated deoxyhemoglobin on-transition kinetic, with a shorter time delay, time constant, and mean response time than the OFF condition. Lactate concentration was higher during stimulation. No differences were found for total oxygenation index and pulmonary oxygen kinetics. Local application of a precise PEMF stimulation can increase the rate of the muscle O2 extraction and utilization. These changes were not accompanied by faster oxygen kinetics, reduced oxygen slow component, or reduced blood lactate level. It seems that oxygen consumption is more influenced by exercise involving large muscle mass like cycling, whereas PEMF might only act at the local level.
1. Introduction
Pulsed electromagnetic field (PEMF) therapies were first approved for human use by the US Food and Drug Administration (FDA) in 1979. Noninvasive PEMF therapies yield several benefits in the treatment of acute and chronic tissue inflammation [1]. In the cellular culture of human skin, PEMF was able to mediate gene expression involved in the acute phases of inflammations [2]. The stimulation led to a reduction in pro-inflammatory cytokines, such as interleukin 1-beta, with the increment of enzymes involved in the removal of reactive oxygen species. This might explain the positive influence of PEMF in the treatment of delayed onset muscle soreness (DOMS) after exercise [3]. Regarding tissue oxygenation and microcirculation in rat muscles, PEMF was able to enhance vascularization and increased the diameter of arterioles after 2 min of acute stimulation [4]. Moreover, Rolando et al. [5] showed enhanced angiogenesis after 12 weeks of stimulation, with a significant increase in neovascularization. In a cell-free preparation, 10 min of PEMF treatment was able to increase the rate of hemoglobin deoxygenation, observable up to 150 min after stimulation [6]. Such responses could be caused by a modulation of nitric oxide through the interaction between PEMFs and Ca2+/nitric oxide/cGMP/protein kinase G signal [7]. Angiogenesis would be generated from the prolonged stretch of vessel walls in response to the release and to the vasodilatory effect of nitric oxide [8]. In adult people, it has been found that PEMF could affect peripheral resistance and microcirculation, with a decrease in systolic blood pressure [9]. Mayrovitz and Larsen [10] explored the consequence of a PEMF exposure (27.12 MHz, 600 pulses/s, 0.1 mT) on human skin microcirculation. A laser doppler probe was positioned on both forearms of healthy participants. It was shown that 40 min of stimulation augmented significantly blood perfusion in the exposed arm (by 29%) and unchanged perfusion in the control arm. Additional use of the pulse sequence used by Mayrovitz and Larsen was efficacious in rising blood perfusion in peri-ulcer skin microcirculation, enhancing capillary blood velocity and diameter in diabetic patients [11,12].
To the best of our knowledge, no investigation has evaluated the effects of PEMF on the human muscle tissue level during exercise, and, more precisely, on factors of endurance capacity that are related to performance—such as faster oxygen kinetics, reduced oxygen slow component, or reduced blood lactate level—at high submaximal workload. The rate at which pulmonary O2 uptake increases after the onset of exercise is a determinant of sport performance and an indicator of well-done state of oxidative energetic system activity [13]. Specifically, a faster increase in VO2 after the onset of exercise indicates a greater muscle O2 utilization is a feature common in elite athletes and trained subjects due to the lower mismatch between muscle oxygen delivery and utilization [14].
Considering the influence of PEMF on microcirculation, vascularization, and tissue oxygenation, seen above, and given that endurance exercise changes the microvascular oxygenation profile following the onset of contractions [15], we sought to evaluate the effect of PEMF stimulation on muscle tissue oxygenation and its influence on pulmonary oxygen kinetics during heavy aerobic exercise in cyclists. We collected both tissue oxygenation index (TOI) and deoxyhemoglobin (HHb) values, which are influenced by skin blood flow in the former, and reflect the rate of the muscle O2 extraction and utilization in the latter [16]. We hypothesized that PEMF stimulation should result in higher O2 muscle supply during exercise through increased O2 release and uptake. Moreover, we expected faster HHb kinetics due to the increased muscle O2 availability leading to faster adaption of workload and a decrease in O2 deficit related to the metabolic demand.
2. Materials and Methods
2.1. Subjects and Design
Experiments were performed on 18 male cyclists (mean ± SD: age 21.6 ± 4.7 years; body mass index 22.7 ± 3.3 kg/m2; VO2peak 55.2 ± 9.7 mL/min/kg; weight 71.4 ± 11.8 kg; height 177.3 ± 6.4 cm). All athletes were volunteers, healthy, and non-smokers, and none of them were taking medications or supplements. None of the cyclists reported a physical deficit or muscular injury at the time of the study. All subjects received a verbal explanation of the experimental procedures, and informed consent was obtained before the beginning of recordings. In agreement with the Declaration of Helsinky, the experimental protocol was approved by our University Institutional Ethic Committee. The study design was a single-blind, randomized controlled trial.
