Isolation and Identification of Two Potent Phytotoxic Substances from Afzelia xylocarpa for Controlling Weeds
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material and Model Plants
2.2. Preparation of the A. xylocarpa Leaf Extracts
2.3. Bioassay Procedure
2.4. Isolation and Identification of the Active Substances
2.5. Biological Activity of the Isolated Active Substances
2.6. The Synergistic Effects of a Mixture of the Two Isolated Substances
2.7. Statistical Analysis
3. Results and Discussion
3.1. Biological Activity of the Aqueous Methanol Extracts of A. xylocarpa Leaves
3.2. Isolation and Identification of the Active Substances in the A. xylocarpa Leaf Extracts
3.3. Biological Activity of Vanillic Acid and Trans-Ferulic Acid
3.4. The Synergistic Effects of the Mixture of Vanillic Acid and Trans-Ferulic Acid
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Qasem, J.R.; Foy, C.L. Weed allelopathy, its ecological impacts and future prospects. J. Crop. Prod. 2001, 4, 43–119. [Google Scholar] [CrossRef]
- Oerke, E.C. Crop losses to pests. J. Agric. Sci. 2006, 144, 31–43. [Google Scholar] [CrossRef]
- Jabran, K.; Mahajan, G.; Sardana, V.; Chauhan, B.S. Allelopathy for weed control in agricultural systems. J. Crop. Prot. 2015, 72, 57–65. [Google Scholar] [CrossRef]
- Aktar, M.W.; Sengupta, D.; Chowdhury, A. Impact of pesticides use in agriculture: Their benefits and hazards. Interdisc. Toxicol. 2009, 2, 569–596. [Google Scholar] [CrossRef] [PubMed]
- Bhardwaj, T.; Sharma, J.P. Impact of pesticides application in agricultural industry: An Indian scenario. Int. J. Agric. Food Sci. Technol. 2013, 4, 817–822. [Google Scholar]
- Awan, T.H.; Cruz, P.C.S.; Chauhan, B.S. Agronomic indices, growth, yield-contributing traits, and yield of dry-seeded rice under varying herbicides. Field Crop. Res. 2015, 177, 15–25. [Google Scholar] [CrossRef]
- Délye, C. Unraveling the genetics bases of non-target-site-based resistance (NTSR) to herbicides: A major challenge for weed science in the forthcoming decade. Pest Manag. Sci. 2013, 69, 176–187. [Google Scholar] [CrossRef] [PubMed]
- Bari, I.N.; Kato-Noguchi, H. Phytotoxic effects of Cerbera manghas L. leaf extracts on seedling elongation of four monocot and four dicot test species. Acta Agrobot. 2017, 70, 1720. [Google Scholar] [CrossRef]
