Abstract
Background: It has been proven that the antihypertensive agent nifedipine can cause gingival overgrowth as a side effect. The aim of this study was to analyze the effects of pharmacological treatment with nifedipine on human gingival fibroblasts activity, investigating the possible pathogenetic mechanisms that lead to the onset of gingival enlargement. Methods: The expression profile of 57 genes belonging to the “Extracellular Matrix and Adhesion Molecules” pathway, fibroblasts’ viability at different drug concentrations, and E-cadherin levels in treated fibroblasts were assessed using real-time Polymerase Chain Reaction, PrestoBlue™ cell viability test, and an enzyme-linked immunoassay (ELISA), respectively. Results: Metalloproteinase 24 and 8 (MMP24, MMP8) showed significant upregulation in treated cells with respect to the control group, and cell adhesion gene CDH1 (E-cadherin) levels were recorded as increased in treated fibroblasts using both real-time PCR and ELISA. Downregulation was observed for transmembrane receptors ITGA6 and ITGB4, the basement membrane constituent LAMA1 and LAMB1, and the extracellular matrix protease MMP11, MMP16, and MMP26. Conclusions: The obtained data suggested that the pathogenesis of nifedipine-induced gingival overgrowth is characterized by an excessive accumulation of collagen due to the inhibition of collagen intracellular and extracellular degradation pathways.
1. Introduction
The most frequent risk factor for myocardial infarction, congestive heart failure, and stroke is hypertension [1,2]. Nifedipine is used as an antihypertensive and antianginal agent, providing vascular smooth muscle relaxation and negative ionotropic and chronotropic effects on. This drug is able to inhibit voltage-dependent L-type calcium channels in vascular smooth muscles and myocardial cells, preventing the entry of calcium ions. The reduction of intracellular calcium level causes a decrease of peripheral arterial vascular resistance and the dilatation of coronary arteries. These conditions result in systemic blood pressure reduction and in myocardial oxygen delivery increase [3,4,5,6]. The systematic review and meta-analysis by Ross et al. [7] analyzed the side effects of antihypertensive agents on 28,922 patients, reporting that calcium channel blockers are most associated with neurologic (17.6%), cardiovascular (11.9%), respiratory (7.8%), and dermatologic (6.8%) adverse events. The literature also proved gingival overgrowth (GO) to be a further side effect associated with the administration of calcium channel blockers, including nifedipine. The cross-sectional study by Miranda et al. [8] recorded a significantly higher prevalence of gingival enlargement in 65 patients treated with nifedipine compared with the control group, and it also assessed, in line with other studies, that gingival lesions were more severe in the vestibular area of inferior and anterior teeth [9,10].
The onset of gingival overgrowth is not only associated with antihypertensive therapy, but also with anticonvulsant and immunosuppressant drugs’ chronic administration: the prevalence of drug-induced gingival enlargement (DIGO) was demonstrated to be around 50–70%, 30%, and 25–30% in the case of phenytoin [11,12], nifedipine [8], and cyclosporine, respectively [12]. Clinical manifestation of GO is similar for all the cited drugs: it usually manifests itself during the first 3 months of the therapy, reaching its widest expression after 9–12 months [13]. The study of 2012 by Miranda et al. [14] described the manifestation of gingival lesions, evaluating two clinical indices, i.e., vertical and horizontal overgrowth: GO occurs at first in the area of interdental papilla, as localized nodullary enlargement (horizontal growth), and it afterwards expands to the dental crown (vertical growth), interfering with mastication. The severity of drug-induced gingival enlargement (DIGO) seems to be influenced by plaque scores and gingival inflammation [15]. In particular, with reference to nifedipine, some studies demonstrated that the onset and the development of GO depends on high plaque index, administration of high drug doses, and genetic factors [16,17]. Moreover, Trackman et al. assessed that the characteristics of GO induced by nifedipine, phenytoin, and ciclosporin are different: gingival lesions associated with anticonvulsants are the most fibrotic, gingival tissue in patients treated with cyclosporin appears highly inflamed, while lesions related to nifedipine administration have intermediate characteristics [18].