2.2. Experimental Procedures
Each participant visited our laboratory 5 times over a 4-wk period, during which we performed different recordings. During visit 1, we recorded an incremental test to exhaustion on a cycle-ergometer (H-300-R Lode), necessary to individualize the workload for the succeeding recording sessions through the detection of ventilatory threshold (VT) and peak oxygen consumption (VO2peak). Participants performed 5 min of warm-up cycling at 50 W, after which the work rate, starting at 80 W, was increased by 20 W/min until the volitional exhaustion [17,18]. Participants cycled at 70–80 rpm and this pedal rate was reproduced in subsequent constant-load exercises. Breath by breath pulmonary gas exchange values were recorded non-stop during the incremental exercise tests and averaged every 1 s. The VO2peak was taken as the highest 20 s mean value attained until volitional exhaustion. VT was obtained considering: (i) the first unbalanced rise in CO2 production (VCO2) from visual examination of individual plots of VCO2 vs. VO2; (ii) an increase in expired ventilation (VE/VO2) with no increase in VE/VCO2; and (iii) an increase in end-tidal O2 tension with no fall in end-tidal CO2 tension. The data recorded during the incremental test were used to calculate the work rates used during the subsequent constant-load exercise tests. Precisely, the workload for each athlete (mean ± SD: 308.2 ± 61.8 watt) was set to ~50% of the difference between power reached at ventilatory threshold (VT) and at VO2peak (~50% Δ VT-VO2peak). Expired gas was recorded using the Quark b2 breath-by-breath metabolic system (Cosmed, Rome, Italy).
During the following four visits, athletes performed 6 min of heavy constant-load exercise both during active (ON) and inactive (OFF) PEMF stimulation. The four sessions were performed in random order. Two PEMF loop-antenna devices (Torino II, Rio Grande Neurosciences, USA) were positioned on the right leg, one at the upper and one at the lower thigh level. The PEMF waveform consisted of a pulse-burst modulated 27.12 MHz sinusoidal carrier, with a 2 ms burst width repeated at 2 Hz, with peak magnetic field at the center of the loop 5 ± 1 μT. The two circular 20 cm loops were applied on the thigh in both experimental conditions (ON and OFF) because the stimulation was not perceived by the subjects at the cutaneous level (single-blind).
Heavy constant-load exercise was performed on the same cycle-ergometer, under a standardized procedure, in a quiet chamber with a steady and comfortable temperature (22 °C), at the similar day time (9:00–12:00 AM) to prevent circadian effect [19,20]. Subjects were asked to refrain from caffeine intake before the tests and were recommended to avoid physical activity and alcohol in the 12h prior the test. After 10 min of free warm-up and 5 min of rest, the athletes started the recording session with 1 min of unloaded cycling (baseline), followed by an instantaneous increase in the individualized power, which was attained in ~3s. Pulmonary O2 uptake was collected with the Quark b2 breath by breath metabolic system (Cosmed, Rome, Italy); meanwhile, for muscle oxygenation we used a near-infrared spectroscopy (NIRS, NIMO, Nirox s.r.l. Italy), a noninvasive and validated method which used different light absorptions of hemoglobin (Hb) and myoglobin related to their oxygen saturation [21].
To record HHb and TOI values during cycling, the NIRS probe, with a continuous wave with a sampling rate set at 40 Hz, with an emitter and detector pair, was firmly located on the skin above the lower third of the right vastus lateralis (VL) muscle (about 12 cm upon the proximal border of patella and 3 cm lateral to the midline of the thigh), and held with a band of Velcro straps [22]. Elastic strappings were placed around the muscle probe to avoid corruption from localized light. Pen marks were made above the skin to indicate the boundaries of the plastic spacer to check for any movements of the probe during cycling. Before starting the trial, we measured the skinfold thickness of VL by a caliper (Holtain) in order to carry out fat layer correction [23].
A blood sample was performed from the right ear lobe for lactate measurements (Lactate Scout, SensLab, Germany) at the baseline (before the beginning of each trial) and at the third minute of the constant-load exercise, in both experimental conditions.
2.3. Data Analysis
Breath by breath VO2 values acquired in the several repetitions of the same constant load exercise (ON; OFF) were time aligned, interpolated on a second-by-second basis and then superimposed for each athlete. Then, we averaged VO2 values every second and used it for kinetics analysis. The same procedure was followed for HHb values. Data collected during the first 20 s of the on-transition (equivalent to the “cardio-dynamic phase”) were removed from the analysis. The baseline (unloaded cycling) of HHb and VO2 was defined as the mean value measured 30 s before transition. In order to evaluate VO2 and HHb on-kinetics, data were fitted by nonlinear regression functions:
where VO2(t) is the pulmonary oxygen uptake during the entire trial; HHb(t) is the value of deoxyhemoglobin of vastus lateralis during the entire trial; VO2bas is the pulmonary oxygen uptake at the baseline; HHbbas is the value of deoxyhemoglobin of vastus lateralis at the baseline; Ap is the amplitude of the primary component, as the difference between VO2 or HHb at the baseline with the VO2 or HHb reached at the steady-state (as mean of the last 30 s); TDp is the time delay of the primary component; τp is the time constant (tau) of the primary component; and As is the amplitude of the slow component, as the difference between VO2 or HHb reached at the steady-state with the VO2 or HHb mean values of the last 30 s of exercise (as usually quantified by calculating the difference between 3 and 6 min of constant load exercise). TDs is the time delay of the slow component and τs is the time constant (tau) of the slow component. We measured the magnitude and the percent contribution of the slow component to the total amplitude of the response.
