Abstract
Daegunjoong-tang (DGJT) is an oriental medicine consisting of four medicinal herbs (Zingiber officinale Rosc., Panax ginseng C.A.Mey., Oryza sativa L., and Zanthoxylum schinifolium Sieb. et Zucc.) that is used to treat intestinal- and cancer-related diseases. In this study, a protocol for quality control of DGJT based on reverse-phase high-performance liquid chromatography (HPLC) and liquid chromatography tandem mass spectrometry (LC–MS/MS) analysis were developed. In HPLC analysis, the marker analytes (hyperoside, quercitrin, ginsenoside Rg1, and 6-gingerol) were separated, verified, and quantified using a mobile phase of 0.1% (v/v) aqueous formic acid–0.1% (v/v) formic acid in acetonitrile system, and a C18 reverse-phase column (4.6 mm × 250 mm, particle size; 5 m) maintained at 40 °C. In LC–MS/MS analysis, all analytes were separated using a Waters Acquity UPLC BEH C18 column (2.1 mm × 100 mm, particle size; 1.7 μm). Using the developed HPLC and LC–MS/MS methods, the four marker analytes were found in the samples at 0.95–13.86 mg/g (HPLC) and 0.27–2.42 mg/g (LC–MS/MS). The assay will be useful for evaluating the quality of DGJT.
1. Introduction
Daegunjoong-tang (DGJT; Daikenchuto in Japanese; Da-Jian-Zhong-Tang in Chinese) is a herbal medicine prescription first recorded in Gumgoeyorak (金匱要略, Jin Gui Yao Lue in Chinese), a famous traditional oriental medicine book written by Zhongjing Zhang in the Han Dynasty, and consists of four herbal medicines, Zingiber officinale Rosc., Panax ginseng C.A.Mey., Oryza sativa L., and Zanthoxylum schinifolium Sieb. et Zucc. [1]. According to Gumgoeyorak, this prescription was used for patients with medical conditions and symptoms such as abdominal cramps, abdominal pain, fatigue, poor appetite, chills, and pale complexion [1]. Z. officinale promotes antispasmodic action and secretion of digestive juices, and P. ginseng has antibacterial, anticancer, and antiulcer effects [2]. O. sativa replenishes sugar to smooth the energy metabolism of cells, and Z. schinifolium promotes anthelmintic action, digestion, and absorption, and has antifungal, antibacterial, and antipain activities [2]. Each herbal medicine contains a range of compound types including phenolic compounds, gingerols, and shogaols in Z. officinale [3]; triterpenoid saponins and ginsenosides in P. ginseng [4]; polysaccharides and maltose in O. sativa [5]; and essential oils oleic acid, palmitic acid, and estragole, flavonoid glycosides hyperoside and quercitrin, and the alkaloids schinifolin in Z. schinifolium [6,7,8].
DGJT is one of the most prescribed Kampo medicines in Japan, and various biological activities have been reported; these include anticancer action [9,10], improved intestinal blood flow [11,12], and beneficial effects against diseases of the digestive system such as gastric ulcer, enteritis, bowel motility, and ileus [13,14,15,16]. Based on these biological activities, several clinical studies have also been conducted that examined diseases related to postoperative paralytic ileus [17,18], postoperative constipation [19,20], and postoperative intestinal obstruction [21,22]. Various nonclinical/clinical efficacy studies have been reported, but the current analysis methods for quality assessment of DGJT have not been validated. Therefore, it is necessary to establish an analysis method for the quality control of DGJT.
In this study, a simultaneous reverse-phase high-performance liquid chromatography (RP–HPLC) and liquid chromatography tandem mass spectrometry (LC–MS/MS) multiple reaction monitoring (MRM) methods were developed for the quality control of DGJT using four marker components: hyperoside (from Z. schinifolium), quercitrin (from Z. schinifolium), ginsenoside Rg1 (from P. ginseng), and 6-gingerol (from Z. officinale).
