Development of a Component-Level Hydrogen Transport Model with OpenFOAM and Application to Tritium Transport Inside a DEMO HCPB Breeder
Abstract
1. Introduction
2. OpenFOAM Tritium Transport Model Upgrades
2.1. Boundary Condition (BC)Formalism
2.2. Two Species BC Correlation
2.3. Porosity Model
- all volumetric source terms for hydrogen have to be divided by a factor of ε.
- the interface mass transfer has to be corrected by a factor of 1/ε on the fluid side.
- advective transport enhances by a factor of 1/ε.
- diffusive transport reduces by a factor of ε/δ.
2.4. Residence Time Release Model
2.5. Integration of Model Upgrades
3. DEMO HCPB Single Pin Simulation Setup
3.1. HCPB Pin Design
3.2. OpenFOAM Model
4. OpenFOAM Simulation Results for a Single DEMO HCPB Fuel Pin
- permeation regime (diffusion-limited vs. surface-limited)
- single- vs. two-species scenarios
- purge gas composition (with and without additional 300 Pa H2 in purge gas)
- purge gas pressure (2 bar vs. 80 bar)
- species-specific rate constants (different recombination constant for mixed species molecule HT)
4.1. Diffusion-Limited Cases
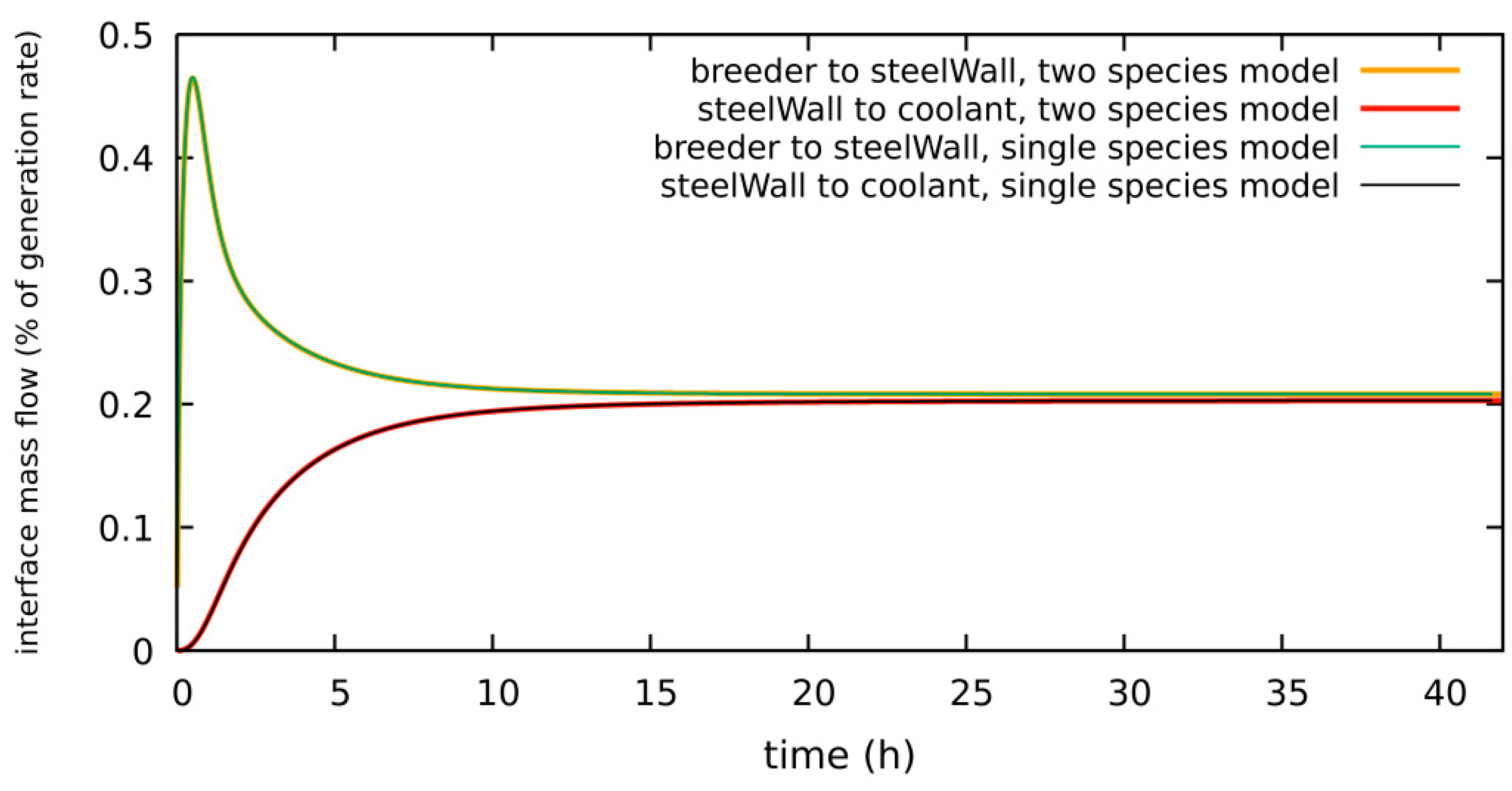
4.1.1. Low/No H2 System, Diffusion-Limited, Low H2 in Purge Gas, Single- vs. Two-Species Correlation