VO2(t) = VO2bas + Ap * [1 − e − (t − TDp)/τp] + As * [1 − e − (t −TDs/τs)]
HHb(t) = HHbbas + Ap * [1 − e − (t − TDp)/τp] + As * [1 − e − (t −TDs/τs)]
We also calculated the gain of VO2, as the increase in VO2 above baseline to the reached steady-state and corrected for individualized workload (WL), according to this equation:
Gain = (VO2 [150sec–180sec] − VO2bas)/WL
The model parameters were obtained from the least-squares nonlinear regression, in which the convergence criteria were satisfied by minimizing the sum of squared error. Although many powerful and dedicated software packages have been developed for regression analysis, we used the Solver add-in bundled with Microsoft Excel [24,25,26].
The TOI values of the vastus lateralis during baseline, at 60 s, and at 180 s (±15 s) of the constant-load exercise were subsequently calculated.
2.4. Statistical Analysis
All data are shown as means ± SEM. Values were compared with a Paired sample t-test with means considered significantly different at p < 0.05. To determine the magnitude of the stimulation effects, effect sizes (ES) were calculated as the mean difference standardized by the between subject standard deviation and interpreted according to the following thresholds: <0.20; small, >0.20–0.60; moderate, >0.60–1.20; large, >1.20–2.00; very large, >2.00–4.00; extremely large, >4.00 [27]. Data were analyzed with SPSS v22.0 (IBM, New York, NY, USA).
3. Results
Table 1 and Table 2 show the mean values for VO2 and for HHb kinetics recorded in both experimental conditions. For VO2 kinetics, analysis have not shown any significant differences between conditions (ON vs. OFF). VO2 on-kinetics analysis for a representative subject is presented in Figure 1.

Table 1.
Mean values (±SEM) of VO2 on-kinetics recorded during constant load exercise in both conditions (PEMF OFF; PEMF ON). Base, baseline; Ss, steady-state; Ap, amplitude of primary component; TDp, time delay for primary component; τp, tau for primary component; As, amplitude of slow component; TDs, time delay for slow component; τs, tau for slow component; MRTp, mean response time for primary component; MRTs, mean response time for slow component; Sc, magnitude of slow component.

Table 2.
Mean values (± SEM) of HHb on-kinetics recorded during constant-load exercise in both conditions (PEMF OFF; PEMF ON). Base, baseline; Ss, steady-state; Ap, amplitude of primary component; TDp, time delay for primary component; τp, tau for primary component; As, amplitude of slow component; TDs, time delay for slow component; τs, tau for slow component; MRTp, mean response time for primary component; MRTs, mean response time for slow component; Sc, magnitude of slow component. Asterisk indicates significant differences at p < 0.05.
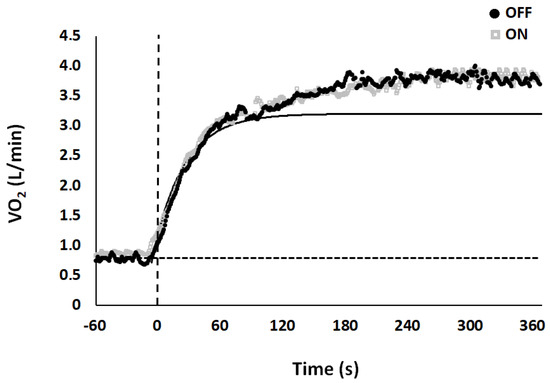
Figure 1.
Model fit for VO2 kinetics between conditions, OFF (black dot) and ON (grey square) for a typical subject. Vertical dotted line at time 0 represents the transition from unloaded cycling (baseline) to constant load exercise. Horizontal dashed line represents the baseline. Data points are average values calculated each second.