The increase of the volume of gingival tissues represents the consequence of the connective tissue response: the alterations in cellular and extracellular matrix (ECM) metabolisms leads to an excessive storage of extracellular matrix proteins (collagens or amorphous ground substance) [15,19]. Calcium channel blockers can inhibit the intracellular calcium uptake, an event that leads to the stimulation of gingival fibroblasts, which are mainly involved in the synthesis and enzymatic degradation of ECM proteins. The pathogenetic hypothesis of DIGO is based on the presence of genetically predisposed gingival fibroblasts, which produce enormous quantity of collagen, consequently causing a disproportion in the collagen synthesis-degradation mechanism [13,20]. Some authors demonstrated otherwise that the storage of collagen in DIGO is the result of the inhibition of the collagen degradation process [21].
Objectives
This study aimed to analyze the effects of pharmacological treatment with nifedipine on human gingival fibroblasts’ activity, investigating the possible pathogenetic mechanisms that lead to the onset of gingival enlargement. The expression profile of 57 genes belonging to the “Extracellular Matrix and Adhesion Molecules” pathway, fibroblasts’ viability at different drug concentrations, and E-cadherin levels in treated fibroblasts were assessed.
2. Materials and Methods
2.1. Primary Human Fibroblast Cells’ Culture
Human gingival fibroblasts were purchased from ATCC® Cell Lines. Cells at the second passage were cultured in Dulbecco’s Modified Eagle Medium (DMEM) (Sigma Aldrich, Inc., St. Louis, MO, USA) supplemented with 10% fetal calf serum and antibiotics (penicillin 100 U/mL and streptomycin 100 micrograms/mL, Sigma Aldrich, Inc., St. Louis, MO, USA). Cells were replicated for subsequent experiments.
2.2. Cell Viability Test
Human gingival fibroblasts were seeded into 96-well plates at a density of 104 cells per well containing 100 µL of cell culture medium and incubated for 24 h to allow cell adherence.
A stock solution of nifedipine (Nifedicor, Meda Pharma Spa, Mylan, Italy) was prepared dissolving 1 mg of nifedipine powder in 1 mL of absolute ethanol.
The stock solution of Nnifedipine (1 mg/mL) was dissolved in DMEM medium to prepare serial dilution at the concentrations of 5000, 2000, 1000, 500, and 100 ng/mL.
The vehicle absolute ethanol dissolved in the cell culture medium at the same concentration used to prepare the serial dilutions was used as a negative control.
After 24 h of incubation, cell viability was measured using PrestoBlue™ Reagent Protocol (Invitrogen, Carlsbad, CA, USA) according to the manufacturer’s instructions, as previously described [22].
2.3. Cell Treatment
Once semi-confluence was reached (about 70% of confluence), cells were detached from flasks by trypsin-ethylenediaminetetraacetic acid (EDTA) and seeded at a density of 1 × 105 cells/mL into 9 cm2 (3 mL) wells. Cells were washed two times with phosphate-buffered saline (PBS) and incubated for 16 h at 37 °C with serum-free DMEM. After serum starvation, cells were treated for 24 h with 1000 ng/mL nifedipine solution prepared in DMEM supplemented with 2% FBS, antibiotics and amino acids. Absolute ethanol dissolved in the cell culture medium at the same concentration used to prepare the nifedipine solution treatment was used as a negative control.
Three biological replicates were performed.
Cells were maintained in a humidified atmosphere of 5% CO2 at 37 °C. After the end of the treatment, cells were sub-confluent (about 90% of confluence) and RNA was extracted from the cells.
2.4. RNA Isolation, Reverse Transcription, and Quantitative Real-Time Polymerase Chain Reaction
Total RNA was isolated from cells using the GenElute mammalian total RNA purification miniprep kit (Sigma-Aldrich) according to manufacturer’s instructions. Pure RNA was quantified by a NanoDrop 2000 spectrophotometer (Thermo Scientific, Waltham, MA, USA).
cDNA synthesis was performed starting from 500 ng of total RNA, using PrimeScript RT Master Mix (Takara Bio Inc.). The reaction was incubated at 37 °C for 15 min and inactivated by heating at 70 °C for 10 s.
cDNA was amplified by real-time quantitative PCR using the ViiA™ 7 System (Applied Biosystems, Foster City, CA, USA).