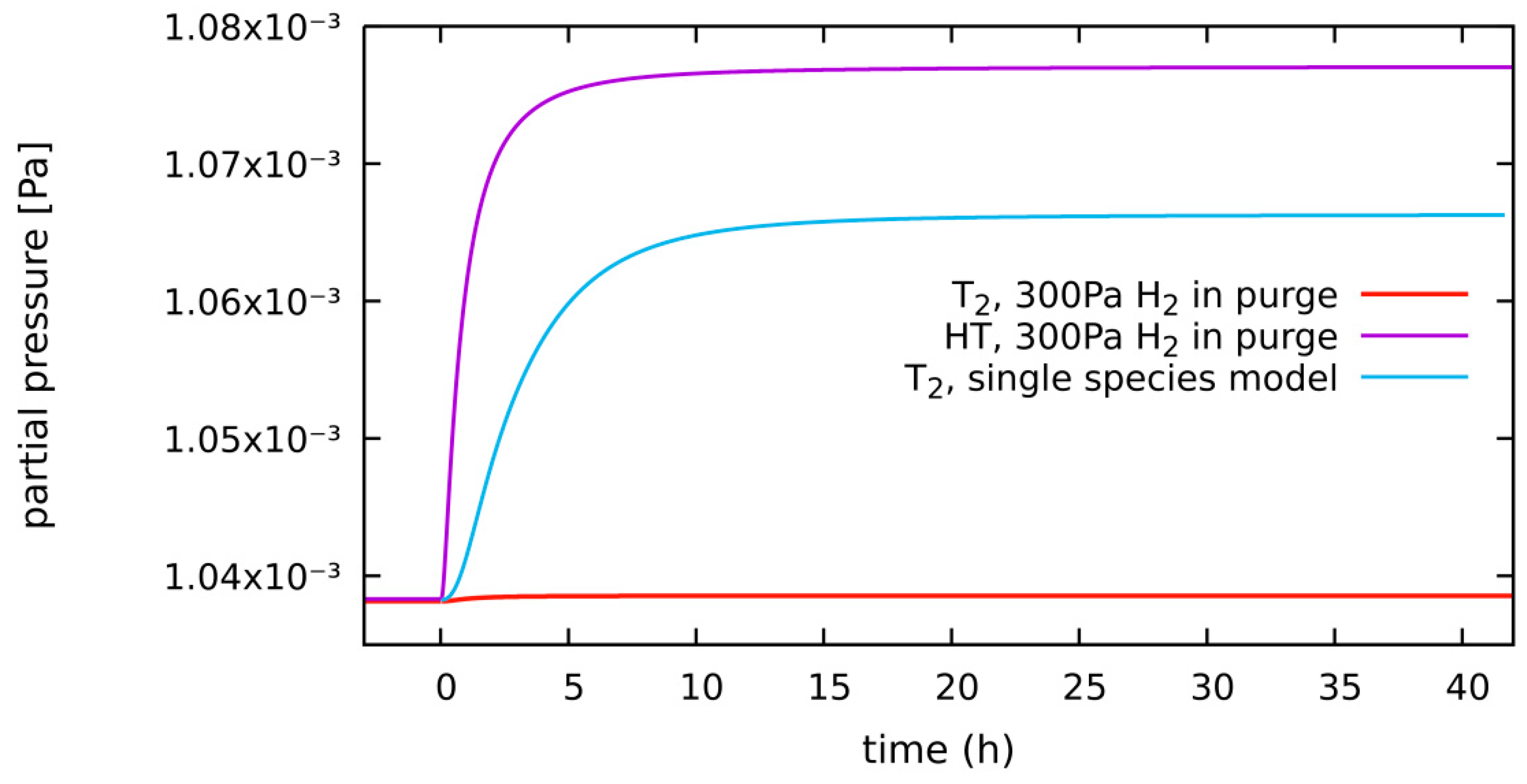
4.1.2. HT System, Diffusion-Limited, 300 Pa H2 in Purge Gas
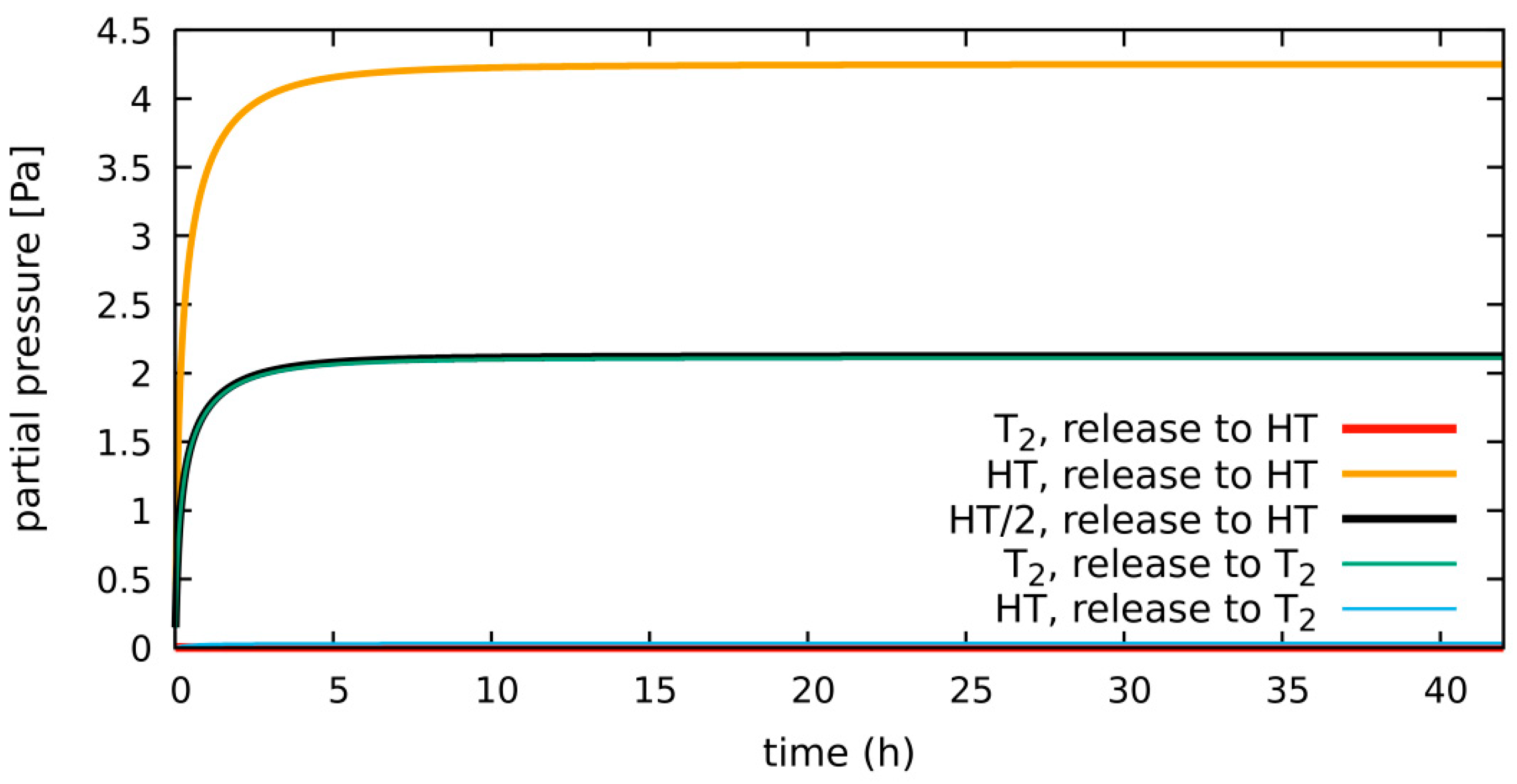
4.1.3. HT System, Diffusion-Limited, 300 Pa H2 in Purge Gas, HT vs. T2 as the Released Species
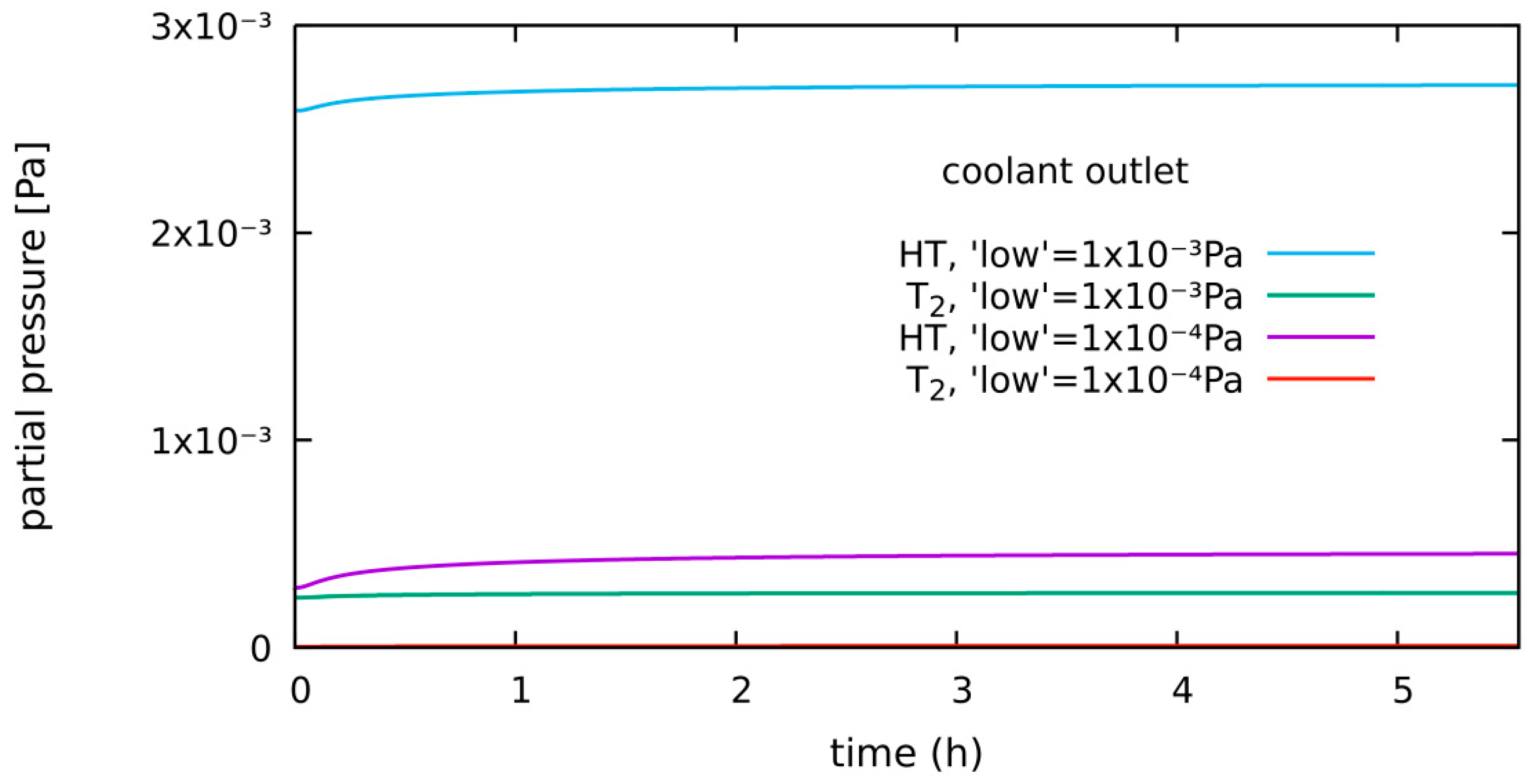
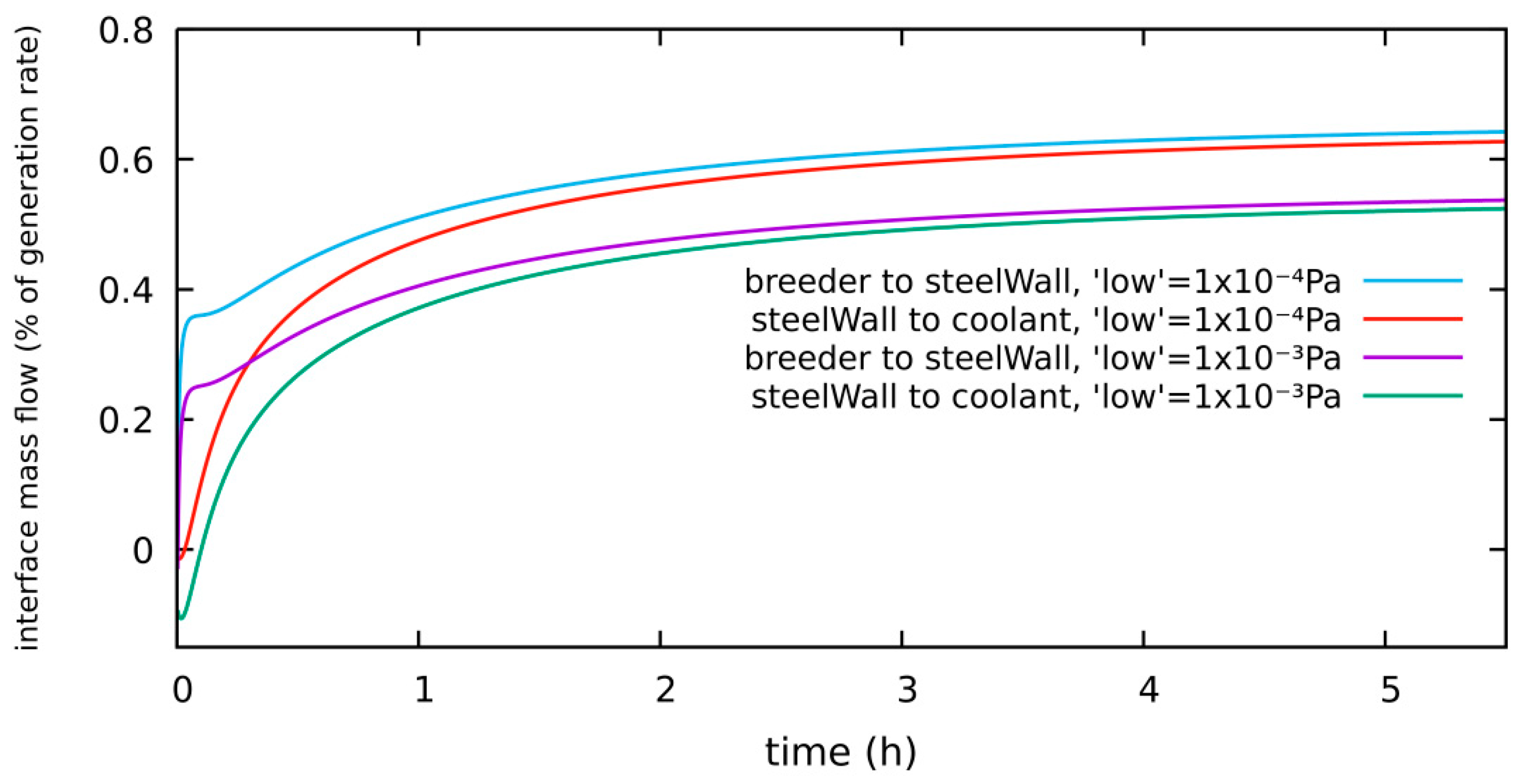
4.1.4. HT System, Diffusion-Limited, 300 Pa H2 in Purge, New “Low” Level: 1 × 10−4 Pa
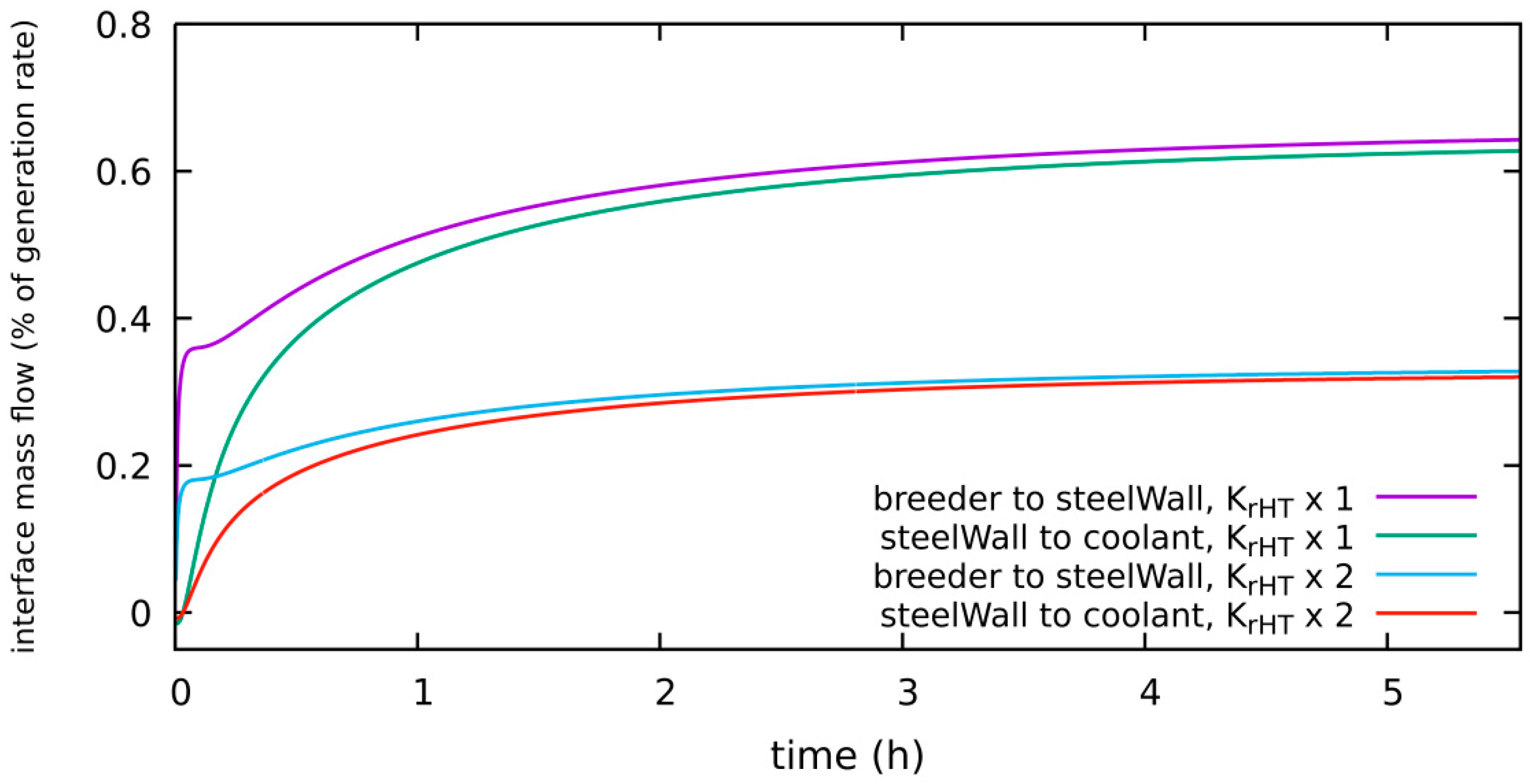
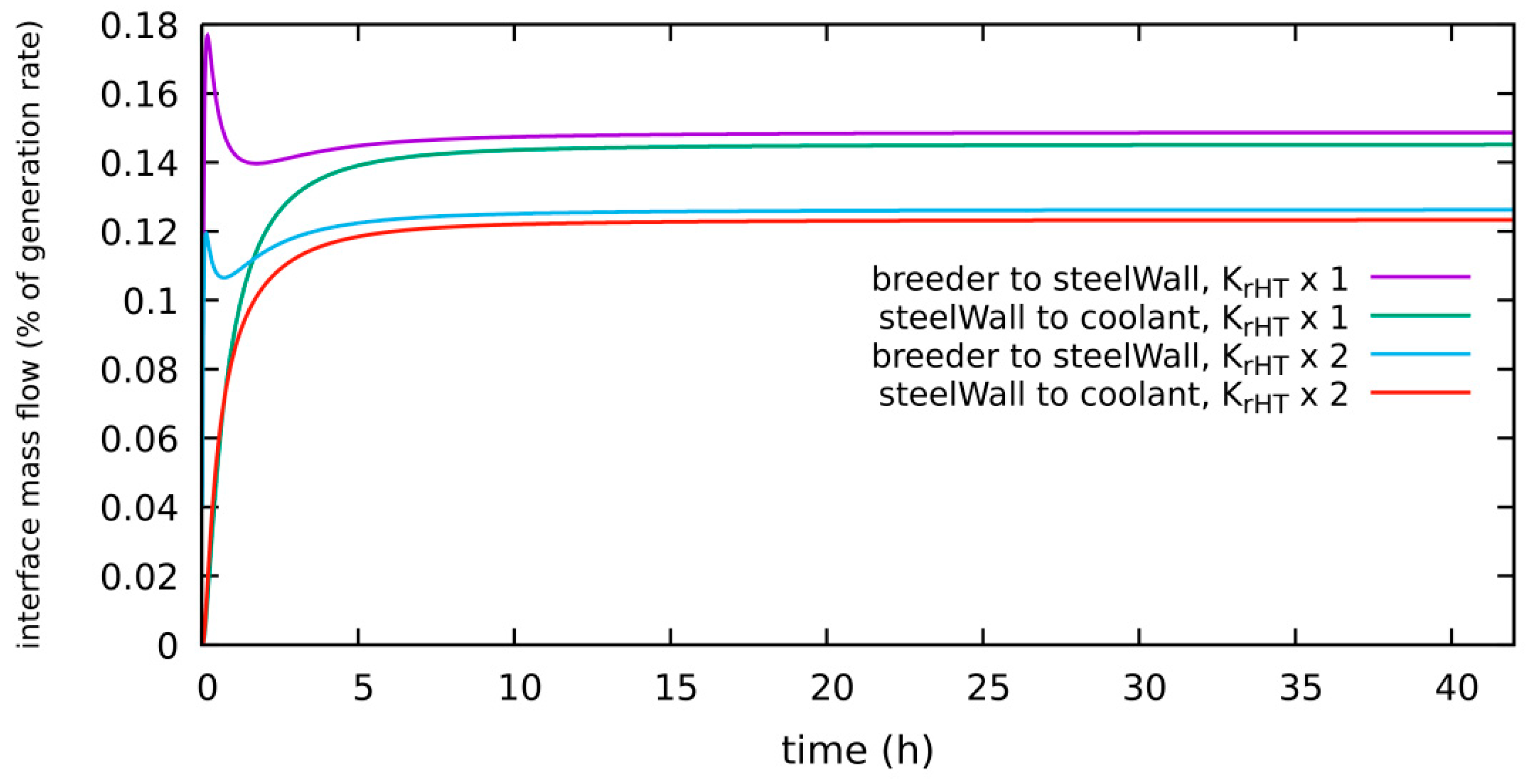
4.1.5. HT System, Diffusion-Limited, 300 Pa H2 in Purge Gas, New “Low” Level: 1 × 10−4 Pa, Kr_HT × 2
4.1.6. Purge Gas 80 Bar with Equal Mass Flow, Diffusion-Limited
4.1.7. Purge Gas 80 Bar with Equal Volumetric Flow, Diffusion-Limited
4.2. Surface-Limited Cases
4.2.1. No/Low H2 System, Surface-Limited, No/Low H2 in Purge Gas, Single- vs. Two- Species Correlation
4.2.2. HT System, Surface-Limited, 300 Pa H2 in Purge Gas
4.2.3. HT System, Surface-Limited, 300 Pa H2 in Purge Gas, HT vs. T2 as the Released Species
4.2.4. HT System, Surface-Limited, 300 Pa H2 in Purge Gas, Kr_HT × 2
4.2.5. Purge Gas 80 Bar with Equal Mass Flow, Surface-Limited
5. Summary
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Zhou, G.; Kang, Q.; Hernández, F.A.; D’Amico, S.; Kiss, B. Thermal hydraulics activities for consolidating HCPB breeding blanket of the European DEMO. Nucl. Fusion 2020, 60, 096008. [Google Scholar] [CrossRef]
