Abstract
Sleep inertia (SI) refers to a complex psychophysiological phenomenon, observed after awakening, that can be described as the gradual recovery of waking-like status. The time course of cognitive performance dissipation in an everyday life condition is still unclear, especially in terms of the sleep stage at awakening (REM or NREM-stage 2) and the relative effects on performance. The present study aimed to investigate the SI dissipation in different memory performances upon spontaneous morning awakening after uninterrupted nighttime sleep. Eighteen young adults (7 females; mean age 24.9 ± 3.14 years) spent seven non-consecutive nights (one baseline, three REM awakenings and three St2 awakenings) in the laboratory under standard polysomnographic (PSG) control. Participants were tested after three REM awakenings and three St2 awakenings, and three times at 11:00 a.m. as a control condition. In each testing session, participants filled in the Global Vigor and Affect Scale and carried out one memory task (episodic, semantic, or procedural task). For each condition, participants were tested every 10 min within a time window of 80 min. In accordance with previous studies, SI affected subjective alertness throughout the entire time window assessed. Moreover, SI significantly affected performance speed but not accuracy in the semantic task. With reference to this task, the SI effect dissipated within 30 min of awakening from REM, and within 20 min of awakening from St2. No significant SI effect was observed on episodic or procedural memory tasks.
1. Introduction
Sleep drunkenness [1], post-dormital sleepiness [2], or sleep inertia [3], as it is currently named, refers to a complex psychophysiological phenomenon, observed after awakening, that can be described as the gradual recovery of waking-like status [4]. This transition is not based on an all-or-nothing process, but acts according to slow and progressive mechanisms [5]. The first study on sleep inertia (SI) dates back to 1961 [6], and over the past 60 years, a considerable number of experiments has been carried out on this topic [4,7]. SI magnitude, severity, and duration depend on several factors, such as prior sleep duration and deprivation [8,9], sleep stage of awakening [10,11,12], and the time of day at which awakening takes place [13], as well as the type of task participants are accomplishing [14,15]. The duration and time course of SI is one of the most controversial aspects in the literature on SI [7,16], in particular regarding everyday activities in the normal sleep-wake condition.
Cognitive processes are diversely sensitive to SI [7,17], but it is still not clear whether all, and which, cognitive processes are mostly involved. Complex tasks seem to be less sensitive to SI compared to simpler ones [18]; in particular, strategic mechanisms seem to contrast a physiological condition of low arousal better than automatic ones, even though this datum has not been confirmed in subsequent studies [15,19,20]. Moreover, some higher-order cognitive processes are differentially sensitive to different sleep-wake regulatory processes [21]. The difference among the studies concerning the time course of SI can derive from methodological standpoints. First, only a few studies have investigated the time course of SI dissipation by measuring performances at regular time intervals and for a long time [22]. Indeed, most authors have typically made only one performance assessment after awakening (e.g., [23]). Therefore, SI has been reported to be short-lasting, rarely exceeding 30 min [18,24]. Second, the SI effect in some studies was measured by comparing post-sleep performances with pre-sleep ones, while in other studies it was evaluated by recording the time needed for the performance to level off during the post-sleep period [7]. Third, SI varies according to the performance indexes (i.e., accuracy and/or speed) utilized. In some conditions, SI affects speed but not accuracy [25,26], whereas both indexes decreased when sleep loss was associated with SI [24]. Finally, experimental data show differing results regarding the effect of sleep stage at awakening on SI. There is general agreement regarding a strong SI effect upon awakening from Slow Wave Sleep (SWS) [7,27]. In some studies, SI did not significantly differ as a function of waking up during stage-2 (St2) vs. Rapid Eye Movement (REM) sleep [28]. Other studies reported a more deteriorated performance after awakening from St2 as compared to REM sleep [28,29]. Thus, the SI effect on cognitive performance according to the sleep stage at awakening is still a matter of debate and further studies are needed.
It is relevant to note that SI has been studied in applied fields (i.e., emergency conditions, shift work schedules, sleep disorders). Thus, participants have been tested in particular sleep conditions, such as sleep deprivation, sleep fragmentation, short naps, or SI during the first or second part of the night. By way of contrast, very few studies have investigated SI in a normal sleep-wake cycle condition [15,28,29,30]. Importantly, the studies report different findings, restricting the generalizability of results.
In light of the above-mentioned issues, the main purpose of this research is to evaluate an SI effect on memory performance after an uninterrupted night’s sleep. As far as cognitive functions are concerned, in this study, we focused on memory performances. Mnemonic functions have been investigated sporadically. Only a few specific memories have been assessed, but results have never been replicated in comparable experimental conditions. Research focusing on mnemonic performances has been carried out, in particular, after sleep deprivation [31,32] or in relation to sleep stages at awakening [33,34,35,36,37]. We investigated the effect of the SI time course on three long-term memory systems: semantic, episodic, and procedural [38,39].
We also collected data on subjective alertness as a control measure, given that subjective alertness could affect cognitive performance [40,41,42,43,44].
We expected low levels of arousal and vigilance to produce an important decrease in speed and performance accuracy immediately after awakening.