For HHb kinetics, we found that HHb tended to be higher (mean: 25.63 ± 4.1 vs. 23.21 ± 5.5 uM for ON and OFF, respectively, p = 0.062; d = 0.50) when subjects were stationary on the bike, just before the baseline started. Analysis showed a significant difference between mean values at steady-state (t(17) = −1.751; p = 0.049; ES = 0.17-small), for the amplitude (t(17) = −2.306; p = 0.017; ES = 0.24-moderate), TD (t(17) = 2.609; p = 0.009; ES = 0.33-moderate), τp (t(17) = 2.296; p = 0.017; ES = 0.28-moderate), and MRTp (t(17) = 3.531; p < 0.01; ES = 0.41-moderate) of the primary component, and for TD (t(17) = 1.760; p = 0.048; ES = 0.2-moderate) of the slow component. HHb on-kinetics analysis for a representative subject is presented in Figure 2. There were no differences in TOI values between conditions (p > 0.05; Figure 3).
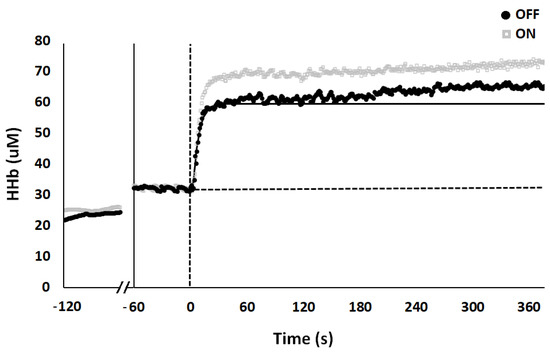
Figure 2.
Model fit for HHb kinetics between conditions, OFF (black dot) and ON (grey square) for a typical subject. The first 60 s represent HHb values recorded when subject was stationary on the bike, just before baseline values (subsequent 60 s). Vertical dotted line at time 0 represents the transition from unloaded cycling (baseline) to constant load exercise. Horizontal dashed line represents baseline. Data points are average values calculated each second.
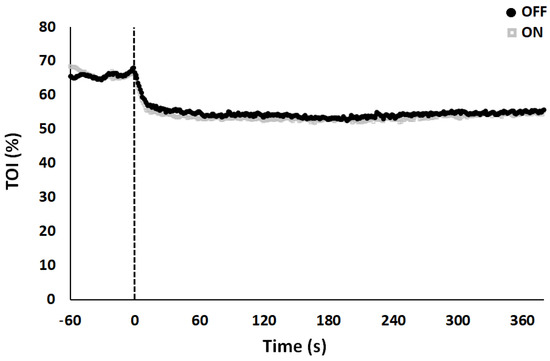
Figure 3.
Vastus lateralis tissue oxygenation index (TOI) kinetics following acute PEMF stimulation (ON) and sham condition (OFF). Vertical dotted line at time 0 represents the transition from unloaded pedaling (baseline at 0 W) to constant-load exercise. Data points are average values (%) calculated each second.
Finally, blood lactate (mmol/L) measured at the third minute of the constant-load exercise was significantly higher (t(17) = −5.14; p < 0.001; ES 0.50-moderate) during stimulation (10.37 ± 0.68 mmol/L) compared to OFF (7.52 ± 0.46 mmol/L) condition.
4. Discussion
The main result of this study is that PEMF stimulation, applied during heavy constant-load exercise, increases the velocity and the quantity of muscle O2 available. The greater muscle O2 extraction induced by stimulation accelerated the HHb kinetics without affecting the tissue oxygenation index or the pulmonary VO2 kinetics. Different studies have established the incidence of a good correlation between the HHb signal recorded with NIRS and the fractional O2 extraction in animals and in exercising human muscles, and the concept is further strengthened only if the tissue oxygenation index remains constant. This is because TOI includes oxyhemoglobin (HbO2), which is more significantly influenced by skin blood flow, occurring for thermoregulatory reasons due to exercise with respect to HHb (for a review see [16]).