- Hernández, F.A.; Pereslavtsev, P.; Zhou, G.; Kang, Q.; D’Amico, S.; Neuberger, H.; Boccaccini, L.V.; Kiss, B.; Nádasi, G.; Maqueda, L.; et al. Consolidated design of the HCPB Breeding Blanket for the pre-Conceptual Design Phase of the EU DEMO and harmonization with the ITER HCPB TBM program. Fusion Eng. Des. 2020, 157, 111614. [Google Scholar] [CrossRef]
- Alberghi, C.; Candido, L.; Testoni, R.; Utili, M.; Zucchetti, M. Magneto-convective effect on tritium transport at breeder unit level for the WCLL breeding blanket of DEMO. Fusion Eng. Des. 2020, 160, 111996. [Google Scholar] [CrossRef]
- Candido, L.; Testoni, R.; Utili, M.; Zucchetti, M. Tritium transport model at breeder unit level for WCLL breeding blanket. Fusion Eng. Des. 2019, 146, 1207–1210. [Google Scholar] [CrossRef]
- Zhao, X.; Ni, M.; Nie, B.; Zhang, B.; Chen, L.; Huang, K.; Liu, S. 3D tritium transport analysis for WCCB blanket based on COMSOL. Fusion Eng. Des. 2020, 151, 111405. [Google Scholar] [CrossRef]
- Ying, A.; Zhang, H.; Merrill, B.; Ahn, M.-Y.; Cho, S. Breeding blanket system design implications on tritium transport and permeation with high tritium ion implantation: A MATLAB/Simulink, COMSOL integrated dynamic tritium transport model for HCCR TBS. Fusion Eng. Des. 2018, 136, 1153–1160. [Google Scholar] [CrossRef]