2. Materials and Methods
2.1. Plant Materials
The three raw materials, Z. schinifolium, P. ginseng, and Z. officinale, (Table S1) were purchased from Kwangmyungdag Medicinal Herbs (Ulsan, Korea) and used after morphological verification by Dr. Goya Choi, Herbal Medicine Resources Research Center, Korea Institute of Oriental Medicine (KIOM, Naju, Korea) according to the National Institute of Food and Drug Safety Evaluation guidelines [23]. Each raw material (2018–KE65–1 to 2018–KE65–3) have been deposited at the KIOM.
2.2. Chemicals and Reagents
Four marker analytes (Figure S1) were supplied from manufacturers specializing in natural product standards: 6-gingerol (Cat. No. 076-05901, 98.3%) from Fujifilm Wako Pure Chemical Co. (Osaka, Japan), ginsenoside Rg1 (Cat. No. BP0664, 99.3%) and hyperoside (Cat. No. BP0753, 98.7%) from Chengdu Biopurify Phytochemicals Ltd. (Chengdu, China), and quercitrin (Cat. No. CFN98850, 98.4%) from Wuhan ChemFaces Biochemical Co., Ltd. (Wuhan, China).
Solvents methanol (CAS No. 67-56-1, >99.9%), acetonitrile (CAS No. 75-05-8), and distilled water (CAS No. 7732-18-5) were purchased from J.T.Baker (for HPLC, Phillipsburg, NJ, USA) and ThermoFisher Scientific (for LC–MS, Korea, Seoul). Formic acid (CAS No. 64-18-6, 98–100%) and ammonium formate (CAS No. 540-69-2, 99.0%) were supplied from Merck KGaA (Darmstadt, Germany) and Kanto Chemicals (Tokyo, Japan) as the ACS reagent, respectively.
2.3. DGJT Sample Extraction
To prepare DGJT samples for HPLC analysis, as shown in Table S1, Z. schinifolium (1.25 kg), P. ginseng (1.25 kg), and Z. officinale (2.5 kg) were mixed (total 5.00 kg) in a weight ratio (w/w), 50 L of distilled water was added, and the mixture was extracted under pressure at 100 °C for 2 h. Thereafter, the extract was filtered through a testing sieve (pore size 53 μm, Cheunggye Co., Ltd., Gunpo, Korea) and freeze-dried with an LP110R freeze-dryer (IlShinBioBase, Dongducheon, Korea) to obtain 606.7 g (yield 12.1%) of a powder sample. The prepared DGJT sample was stored at −20 °C until analysis and further study.
2.4. HPLC Quantification of the Four Marker Analytes
Quantification of hyperoside, quercitrin, ginsenoside Rg1, and 6-gingerol in DGJT samples was performed based on previous protocols [24,25]. Briefly, HPLC analysis was conducted with a Prominence LC-20A series equipped with a photodiode array (PDA) detector (Kyoto, Japan), that were controlled with LabSolution software (Ver. 5.53, SP3, Kyoto, Japan); detailed operating conditions are shown in Table S2.
A test solution for quantitative analysis of the four markers in DGJT was obtained by ultrasonic extraction for 60 min of a sample at a concentration of 10 mg/mL in 70% methanol. Standard solutions of each analyte were prepared at a concentration of 1 mg/mL using methanol, and then stored in a refrigerator until use. All solutions were analyzed after filtering through a 0.2 μm polypropylene membrane syringe filter (Pall Life Sciences, Ann Arbor, MI, USA).
2.5. System Suitability
System suitability parameters such as retention factor (k′), relative retention (α), resolution (Rs), number of theoretical plates (N), and tailing factor (Tf) were evaluated and used to assess the analysis method for quality control of DGJT using HPLC.
2.6. Method Validation of Developed Assay
For verification of the developed analysis method, the linearity, limit of detection (LOD), limit of quantification (LOQ), accuracy, and precision were evaluated according to the International Conference on Harmonization guidelines [26] as in previous studies [24,25].