- Poonpaiboonpipat, T.; Poolkum, S. Utilization of Bidens pilosa var. radiata (Sch. Bip.) Sherff integrated with water irrigation for paddy weed control and rice yield production. Weed Biol. Manag. 2019, 19, 31–38. [Google Scholar] [CrossRef]
- Kyaw, E.H.; Kato-Noguchi, H. Allelopathic potential of Acacia pennata (L.) Willd. leaf extracts against the seedling growth of six test plants. Not. Bot. Horti Agrobot. Cluj-Napoca 2020, 48, 1534–1542. [Google Scholar] [CrossRef]
- Singh, N.B.; Pandey, B.N.; Singh, A. Allelopathic effects of Cyperus rotundus extract in vitro and ex vitro on banana. Acta Physiol. Plant 2009, 31, 633–638. [Google Scholar] [CrossRef]
- Abbas, T.; Tanveer, A.; Khaliq, A.; Safdar, M.E. Comparative allelopathic potential of native and invasive weeds in rice ecosystem. Pak. J. Weed. Sci. Res. 2016, 22, 269–283. [Google Scholar]
- Rice, E.L. Allelopathy, 2nd ed.; Academic Press: Orlando, FL, USA, 1984; pp. 67–68. [Google Scholar]
- Chon, S.U.; Nelson, C.J. Allelopathy in compositae plants. Agron. Sustain. Dev. 2010, 30, 349–358. [Google Scholar] [CrossRef]
- Teerarak, M.; Charoenying, P.; Laosinwattana, C. Physiological and cellular mechanisms of natural herbicide resource from Aglaia odorata Lour. on bioassay plants. Acta Physiol. Plant 2012, 34, 1277–1285. [Google Scholar] [CrossRef]
- Cheng, F.; Cheng, Z. Research progress on the use of plant allelopathy in agriculture and the physiological and ecological mechanisms of allelopathy. Front. Plant Sci. 2015, 6, 1020. [Google Scholar] [CrossRef]
- Vyvyan, J.R. Allelochemicals as leads for new herbicides and agrochemicals. Tetrahedron 2002, 58, 1631–1646. [Google Scholar] [CrossRef]
- Dayan, F.E.; Cantrell, C.L.; Duke, S.O. Natural products in crop protection. Bioorg. Ned. Chem. 2009, 17, 4022–4034. [Google Scholar] [CrossRef]
- Kato-Noguchi, H.; Suwitchayanon, P.; Boonmee, S.; Iwasaki, A.; Suenaga, K. Plant growth inhibitory activity of the extracts of Acmella oleracea and its growth inhibitory substances. Nat. Prod. Commun. 2019, 14, 1934578X19858805. [Google Scholar] [CrossRef]
- Islam, M.S.; Zaman, F.; Iwasaki, A.; Suenaga, K.; Kato-Noguchi, H. Phytotoxic potential of Chrysopogon aciculatus (Retz.) Trin. (Poaceae). Weed Biol. Manag. 2019, 19, 51–58. [Google Scholar] [CrossRef]
- Bari, I.N.; Kato-Noguchi, H.; Iwasaki, A.; Suenaga, K. Allelopathic potency and an active substance from Anredera cordifolia (Tenore) Steenis. Plants 2019, 8, 134. [Google Scholar] [CrossRef]