All PCR reactions were performed in a 20 µL volume. Each reaction contained 10 µL of 2x qPCRBIO SYGreen Mix Lo-ROX (Pcrbiosystems), 400 nM concentration of each primer, and cDNA.
Custom primers belonging to the “Extracellular Matrix and Adhesion Molecules” pathway were purchased from Sigma Aldrich. All experiments were performed including non-template controls to exclude reagents’ contamination. PCR was performed including two analytical replicates.
The amplification profile was initiated by 10 min incubation at 95 °C, followed by two-step amplification of 15 s at 95 °C and 60 s at 60 °C for 40 cycles. As a final step, a melt curve dissociation analysis was performed [22,23,24,25].
2.5. Statistical Analysis
RPL13 is the reference gene used in this analysis and was selected by preliminary test among three housekeeping genes. The expression of RPL13 appeared more consistent with the amount of RNA input. Gene expression quantification was conducted with the delta/delta Ct calculation method [26] and RPL13 was used as a reference gene to normalize the gene expression levels. After normalization, quantification cycles of treated cells and control were compared by a paired sample t test. Mean expression levels of treated cells were calculated as fold changes relative to the expression of untreated cells with the delta/delta calculation method.
2.6. Detection of E-Cadherin Levels by Enzyme-Linked Immunosorbent Assay
E-cadherin levels were measured by sandwich enzyme-linked immunoassay (ELISA) after fibroblast treatment with nifedipine, by using a commercial kit, Human E-cadherin ELISA KIT (Bioassay Technology Laboratory, Shanghai, China), which uses monoclonal antibodies directed against distinct epitopes of human E-cadherin (E-Cad).
The plate has been pre-coated with human E-Cad antibody. Samples were added to these wells and binds to antibodies coated on the wells. Then biotinylated human E-Cad antibody was added and binds to E-Cad in the sample. Then, the secondary antibody streptavidin- horseradish peroxidase was added and binds to the biotinylated E-Cad antibody. After incubation, unbound streptavidin-HRP was washed away during the washing step. Substrate solution was then added, and color develops in proportion to the amount of human E-Cad. The reaction was terminated by addition of acidic stop solution and absorbance is measured at 450 nm by an automated microplate reader (Sunrise™, Tecan Trading AG, Switzerland). E-cadherin levels were expressed as ng E-Cad/ng of total protein.
3. Results
PrestoBlue™ cell viability test was conducted to determine the optimal concentration of nifedipine to be used for cell treatment that did not significantly affect cell viability. PrestoBlue™ data showed that nifedipine, regardless of treatment concentration, had no effect on cell viability compared to that of untreated cells (Figure 1). Considering the percentage of viability cells, the concentration of 1000 ng/ul was assumed as not lethal but sufficient to induce a significant response.
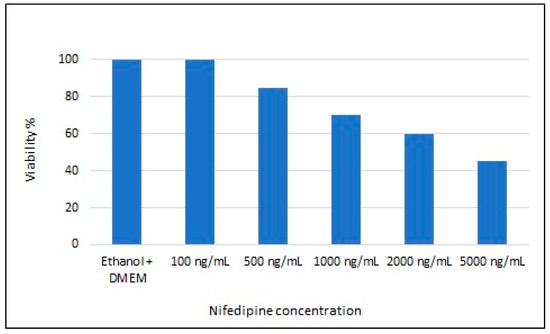
Figure 1.
Nifedipine effect on cell viability.
Based on this test, the optimal concentration used for the treatment was 1000 ng/mL.