2.7. LC–MS/MS System and Conditions for Quantification of the 4 Markers
Quantification of the 4 markers in the DGJT sample was performed using an Acquity UPLC H-Class system (Waters, Milford, MA, USA) with a TQ-XS system controlled by MassLynx software (version 4.1, Waters). A Waters Acquity UPLC BEH C18 column (2.1 mm × 100 mm, 1.7 μm) was used to separate the marker compounds with a distilled water containing 0.1% (v/v) formic acid and 5 mM ammonium formate (solvent A)–acetonitrile (solvent B) system. The mobile phase was eluted at 0.3 mL/min. The gradient conditions were the following: 20% solvent B at initial to 0.1 min, 20–95% solvent B at 0.1 to 14.0 min, 95–100% solvent B at 14.0 to 15.0 min, 100–20% solvent B at 15.0 to 15.1 min, and 20% solvent B at 15.1–18.0 min. The temperature of the column and sample tray were maintained at 45 and 5 °C, respectively. Quantification was conducted using the MRM mode. Capillary voltage, source temperature, desolvation temperature, desolvation gas flow, and cone gas flow were set at 3.0 kV, 150 °C, 500 °C, 700 L/h, and 50 L/h, respectively. Other parameters such as collision energy, cone voltage, and transition for LC–MS/MS MRM analysis of each marker are shown in Table S3.
3. Results and Discussion
3.1. Identification of the Main Component of Each Herbal Medicine
As described in the previous study [27], O. sativa, which contains mainly saccharride (mostly maltose), was excluded. In order to select marker analytes for quality control of DGJT using constituents of three herbal medicines other than O. sativa, the main components of each medicine were investigated, compared, and analyzed as follows: 6-gingerol from Z. officinale [3,28]; ginsenoside Rb1 and ginsenoside Rg1 from P. ginseng [4,28]; and xeroboside, rutin, hyperoside, ferulic acid, foeniculin, quercitrin, isorhamnetin-7-glucoside, 3,4,5-trihydroxy-7,4′-dimethoxyflavone, dictamnin, and schinifolin from Z. schinifolium [6,7,8,29] (Figure S2). All the components were analyzed using HPLC–PDA (PDA simultaneously scanned from 190 to 400 nm) using a mobile phase of distilled water–acetonitrile, with both phases containing 0.1% (v/v) formic acid.
3.2. Selection of Marker Analytes for Quality Standardization
HPLC chromatograms for the 13 main components listed in Section 3.1 are shown in Figure S3. From these, four components (hyperoside, quercitrin, ginsenoside Rg1, and 6-gingerol) were clearly identified in the DGJT 70% methanol extract, and these were selected as marker analytes for quality control of DGJT samples (Figure S1) and for subsequent studies.
3.3. Establishment of Optimal Analysis Conditions
The optimal HPLC analysis conditions were determined by comparing a selection of reverse-phase C18 columns (I. D. 4.6 mm × length 250 mm, particle size 5 μm) such as Phenomenex, Waters, Shiseido, and YoungJin Biochrom Co., and column temperatures (30, 35, 40, and 45 °C) using the four selected marker analytes. The optimal conditions were established to be the use of a Waters’ SunFire C18 column (Milford, MI, USA), maintained at 40 °C, and gradient elution using distilled water and acetonitrile, both containing 0.1% (v/v) formic acid (Table S2). The four markers, hyperoside, quercitrin, ginsenoside Rg1, and 6-gingerol, were eluted at 14.15, 16.04, 17.87, and 31.24 min, respectively. A representative HPLC chromatogram of the standard and test solutions is shown in Figure 1.

Figure 1.
Representative HPLC chromatograms of (A) a standard solution and (B) 70% methanol extract of Daegunjoong-tang (DGJT) samples, at 203 and 255 nm. Hyperoside (1), quercitrin (2), ginsenoside Rg1 (3), and 6-gingerol (4).