- Rob, M.; Iwasaki, A.; Suzuki, R.; Suenaga, K.; Kato-Noguchi, H. Garcienone, a novel compound involved in allelopathic activity of Garcinia Xanthochymus hook. Plants 2019, 8, 301. [Google Scholar] [CrossRef] [PubMed]
- El-Derek, M.H.; Hess, F.D. Inhibited mitotic entry is the cause of growth inhibition by cinmethylin. Weed Sci. 1986, 34, 684–688. [Google Scholar] [CrossRef]
- Grossmann, K.; Hutzler, J.; Tresch, S.; Christiansen, N.; Looser, R.; Ehrhardt, T. On the mode of action of the herbicides cinmethylin and 5-benzyloxymethyl-1, 2-isoxazolines: Putative inhibitors of plant tyrosine aminotransferase. Pest Manag. Sci. 2012, 68, 482–492. [Google Scholar] [CrossRef] [PubMed]
- Dayan, F.E.; Romagni, J.G.; Duke, S.O. Investigating the mode of action of natural phytotoxins. J. Chem. Ecol. 2000, 26, 2079–2094. [Google Scholar] [CrossRef]
- Boonmee, S.; Iwasaki, A.; Suenaga, K.; Kato-Noguchi, H. Evaluation of phytotoxic activity of leaf and stem extracts and identification of a phytotoxic substance from Caesalpinia mimosoides Lamk. Theor. Exp. Plant Physiol. 2018, 30, 129–139. [Google Scholar] [CrossRef]
- Krumsri, R.; Iwasaki, A.; Suenaga, K.; Kato-Noguchi, H. Assessment of allelopathic potential of Dalbergia cochinchinensis Pierre and its growth inhibitory substance. Emir. J. Food. Agric. 2020, 32, 513–521. [Google Scholar] [CrossRef]
- Wink, M. Evolution of secondary metabolites in legumes (Fabaceae). S. Afr. J. Bot. 2013, 89, 164–175. [Google Scholar] [CrossRef]
- El-Gawad, A.M.A.; El-Amier, Y.A.; Bonanomi, G. Allelopathic activity and chemical composition of Rhynchosia minima (L.) DC. essential oil from Egypt. Chem. Biodivers. 2018, 15, e1700438. [Google Scholar] [CrossRef]
- Quddus, M.S.; Bellairs, S.M.; Wurm, P.A. Acacia holosericea (Fabaceae) litter has allelopathic and physical effects on mission grass (Cenchrus pedicellatus and C. polystachios) (Poaceae) seedling establishment. Aust. J. Bot. 2014, 62, 189–195. [Google Scholar] [CrossRef]
- Hussain, M.I.; El-Sheikh, M.A.; Reigosa, M.J. Allelopathic Potential of Aqueous Extract from Acacia melanoxylon R. Br. on Lactuca sativa. Plants 2020, 9, 1228. [Google Scholar] [CrossRef]
- Pakkad, G.; Ueno, S.; Yoshimaru, H. Isolation and characterization of microsatellite loci in an endangered tree species, Afzelia xylocarpa (Kurz) Craib (Caesalpinioideae). Mol. Ecol. Resour. 2009, 9, 880–882. [Google Scholar] [CrossRef]