The gene expression profile of 57 genes belonging to the “Extracellular Matrix and Adhesion Molecules” pathway was analyzed using real-time PCR. Table 1 reports the list of genes and their fold change after treatment with nifedipine. Bold fonts indicate significant variation of gene expression level: fold change ≥ 2 and p-value ≤ 0.05 for upregulated genes, and fold change ≤ 0.5 and p-value ≤ 0.05 for significantly downregulated genes.

Table 1.
Genes belonging to the “Extracellular Matrix and Adhesion Molecules” pathway analyzed using real-time Polymerase Chain Reaction. Fold changes represent the expression levels of genes after 24 h treatment with nifedipine, as compared with untreated cells (n = 3). SD = standard deviation.
Table 2 reports only the significantly deregulated genes after nifedipine treatment.

Table 2.
Significant gene expression levels after 24 h treatment with nifedipine, as compared with untreated cells (n = 3).
Genes significantly upregulated in treated cells with respect to the control were CDH1 (E-cadherin), belonging to cell adhesion genes, and MMP24 and MMP8, involved in extracellular matrix deposition.
The CDH1 (E-cadherin) levels measured by enzyme linked immunoassay (ELISA) after nifedipine treatment showed an increase of CDH1 levels (5.34-fold ± 0.19) in treated fibroblasts vs. untreated control, confirming the gene expression results obtained by real-time PCR.
Among the significant downregulated genes induced by the treatment, there were transmembrane receptor ITGA6 and ITGB4, the basement membrane constituent LAMA1 and LAMB1, and the extracellular matrix protease MMP11, MMP16, and MMP26.
Figure 2 shows the gene expression profile of the significant deregulated genes belonging to the “Extracellular Matrix and Adhesion Molecules” pathway.
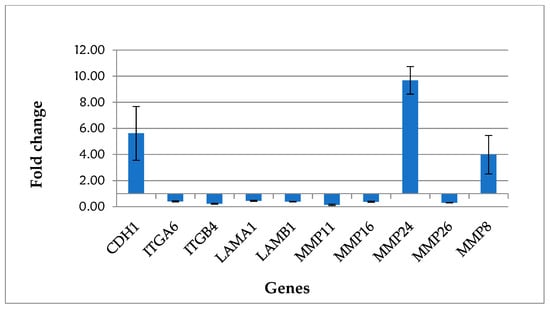
Figure 2.
Gene expression profile of fibroblasts treated with nifedipine.
4. Discussion
Gingival enlargement occurs as an adverse reaction in patients treated with calcium channel blocker agents (CCBs). It has been reported that DIGO is the most frequent periodontal side effect of CCBs, leading to mastication and speech problems, altering aesthetics, complicating oral hygiene maneuvers, and increasing the risk of bacterial infection [27]. CCBs take action in calcium metabolism: these drugs can decrease cellular Ca2+ influx and consequently reduce the uptake of folic acid, leading to a decreased synthesis of active collagenase, implicated in collagen degradation. Collagen degradation inhibition results in collagen accumulation [28,29]. In our study, fibroblasts were treated with nifedipine solution at a concentration of 1000 ng/mL for 24 h, and the obtained data showed a significant downregulation of genes coding for metalloproteinase (MMP) 11, 16, and 26. MMPs are enzymes involved in the connective tissue catabolic turnover, whose activation inhibition leads to connective tissue accumulation [30,31]. These results confirm that the increase of connective tissue collagen fibers induced by CCBs derives from the inhibition of collagen extracellular degradation pathways rather than from the increase in its production [21]. Downregulation was also observed for integrins ITGB4 and ITGA6, transmembrane cell-matrix adhesion receptors in fibroblasts that are responsible for collagen phagocytosis (collagen intracellular degradation pathway). The inhibition of MMPs expression occurs not only in nifedipine-induced GO, but it is also observable in gingival enlargement associated with immunosuppressants administration. The research of Lauritano et al. [32] recorded a downregulation of MMP8, MMP11, MMP15, MMP16, and MMP24 in gingival fibroblasts treated with cyclosporine and mycophenolate mophetil solutions. Many other authors demonstrated that anticonvulsant and immunosuppressant drugs are able to reduce the expression of α and β integrins or to interfere with collagen adhesion, thanks to pro-inflammatory cytokines action and, consequently, altering collagen phagocytosis mechanisms [33,34,35]. Kataoka et al. demonstrated, in particular, that the lack of collagen degradation, in a sample of 20-day-old rats treated with nifedipine, was associated with the storage of Type I collagen [36]. According to Kim et al. [37], nifedipine-induced gingival enlargement is characterized by an increase in Transforming growth factor -β signaling, known as a mediator of fibrotic processes and able to regulate extracellular matrix remodeling and inflammatory response [36,38]. The authors of this study demonstrated that TGF-β, in nifedipine-induced GO, causes an upregulation of gingival fibroblasts periostin, a matricellular protein involved in functional and structural regulation of connective tissue [39]. TGF-β also stimulates fibroblastic population and ECM deposit of fibronectin and glycosaminoglycans [40]. According to the literature, interleukin-1β (IL-1β) and IL-6 have been demonstrated to play a pathogenic role in drug-induced gingival fibrosis [12,41]. The authors of our study also assessed CDH1 (E-cadherin) level by performing an enzyme-linked immunoassay (ELISA): an increase of its levels was recorded in treated fibroblasts vs. untreated control, confirming the gene expression results obtained by real-time PCR. Cadherins 1 are calcium-dependent cell adhesion proteins and are involved in mechanisms regulating cell–cell adhesion, mobility, and proliferation of epithelial cells [42]. This data is in contrast with those provided by Kantarci et al. [43], who studied the effect of phenytoin, cyclosporine, and nifedipine administration on gingival tissues’ basement membranes. Kantarci et al. [43], according to other authors [44], demonstrated that DIGO shows signs of the “epithelial to mesenchymal transition” (EMT) process, in which epithelial cells differentiate into fibrogenic fibroblast-like cells, losing epithelial cell–cell or cell–ECM contacts and migrating into connective tissue. This fibrotic enlargement is supported by TGF-β1, that stimulates collagen accumulation and deposition by fibroblasts, by increasing the expression of connective tissue growth factor (CTGF) and that leads to the EMT phenomenon.
5. Conclusions
Gingival overgrowth has been proven to be an adverse reaction in patients taking the CCB agent nifedipine. Enzyme-linked immunoassay (ELISA) and the expression profile analysis of 57 genes belonging to the “Extracellular Matrix and Adhesion Molecules” pathway in treated fibroblasts highlighted increased CDH1 levels and a downregulation of MMP11, MMP16, and MMP26, and of integrins ITGB4 and ITGA6, respectively. This data suggested that the pathogenesis of nifedipine-induced GO is characterized by an excessive accumulation of collagen due to the inhibition of collagen intracellular and extracellular degradation pathways.
Author Contributions
D.L., conceptualization; G.M., data curation and writing—review; F.D.V. and A.P., investigation; F.C., validation; M.P., supervision. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Psaty, B.M.; Smith, N.L.; Siscovick, D.S.; Koepsell, T.D.; Weiss, N.S.; Heckbert, S.R.; Lemaitre, R.N.; Wagner, E.H.; Furberg, C.D. Health Outcomes Associated With Antihypertensive Therapies Used as First-Line AgentsA Systematic Review and Meta-analysis. JAMA 1997, 277, 739–745. [Google Scholar] [CrossRef] [PubMed]
- Whelton, P.K.; Carey, R.M.; Aronow, W.S.; Casey, D.E.; Collins, K.J.; Dennison Himmelfarb, C.; DePalma, S.M.; Gidding, S.; Jamerson, K.A.; Jones, D.W.; et al. ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the Prevention, Detection, Evaluation, and Management of High Blood Pressure in Adults: Executive Summary: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Hypertension 2018, 71, 1269–1324. [Google Scholar] [PubMed]
- Striessnig, J.; Ortner, N.; Pinggera, A. Pharmacology of L-type Calcium Channels: Novel Drugs for Old Targets? Curr. Mol. Pharmacol. 2015, 8, 110–122. [Google Scholar] [CrossRef] [PubMed]
- Godfraind, T. Discovery and Development of Calcium Channel Blockers. Front. Pharmacol. 2017, 8, 286. [Google Scholar] [CrossRef] [PubMed]
- van Geijn, H.P.; Lenglet, J.E.; Bolte, A.C. Nifedipine trials: Effectiveness and safety aspects. BJOG Int. J. Obstet. Gynaecol. 2005, 112, 79–83. [Google Scholar] [CrossRef]
- Khan, K.M.; Patel, J.; Schaefer, T.J. Nifedipine. StatPearls Publishing, Treasure Iland (FL). Available online: https://www.ncbi.nlm.nih.gov/books/NBK537052/ (accessed on 14 December 2020).