2. Materials and Methods
2.1. Participants
Eighteen participants, 7 females and 11 males, aged 24.9 ± 3.14, took part in the experimental protocol and were selected as paid (25 euros per night) volunteers for the study. They signed an informed consent form before participating in the study. None worked flexi-time or night shifts. Exclusion criteria included sleep disorders, serious or acute illness, psychopharmaceutical drug use, disabilities that would interfere with or restrict mobility, being an evening- or morning-type, and left-handedness. As well as taking part in an interview, participants filled in several questionnaires in order to assess whether they met the exclusion criteria: in particular, they filled in the Morningness-Eveningness Questionnaire [45], the General Health Questionnaire [46], the Sleep Disorders Questionnaire [47], the State-Trait Anxiety Inventory (STAI) [48], the Self-Rating Depression Scale (ZUNG) [49], and the Edinburgh Handedness Inventory [50]. Participants were instructed to keep a regular sleep-wake schedule before and during the experiment. The usual schedules of sleep onset and awakening of participants were monitored by sleep log [51] during the week preceding the experiment. These data were used to personalize the approximate time of the experimental awakening. During the following 6 weeks, participants were invited to continue to fill in a daily sleep log to check the regularity of their sleep-wake cycle throughout the experiment.
2.2. Design and Procedure
Participants were tested in two experimental conditions (morning awakening from REM sleep and from St2 sleep), and in a diurnal control condition at 11:00 a.m. Each participant spent seven (one adaptation night and six experimental nights) non-consecutive nights (one a week) in the laboratory of Psychophysiology of Sleep and Dream at the Department of Psychology “Renzo Canestrari” of the University of Bologna (Bologna, Italy). The choice to perform the adaptation night in the laboratory was based on the fact that participants had to experience sleep in a laboratory room with electrodes. Thus, the subsequent experimental nights were not influenced by the specific setting of the laboratory. In addition, the adaptation night was used to assess the macrostructure of sleep night of each participant. To reach this goal, polysomnographic recordings were obtained using the Grass Heritage TM PSG System with Gamma TM version 4.2 software (Grass Telefactor, West Warwick, RI, USA). Unipolar electroencephalograms were recorded according to the international 10–20 system: Fz-A1, Cz-A1, C3-A2, and Oz-A1. A submental electromyogram and two unipolar electro-oculograms were also recorded. Electrode impedance was kept below 5KΩ. Sleep records were visually scored in 30 s epochs according to Rechtschaffen and Kales’ [52] standard scoring criteria by two trained scorers. Inter-rater reliability for the two scorers was 95.4%.
At the awakening from the adaptation night, no cognitive tasks or subjective evaluation of alertness was performed. The experimental nights were divided into two conditions according to sleep stage before awakening: three awakenings in REM sleep and three awakenings in St2. The order of experimental conditions was counterbalanced among participants. Experimental awakenings occurred in the morning after an undisturbed night of sleep and in a time window matching the participant’s sleep habits. Participants were awakened after 10 min elapsed in REM or St2 sleep. After the awakening, each participant took part in nine experimental trials during an overall interval of 80 min. The tasks were administered at regular intervals of 10 min (T0, immediately after awakening; T1, 10 min after awakening; T2, 20 min after awakening; T3, 30 min after awakening; T4, 40 min after awakening; T5, 50 min after awakening; T6, 60 min after awakening; T7, 70 min after awakening; T8, 80 min after awakening). In each interval (T0–T8), only one cognitive task was administered individually, according to the Latin Square design. This schedule of task administration reduced a possible learning and/or fatigue effect. At the end of the task execution, participants always filled in a subjective alertness scale.
The same task schedule was carried out in the three diurnal (one a week) control sessions. Diurnal sessions were performed in the laboratory from 11:00 a.m. Half the sample participated firstly in the diurnal condition sessions and then in the experimental night trials, while the opposite order was carried out for the other half of the sample. The choice of three REM and three St2 experimental nights, as well as three diurnal control sessions, were based on the assumption that three different memory systems were assessed, and thus, during each condition only one task (out of three) was performed, requiring three different sessions for each experimental condition (REM and St2 awakenings and diurnal condition) to complete all tasks for each condition.
2.3. Tasks
All cognitive tasks were computerized.
Lexical decision task (semantic memory system). Nine lists were created, each made up of 80 stimuli that included 40 words and 40 non-words. The words were related to concrete objects and items and were balanced across lists on the basis of length (number of letters and syllables) and frequency (expressed in the percentage of appearance and usage in the Italian culture). Each stimulus appeared in white against a black foreground and they were written in Courier New font 30. The participants were instructed to press the spacebar on a keyboard as fast as possible when they detected an Italian word on the screen; when presented with a non-word, they were required to avoid any response. In any case, both word and non-word stimuli remained on the screen for 500 ms. In order to assess to what extent semantic memory was automatically activated through the embedding of semantic priming, 10 prime words and 10 target words were included in the Italian words section of the list; each section, therefore, contained 20 related words (the 10 prime-target pairs) and 20 unrelated words. The prime words were semantically related to, and immediately preceded by, the target words, e.g., prime: bambola (doll), target: palla (ball). Unrelated words might be pasta (pasta) and arte (art). The words were balanced across lists in terms of length and frequency. The non-words (for example, “namoi”) were legible stimuli but were meaningless in Italian. These non-word stimuli were created modifying the words used in a list (e.g., for non-words in list 1 we modified the words of list 4, just changing the order of vowels and consonants). In the task, a white fixation cross appeared for 500 ms in the center of the screen, and then a stimulus (word or non-word) appeared in the center of the screen. After a participant’s response or elapsed time, a new trial started. In each list, a pseudorandom presentation of the stimuli was created with the following rules: the target stimulus appeared after its relative prime stimulus and no more than three consecutive non-words were presented. All task lasted about 10 min. We measured the mean RTs for word recognition, priming effect, i.e., the RT difference between the recognition of the prime word and the target word, and accuracy expressed as a percentage.