- Carella, E.; Moreno, C.; Urgorri, F.R.; Rapisarda, D.; Ibarra, A. Tritium modelling in HCPB breeder blanket at a system level. Fusion Eng. Des. 2017, 124, 687–691. [Google Scholar] [CrossRef]
- Pan, L.; Chen, H.; Zeng, Q. Tritium transport analysis of HCPB blanket for CFETR. Fusion Eng. Des. 2016, 113, 82–86. [Google Scholar] [CrossRef]
- Franza, F.; Boccaccini, L.V.; Demange, D.; Ciampichetti, A.; Zucchetti, M. Tritium Transport Issues for Helium-Cooled Breeding Blankets. IEEE Trans. Plasma Sci. 2014, 42, 1951–1957. [Google Scholar] [CrossRef]
- Pasler, V.; Arbeiter, F.; Klein, C.; Klimenko, D.; Schlindwein, G.; von der Weth, A. Development and verification of a component-level hydrogen transport model for a DEMO-like HCPB breeder unit with OpenFOAM. Fusion Eng. Des. 2018, 127, 249–258. [Google Scholar] [CrossRef]
- Ambrosek, J.; Longhurst, G.R. Verification and Validation of TMAP 7; INEEL/EXT-04-01657; Idaho National Laboratory: Idaho Falls, ID, USA, 2004. [Google Scholar]
- Kwast, H.; Conrad, R.; May, R.; Casadio, S.; Roux, N.; Werle, H. The behaviour of ceramic breeder materials with respect to tritium release and pellet/pebble mechanical integrity. J. Nucl. Mater. 1994, 212–215, 1010–1014. [Google Scholar] [CrossRef]