- Cai, X.; Liu, J.X.; Song, Q.S.; Chen, K.L.; Lu, Z.Y.; Zhang, Y.M. Chemical constituents of Afzelia xylocarpa. Chem. Nat. Compd. 2018, 54, 764–765. [Google Scholar] [CrossRef]
- Akah, P.A.; Okpi, O.; Okoli, C.O. Evaluation of the anti-inflammatory, analgesic and antimicrobial activities of bark of Afzelia africana. Niger. J. Nat. Prod. Med. 2007, 11, 48–52. [Google Scholar] [CrossRef]
- Oyedemi, S.O.; Adewusi, E.A.; Aiyegoro, O.A.; Akinpelu, D.A. Antidiabetic and haematological effect of aqueous extract of stem bark of Afzelia africana (Smith) on streptozotocin-induced diabetic Wistar rats. Asian. Pac. J. Trop. Med. 2011, 1, 353–358. [Google Scholar] [CrossRef]
- Akinpelu, D.A.; Aiyegoro, O.A.; Okoh, A.I. The in vitro antioxidant property of methanolic extract of Afzelia africana (Smith.). J. Med. Plant Res. 2010, 4, 2022–2027. [Google Scholar] [CrossRef]
- Pellissier, F.; Gallet, C.; Souto, X.C. Allelopathic interactions in forest ecosystems. In Allelopathy: From Molecules to Ecosystems; Reigosa, M.J., Nuria, P., Sanchez-Moreiras, A.M., Gonzales, L., Eds.; Science Publishers: Enfield, NH, USA, 2002; pp. 257–269. [Google Scholar]
- Kimura, F.; Sato, M.; Kato-Noguchi, H. Allelopathy of pine litter: Delivery of allelopathic substances into forest floor. J. Plant Biol. 2015, 58, 61–67. [Google Scholar] [CrossRef]
- Phuong, D.L.; Thuy, N.T.; Long, P.Q.; Kuo, P.C.; Thang, T.D. Composition of fatty acids, tocopherols, sterols, total phenolics, and antioxidant activity of seed oils of Afzelia xylocarpa and Cassia fistula. Chem. Nat. Compd. 2019, 55, 242–246. [Google Scholar] [CrossRef]
- Thanh, N.D.; Nghia, N.H. Genetic diversity of Afzelia xylocarpa (Kurz) Craib in Vietnam based on analyses of chloroplast markers and random amplified polymorphic DNA (RAPD). Afr. J. Biotechnol. 2012, 11, 14529–14535. [Google Scholar] [CrossRef]
- Yu, J.Q.; Shou, S.Y.; Qian, Y.R.; Zhu, Z.Z.; Hu, W.H. Autotoxic potential of cucurbit crops. Plant Soil. 2000, 223, 149–153. [Google Scholar] [CrossRef]
- Zhou, X.; Zhang, J.; Pan, D.; Ge, X.; Jin, X.; Chen, S.; Wu, F. p-Coumaric can alter the composition of cucumber rhizosphere microbial communities and induce negative plant-microbial interactions. Biol. Fertil. Soils. 2018, 54, 683. [Google Scholar] [CrossRef]
- Noguchi, K.; Ohno, O.; Suenaga, K.; Laosinwattana, C. A potent phytotoxic substance in Aglaia odorata Lour. Chem. Biodivers. 2016, 13, 549–554. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.S.; Iwasaki, A.; Suenaga, K.; Kato-Noguchi, H. 2-Methoxystypandrone, a potent phytotoxic substance in Rumex maritimus L. Theor. Exp. Plant. Physiol. 2017, 29, 195–202. [Google Scholar] [CrossRef]