Word list free recall (episodic memory system). A single word, in white color against a black foreground written in Courier New 30, was presented centrally on the screen for 500 ms. The participants were instructed to read the word presented and write it on a sheet of paper. When this was done, they had to press the spacebar, after which another word appeared on the screen. Thus, each word appeared for a short duration, but participants had enough time to write the word and to decide when a new word was presented, by pressing the spacebar. This procedure was repeated for all 15 words. Each word was disyllabic (e.g., naso [nose], prete [priest], or lardo [lard]), referred to objective items (no abstract concepts were used), and balanced for frequency (see before) across lists. At the end of the word list, the participants were instructed to read all the words they had written in a serial order, and to hand over the sheet at the end. After a one-minute break, the experimenter gave the start signal, and the participants had to recall the words in a free order for 3 min. For this task, nine lists were created. Given that participants determined the timing of appearance of words, this task lasted about 10 min. In this task, we counted the number of correctly recalled words.
Finger tapping task (procedural memory system). The participants had to learn a sequence of five keys using at least three fingers of their left hand (non-dominant hand). We created nine sequences. Each sequence, composed of five keys, was made up of a combination of four arrow keys on the keyboard. In each sequence, the first and last keys were identical. The task included a learning phase and a recall phase. In the first phase, the participants had to learn the sequence and were instructed to press the five keys in the right order. The sequence remained at the center of screen (i.e., participants saw the sequence of figured white arrows against a black foreground: Up arrow, Left arrow, Right arrow, Down arrow, Up arrow) and visual feedback was given. When the participants pressed the correct key, a green dot appeared while a red dot appeared when the wrong key was pressed. In case of error, individuals had to start the sequence again. The learning phase ended when the participant pressed the correct sequence 50 times (about 5 min). After a break of 1 min, the recall phase started. In this phase, the sequence was not present on the screen, while the visual feedback was maintained. Specifically, participants saw a black screen and after each key press, a green or red dot appeared. The task ended when the participant had finished 50 sequences (about 5 min). We counted the mean time needed to complete a correct sequence (the total time to complete all corrected sequences divided by the number of corrected sequence) and the number of correct sequences in the recalling phase.
Given that for each cognitive task we created nine different list, we randomly presented each list in each testing session for every participant. For example, for one participant, the list order was 1, 7, 4, 2, 8, 5, 3, 9, and 6, whereas for another participant, the list order was 7, 4, 1, 8, 5, 2, 9, 6, and 3.
Subjective alertness The Global Vigor and Affect Scale (GVA) [53] was used to assess subjective alertness. The answers to four visual analogue items (vigor, tiredness, effort required for everyday tasks, and sleepiness) were combined to calculate global alertness. Higher scores indicate greater alertness.
2.4. Data Analysis
For each measure, we performed a two-way ANOVA with experimental condition (three levels, wake, awakening from REM, and awakening from St.2) and experimental trials (nine levels, from T0 to T8) as within-subject factors. The significant main effects or interactions between factors were further analyzed using the Scheffé post-hoc test. In order to further clarify the interaction, we performed a one-way ANOVA within each experimental condition, considering the experimental trial (nine levels) as the within-subjects factor. Finally, we also performed a one-way ANOVA for each experimental trial considering the experimental condition (three levels) as the within-subjects factor. We set the significance level at p < 0.05.
3. Results
3.1. PSG Data
Table 1 shows the PSG data. As can be observed, no significant differences were found between sleep conditions.

Table 1.
Means and standard deviations of sleep parameters in experimental awakenings.
3.2. Lexical Decision Task
We analyzed RTs of valid trial. In addition, we discarded, for each participant and each list, all RTs above or below 2.5 standard deviations as outliers (about 3%). Data relating to the response speed in the lexical decision task are shown in Table 2. The ANOVA on reaction time revealed that experimental condition reached significance (F(2,34) = 7.55, p < 0.001). The wake condition obtained a lower reaction time (mean = 590 ms) in comparison with both REM (mean = 636 ms; p < 0.05) and St.2 awakenings (mean = 645 ms; p < 0.005) (Scheffé post-hoc test). A significant session effect was also found (F(8,136) = 3.09, p < 0.005). The Scheffé post-hoc test did not show any significant differences between the trials. The interaction between condition and trial reached statistical significance (F(16,272) = 2.64, p < 0.001). In particular, the mean reaction time for the wake condition at t0 was significantly lower than for the REM condition (p < 0.005). We performed a further one-way ANOVA for each trial using the experimental condition (three levels, wake, REM, and St.2) as a within-subjects factor. In this case, we observed a significant result at t0 (F(2,34) = 14.7, p < 0.00001), t1 (F(2,34) = 5.81, p < 0.01), t2 (F(2,34) = 5.64, p < 0.001), and t4 (F(2,34) = 3.51, p < 0.05). Based on the Scheffé post-hoc test, reaction times in the wake condition were faster in comparison to those in the REM condition at t0 (p < 0.00001) and t2 (p < 0.01); faster RTs were also observed in the wake condition compared to St.2 at t0 (p < 0.001), t1 (p < 0.01), and t2 (p < 0.05). The mean RT for prime words was 646 ms, while for target words, the mean RT was 483 ms. Thus, a general priming effect was found; however, the ANOVA did not display any significant effect or interaction.

Table 2.
Mean values of reaction times in the lexical decision task for experimental conditions (wake, REM awakening, St2 awakening) and experimental trials (from t0 to t8). The last column shows the F values of ANOVAs within condition. The F values of ANOVAs within trial are reported in the last row.
