Enzymatic Synthesis and Characterization of Different Families of Chitooligosaccharides and Their Bioactive Properties
Abstract
1. Introduction
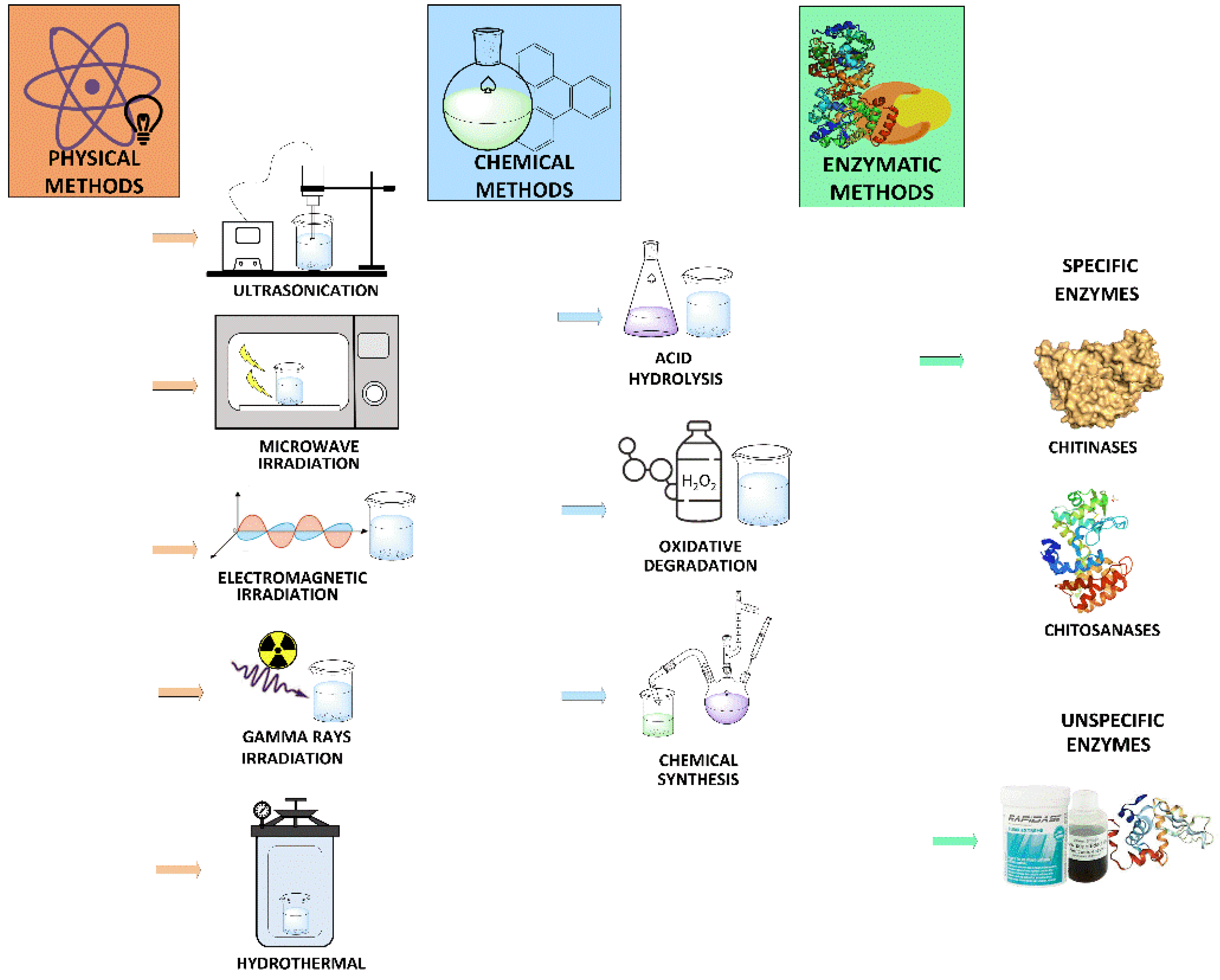
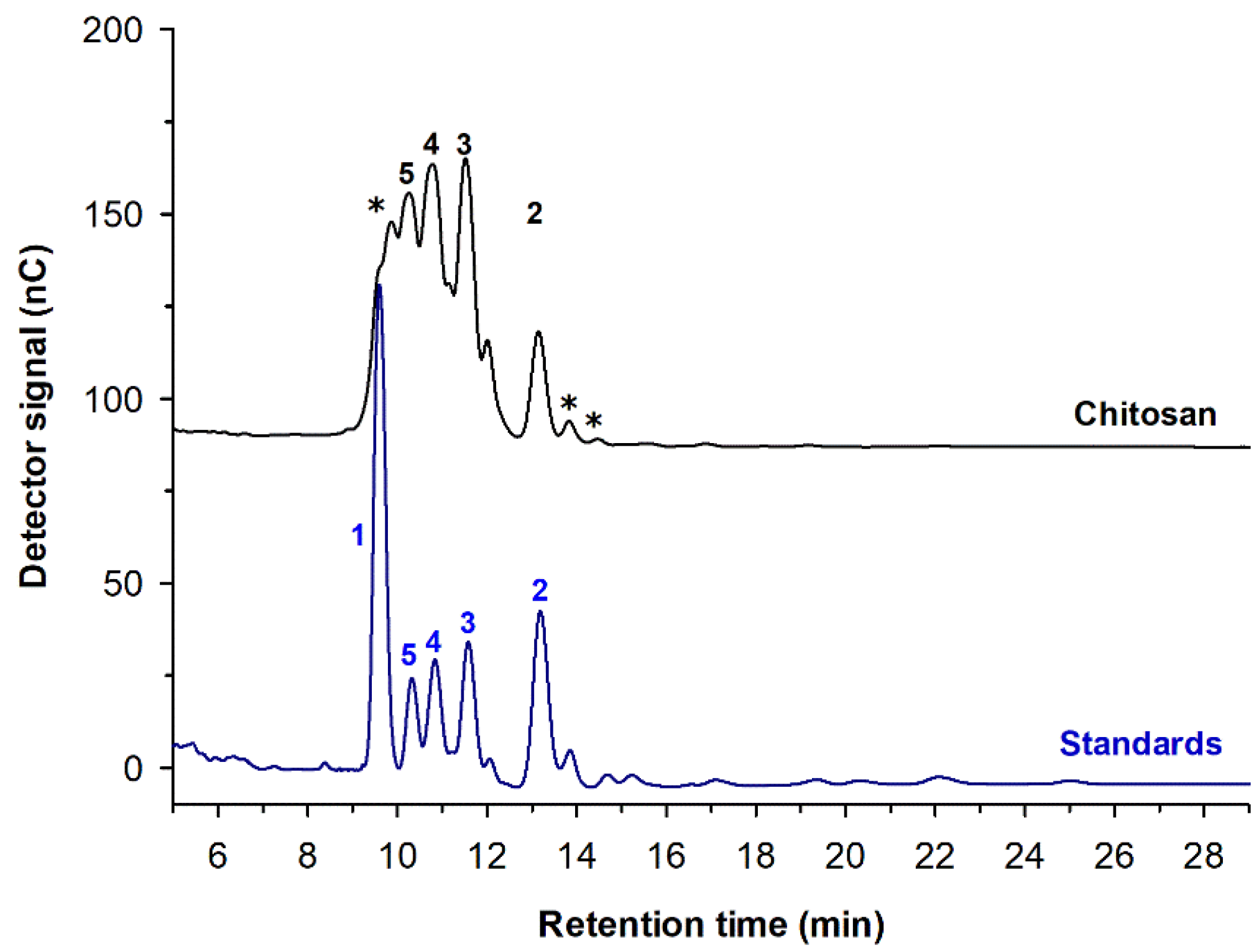