- Ross, S.D.; Akhras, K.S.; Zhang, S.; Rozinsky, M.; Nalysnyk, L. Discontinuation of antihypertensive drugs due to adverse events: A systematic review and meta-analysis. Pharmacother. J. Hum. Pharmacol. Drug Ther. 2001, 21, 940–953. [Google Scholar] [CrossRef] [PubMed]
- Miranda, J.; Brunet, L.; Roset, P.; Berini, L.; Farré, M.; Mendieta, C. Prevalence and Risk of Gingival Enlargement in Patients Treated With Nifedipine. J. Periodontol. 2001, 72, 605–611. [Google Scholar] [CrossRef]
- Sauget, P.; Monteil, R.A.; Morand, P.; Loubiere, R.; Lapalus, P.; Haudebourg, C. Gingival hyperplasia secondary to the use of calcium antagonists: Analysis. J. Boil. Buccale 1992, 20, 25–32. [Google Scholar]
- Lucas, R.M.; Howell, L.P.; Wall, B.A. Nifedipine-Induced Gingival Hyperplasia: A Histochemical and Ultrastructural Study. J. Periodontol. 1985, 56, 211–215. [Google Scholar] [CrossRef]
- Brunet, L.; Miranda, J.; Roset, P.; Berini, L.; Farré, M.; Mendieta, C. Prevalence and risk of gingival enlargement in patients treated with anticonvulsant drugs. Eur. J. Clin. Investig. 2001, 31, 781–788. [Google Scholar] [CrossRef]
- Dongari-Bagtzoglou, A. Informational Paper: Drug-Associated Gingival Enlargement. J. Periodontol. 2004, 75, 1424–1431. [Google Scholar] [CrossRef]
- Ramírez-Rámiz, A.; Brunet-Llobet, L.; Lahor-Soler, E.; Miranda-Rius, J. On the Cellular and Molecular Mechanisms of Drug-Induced Gingival Overgrowth. Open Dent. J. 2017, 11, 420–435. [Google Scholar] [CrossRef]
- Miranda, J.; Brunet, L.; Roset, P.; Farre, M.; Mendieta, C. Reliability of two measurement indices for gingival enlargement. J. Periodontal Res. 2012, 47, 776–782. [Google Scholar] [CrossRef]
- Bharti, V.; Bansal, C. Drug-induced gingival overgrowth: The nemesis of gingiva unravelled. J. Indian Soc. Periodontol. 2013, 17, 182–187. [Google Scholar] [CrossRef] [PubMed]
- Eslami, M.; Baghaii, F.; Jalayer Nadery, N. An Investigation on gingival hyperplasia induced by nifedipine. J. Dent. 2004, 1, 33–37. [Google Scholar]
- Sunil, P.M.; Nalluswami, J.S.; Sanghar, S.J.; Joseph, I. Nifedipine-induced gingival enlargement: Correlation with dose and oral hygiene. J. Pharm. Bioallied Sci. 2012, 4, S191–S193. [Google Scholar] [CrossRef] [PubMed]
- Trackman, P.; Kantarci, A. Molecular and clinical aspects of drug-induced gingival overgrowth. J. Dent. Res. 2015, 94, 540–546. [Google Scholar] [CrossRef] [PubMed]
- Kanno, C.M.; De Oliveira, J.A.; Ervolino, E.; Soubhia, A.M.P. Effects of cyclosporin, nifedipine and phenytoin on gingival myofibroblast transdifferentiation in monkeys. J. Appl. Oral Sci. 2018, 27, e20180135. [Google Scholar] [CrossRef]
- Dannewitz, B.; Edrich, C.; Tomakidi, P.; Kohl, A.; Gabbert, O.; Steinberg, T.; Staehle, H.-J. Elevated levels of gene expression for collagen and decorin in human gingival overgrowth. J. Clin. Periodontol. 2006, 33, 510–516. [Google Scholar] [CrossRef]