As regards accuracy (i.e., percentage of correct responses), we performed a three-way ANOVA with experimental condition (wake, awakening from REM, and awakening from St.2), experimental trial (from T0 to T8), and target (words and non-words) as variables (see Figure 1). As regards the first two factors, no significant effects were found. On the contrary, we observed a significant difference between the accuracy of words (99.5%) and non-words (96.9%) (F(1,17) = 26.91; p < 0.0001). No interactions reached statistical significance. In the analysis for the trial, we detected a significant effect at t2 for non-words (F(2,34) = 3,4; p < 0.05). No post-hoc comparisons were significant.
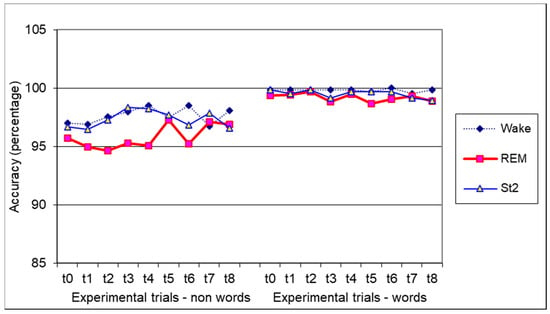
Figure 1.
Accuracy (expressed as percentage) in the lexical decision task for non-words and words. Means are shown.
3.3. Episodic Learning Task
In this task, we coded the number of correct words recalled by the participants (Table 3). The ANOVA revealed that experimental condition reached significance (F(2,34) = 3.82, p < 0.05). The wake condition was characterized by the higher number of correctly recalled words (mean = 11.5) in comparison with both REM awakening (mean = 10.2) and St.2 awakening (mean = 10.4), although none of these differences was statistically significant in the Scheffé post hoc test. In the analysis for trials (Table 3), we obtained significant results at t0 (F(2,34) = 3.37; p < 0.05), t5 (F(4,57) = 4.57; p < 0.01), and t6 (F(3,76) = 3.37; p < 0.05). The post-hoc analyses revealed that the wake condition presented a significantly higher number of correctly recalled words compared to REM awakening at t5 (p < 0.05) and St2 awakening at t6 (p < 0.05) (Scheffé post hoc test).

Table 3.
Mean number of correctly recalled words in the episodic learning task for experimental conditions (wake, REM awakening, St2 awakening) and experimental trials (from t0 to t8). The last column shows the F values of ANOVAs within condition. The F values of ANOVAs within trial are reported in the last row.
3.4. Finger Tapping Task
The number of correct sequences in the recall phase was very high in all conditions: wake (45.15), REM awakening (44.27), and St2 awakening (45.74). The ANOVAs did not show any significant main effects or interactions. The trial by trial ANOVA showed a significant effect at t8 (F(2,34) = 3.4; p < 0.05). No significance was detected with the Scheffé post-hoc test.
As regards the speed of execution, we considered the mean time (in seconds) of each correct sequence in the recall phases. The mean time for a correct sequence was 4.03 s for wake, 4.43 s for REM awakening, and 4.34 s for St2 awakening. The ANOVAs did not show any significant main effects or interactions (Figure 2). The trial by trial ANOVA did not show any significant results.
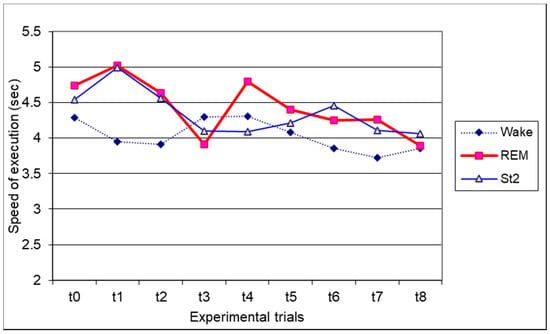
Figure 2.
Graphical representation of the interaction between the experimental condition and experimental trial of the speed of execution (in sec) of the finger tapping task. Means are reported.
3.5. Subjective Alertness
Data concerning subjective alertness are shown in Figure 3. The ANOVA on subjective alertness revealed that experimental condition reached significance (F(2,34) = 24.4, p < 0.00001). The wake condition obtained a higher subjective alertness score (mean = 76.18) in comparison with both REM awakening (mean = 51.45; p < 0.000001) and St.2 awakening (mean = 57.60; p < 0.0001) (Scheffé post-hoc test). A significant trial effect was also found (F(8,136) = 16.22, p < 0.0000001). The Scheffé post-hoc test showed that the mean score significantly and linearly increased from T0 to T8. The interaction between condition and trial also reached statistical significance (F(16,272) = 18.52, p < 0.0000001). In particular, the mean subjective alertness score for the wake condition at t0, t1, t2, t3, t4, t5, t6, and t7 was significantly higher in comparison with that of both the REM and St2 conditions. At t8, alertness in the wake condition was higher only in comparison to the REM condition. REM subjective alertness never significantly differed from that of St2.
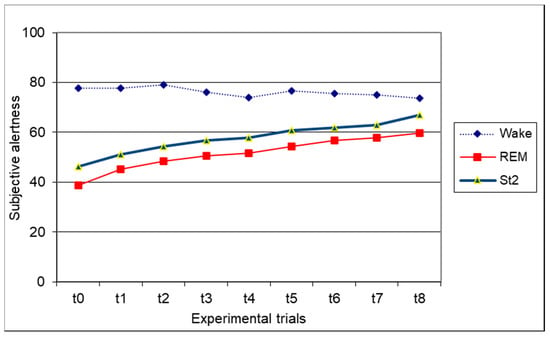
Figure 3.
Graphical representation of the interaction between the experimental condition and experimental trial of subjective alertness. Mean values are reported.