During PEMF stimulation, we observed a tendency toward a greater change and faster kinetic of HHb concentration. PEMF was able to induce a muscle O2 availability, but at the same time, the stimulation was not sufficient enough to speed up and change the pulmonary VO2 on-transition kinetics. These variations at the muscle level were not accompanied by an improvement in endurance performance, such as the changes of the VO2 on-kinetics (speeding up of its phase II with the decrease in the slow component). This result can be clarified by the fact that the maximal cardiac output places a limitation on muscle blood flow and oxygen delivery to muscles employed during high intensity constant-load exercise that involves large muscle mass (i.e., during cycling), whereas PEMF might act only at the local level. Some authors found that the lack of faster oxygen kinetics in conditions where oxygen transport has been augmented is evidence that a metabolic mechanism, independent of oxygen, limits oxidative phosphorylation at the exercise onset (for more information see [28]). Given that our data demonstrate an accelerated O2 release at muscle level, the higher metabolism and/or the recruitment of type II muscle fibers at the onset of heavy exercise could be the procedure responsible for the slower oxygen kinetics [29]. Type II muscle fibers exhibit higher O2 costs during cycling and show slower VO2 on-kinetics in comparison to type I oxidative fibers, which might explain the slower VO2 responses. These results reinforce the concept that slower VO2 on-kinetics may be linked not only to the intracellular components related to aerobic metabolism, but also connected to the divergence between oxygen delivery and utilization at tissue level [30]. A faster modification of blood flow or the removal of limitations related to O2 diffusion at the periphery did not accelerate the VO2 kinetics response in isolated muscle [31]. According to Murias et al. [30], our findings propose that when τs is fewer than 20 s, oxygen delivery and utilization are well-matched, so additional provision of O2 may not result in quicker VO2 kinetics. In fact, when the rate of adjustment in pulmonary VO2 on-kinetics was reduced to the level that it became comparable to the rate of adjustment of HHb on-kinetics, no additional accelerating of VO2 kinetics was detected with supplementary training [32]. Together, these values support the role of microvascular O2 delivery as a constraint to muscle O2 utilization, meaning that there could be a point above which VO2 kinetics is limited primarily by the rate of adjustment of O2 delivery within the tissues. Importantly, accelerating the time course of the matching between muscle O2 delivery to O2 utilization can speed up the rate of adjustment when the time constant is longer than 20 s; nevertheless, when it is less than 20 s, it would appear that the modifications of VO2 are principally administered by intracellular processes [30].
The amplitude of the HHb primary component is interrelated to muscle O2 release and homogeneity of perfusion. Based on a previous study [4], we hypothesized that PEMF stimulation enhanced microcirculation through an increase in arteriole diameter. In addition, we supposed that the interaction between stimulation and Ca2+ /NO/cGMP/protein kinase G led to a modulation of nitric oxide (NO) signal, which facilitates the release of O2 in muscles during contraction by enhancing muscle microvascular oxygenation [7,33]. As postulated by Fick’s law of diffusion, the oxygen pressure in the microvasculature (i.e., muscle PO2mv) is the driving force for blood–myocyte O2 transfer. During metabolic demand, the skeletal muscle PO2mv on-kinetics is determined by the dynamic matching between oxygen delivery and utilization (i.e., QO2/VO2 ratio) [34]. Previous reports show that changes in NO levels influence muscle PO2mv during transitions of metabolic demand, suggesting that augmented NO-mediated function could, at least in part, enhance muscle microvascular oxygenation in trainers [35]. More recently, Cocksedge et al. [36] found that beetroot juice (supplements rich of NO3−) has no effect on TOI, VO2 kinetics, and exercise tolerance when severe intensity exercise (similar to our results; 3.49 vs. 3.46 L/min) occurred in normoxia with respect to hypoxia. The authors concluded that NO supplementation during severe exercise is related to the level of muscle deoxygenation sustained during such exercise intensity. Besides, it is probable that NO supplementation has a major role during exercise conducted in altitude, in persons with higher type II muscle fibers, and in populations with impairments in muscle oxygen delivery. The possible release of NO is a significant factor of the metabolic inertia to the VO2 dynamics during exercise conducted at a higher intensity. The specific mechanism by which NO contributes to the metabolic inertia at exercise onset is unclear but, in vitro, it has been verified that NO competes with O2 at the mitochondrial level [37]. Although our results cannot discriminate between ‘‘metabolic’’ and ‘‘O2 delivery’’ factors, which reduce VO2 on-kinetics, we can suppose that intracellular features would slow the VO2 on-kinetics to the level detected in the present study, but more studies are needed to explore this phenomenon.
Blood lactate level, recorded at the third minute of exercise, was significantly higher during stimulation, suggesting a possible influence of PEMF on muscle activity and on the glycolytic metabolism of type-II muscular fibers [38]. This effect could be caused by the change of membrane permeability and Ca2+ channel conduction, enhancing ion flux and cellular concentrations [39] that improve contraction mechanisms during exercise. A recent study [40] has showed that NO3− changes contractile properties and metabolic and/or vascular control in fast-twitch muscle fibers. Precisely, it has been found that NO supplementation speeds up pulmonary VO2 and muscle HHb kinetics during the transition from moderate to severe exercise intensity compared to placebo juice. Given that this intensity would be expected to recruit type II muscle fibers, Breese et al. concluded that NO supplementation could have precise effects on metabolic and/or vascular control in fast-twitch human muscle fibers, in accordance with previous research in rodents [41]. Surprisingly, this was not associated with a reduced blood lactate level. As expected, these selective VO2 and HHb effects should have provided the mechanistic basis (increase the O2 delivery to O2 utilization ratio, with enhanced oxidative function) for the reduced blood lactate level, as it was found in rodents [41]. Breese et al. did not do an explanation. We do not know why, in our study, lactate was not reduced (but increased) with respect to the sham stimulation. We could hypothesize that PEMF increased the magnitude of muscular response, especially the activity of the type-II muscular fibers, as typically recruited when intensity of the exercise exceeds ventilatory threshold, and related to the rise of VO2 slow component [42]. Moreover, the lactate is crucial for muscles to make cytosolic NAD+ and necessary to ATP regeneration from glycolysis, protecting muscles from acidosis. Lactate utilizes two protons necessary to delay acidosis, promoting proton elimination from muscles [43].