- Xuan, T.D.; Shinkichia, T.; Khanh, T.D.; Chung, M. Biological control of weeds and plant pathogens in paddy rice by exploiting plant allelopathy: An overview. Crop Prot. 2005, 24, 197–206. [Google Scholar] [CrossRef]
- Krumsri, R.; Boonmee, S.; Kato-Noguchi, H. Evaluation of the allelopathic potential of leaf extracts from Dischidia imbricata (Blume) Steud. on the seedling growth of six test plants. Not. Bot. Horti Agrobot. Cluj-Napoca 2019, 47, 1019–1024. [Google Scholar] [CrossRef]
- Boonmee, S.; Iwasaki, A.; Suenaga, K.; Kato-Noguchi, H. Identification of 6, 7-dimethoxychromone as a potent allelochemical from Jatropha podagrica. Nat. Prod. Commun. 2018, 13, 1934578X1801301126. [Google Scholar] [CrossRef]
- Zaman, F.; Islam, M.S.; Kato-Noguchi, H. Allelopathic activity of the Oxalis europea L. extracts on the growth of eight test plant species. Res Crop. 2018, 19, 304–309. [Google Scholar] [CrossRef]
- Poonpaiboonpipat, T.; Jumpathong, J. Evaluating herbicidal potential of aqueous–ethanol extracts of local plant species against Echinochloa crus-galli and Raphanus sativus. Int. J. Agric. Biol. 2019, 21, 648–652. [Google Scholar] [CrossRef]
- Rob, M.M.; Kato-Noguchi, H. Study of the allelopathic activity of Garcinia pedunculata Roxb. Plant Omics 2019, 12, 31–36. [Google Scholar] [CrossRef]
- Boonmee, S.; Suwitchayanon, P.; Krumsri, R.; Kato-noguchi, H. Investigation of the allelopathic potential of Nephrolepis cordifolia (L.) C. Presl against dicotyledonous and monocotyledonous plant species. Environ. Control. Biol. 2020, 58, 71–78. [Google Scholar] [CrossRef]
- Krumsri, R.; Kato-Noguchi, H.; Poonpaiboonpipat, T. Allelopathic effect of Sphenoclea zeylanica Gaertn. on rice (Oryza sativa L.) germination and seedling growth. Aust. J. Crop Sci. 2020, 14, 1450–1455. [Google Scholar] [CrossRef]
- Hossen, K.; Iwasaki, A.; Suenaga, K.; Kato-Noguchi, H. Phytotoxic Activity and Growth Inhibitory Substances from Albizia richardiana (Voigt.) King & Prain. Appl. Sci. 2021, 11, 1455. [Google Scholar] [CrossRef]
- Suwitchayanon, P.; Ohno, O.; Suenaga, K.; Kato-Noguchi, H. Phytotoxic property of Piper retrofractum fruit extracts and compounds against the germination and seedling growth of weeds. Acta Physiol. Plant 2019, 41, 33. [Google Scholar] [CrossRef]