4. Discussion
The present study aimed to investigate the effect of SI and its dissipation on long-term memory systems (semantic, episodic, and procedural) after an uninterrupted nocturnal sleep. According to the taxonomy of memory proposed by Schacter and Tulving [39], semantic memory contains all the general knowledge, language, symbols, and encyclopedic information about the world; episodic memory involves the conscious recollection of personally experienced events; and procedural memory mainly concerns motor skills, which are learned with simple exercise. The importance of the SI-memory relationship is evident since memory processes play a crucial role in reinstating the levels of cognitive efficiency of wakefulness.
Specifically, our purpose was to evaluate: (a) the SI effect after an uninterrupted night’s sleep; (b) the time course of SI dissipation by assessing performance at regular intervals for a total time of 80 min; (c) the SI effect assessed by comparing post-sleep performances with diurnal performance collected in the same conditions; and (d) the SI effect in relation to sleep stage of awakening (St2 vs. REM sleep). The main results showed an SI effect on the speed execution of the cognitive tasks and on subjective alertness, independently from sleep stage at awakening.
Regarding the SI effect after an uninterrupted night’s sleep, we did not find a systematic SI effect on different memory systems. Generally, these results partly confirm that mental functions are diversely sensitive to SI [4,54,55]. On this basis, it is possible to consider the results separately according to the different and independent memory systems [56].
In the semantic memory task, we observed an SI effect on RTs for lexical decisions during the first 20 min: participants were slower at detecting an Italian word until the fourth session. With regard to accuracy, we did not find any effects. Our results agreed with those of Balkin and Badia [57], suggesting that performance speed and accuracy are differentially affected by SI during the sleep-wake transition [4,22,55]. This finding could indicate that RT is an index that is more sensitive to the SI effect. More probably, speed and accuracy could be reliable indices when a sleep deprivation effect is being measured. Indeed, Miccoli et al. [58] found that both sleepiness and SI determined an increase in RTs, but after a total sleep deprivation (i.e., higher sleepiness while SI was absent), the number of lapses (i.e., accuracy) increased.
The results obtained from the episodic memory task could be problematic. Indeed, we expected poor performance due to the difficulty of the task. Instead, the episodic recall, in which time of processing was not assessed, did not prove to be affected by SI. The most probable alternative interpretation is that episodic recovery is a highly complex task that could produce a very high cognitive engagement (both encoding and rehearsal processes involve attention, execution operations, deliberate effort, etc.). As regards procedural memory, no SI effect was observed in the finger tapping task, in line with a previous study [20]. Taken together, these results could indicate that long-term memory systems are differently affected by SI.
As previously stated, the participants reached a high accuracy level in all cognitive tasks. This is an interesting result, considering that the subjective alertness in experimental conditions did not reach the control value throughout the whole testing session (i.e., 80 min). Our result regarding subjective alertness was in line with previous studies [24,40,41,42], but we described a linear increase in subjective alertness after awakening instead of an asymptotic pattern [40,59,60]. This discrepancy could be explained by the fact that our data collection was carried out every 10 min from awakening, whereas others, such as Jewett et al. [40], tested their participants every 10 min during the first hour, and then every 30 min.
As regards the effect of sleep stage at awakening on SI, the present study did not show any differences. Jewett et al. [40] showed no SI as a function of waking up during St2 vs. REM sleep, after a normal night’s sleep. Moreover, we did not find sleep or SI effects on semantic priming, casting doubt upon the more recent theories of a different effect of REM and NREM sleep on semantic memory [35,61,62]. Another study shows that the sleep stage before awakening influences the magnitude of sleep inertia, with deeper sleep with high delta power being associated with subsequent performance impairment and increased latency of the P300 event-related potential (ERP) [63]. Taken together, the data available so far do not clarify whether sleep inertia is simply an epiphenomenon of the carryover from a preceding sleep state or a precise physiological process that ensures a gradual transition from sleep to waking [64].
We would like to add a consideration regarding a possible dissociation between subjective alertness and memory performance. In the literature, it has been reported that subjective alertness may in part moderate an individual’s global assessment of performance [65], but that performance rhythms are not directly mediated by levels of subjective alertness [66]. Recent studies have clarified that SI is part of a complex transition of levels of consciousness, namely from different sleep stages to wakefulness [67,68]. This condition is accompanied by different regional activation/deactivation patterns, possibly reflecting a functional differentiation between cortical/subcortical areas that, in turn, influences human consciousness during the transitional states [69]. Within this perspective, our data bring evidence in favor of a dissociation between systems that regulate subjective vigilance and systems predisposed to memory efficiency.
From an adaptive point of view, it is interesting to underline the fact that the procedural memory system already seems to perform well immediately after the last morning awakening. It could guarantee an already effective routine behavior for the new day that is about to begin. For example, many of us, despite being subjectively sleepy, are able to complete the procedures to prepare a hot cup of coffee in the morning as soon as we wake up.
Globally, the present research confirms that the SI effect and its dissipation depend on the type of task used and they influence the speed of information processing more directly than the accuracy. These results further confirm the importance of considering the SI effect when planning sleep logistics for personnel who may have to perform complex tasks quickly after an abrupt awakening.
Further research development could be addressed to the study of memory by introducing different tasks. It would also be interesting to evaluate working memory to establish a more united framework in terms of the memory processes in this transition phase.
Author Contributions
Conceptualization, M.O. and V.N.; methodology, M.O. and V.N.; formal analysis, V.N.; investigation, M.O., M.F., L.T., M.M. and V.N.; data curation, V.N.; writing—original draft preparation, M.O. and V.N.; writing—review and editing, M.O., M.F., L.T., M.M. and V.N.; supervision, V.N. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by MIUR, grant number PRIN 2004-004113175.