- Pereslavtsev, P.; Hernández, F.A.; Zhou, G.; Lu, L.; Wegmann, C.; Fischer, U. Nuclear analyses of solid breeder blanket options for DEMO: Status, challenges and outlook. Fusion Eng. Des. 2019, 146, 563–567. [Google Scholar] [CrossRef]
- Franza, F.; Boccaccini, L.; Ciampichetti, A.; Zucchetti, M. Tritium transport analysis in HCPB DEMO blanket with the FUS-TPC code. Fusion Eng. Des. 2013, 88, 2444–2447. [Google Scholar] [CrossRef]
- Esteban, G.; Peña, A.; Urra, I.; Legarda, F.; Riccardi, B. Hydrogen transport and trapping in EUROFER’97. J. Nucl. Mater. 2007, 367–370, 473–477. [Google Scholar] [CrossRef]
- Esteban, G.; Perujo, A.; Sedano, L.; Mancinelli, B. The surface rate constants of deuterium in the reduced activating martensitic steel OPTIFER-IVb. J. Nucl. Mater. 2000, 282, 89–96. [Google Scholar] [CrossRef]
- Tosti, S. “Membranes and Membrane Reactors for Tritium Separation”, namely pages 209 ff. In Tritium in Fusion; Tosti, S., Ghirelli, N., Eds.; Nova Science Publishers Inc.: Hauppauge, NY, USA, 2013; ISBN 978-1-62417-270-0. [Google Scholar]
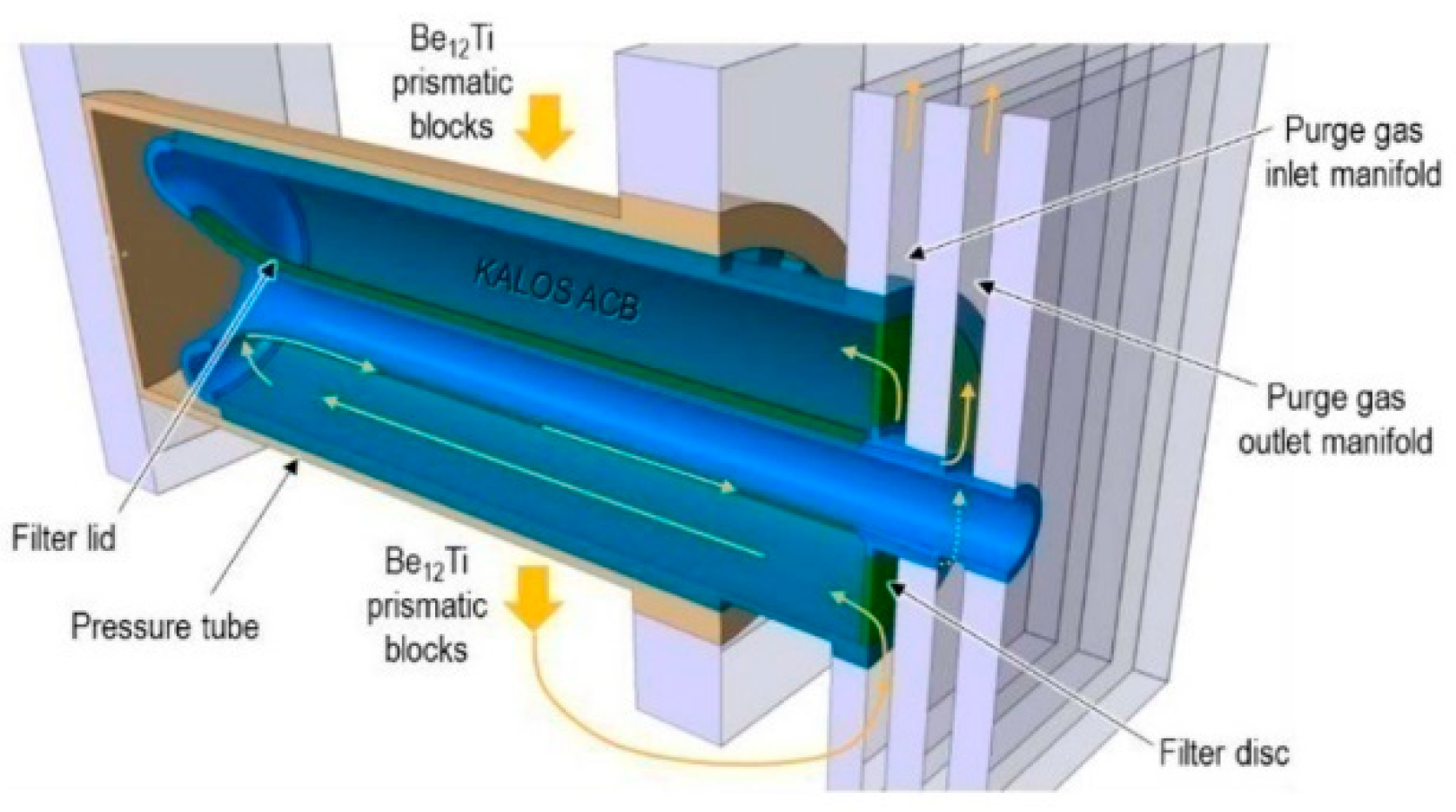
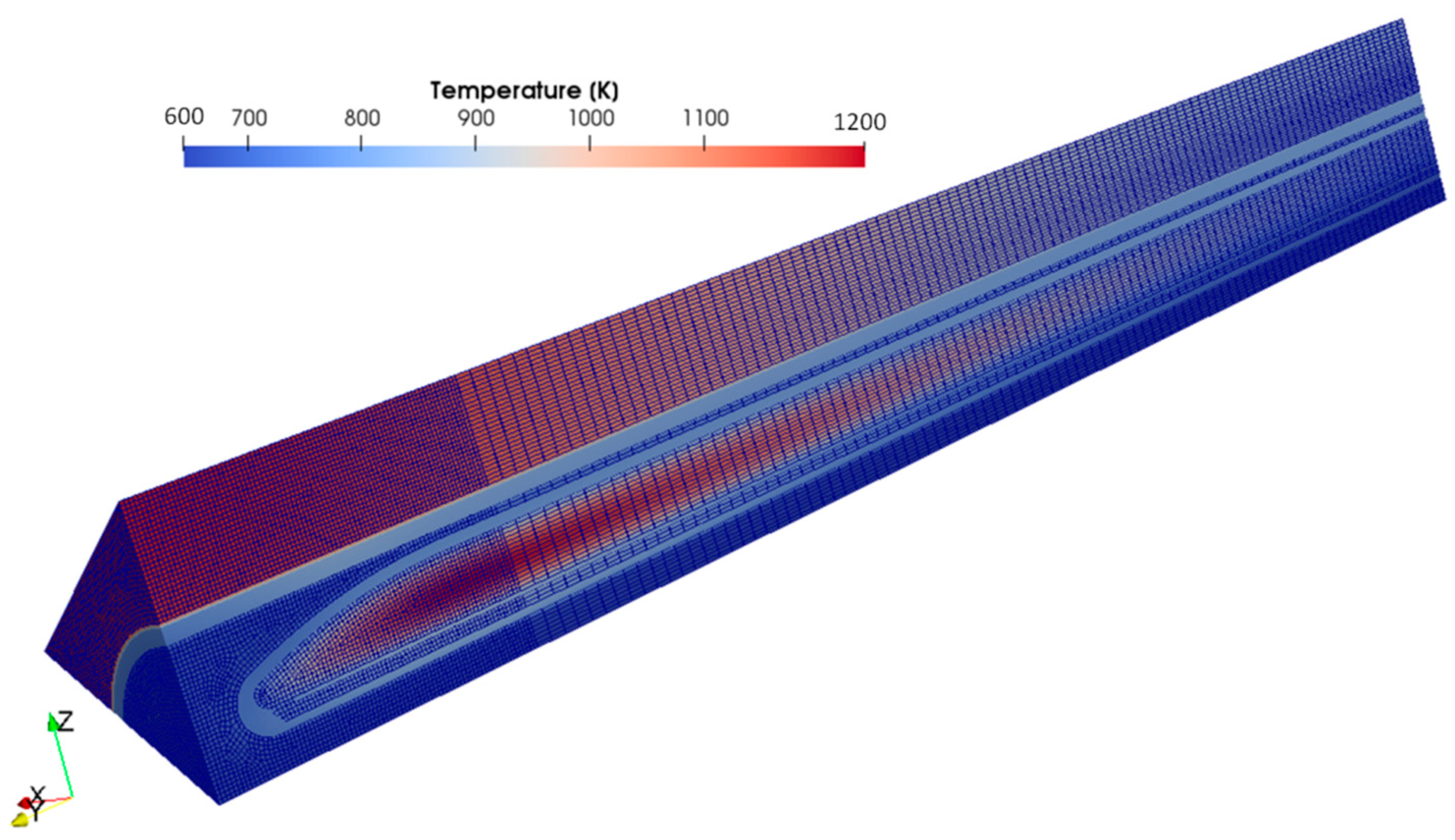