- Namkeleja, H.S.; Tarimo, M.T.; Ndakidemi, P.A. Allelopathic effects of Argemone mexicana to growth of native plant species. Am. J. Plant Sci. 2014, 5, 1336–1344. [Google Scholar] [CrossRef]
- Pu, W.; Wang, D.; Zhou, D. Structural characterization and evaluation of the antioxidant activity of phenolic compounds from Astragalus taipaishanensis and their structure-activity relationship. Sci. Rep. 2015, 5, 13914. [Google Scholar] [CrossRef]
- Sethupathy, S.; Ananthi, S.; Selvaraj, A.; Shanmuganathan, B.; Vigneshwari, L.; Balamurugan, K.; Pandian, S.K. Vanillic acid from Actinidia deliciosa impedes virulence in Serratia marcescens by affecting S-layer, flagellin and fatty acid biosynthesis proteins. Sci. Rep. 2017, 7, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Ghareib, H.R.A.; Abdelhamed, M.S.; Ibrahim, O.H. Antioxidative effects of the acetone fraction and vanillic acid from Chenopodium muraleon tomato plants. Weed Biol. Manag. 2010, 10, 64–72. [Google Scholar] [CrossRef]
- Domínguez, C.R.; Dominguez Avila, J.A.; Pareek, S.; Villegas Ochoa, M.A.; Ayala Zavala, J.F.; Yahia, E. Content of bioactive compounds and their contribution to antioxidant capacity during ripening of pineapple (Ananas comosus L.) cv. Esmeralda. J. Appl. Bot. Food Qual. 2018, 91, 61–68. [Google Scholar] [CrossRef]
- Archivio, D.M.; Filesi, C.; Di Benedetto, R.; Gargiulo, R.; Giovannini, C.; Masella, R. Polyphenols, dietary sources and bioavailability. Ann. Ist. Super. Sanita 2007, 43, 348–361. [Google Scholar]
- Zavala-López, M.; Flint-García, S.; García-Lara, S. Compositional variation in trans-ferulic, p-coumaric, and diferulic acids levels among kernels of modern and traditional maize (Zea mays L.) hybrids. Front. Nutr. 2020, 7, 600747. [Google Scholar] [CrossRef] [PubMed]
- Trombino, S.; Cassano, R.; Ferrarelli, T.; Barone, E.; Picci, N.; Mancuso, C. Trans-ferulic acid-based solid lipid nanoparticles and their antioxidant effect in rat brain microsomes. Colloids Surf. B Biointerfaces 2013, 109, 273–279. [Google Scholar] [CrossRef]
- Granata, G.; Consoli, G.M.; Nigro, R.L.; Geraci, C. Hydroxycinnamic acids loaded in lipid-core nanocapsules. Food Chem. 2018, 245, 551–556. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Haig, T.; Pratley, J.; Lemerle, D.; An, M. Allelochemicals in wheat (Triticum aestivum L.): Variation of phenolic acids in shoot tissues. J. Chem. Ecol. 2001, 27, 125–135. [Google Scholar] [CrossRef]