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki, adopted as ethical reference by the Department of Psychology of the University of Bologna.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The data presented in this study are available on reasonable request from the corresponding author. The data are not publicly available due to privacy reason.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
References
- Broughton, R.J. Sleep disorders: Disorders or arousal? Science 1968, 159, 1070–1078. [Google Scholar] [CrossRef] [PubMed]
- Association of Sleep Disorders Centers. Diagnostic Classification of Sleep and Arousal Disorders, 1st Ed; Prepared by the Sleep Disorders Classification Committee, H.P.; Roffwarg, Chairman. Sleep 1979, 2, 1–137. [Google Scholar] [CrossRef]
- Lubin, A.; Hord, D.; Tracy, M.L.; Johnson, L.C. Effects of exercise, bedrest and napping on performance decrement during 40 hours. Psychophysiology 1976, 13, 334–339. [Google Scholar] [CrossRef]
- Tassi, P.; Muzet, A. Sleep inertia. Sleep Med. Rev. 2000, 4, 341–353. [Google Scholar] [CrossRef] [PubMed]
- Trotti, L.M. Waking up is the hardest thing I do all day: Sleep inertia and sleep drunkenness. Sleep Med. Rev. 2017, 35, 76–84. [Google Scholar] [CrossRef]
- Hartman, B.O.; Langdon, D.E.; McKenzie, R.E. Performance on Sudden Awakening; USAF School of Aerospace Medicine Report: San Antonio, TX, USA, 1961. [Google Scholar]
- Hilditch, C.J.; McHill, A.W. Sleep Inertia: Current insights. Nat. Sci. Sleep 2019, 11, 155–165. [Google Scholar] [CrossRef] [PubMed]
- McHill, A.; Hull, J.; Czeisler, C.; Klerman, E. The effect of chronic sleep restriction and prior sleep duration on sleep inertia measured using cognitive performance. Sleep Med. 2017, 40, 163. [Google Scholar] [CrossRef]
- Peter-Derex, L.; Magnin, M.; Bastuji, H. Heterogeneity of arousals in human sleep: A stereo-electroencephalographic study. NeuroImage 2015, 123, 229–244. [Google Scholar] [CrossRef] [PubMed]
- Hilditch, C.; Centofanti, S.; Dorrian, J.; Banks, S. A 30-minute, but not a 10-minute nighttime nap is associated with sleep inertia. Sleep 2016, 39, 675. [Google Scholar] [CrossRef] [PubMed]
- Marzano, C.; Ferrara, M.; Moroni, F.; De Gennaro, L. Electroencephalographic sleep inertia of the awakening brain. Neuroscience 2010, 176, 308–317. [Google Scholar] [CrossRef]
- Gorgoni, M.; Ferrara, M.; D’Atri, A.; Lauri, G.; Scarpelli, S.; Truglia, I.; De Gennaro, L. EEG topography during sleep inertia upon awakening after a period of increased homeostatic sleep pressure. Sleep Med. 2015, 16, 883–890. [Google Scholar] [CrossRef] [PubMed]
- Scheer, F.A.; Shea, T.J.; Hilton, M.F.; Shea, S.A. An endogenous circadian rhythm in sleep inertia results in greatest cognitive impairment upon awakening during the biological night. J. Biol. Rhythm. 2008, 23, 353–361. [Google Scholar] [CrossRef]
- Ritchie, H.K.; Burke, T.M.; Dear, T.B.; McHill, A.W.; Axelsson, J.; Wright, K.P.J. Impact of sleep inertia on visual selective attention for rare targets and the influence of chronotype. J. Sleep Res. 2017, 26, 551–558. [Google Scholar] [CrossRef]
- Hilditch, C.J.; Dorrian, J.; Banks, S. Time to wake up: Reactive countermeasures to sleep inertia. Ind. Health 2016, 54, 528–541. [Google Scholar] [CrossRef]
- Ferrara, M.; De Gennaro, L.; Bertini, M. Time-course of sleep inertia upon awakening from nighttime sleep with different sleep homeostasis conditions. Aviat. Space Environ. Med. 2000, 71, 225–229. [Google Scholar]
- Wertz, A.T.; Ronda, J.M.; Czeisler, C.A.; Wright, K.P.J. Effects of sleep inertia on cognition. JAMA 2006, 295, 163–164. [Google Scholar] [CrossRef]
- Seminara, J.L.; Shavelson, R.J. Effectivness of space crew performance subsequent to sudden sleep arousal. Aerosp. Med. 1969, 40, 723–727. [Google Scholar] [PubMed]
- Ferrara, M.; De Gennaro, L.; Casagrande, M.; Bertini, M. Auditory arousal thresholds after selective slow-wave sleep deprivation. Clin. Neurophysiol. 1999, 110, 2148–2152. [Google Scholar] [CrossRef]
- Ferrara, M.; De Gennaro, L.; Casagrande, M.; Bertini, M. Selective slow-wave sleep deprivation and time-of-night effects on cognitive performance upon awakening. Psychophysiology 2000, 37, 440–446. [Google Scholar] [CrossRef] [PubMed]
- Burke, T.M.; Scheer, F.A.; Ronda, J.M.; Czeisler, C.A.; Wright, K.P., Jr. Sleep inertia, sleep homeostatic and circadian influences on higher-order cognitive functions. J. Sleep Res. 2015, 24, 364–371. [Google Scholar] [CrossRef] [PubMed]
- Ferrara, M.; De Gennaro, L. The sleep inertia phenomenon during the sleep-wake transition. Theoretical and operational issues. Aviat. Space Environ. Med. 2000, 71, 843–848. [Google Scholar]
- Naitoh, P.; Kelly, T.; Babkoff, H. Sleep inertia, best time not to wake up? Chronobiol. Int. 1993, 10, 109–118. [Google Scholar] [CrossRef] [PubMed]
- Dinges, D.F. Are you awake? Cognitive Performance and Reverie During the Hypnopompic State. In Sleep and Cognition; Bootzin, R., Kihlstrom, J., Schacter, D., Eds.; American Psychological Society: Washington, DC, USA, 1990; pp. 159–175. [Google Scholar]