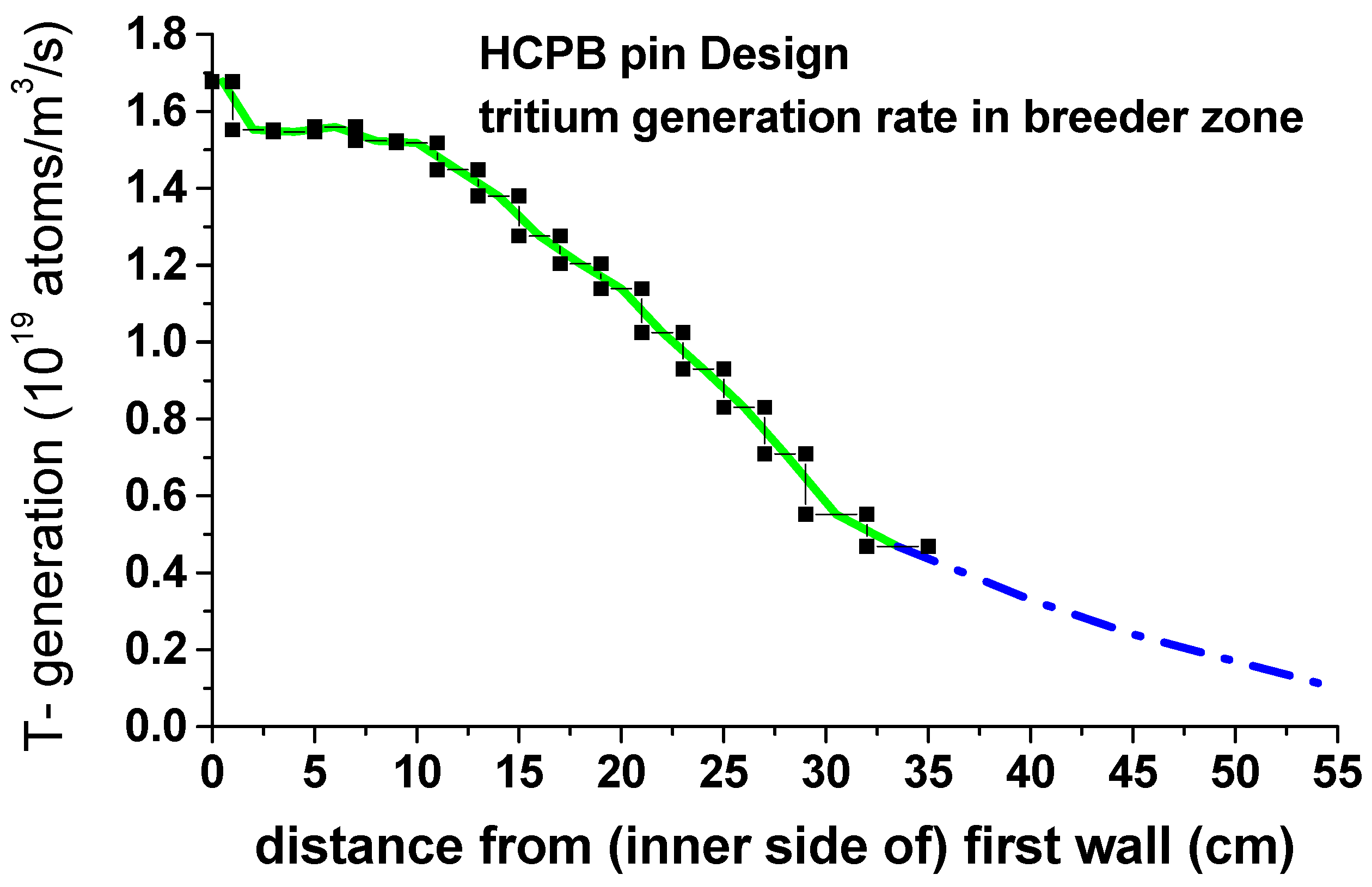
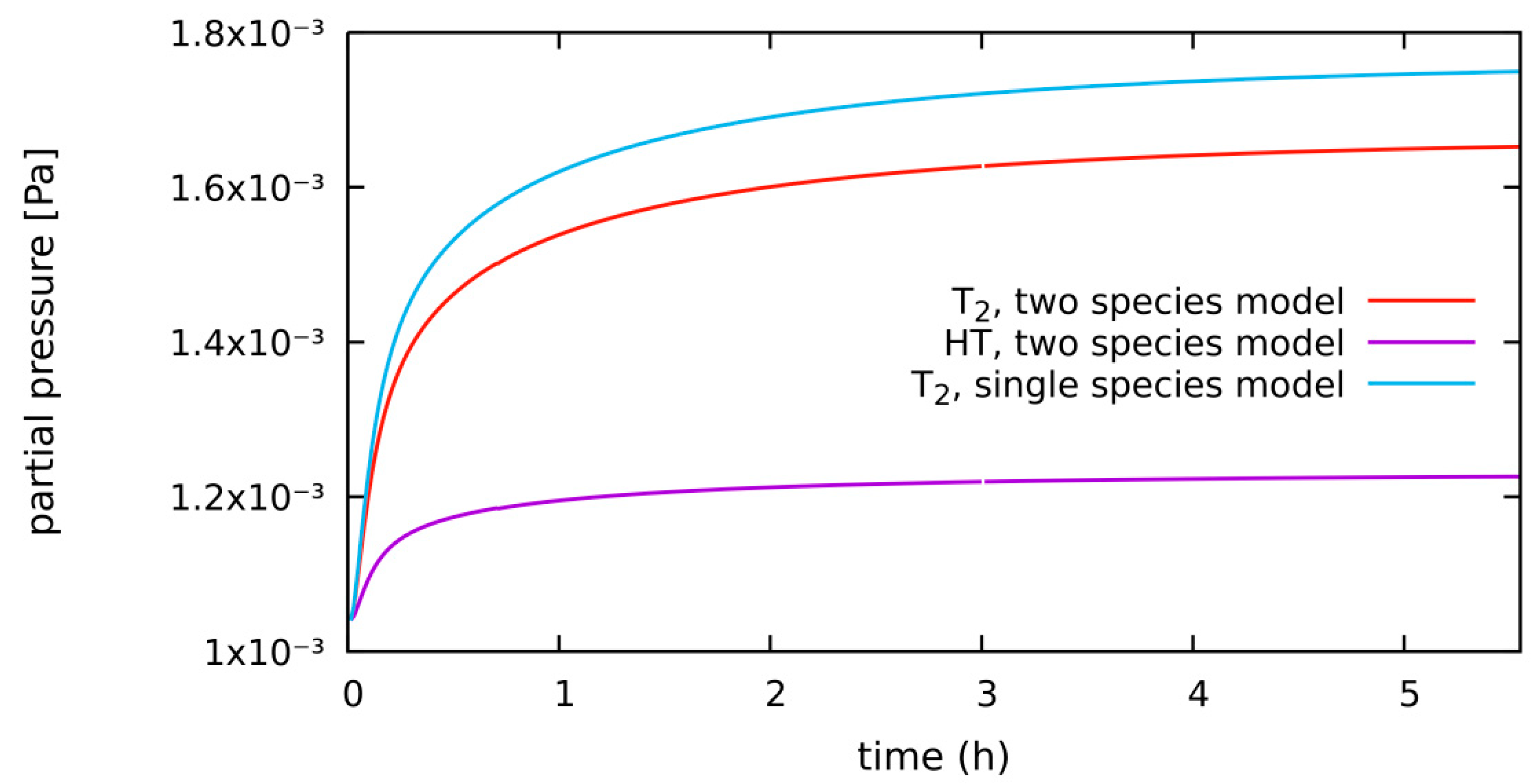










| Hydrogen Species | H, T, H2, T2, HT |
|---|---|
| operating temperature | imported CFX temperature field [1] |
| pebble bed porosity (void fraction) | 0.36 |
| coolant | 80 bar He, inlet speed 20 m/s, pin coolant mass flow = 2.405 × 10−2 kg/s |
| purge gas | 2 bar He, inlet speed 1 cm/s pin purge gas mass flow = 2.75 × 10−6 kg/s isotropic Darcy parameter for porous flow in flow equations = 7 × 10 6 m−2 |
| H2, HT, T2 purge + coolant inlet BC and initial conditions | 1 × 10−3 Pa (sometimes named “no/low” H2 content in text) |
| H+T initial concentration in steel/EUROFER before prerun | 4.4 × 10 19 atoms/m3 (Sieverts equilibrium for around 800K vs. 1 × 10−3 Pa T2 for EUROFER) |
| tritium generation rate | 1D-MCNP simulation (figure 3.4) [13] |
| tritium residence time [14] | (note that this is not yet ACB but still Li4OSi4 pebbles data!) |
| H+T Sieverts constant [15] | |
| H+T rate constants [16] in surface- limited cases | |
| H+T rate constants in diffusion- limited cases | Surface-limited rate constants × 105 with same Ks |
| tritium diffusion coefficient [15] |
| Permeation Regime | Purge Gas | Permeation to Coolant | Pin Wall T Inventory | Case/Section | Comment |