- Kanno, C.M.; Oliveira, J.A.; Garcia, J.F.; Castro, A.L.; Crivelini, M.M. Effects of Cyclosporin, Phenytoin, and Nifedipine on the Synthesis and Degradation of Gingival Collagen in Tufted Capuchin Monkeys (Cebus apella): Histochemical and MMP-1 and -2 and Collagen I Gene Expression Analyses. J. Periodontol. 2008, 79, 114–122. [Google Scholar] [CrossRef]
- Lauritano, D.; Palmieri, A.; Lucchese, A.; Di Stasio, D.; Moreo, G.; Carinci, F. Role of Cyclosporine in Gingival Hyperplasia: An In Vitro Study on Gingival Fibroblasts. Int. J. Mol. Sci. 2020, 21, 595. [Google Scholar] [CrossRef]
- Lauritano, D.; Lucchese, A.; Di Stasio, D.; Della Vella, F.; Cura, F.; Palmieri, A.; Carinci, F. Molecular Aspects of Drug-Induced Gingival Overgrowth: An In Vitro Study on Amlodipine and Gingival Fibroblasts. Int. J. Mol. Sci. 2019, 20, 2047. [Google Scholar] [CrossRef]
- Lauritano, D.; Moreo, G.; Limongelli, L.; Tregambi, E.; Palmieri, A.; Carinci, F. Drug-Induced Gingival Overgrowth: A Pilot Study on the Effect of Diphenylhydantoin and Gabapentin on Human Gingival Fibroblasts. Int. J. Environ. Res. Public Heal. 2020, 17, 8229. [Google Scholar] [CrossRef]
- Pre-treatment with berberine enhances effect of 5-fluorouracil and cisplatin in HEP2 laryngeal cancer cell line. J. Biol. Regul. Homeost. Agents. 2018, 32 (2 Suppl. 1), 167–177.
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2-ΔΔCT Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Umeizudike, K.A.; Olawuyi, A.B.; Umeizudike, T.I.; Olusegun-Joseph, A.D.; Bello, B.T. Effect of Calcium Channel Blockers on Gingival Tissues in Hypertensive Patients in Lagos, Nigeria: A Pilot Study. Contemp. Clin. Dent. 2017, 8, 565–570. [Google Scholar] [CrossRef] [PubMed]
- Barclay, S.; Thomason, J.M.; Idle, J.R.; Seymour, R.A. The incidence and severity of nifedipine-induced gingival overgrowth. J. Clin. Periodontol. 1992, 19, 311–314. [Google Scholar] [CrossRef] [PubMed]
- Livada, R.; Shiloah, J. Calcium channel blocker-induced gingival enlargement. J. Hum. Hypertens. 2014, 28, 10–14. [Google Scholar] [CrossRef] [PubMed]
- Brown, R.S.; Arany, P.R. Mechanism of drug-induced gingival overgrowth revisited: A unifying hypothesis. Oral Dis. 2015, 21, e51–e61. [Google Scholar] [CrossRef]
- Wang, X.; Khalil, R.A. Matrix metalloproteinases, vascular remodeling, and vascular disease. Adv. Pharmacol. 2018, 81, 241–330. [Google Scholar] [CrossRef] [PubMed]
- Lauritano, D.; Moreo, G.; Limongelli, L.; Palmieri, A.; Carinci, F. Drug-Induced Gingival Overgrowth: The Effect of Cyclosporin A and Mycophenolate Mophetil on Human Gingival Fibroblasts. Biomedicines 2020, 8, 221. [Google Scholar] [CrossRef]