5. Conclusions
The present study reinforces the theory that the local application of a precise PEMF waveform can stimulate the rate of muscle oxygen extraction and utilization. These significant changes at the muscle level were not supported by an improvement in the predictable estimators of endurance performance, such as changes in the VO2 on-kinetics (speeding up of the primary phase and decrease in the slow component). PEMF stimulation could be used to augment muscle O2 availability during training sessions. It remains to be elucidated why stimulation did not speed up the VO2 on-transition kinetics and did not reduce (but increased) lactate levels. Further investigations are needed in terms of stimulation parameters (e.g., time, frequency, duration) and different exercise protocols. Moreover, although we have applied it to athletes, it could be important to direct future studies on patients and older adults whose agility and life quality are limited by an impairment in oxygen delivery and utilization. These people could enormously benefit from therapeutic approaches that increase their oxygen availability, a valuable aim for forthcoming investigations.
Author Contributions
Conceptualization, M.R. and A.P.; methodology, A.T.; software, A.M.; validation, A.P. and M.R.; formal analysis, A.T.; investigation, A.T., S.T., D.M., and F.C.; resources, M.R. and A.P.; data curation, A.T.; Writing—Original draft preparation, A.T.; Writing—Review and editing, A.P.; project administration, M.R.; funding acquisition, M.R. and A.P. All authors have read and agreed to the published version of the manuscript.
Funding
Supported by the University of Bologna, RFO 2018.
Institutional Review Board Statement
This study was approved by the Bioethics Committee of the University of Bologna in accordance with the Declaration of Helsinki. The protocol was approved.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
Data sharing is not applicable to this article because of the consent provided by participants on the use of confidential data.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Pesce, M.; Patruno, A.; Speranza, L.; Reale, M. Extremely low frequency electromagnetic field and wound healing: Implication of cytokines as biological mediators. Eur. Cytokine Netw. 2013. [Google Scholar] [CrossRef]
- Kubat, N.J.; Moffett, J.; Fray, L.M. Effect of pulsed electromagnetic field treatment on programmed resolution of inflammation pathway markers in human cells in culture. J. Inflamm. Res. 2015. [Google Scholar] [CrossRef]
- Jeon, H.S.; Kang, S.Y.; Park, J.H.; Lee, H.S. Effects of pulsed electromagnetic field therapy on delayed-onset muscle soreness in biceps brachii. Phys. Ther. Sport 2015. [Google Scholar] [CrossRef]
- Smith, T.L.; Wong-Gibbons, D.; Maultsby, J. Microcirculatory effects of pulsed electromagnetic fields. J. Orthop. Res. 2004. [Google Scholar] [CrossRef]
- Roland, D.; Ferder, M.; Kothuru, R.; Faierman, T.; Strauch, B. Effects of pulsed magnetic energy on a microsurgically transferred vessel. Plast. Reconstr. Surg. 2000, 105, 1371–1374. [Google Scholar] [CrossRef] [PubMed]
- Muehsam, D.; Lalezari, P.; Lekhraj, R.; Abruzzo, P.; Bolotta, A.; Marini, M.; Bersani, F.; Aicardi, G.; Pilla, A.; Casper, D. Non-Thermal Radio Frequency and Static Magnetic Fields Increase Rate of Hemoglobin Deoxygenation in a Cell-Free Preparation. PLoS ONE 2013. [Google Scholar] [CrossRef]
- Pall, M.L. Electromagnetic fields act via activation of voltage-gated calcium channels to produce beneficial or adverse effects. J. Cell. Mol. Med. 2013, 17, 958–965. [Google Scholar] [CrossRef] [PubMed]
- Milkiewicz, M.; Brown, M.D.; Egginton, S.; Hudlicka, O. Association between shear stress, angiogenesis, and VEGF in skeletal muscles in vivo. Microcirculation 2001. [Google Scholar] [CrossRef]
- Rikk, J.; Finn, K.J.; Liziczai, I.; Radák, Z.; Bori, Z.; Ihász, F. Influence of pulsing electromagnetic field therapy on resting blood pressure in aging adults. Electromagn. Biol. Med. 2013, 32, 165–172. [Google Scholar] [CrossRef]