- Naitoh, P.; Angus, R.G. Napping and human functioning during prolonged work. In Chronobiological, Behavioral and Medical Aspects of Napping; Dinges, D.F., Broughton, R.J., Eds.; Raven Press: New York, NY, USA, 1989; pp. 221–246. [Google Scholar]
- Tassi, P.; Nicolas, A.; Dewasmes, G.; Eschenlauer, R.; Ehrhart, J.; Salame, P.; Muzet, A.; Libert, J.P. Effects of noise on sleep inertia as a function of circadian placement of a one-hour nap. Percept. Mot. Ski. 1992, 75, 291–302. [Google Scholar] [CrossRef] [PubMed]
- Hilditch, C.J.; Dorrian, J.; Banks, S. A review of short naps and sleep inertia: Do naps of 30 min or less really avoid sleep inertia and slow wave sleep? Sleep Med. Rev. 2017, 32, 176–190. [Google Scholar] [CrossRef] [PubMed]
- Cavallero, C.; Versace, F. Stage at awakening, sleep inertia and performance. Sleep Res. Online 2003, 5, 89–97. [Google Scholar]
- Silva, E.J.; Duffy, J.F. Sleep inertia varies with circadian phase and sleep stage in older adults. Behav. Neurosci. 2008, 122, 928–935. [Google Scholar] [CrossRef]
- Dinges, D.F.; Orne, M.T.; Orne, E.C. Assessing performance upon abrupt awakening from naps during quasi-continuous operations. Behav. Res. Methods Instrum. Comput. 1985, 17, 37–45. [Google Scholar] [CrossRef]
- Akerstedt, T.; Gillberg, M. Effects of sleep deprivation on memory and sleep latencies in connection with repeated awakening from sleep. Psychophysiology 1979, 16, 49–52. [Google Scholar] [CrossRef]
- Rosa, R.R.; Bonnet, M.H.; Warm, J.S. Recovery of performance during sleep following sleep deprivation. Psychophysiology 1983, 20, 152–159. [Google Scholar] [CrossRef]
- Stones, M.J. Memory performance after arousal from different sleep stages. Br. J. Psychol. 1977, 68, 177–181. [Google Scholar] [CrossRef]
- Grosvenor, A.; Lack, L.C. The effect of sleep before or after learning on memory. Sleep 1984, 7, 155–167. [Google Scholar] [CrossRef]
- Stickgold, R.; Scott, L.; Rittenhouse, C.; Hobson, J.A. Sleep-induced changes in associative memory. J. Cogn. Neurosci. 1999, 11, 182–193. [Google Scholar] [CrossRef]
- Kolff, M.; Hofman, W.; Kerkhof, G.; Coenen, A. Effects on sleep-wake states on reaction times and priming effects in a semantic priming paradigm. Sleep Hypn. 2003, 5, 72–77. [Google Scholar]
- Kolff, M.; Hofman, W.; Kerkhof, G.; Coenen, A. The time course of sleep inertia in a semantic priming paradigm. Sleep Hypn. 2003, 5, 78–82. [Google Scholar]
- Tulving, E. How many memory systems are there? Am. Psychol. 1985, 40, 385–398. [Google Scholar] [CrossRef]
- Schacter, D.L.; Tulving, E. (Eds.) Memory Systems; The MIT Press: Cambridge, MA, USA, 1994. [Google Scholar]
- Jewett, M.E.; Wyatt, J.K.; Ritz-De Cecco, A.; Khalsa, S.B.; Dijk, D.J.; Czeisler, C.A. Time course of sleep inertia dissipation in human performance and alertness. J. Sleep Res. 1999, 8, 1–8. [Google Scholar] [CrossRef]
- Ikeda, H.; Hayashi, M. The effect of self-awakening from nocturnal sleep on sleep inertia. Biol. Psychol. 2010, 83, 15–19. [Google Scholar] [CrossRef] [PubMed]
- Ikeda, H.; Hayashi, M. Effect of sleep inertia on switch cost and arousal level immediately after awakening from normal nocturnal sleep. Sleep Biol. Rhythm. 2008, 6, 120–125. [Google Scholar] [CrossRef]
- Matchock, R.L.; Mordkoff, J.T. Visual attention, reaction time, and self-reported alertness upon awakening from sleep bouts of varying lengths. Exp. Brain Res. 2007, 178, 228–239. [Google Scholar] [CrossRef] [PubMed]
- Natale, V.; Alzani, A.; Cicogna, P.C. Cognitive efficiency and circadian typologies: A diurnal study. Personl. Individ. Differ. 2003, 35, 1089–1105. [Google Scholar] [CrossRef]
- Horne, J.; Ostberg, O. A self-assessment questionnaire to determine morningness-eveningness in human circadian rhythm. Int. J. Chronobiol. 1976, 4, 97–110. [Google Scholar]
- Goldberg, D.P.; Blackwell, B. Psychiatric illness in general practice: A detailed study using a new method of case identification. Br. Med. J. 1970, 2, 439–443. [Google Scholar] [CrossRef] [PubMed]
- Violani, C.; Devoto, A.; Lucidi, F.; Lombardo, C.; Russo, P.M. Validity of a short insomnia questionnaire: The SQD. Brain Res. Bull. 2004, 63, 415–421. [Google Scholar] [CrossRef] [PubMed]
- Spielberger, C.D.; Gorsuch, R.L.; Lushene, R.D. STAI Manual; Consulting Psychologist: Palto Alto, CA, USA, 1970. [Google Scholar]
- Zung, W.W. A Self-Rating Depression Scale. Arch. Gen. Psychiatry 1965, 12, 63–70. [Google Scholar] [CrossRef]
- Oldfield, R.C. The assessment and analysis of handedness: The Edinburgh Inventory. Neuropsychologia 1971, 9, 97–113. [Google Scholar] [CrossRef]
- Natale, V.; Leger, D.; Bayon, V.; Erbacci, A.; Tonetti, L.; Fabbri, M.; Martoni, M. The Consensus SleepDiary: Quantitative criteria for primary insomnia diagnosis. Psychosom. Med. 2015, 77, 413–418. [Google Scholar] [CrossRef] [PubMed]
- Rechtschaffen, A.; Kales, A. A Manual of Standardized Terminology, Techniques and Scoring System for Sleep Stages of Human Subjects; National Institute of Health: Washington, DC, USA, 1968.