- Sampietro, D.A.; Vattuone, M.A.; Isla, M.I. Plant growth inhibitors isolated from sugarcane (Saccharum officinarum) straw. J. Plant Physiol. 2006, 163, 837–846. [Google Scholar] [CrossRef]
- Tuyen, P.T.; Xuan, T.D.; Tu Anh, T.T.; Mai Van, T.; Ahmad, A.; Elzaawely, A.A.; Khanh, T.D. Weed suppressing potential and isolation of potent plant growth inhibitors from Castanea crenata Sieb. et Zucc. Molecules 2018, 23, 345. [Google Scholar] [CrossRef] [PubMed]
- Jmii, G.; Molinillo, J.M.; Zorrilla, J.G.; Haouala, R. Allelopathic activity of Thapsia garganica L. leaves on lettuce and weeds, and identification of the active principles. S. Afr. J. Bot. 2020, 131, 188–194. [Google Scholar] [CrossRef]
- Zakaria, W.; Razak, A.R. Effects of groundnut plant residues on germination and radicle elongation of four crop species. Pertanika 1990, 13, 297–302. [Google Scholar]
- Kobayashi, K. Factors affecting phytotoxic activity of allelochemicals in soil. Weed Biol. Manag. 2004, 4, 1–7. [Google Scholar] [CrossRef]
- Leishman, M.R.; Wright, I.J.; Moles, A.T.; Westoby, M. The evolutionary ecology of seed size. In Seeds: The Ecology of Regeneration in Plant Communities; Fenner, M., Ed.; CAB International: Wallingford, UK, 2000; pp. 31–57. [Google Scholar] [CrossRef]
- Gan, X.; Hu, D.; Wang, Y.; Yu, L.; Song, B. Novel trans-ferulic acid derivatives containing a chalcone moiety as potential activator for plant resistance induction. J. Agric. Food Chem. 2017, 65, 4367–4377. [Google Scholar] [CrossRef] [PubMed]
- Amin, F.U.; Shah, S.A.; Kim, M.O. Vanillic acid attenuates Aβ 1-42-induced oxidative stress and cognitive impairment in mice. Sci. Rep. 2017, 7, 40753. [Google Scholar] [CrossRef] [PubMed]
- Pezzani, R.; Vitalini, S.; Iriti, M. Bioactivities of Origanum vulgare L.: An update. Phytochem. Rev. 2017, 16, 1253–1268. [Google Scholar] [CrossRef]
- Huang, H.; Ullah, F.; Zhou, D.X.; Yi, M.; Zhao, Y. Mechanisms of ROS regulation of plant development and stress responses. Front. Plant Sci. 2019, 10, 800. [Google Scholar] [CrossRef] [PubMed]
- Jinxiang, C.; Yang, J.; Lanlan, M.; Li, J.; Nasir, S.; Kyung, K.C. Structure-antioxidant activity relationship of methoxy, phenolic hydroxyl, and carboxylic acid groups of phenolic acids. Sci. Rep. 2020, 10, 2611. [Google Scholar] [CrossRef]
- Blum, U. Allelopathic interactions involving phenolic acids. J. Nematol. 1996, 28, 259–267. [Google Scholar] [PubMed]