|---|---|---|---|---|---|
| diffusion-limited | 1 × 10−3 Pa H2 2 bar He | 5% of g.r. | 1.1 g | Section 4.1.1 | single- vs. two-species model verification |
| diffusion-limited | 300 Pa H2 2 bar He | 0.55% of g.r. | 0.17 g | Section 4.1.2 (Section 4.1.3) | Section 4.1.3: T2 vs. HT release |
| diffusion-limited | 300 Pa H2 2 bar He | 0.64% of g.r. | 0.15 g | Section 4.1.4 | low level = 1 × 10−4 Pa |
| diffusion-limited | 300 Pa H2 2 bar He | 0.32% of g.r. | 0.075 g | Section 4.1.5 | Kr_HT × 2, low level = 1 × 10−4 Pa |
| diffusion-limited | 1 × 10−3 Pa H2 80 bar He | 30% of g.r. | 6.6 g | Section 4.1.6 | equal mass flow like 2 bar Section 4.1.1 |
| diffusion-limited | 1 × 10−3 Pa H2 80 bar He | 4.85% of g.r. | 1.1 g | Section 4.1.7 | equal volumetric flow like 2 bar Section 4.1.1 |
| surface-limited | 1 × 10−3 Pa H2 2 bar He | 0.2% of g.r. | 0.15 g | Section 4.2.1 | single- vs. two-species model verification |
| surface-limited | 300 Pa H2 2 bar He | 0.15% of g.r. | 0.19 g | Section 4.2.2 (Section 4.2.3) | Section 4.2.3: T2 vs. HT release |
| surface-limited | 300 Pa H2 2 bar He | 0.12% of g.r. | 0.095 g | Section 4.2.4 | Kr_HT × 2 |