3.4. System Suitability and Method Validation of the Developed HPLC Analytical Method
The stability of the HPLC system for the qualitative and quantitative analysis of marker analytes was assessed by determining the k′, α, N, Rs, and Tf parameters, which were found to be 3.94–9.91, 1.14–1.89, 551122–1122666, 6.94–40.42, and 1.07–1.13, respectively, as shown in Table S4. These values are suitable for both qualitative and quantitative analysis of the marker analytes. In the developed assay, as shown in Table 1, the coefficient of determination (r2) value representing the linearity for quantification of the marker analytes prepared at different concentration levels was 0.9999–1.0000, showing excellent linearity; LOD and LOQ were also calculated as 0.08–0.46 μg/mL and 0.24–1.41 μg/mL, respectively. Recovery tests, which were performed by adding three different concentrations (low, medium, and high) to the DGJT sample for accuracy evaluation, confirmed that all four marker analytes showed a good recovery of 97.01–100.88% (Table 2). The RSD (%) values for the retention time and peak area were measured to be less than 1.0% in repeatability tests performed six times with a standard solution of analytes (Table S5), and the RSD (%) value indicating intraday (for one day) and interday (on three consecutive days) precisions also showed excellent precision values of less than 1.0% (Table 3). The above results show that the HPLC analysis method developed in this study is suitable for both qualitative and quantitative analysis of the markers hyperoside, quercitrin, ginsenoside Rg1, and 6-gingerol in DGJT.

Table 1.
Linear range, regression equation, coefficient of determination (r2), limit of detection (LOD), and limit of quantification (LOQ) of the four marker analytes for the quantification using HPLC (n = 3).

Table 2.
Verification of the extraction recovery (%) of the analysis method developed using the four marker analytes (n = 5).

Table 3.
Verification of the precision of the analysis method developed using the four marker analytes.
3.5. Quantification of the Four Marker Analytes for Quality Control of DGJT Sample Using HPLC
Using the developed and validated HPLC analysis method, the four marker analytes in the prepared DGJT samples were compared to standard compounds with respect to their UV spectra and retention times and finally confirmed via co-injection. Based on this, hyperoside and quercitrin were quantified at 255 nm, and ginsenoside Rg1 and 6-gingerol were quantified at 203 nm. The content of the four marker analytes in the prepared DGJT sample was found to be 0.95–13.86 mg/g (Table 4).

Table 4.
Content of the four marker analytes in DGJT sample determined using HPLC (n = 3).
3.6. Quantification of the Four Marker Compounds by LC–MS/MS
In Table S3, hyperoside, quercitrin, and ginsenoside Rg1 were detected in the negative ion mode ([M−H]–) at m/z 463.2, 447.0, and 799.2, respectively, while 6-gingerol was detected in the positive ion mode ([M+H]+) at m/z 295.3. The precursor ion (Q1) and product ion (Q3) for the LC–MS/MS MRM analysis were set as shown in Table S3 [30,31,32,33]. Various parameters such as linear range, regression equation, r2, LOD, and LOQ for simultaneous determination using the LC–MS/MS MRM method are presented in Table S6. For the quality evaluation of DGJT, quantification of the 4 marker components was performed using the LC–MS/MS MRM method. These markers, hyperoside, quercitrin, ginsenoside Rg1, and 6-gingerol, were detected at 1.63, 2.03, 2.71, and 6.33 min, respectively (Figure 2, Figure S4 and Table S6). The concentrations of 4 marker compounds, hyperoside, quercitrin, ginsenoside Rg1, and 6-gingerol, were 1.07, 0.27, 2.42, and 0.86 mg/g, respectively.
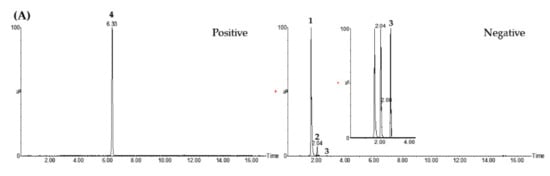

Figure 2.
Total ion chromatograms of the standard solution (A) and DGJT sample (B) by LC–MS/MS MRM method. Hyperoside (1), quercitrin (2), ginsenoside Rg1 (3), and 6-gingerol (4).