- Mayrovitz, H.; Wounds, P.L.-U. Effects of pulsed electromagnetic fields on skin microvascular blood perfusion. Wounds 1992, 4, 197–202. [Google Scholar]
- Mayrovitz, H.N.; Larsen, P.B. A Preliminary Study to Evaluate the Effect of Pulsed Radio Frequency Field Treatment on Lower Extremity Peri-Ulcer Skin Microcirculation of Diabetic Patients. Wounds 1995, 7, 90–93. [Google Scholar]
- Kwan, R.L.C.; Wong, W.C.; Yip, S.L.; Chan, K.L.; Zheng, Y.P.; Cheing, G.L.Y. Pulsed electromagnetic field therapy promotes healing and microcirculation of chronic diabetic foot ulcers. Adv. Ski. Wound Care 2015. [Google Scholar] [CrossRef]
- Burnley, M.; Jones, A.M. Oxygen uptake kinetics as a determinant of sports performance. Eur. J. Sport Sci. 2007, 7, 63–79. [Google Scholar] [CrossRef]
- Koppo, K.; Bouckaert, J.; Jones, A.M. Effects of Training Status and Exercise Intensity on Phase II VO 2 Kinetics. Med. Sci. Sports Exerc. 2004. [Google Scholar] [CrossRef] [PubMed]
- Hirai, D.M.; Copp, S.W.; Holdsworth, C.T.; Ferguson, S.K.; McCullough, D.J.; Behnke, B.J.; Musch, T.I.; Poole, D.C. Skeletal muscle microvascular oxygenation dynamics in heart failure: Exercise training and nitric oxide-mediated function. Am. J. Physiol. Hear. Circ. Physiol. 2014, 306. [Google Scholar] [CrossRef] [PubMed]
- Grassi, B.; Quaresima, V. Near-infrared spectroscopy and skeletal muscle oxidative function in vivo in health and disease: A review from an exercise physiology perspective. J. Biomed. Opt. 2016, 21, 091313. [Google Scholar] [CrossRef] [PubMed]
- Piras, A.; Campa, F.; Toselli, S.; Di Michele, R.; Raffi, M. Physiological responses to partial-body cryotherapy performed during a concurrent strength and endurance session. Appl. Physiol. Nutr. Metab. 2019, 44, 59–65. [Google Scholar] [CrossRef] [PubMed]
- Campa, F.; Piras, A.; Raffi, M.; Trofè, A.; Perazzolo, M.; Mascherini, G.; Toselli, S. The effects of dehydration on metabolic and neuromuscular functionality during cycling. Int. J. Environ. Res. Public Health 2020, 17, 1161. [Google Scholar] [CrossRef] [PubMed]
- Piras, A.; Cortesi, M.; Campa, F.; Perazzolo, M.; Gatta, G. Recovery time profiling after short-, middle-and long-distance swimming performance. J. Strength Cond. Res. 2019, 33, 1408–1415. [Google Scholar] [CrossRef] [PubMed]
- Piras, A.; Gatta, G. Evaluation of the effectiveness of compression garments on autonomic nervous system recovery after exercise. J. Strength Cond. Res. 2017, 31, 1636–1643. [Google Scholar] [CrossRef] [PubMed]
- Mancini, D.M.; Bolinger, L.; Li, H.; Kendrick, K.; Chance, B.; Wilson, J.R. Validation of near-infrared spectroscopy in humans. J. Appl. Physiol. 1994. [Google Scholar] [CrossRef]
- Belardinelli, R.; Barstow, T.J.; Porszasz, J.; Wasserman, K. Skeletal muscle oxygenation during constant work rate exercise. Med. Sci. Sport. Exerc. 1995, 27, 512–519. [Google Scholar] [CrossRef]
- Niwayama, M.; Lin, L.; Shao, J.; Kudo, N.; Yamamoto, K. Quantitative measurement of muscle hemoglobin oxygenation using near-infrared spectroscopy with correction for the influence of a subcutaneous fat layer. Rev. Sci. Instrum. 2000, 71, 4571–4575. [Google Scholar] [CrossRef]
- Walsh, S.; Diamond, D. Non-linear curve fitting using microsoft excel solver. Talanta 1995. [Google Scholar] [CrossRef]
- Brown, A.M. A non-linear regression analysis program for describing electrophysiological data with multiple functions using Microsoft Excel. Comput. Methods Programs Biomed. 2006, 82, 51–57. [Google Scholar] [CrossRef] [PubMed]
- Kemmer, G.; Keller, S. Nonlinear least-squares data fitting in Excel spreadsheets. Nat. Protoc. 2010. [Google Scholar] [CrossRef] [PubMed]
- Hopkins, W.G.; Marshall, S.W.; Batterham, A.M.; Hanin, J. Progressive statistics for studies in sports medicine and exercise science. Med. Sci. Sport. Exerc. 2009, 41, 3–12. [Google Scholar] [CrossRef] [PubMed]
- Grassi, B. Regulation of oxygen consumption at exercise onset: Is it really controversial? Exerc. Sport Sci. Rev. 2001, 29, 134–138. [Google Scholar] [CrossRef]