- Inderjit; Streibig, J.C.; Olofsdotter, M. Joint action of phenolic acid mixtures and its significance in allelopathy research. Physiol. Plant 2002, 114, 422–428. [Google Scholar] [CrossRef] [PubMed]
- Jia, C.; Kudsk, P.; Mathiassen, S.K. Joint action of benzoxazinone derivatives and phenolic acids. J. Agric. Food Chem. 2006, 54, 1049–1057. [Google Scholar] [CrossRef] [PubMed]
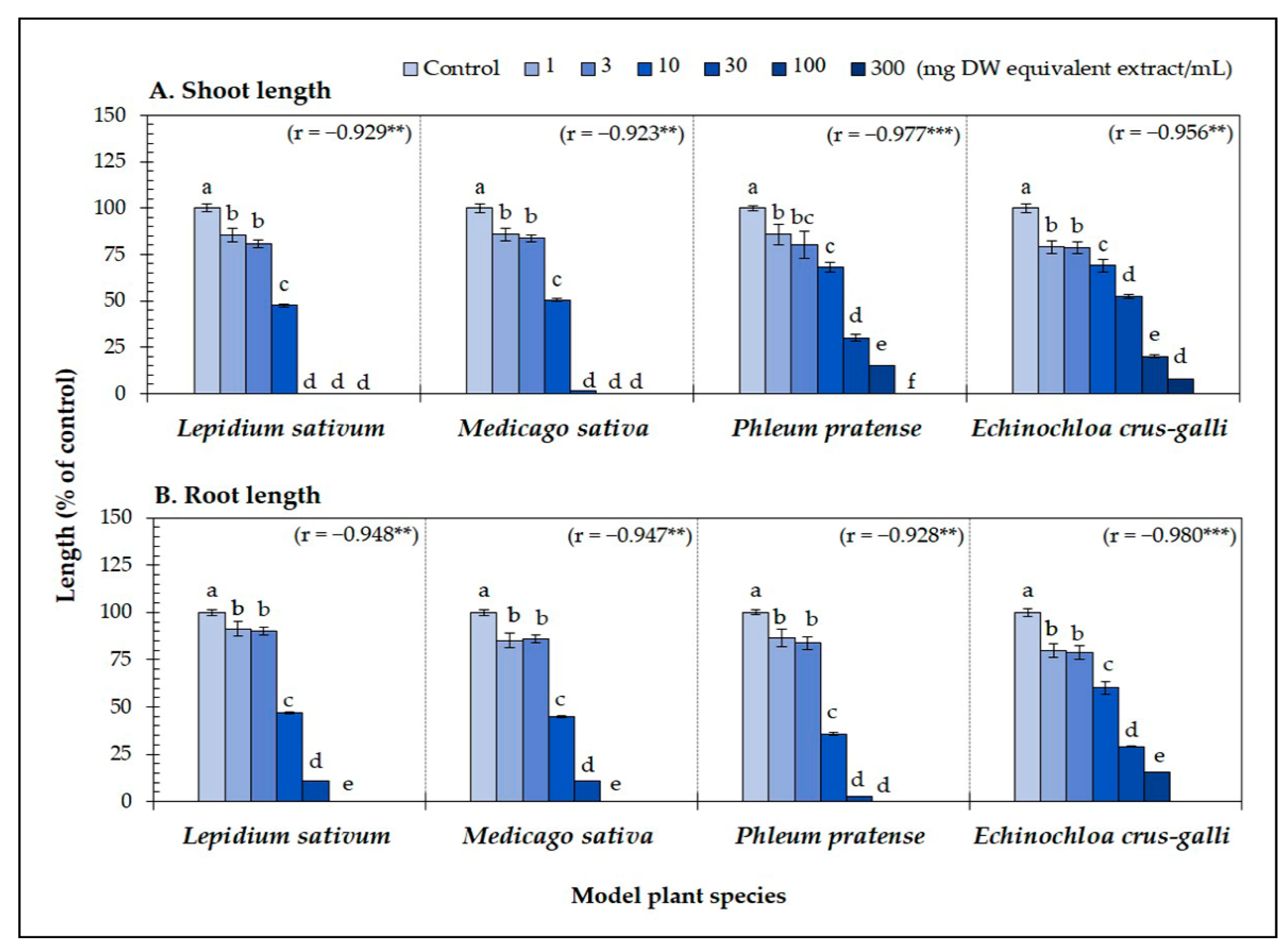
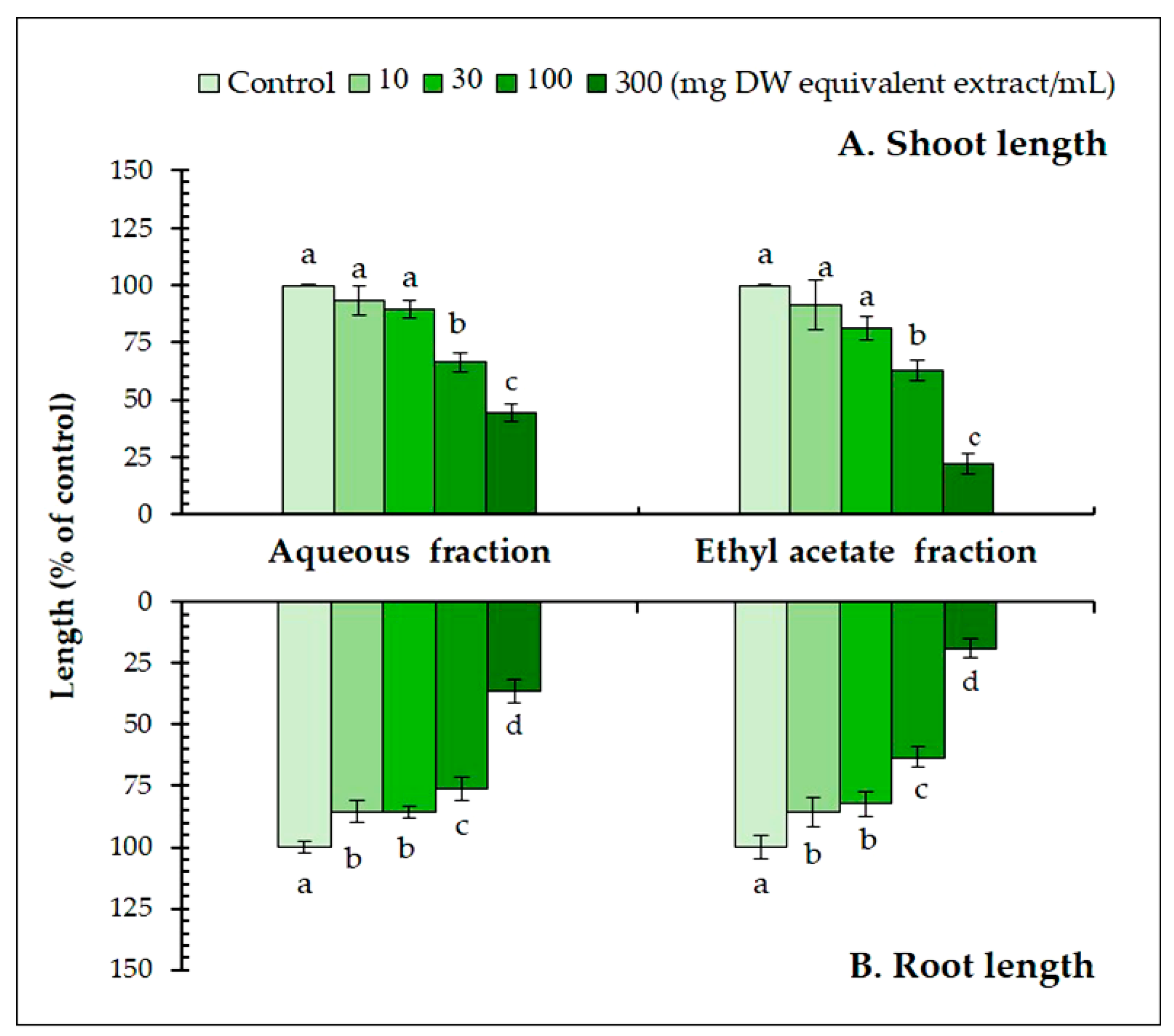
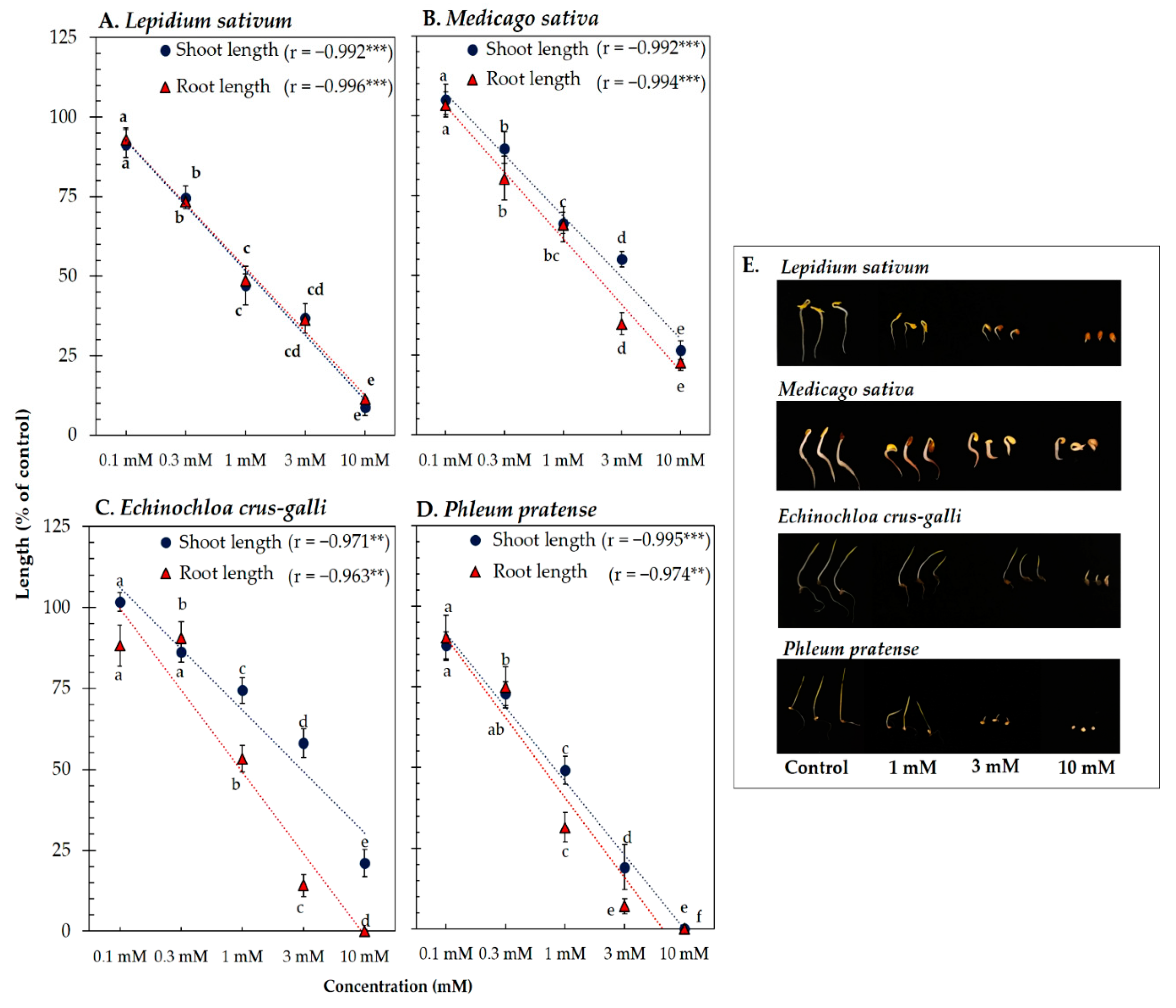

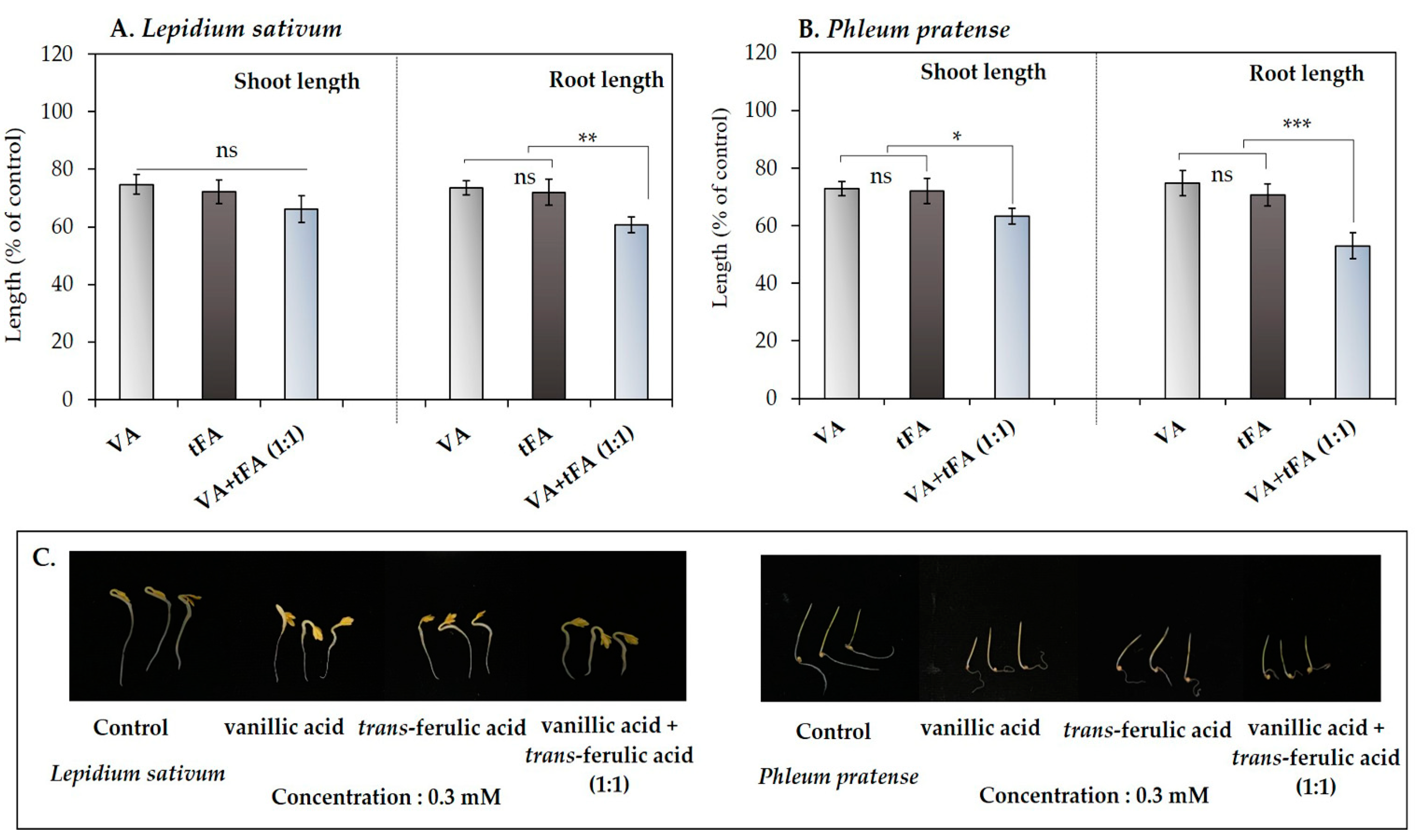
| Test plant species | IC50 values of Afzelia xylocarpa leaf extracts (mg DW equivalent extract/mL) | |
| Shoot length | Root length | |
| Lepidium sativum | 7.78 d | 7.67 c |
| Medicago sativa | 8.50 c | 8.99 b |
| Phleum pratense | 15.28 b | 6.42 d |
| Echinochloa crus-galli | 24.21 a | 12.62 a |
| Test plant species | IC50 values of vanillic acid (mM) | |
| Shoot length | Root length | |
| Lepidium sativum | 0.81 c | 0.93 b |
| Medicago sativa | 2.72 ab | 1.89 a |
| Phleum pratense | 0.80 c | 0.73 c |
| Echinochloa crus-galli | 3.17 a | 1.01 b |
| Test plant species | IC50 values of trans-ferulic acid (mM) | |
| Shoot length | Root length | |
| Lepidium sativum | 0.65 c | 0.76 c |
| Medicago sativa | 2.43 a | 1.40 a |
| Phleum pratense | 0.61 c | 0.42 d |
| Echinochloa crus-galli | 1.10 b | 0.97 b |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Krumsri, R.; Ozaki, K.; Teruya, T.; Kato-Noguchi, H. Isolation and Identification of Two Potent Phytotoxic Substances from Afzelia xylocarpa for Controlling Weeds. Appl. Sci. 2021, 11, 3542. https://doi.org/10.3390/app11083542
Krumsri R, Ozaki K, Teruya T, Kato-Noguchi H. Isolation and Identification of Two Potent Phytotoxic Substances from Afzelia xylocarpa for Controlling Weeds. Applied Sciences. 2021; 11(8):3542. https://doi.org/10.3390/app11083542
Chicago/Turabian StyleKrumsri, Ramida, Kaori Ozaki, Toshiaki Teruya, and Hisashi Kato-Noguchi. 2021. "Isolation and Identification of Two Potent Phytotoxic Substances from Afzelia xylocarpa for Controlling Weeds" Applied Sciences 11, no. 8: 3542. https://doi.org/10.3390/app11083542
APA StyleKrumsri, R., Ozaki, K., Teruya, T., & Kato-Noguchi, H. (2021). Isolation and Identification of Two Potent Phytotoxic Substances from Afzelia xylocarpa for Controlling Weeds. Applied Sciences, 11(8), 3542. https://doi.org/10.3390/app11083542