4. Conclusions
In this study, a simultaneous RP–HPLC and LC–MS/MS MRM analysis methods for efficient quality control of DGJT, which is effective in postoperative intestinal obstruction and postoperative constipation, was developed and verified through linearity, LOD, LOQ, accuracy, and precision. These results provide a framework for quality control monitoring of DGJT as well as other traditional Korean medicines, traditional Chinese medicines, and Kampo medicines.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/app11083437/s1, Figure S1: Chemical structures of the four marker analytes used in the quantitative analysis of DGJT; Figure S2: HPLC chromatogram of constituent herbal medicine and its main components. A: Z. officinale, B: P. ginseng, and C: Z. schinifolium; Figure S3: HPLC chromatograms of the standard solution (A) and 70% methanol extract of DGJT sample (B). Xeroboside (1), rutin (2), hyperoside (3), ferulic acid (4), foeniculin (5), quercitrin (6), isorhametin-7-glucoside (7), ginsenoside Rg1 (8), ginsenoside Rb1 (9), 3,4,5-trihydroxy-7,4’-dimethoxyflavone (10), dictamnin (11), schinifolin (12), 6-gingerol (13); Figure S4: Extracted ion chromatograms of the reference standard (A) and DGJT sample (B) by LC–MS/MS MRM mode. Table S1: Information on the composition of DGJT; Table S2: HPLC parameters for quantification of DGJT; Table S3: MRM parameters for LC–MS/MS analysis of 4 marker compounds in DGJT. Table S4: System suitability for the four marker analytes in the developed HPLC method; Table S5: Repeatability for retention time and peak areas of the four marker analytes using HPLC (n = 6). Table S6: The linear range, regression equation, r2, LODs, and LOQs of the analytes from YPS using LC–MS/MS (n = 3).
Author Contributions
Conceptualization, C.-S.S. and H.-K.S.; performing experiments and analyzing data, and writing—original draft preparation, C.-S.S.; funding acquisition, H.-K.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by a grant from the Korea Institute of Oriental Medicine (grant number KSN2021310).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available in the article (tables and figures).
Conflicts of Interest
The authors have declared no conflict of interest.
References
- Daikenchuto. Available online: https://kampo.ca/herbs-formulas/formulas/daikenchuto/ (accessed on 21 January 2021).
- Daegunjoong-Tang. Available online: http://onrpark.onnuri.co.kr/han/han19.htm (accessed on 22 January 2021).
- Wang, W.; Li, C.Y.; Wen, X.D.; Li, P.; Qi, L.W. Simultaneous determination of 6-gingerol, 8-gingerol, 10-gingerol and 6-shogaol in rat plasma by liquid chromatography-mass spectrometry: Application to pharmacokinetics. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2009, 877, 671–679. [Google Scholar] [CrossRef] [PubMed]
- Shan, S.M.; Luo, J.G.; Huang, F.; Kong, L.Y. Chemical characteristics combined with bioactivity for comprehensive evaluation of Panax ginseng C.A. Meyer in different ages and seasons based on HPLC-DAD and chemometric methods. J. Pharm. Biomed. Anal. 2014, 89, 76–82. [Google Scholar]
- Shinilbooks Committee. The Korean Herbal Pharmacopoeia, 5th ed.; Shinilbooks: Seoul, Korea, 2016; pp. 28–29. [Google Scholar]
- Wang, C.F.; Fan, L.; Tian, M.; Qi, X.S.; Liu, J.X.; Feng, J.B.; Du, S.S.; Su, X.; Wang, Y.Y. Radiosensitizing effect of schinifoline from Zanthoxylum schinifolium Sieb et Zucc on human non-small cell lung cancer A549 cells: A preliminary in vitro investigation. Molecules 2014, 19, 20128–20138. [Google Scholar] [CrossRef] [PubMed]
- Oh, M.; Chung, M.S. Effects of oils and essential oils from seeds of Zanthoxylum schinifolium against foodborne viral surrogates. Evid. Based Complement. Alternat. Med. 2014, 2014, 135797. [Google Scholar] [CrossRef]
- Yamazaki, E.; Inagaki, M.; Kurita, O.; Inoue, T. Antioxidant activity of Japanese pepper (Zanthoxylum piperitum DC.) fruit. Food Chem. 2007, 100, 171–177. [Google Scholar] [CrossRef]