- Monk, T.A. A Visual Analogue Scale technique to measure global vigor and affect. Psychiatry Res. 1989, 27, 89–99. [Google Scholar] [CrossRef]
- Wilkinson, R.T.; Stretton, M. Performance after awakening at different times of night. Psychon. Sci. 1971, 23, 283–285. [Google Scholar] [CrossRef]
- Tassi, P.; Bonnefond, A.; Engasser, O.; Hoeft, A.; Eschenlauer, R.; Muzet, A. EEG spectral power and cognitive performance during sleep inertia: The effect of normal sleep duration and partial sleep deprivation. Physiol. Behav. 2006, 87, 177–184. [Google Scholar] [CrossRef]
- Nyberg, L.; Tulving, E. Classifying human long-term memory: Evidence from converging dissociations. Eur. J. Cogn. Psychol. 1996, 8, 163–183. [Google Scholar] [CrossRef]
- Balkin, T.J.; Badia, P. Relationship between sleep inertia and sleepiness: Cumulative effects of four nights of sleep disruption/restriction on performance following abrupt nocturnal awakenings. Biol. Psychol. 1988, 27, 245–258. [Google Scholar] [CrossRef]
- Miccoli, L.; Versace, F.; Koterle, S.; Cavallero, C. Comparing sleep-loss sleepiness and sleep inertia: Lapses make the difference. Chronobiol. Int. 2008, 25, 725–744. [Google Scholar] [CrossRef]
- Achermann, P.; Werth, E.; Dijk, D.J.; Bobely, A.A. Time course of sleep inertia after nighttime and daytime sleep episodes. Arch. Ital. Biol. 1995, 134, 109–119. [Google Scholar] [PubMed]
- Folkard, S.; Åkerstedt, T. A three-process model of the regulation of alertness-sleepiness. In Sleep, Arousal, and Performance; Broughton, R.J., Ogilvie, R.D., Eds.; Birkhäuser: Boston, MA, USA, 1992; pp. 11–26. [Google Scholar]
- Rauchs, G.; Desgranges, B.; Foret, J.; Eustache, F. The relationships between memory systems and sleep stages. J. Sleep Res. 2005, 14, 123–140. [Google Scholar] [CrossRef] [PubMed]
- Diekelmann, S.; Wilhelm, I.; Born, J. The whats and whens of sleep-dependent memory consolidation. Sleep Med. Rev. 2009, 13, 309–321. [Google Scholar] [CrossRef]
- Bastuji, H.; Perrin, F.; Garcia-Larrea, L. Event-related potentials during forced awakening: A tool for the study of acute sleep inertia. J. Sleep Res. 2003, 12, 189–206. [Google Scholar] [CrossRef] [PubMed]
- Vyazovskiy, V.V.; Cui, N.; Rodriguez, A.V.; Funk, C.; Cirelli, C.; Tononi, G. The Dynamics of Cortical Neuronal Activity in the First Minutes after Spontaneous Awakening in Rats and Mice. Sleep 2014, 37, 1337–1347. [Google Scholar] [CrossRef][Green Version]
- Dorrian, S.; Lamond, N.; Dawson, D. The ability to self-monitor performance when fatigued. J. Sleep Res. 2000, 9, 137–144. [Google Scholar] [CrossRef]
- Monk, T.H.; Buysse, D.J.; Reynolds, C.F.; Berga, S.L.; Jarrett, D.B.; Begley, A.E.; Kupfer, D.J. Circadian rhythms in human performance and mood under constant conditions. J. Sleep Res. 1997, 6, 9–18. [Google Scholar] [CrossRef]
- Balkin, T.J.; Braun, A.R.; Wesensten, N.J.; Jeffries, K.; Varga, M.; Baldwin, P.; Belenky, G.; Herscovitch, P. The process of awakening: A PET study of regional brain activity patterns mediating the re-establishment of alertness and consciousness. Brain 2002, 125, 2308–2319. [Google Scholar] [CrossRef]
- Vallat, R.; Meunier, D.; Nicolas, A.; Ruby, P. Hard to wake up? The cerebral correlates of sleep inertia assessed using combined behavioral, EEG and fMRI measures. NeuroImage 2019, 184, 266–278. [Google Scholar] [CrossRef] [PubMed]
- Massimini, M.; Ferrarelli, F.; Huber, R.; Esser, S.K.; Singh, H.; Tononi, G. Breakdown of cortical effective connectivity during sleep. Science 2005, 309, 2228–2232. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).