- Kataoka, M.; Shimizu, Y.; Kunikiyo, K.; Asahara, Y.; Yamashita, K.; Ninomiya, M.; Morisaki, I.; Oshaki, Y.; Kido, J.-I.; Nagata, T. Cyclosporin A decreases the degradation of type I collagen in rat gingival overgrowth. J. Cell Physiol. 2000, 182, 351–358. [Google Scholar] [CrossRef]
- Kato, T.; Okahashi, N.; Kawai, S.; Kato, T.; Inaba, H.; Morisaki, I.; Amano, A. Impaired Degradation of Matrix Collagen in Human Gingival Fibroblasts by the Antiepileptic Drug Phenytoin. J. Periodontol. 2005, 76, 941–950. [Google Scholar] [CrossRef]
- Kato, T.; Okahashi, N.; Ohno, T.; Inaba, H.; Kawai, S.; Amano, A. Effect of phenytoin on collagen accumulation by human gingival fibroblasts exposed to TNF-alphain vitro. Oral Dis. 2006, 12, 156–162. [Google Scholar] [CrossRef]
- Kataoka, M.; Shimizu, Y.; Kunikiyo, K.; Asahara, Y.; Azuma, H.; Sawa, T.; Kido, J.-I.; Nagata, T. Nifedipine Induces Gingival Overgrowth in Rats Through a Reduction in Collagen Phagocytosis by Gingival Fibroblasts. J. Periodontol. 2001, 72, 1078–1083. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.S.; Jackson-Boeters, L.; Darling, M.R.; Rieder, M.J.; Hamilton, D. Nifedipine Induces Periostin Expression in Gingival Fibroblasts through TGF-beta. J. Dent. Res. 2013, 92, 1022–1028. [Google Scholar] [CrossRef] [PubMed]
- Caja, L.; Dituri, F.; Mancarella, S.; Caballero-Diaz, D.; Moustakas, A.; Giannelli, G.; Fabregat, I. TGF-β and the Tissue Microenvironment: Relevance in Fibrosis and Cancer. Int. J. Mol. Sci. 2018, 19, 1294. [Google Scholar] [CrossRef] [PubMed]
- Hamilton, D.W. Functional role of periostin in development and wound repair: Implications for connective tissue disease. J. Cell Commun. Signal. 2008, 2, 9–17. [Google Scholar] [CrossRef] [PubMed]
- Dreyfuss, J.L.; Veiga, S.S.; Coulson-Thomas, V.J.; Santos, I.A.; Toma, L.; Coletta, R.D.; Nader, H.B. Differences in the expression of glycosaminoglycans in human fibroblasts derived from gingival overgrowths is related to TGF-beta up-regulation. Growth Factors 2009, 28, 24–33. [Google Scholar] [CrossRef]
- Ganesh, P.R. Immunoexpression of interleukin-6 in drug-induced gingival overgrowth patients. Contemp. Clin. Dent. 2016, 7, 140–145. [Google Scholar] [CrossRef]
- National Center for Biotechnology Information. PubChem Protein Summary for NCBI Protein P12830, Cadherin-1. 2021. Available online: https://pubchem.ncbi.nlm.nih.gov/protein/P12830 (accessed on 6 January 2021).
- Kantarci, A.; Nseir, Z.; Kim, Y.-S.; Sume, S.S.; Trackman, P. Loss of Basement Membrane Integrity in Human Gingival Overgrowth. J. Dent. Res. 2011, 90, 887–893. [Google Scholar] [CrossRef]
- Sume, S.S.; Kantarci, A.; Lee, A.; Hasturk, H.; Trackman, P.C. Epithelial to Mesenchymal Transition in Gingival Overgrowth. Am. J. Pathol. 2010, 177, 208–218. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).