- DiMenna, F.J.; Bailey, S.J.; Vanhatalo, A.; Chidnok, W.; Jones, A.M. Elevated baseline VO2 per se does not slow O2 uptake kinetics during work-to-work exercise transitions. J. Appl. Physiol. 2010, 109, 1148–1154. [Google Scholar] [CrossRef][Green Version]
- Murias, J.M.; Spencer, M.D.; Kowalchuk, J.M.; Paterson, D.H. Muscle deoxygenation to VO 2 relationship differs in young subjects with varying τvO 2. Eur. J. Appl. Physiol. 2011. [Google Scholar] [CrossRef]
- Grassi, B.; Gladden, L.B.; Samaja, M.; Stary, C.M.; Hogan, M.C. Faster adjustment of O2 delivery does not affect VO2 on-kinetics in isolated in situ canine muscle. J. Appl. Physiol. 1998, 85, 1394–1403. [Google Scholar] [CrossRef][Green Version]
- Murias, J.M.; Kowalchuk, J.M.; Peterson, D.H. Speeding of Vo2 kinetics with endurance training in old and young men is associated with improved matching of local O2 delivery to muscle O2 utilization. J. Appl. Physiol. 2010. [Google Scholar] [CrossRef] [PubMed]
- Diniz, P.; Soejima, K.; Ito, G. Nitric oxide mediates the effects of pulsed electromagnetic field stimulation on the osteoblast proliferation and differentiation. Nitric Oxide 2002, 7, 18–23. [Google Scholar] [CrossRef]
- Behnke, B.J.; Kindig, C.A.; Musch, T.I.; Koga, S.; Poole, D.C. Dynamics of microvascular oxygen pressure across the rest-exercise transition in rat skeletal muscle. Respir. Physiol. 2001, 126, 53–63. [Google Scholar] [CrossRef]
- Hirai, D.M.; Copp, S.W.; Ferguson, S.K.; Holdsworth, C.T.; McCullough, D.J.; Behnke, B.J.; Musch, T.I.; Poole, D.C. Exercise training and muscle microvascular oxygenation: Functional role of nitric oxide. J. Appl. Physiol. 2012, 113, 557–565. [Google Scholar] [CrossRef] [PubMed]
- Cocksedge, S.P.; Breese, B.C.; Morgan, P.T.; Nogueira, L.; Thompson, C.; Wylie, L.J.; Jones, A.M.; Bailey, S.J. Influence of muscle oxygenation and nitrate-rich beetroot juice supplementation on O2 uptake kinetics and exercise tolerance. Nitric Oxide Biol. Chem. 2020, 99, 25–33. [Google Scholar] [CrossRef]
- Wilkerson, D.P.; Campbell, I.T.; Jones, A.M. Influence of nitric oxide synthase inhibition on pulmonary O2 uptake kinetics during supra-maximal exercise in humans. J. Physiol. 2004, 561, 623–635. [Google Scholar] [CrossRef]
- Sakurai, T.; Satake, A.; Sumi, S.; Inoue, K.; Miyakoshi, J. An Extremely Low Frequency Magnetic Field Attenuates Insulin Secretion from the Insulinoma Cell Line, RIN-m. Bioelectromagnetics 2004. [Google Scholar] [CrossRef]
- Ross, C.L.; Siriwardane, M.; Almeida-Porada, G.; Porada, C.D.; Brink, P.; Christ, G.J.; Harrison, B.S. The effect of low-frequency electromagnetic field on human bone marrow stem/progenitor cell differentiation. Stem Cell Res. 2015, 15, 96–108. [Google Scholar] [CrossRef]
- Breese, B.C.; Mcnarry, M.A.; Marwood, S.; Blackwell, J.R.; Bailey, S.J.; Jones, A.M. Beetroot juice supplementation speeds O2 uptake kinetics and improves exercise tolerance during severe-intensity exercise initiated from an elevated metabolic rate. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2013, 305. [Google Scholar] [CrossRef]
- Ferguson, S.K.; Hirai, D.M.; Copp, S.W.; Holdsworth, C.T.; Allen, J.D.; Jones, A.M.; Musch, T.I.; Poole, D.C. Effects of nitrate supplementation via beetroot juice on contracting rat skeletal muscle microvascular oxygen pressure dynamics. Respir. Physiol. Neurobiol. 2013, 187, 250–255. [Google Scholar] [CrossRef] [PubMed]
- Krustrup, P.; Söderlund, K.; Mohr, M.; Bangsbo, J.; Arch, P.; Krustrup, P.; Söderlund, K.; Mohr, M. The slow com ponent of oxygen uptake during intense sub-maximal exercise in man is associated with additional fi bre recruitment. Pflügers Arch. 2004, 447, 855–866. [Google Scholar] [CrossRef] [PubMed]
- Robergs, R.A.; Ghiasvand, F.; Parker, D. Biochemistry of exercise-induced metabolic acidosis. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2004, 287, R502–R516. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).