| surface-limited | 1 × 10−3 Pa H2 80 bar He | 6% of g.r. | 9.5 g | Section 4.2.5 | equal mass flow like 2 bar Section 4.2.1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pasler, V.; Arbeiter, F.; Klein, C.; Klimenko, D.; Schlindwein, G.; von der Weth, A. Development of a Component-Level Hydrogen Transport Model with OpenFOAM and Application to Tritium Transport Inside a DEMO HCPB Breeder. Appl. Sci. 2021, 11, 3481. https://doi.org/10.3390/app11083481
Pasler V, Arbeiter F, Klein C, Klimenko D, Schlindwein G, von der Weth A. Development of a Component-Level Hydrogen Transport Model with OpenFOAM and Application to Tritium Transport Inside a DEMO HCPB Breeder. Applied Sciences. 2021; 11(8):3481. https://doi.org/10.3390/app11083481
Chicago/Turabian StylePasler, Volker, Frederik Arbeiter, Christine Klein, Dmitry Klimenko, Georg Schlindwein, and Axel von der Weth. 2021. "Development of a Component-Level Hydrogen Transport Model with OpenFOAM and Application to Tritium Transport Inside a DEMO HCPB Breeder" Applied Sciences 11, no. 8: 3481. https://doi.org/10.3390/app11083481
APA StylePasler, V., Arbeiter, F., Klein, C., Klimenko, D., Schlindwein, G., & von der Weth, A. (2021). Development of a Component-Level Hydrogen Transport Model with OpenFOAM and Application to Tritium Transport Inside a DEMO HCPB Breeder. Applied Sciences, 11(8), 3481. https://doi.org/10.3390/app11083481