- Hasebe, T.; Matsukawa, J.; Ringus, D.; Miyoshi, J.; Hart, J.; Kaneko, A.; Yamamoto, M.; Kono, T.; Fujiya, M.; Kohgo, Y.; et al. Daikenchuto (TU-100) Suppresses Tumor Development in the Azoxymethane and APCmin/+ Mouse Models of Experimental Colon Cancer. Phytother. Res. 2017, 31, 90–99. [Google Scholar] [CrossRef]
- Nagato, T.; Toume, K.; Long, L.X.; Hirano, K.; Watanabe, T.; Sekine, S.; Okumura, T.; Komatsu, K.; Tsukada, K. Anticancer effect of a Kampo preparation Daikenchuto. J. Nat. Med. 2016, 70, 627–633. [Google Scholar] [CrossRef]
- Kono, T.; Koseki, T.; Chiba, S.; Ebisawa, Y.; Chisato, N.; Iwamoto, J.; Kasai, S. Colonic Vascular Conductance Increased by Daikenchuto via Calcitonin Gene-Related Peptide and Receptor-Activity Modifying Protein 1. J. Surg. Res. 2008, 150, 78–84. [Google Scholar] [CrossRef]
- Kono, T.; Kaneko, A.; Omiya, Y.; Ohbuchi, K.; Ohno, N.; Yamamoto, M. Epithelial transient receptor potential ankyrin 1 (TRPA1)-dependent adrenomedullin upregulates blood flow in rat small intestine. Am. J. Physiol. Gastrointest. Liver Physiol. 2013, 304, G428–G436. [Google Scholar] [CrossRef]
- Kim, H.K.; Baik, T.H. An experimental study of Daegunchungtang on the gastric ulcer and the function of gastrointestinal tract in rats and mice. J. Korean Med. 1997, 18, 238–250. [Google Scholar]
- Chikakiyo, M.; Shimada, M.; Nakao, T.; Higashijima, J.; Toshikawa, K.; Nishioka, M.; Iwata, T.; Kurita, N. Kampo medicine “Dai-kenchu-to” prevents CPT-11-induced small-intestinal injury in rats. Surg. Today 2012, 42, 60–67. [Google Scholar] [CrossRef]
- Akiho, H.; Nakamura, K. Daikenchuto Ameliorates Muscle Hypercontractility in a Murine T-Cell-Mediated Persistent Gut Motor Dysfunction Model. Digestion 2011, 83, 173–179. [Google Scholar] [CrossRef]
- Tsuchiya, K.; Kubota, K.; Ohbuchi, K.; Kaneko, A.; Ohno, N.; Mase, A.; Matsushima, H.; Yamamoto, M.; Miyano, K.; Uezono, Y.; et al. Transient receptor potential ankyrin 1 agonists improve intestinal transit in a murine model of postoperative ileus. Neurogastroenterol. Motil. 2016, 28, 1792–1805. [Google Scholar] [CrossRef]
- Yaegashi, M.; Otsuka, K.; Itabashi, T.; Kimura, T.; Kato, K.; Fujii, H.; Koeda, K.; Sasaki, A.; Wakabayashi, G. Daikenchuto stimulates colonic motility after laparoscopic-assisted colectomy. Hepatogastroenterology 2014, 61, 85–89. [Google Scholar]
- Okada, K.; Kawai, M.; Hirono, S.; Miyazawa, M.; Shimizu, A.; Kitahata, Y.; Tamaue, H. Perioperative administration of Daikenchuto (TJ-100) reduces the postoperative paralytic ileus in patients with pancreaticoduodenectomy. Hepatogastroenterology 2015, 62, 466–471. [Google Scholar] [CrossRef]
- Arita, R.; Numata, T.; Takayama, S.; Obara, T.; Kikuchi, A.; Ohsawa, M.; Suzuki, A.; Yokota, T.; Kusaba, M.; Yaegashi, N.; et al. Responder Analysis of Daikenchuto Treatment for Constipation in Poststroke Patients: A Subanalysis of a Randomized Control Trial. J. Evid. Based Intergr. Med. 2019, 24, 2515690X19889271. [Google Scholar] [CrossRef]
- Numata, T.; Takayama, S.; Tobita, M.; Ishida, S.; Katayose, D.; Shinkawa, M.; Oikawa, T.; Aonuma, T.; Kaneko, S.; Tanaka, J.; et al. Traditional Japanese Medicine Daikenchuto Improves Functional Constipation in Poststroke Patients. Evid. Based Complement. Altern. Med. 2014, 2014, 231258. [Google Scholar] [CrossRef]
- Oyama, F.; Futagami, M.; Shigeto, T.; Miura, R.; Osawa, Y.; Oishi, M.; Oikiri, H.; Yokoyama, M.; Takabayashi, A.; Yokoyama, Y. Preventive effect of daikenchuto, a traditional Japanese herbal medicine, on onset of ileus after gynecological surgery for malignant tumors. Asia Pac. J. Clin. Oncol. 2020, 16, 254–258. [Google Scholar] [CrossRef]
- Ishizuka, M.; Shibuya, N.; Nagata, H.; Takagi, K.; Iwasaki, Y.; Hachiya, H.; Aoki, T.; Kubota, K. Perioperative administration of traditional Japanese herbal medicine Daikenchuto relieves postoperative ileus in patients undergoing surgery for gastrointestinal cancer: A systematic review and meta-analaysis. Anticancer Res. 2017, 37, 5967–5974. [Google Scholar]
- Lee, K.H. The Dispensatory on the Visual and Organoleptic Examination of Herbal Medicine; National Institute of Food and Drug Safety Evaluation: Seoul, Korea, 2013; pp. 35–507.
- Seo, C.S.; Lee, M.Y. HPLC–PDA and LC–MS/MS Analysis for the Simultaneous Quantification of the 14 Marker Components in Sojadodamgangki-Tang. Appl. Sci. 2020, 10, 2804. [Google Scholar] [CrossRef]
- Seo, C.S.; Shin, H.K. Quantitative Analysis of 18 Marker Components in the Traditional Korean Medicine, Cheongsangbangpung-Tang, Using High-Performance Liquid Chromatography Combined with Photodiode Array Detector. Appl. Sci. 2021, 11, 14. [Google Scholar] [CrossRef]
- International Conference on Harmonization. Guidance for Industry, Q2B, Validation of Analytical Procedures: Methodology; Food and Drug Administration: Rockville, MD, USA, 1996. [Google Scholar]
- Seo, C.S.; Shin, H.K. Phytochemical Analysis of Twelve Marker Analytes in Sogunjung-tang Using a High-Performance Liquid Chromatography Method. Appl. Sci. 2020, 10, 8561. [Google Scholar] [CrossRef]
- Korean Pharmacopoeia Committee. The Korean Pharmacopoeia, 11th ed.; Shinilbooks: Seoul, Korea, 2014; pp. 1776–1857. [Google Scholar]
- Hwang, J.H.; Kwon, H.J.; Oh, Y.J.; Kim, J.H.; Park, Y.D. Determination of flavonoids and xanthoxylin in toothache-tree containing toothpaste using reversed-phase HPLC coupled with photodiode array detector. Int. J. Clin. Prev. Dent. 2012, 8, 231–236. [Google Scholar]
- Qian, Z.M.; Lu, J.; Gao, Q.P.; Li, S.P. Rapid method for simultaneous determination of flavonoid, saponins and polyacetylenes in Folium Ginseng and Radix Ginseng by pressurized liquid extraction and high-performance liquid chromatography coupled with diode array detection and mass spectrometry. J. Chormatogr. A 2009, 1246, 3825–3830. [Google Scholar] [CrossRef]
- Lai, K.M.; Cheng, Y.Y.; Tsai, T.H. Integrated LC-MS/MS analytical systems and physical inspection for the analysis of a botanical herbal preparation. Molecules 2015, 20, 10641–10656. [Google Scholar] [CrossRef]
- Liu, G.; Qiao, S.; Liu, T.; Yu, H.; Wang, W.; Zhou, Y.; Li, Q.; Li, S. Simultaneous determination of 18 chemical constituents in traditional Chinese medicine of antitussive by UPLC–MS-MS. J. Chromatogr. Sci. 2016, 54, 1540–1552. [Google Scholar] [CrossRef]
- Dubber, M.-J.; Sewram, V.; Mshicileli, N.; Shephard, G.S.; Kanfer, I. The simultaneous determination of selected flavonol glycosides and aglycones in Ginkgo biloba oral dosage forms by high-performance liquid chromatography–electrospray ionisation–mass spectrometry. J. Pharm. Biomed. Anal. 2005, 37, 723–731. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).