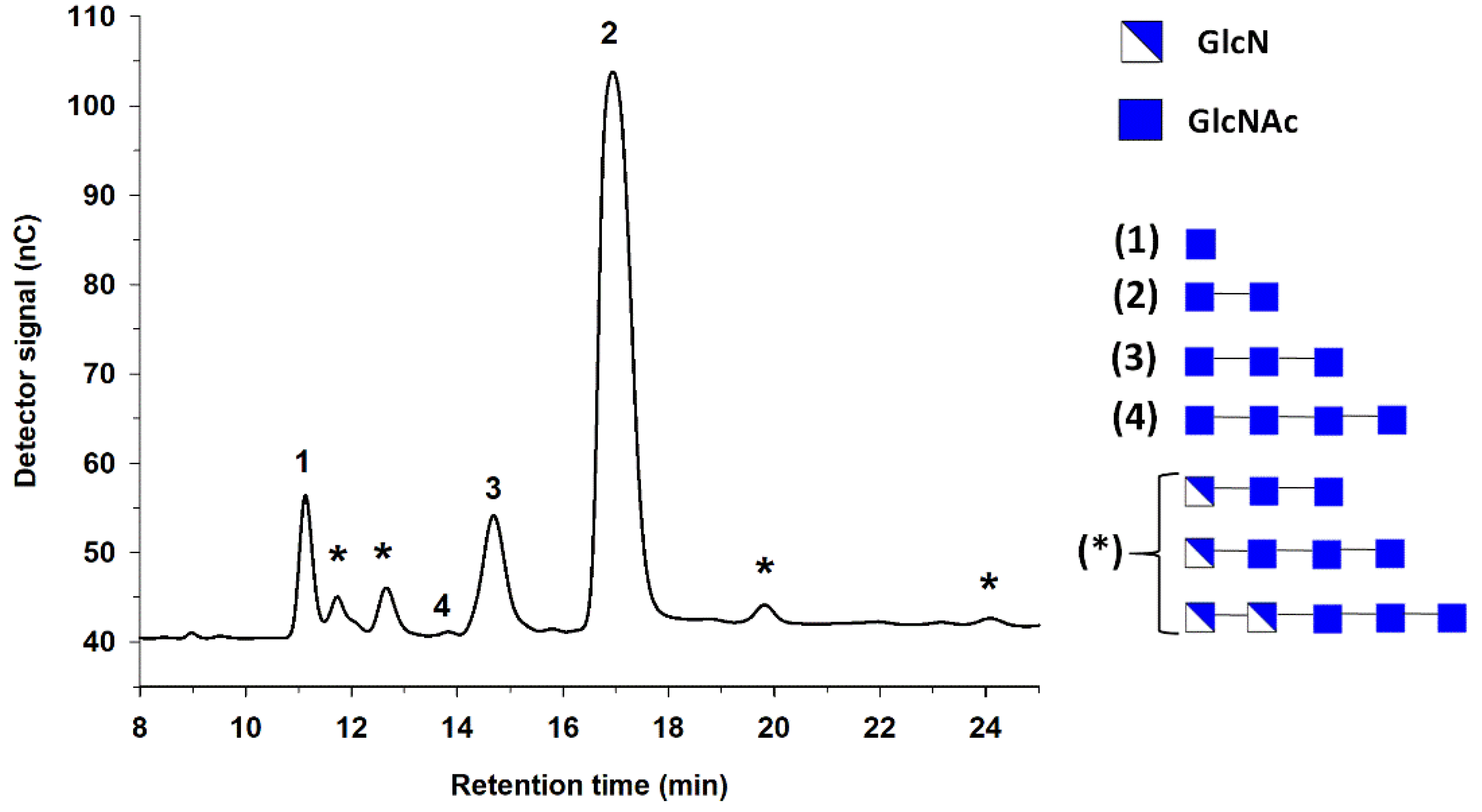
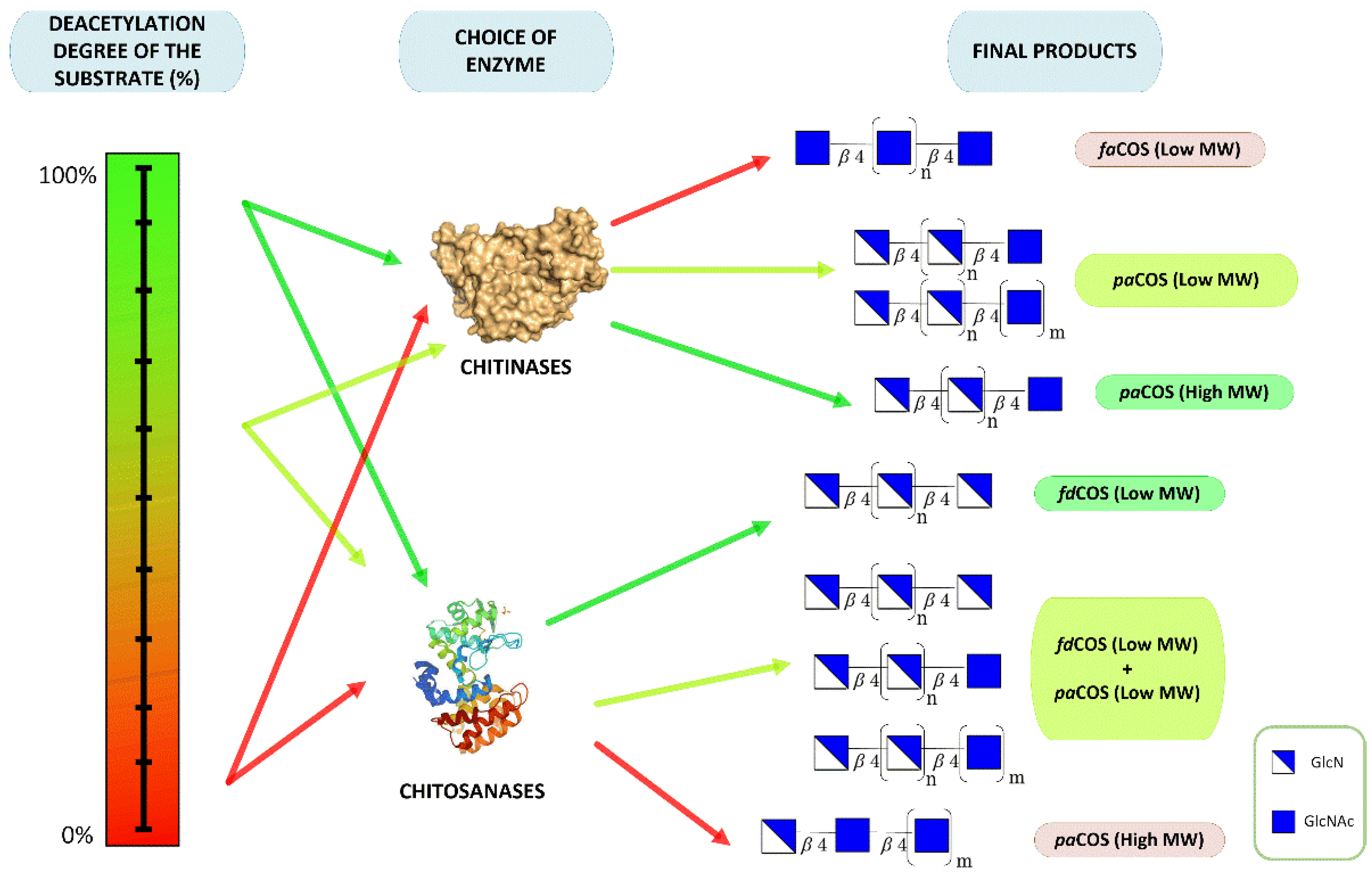
2. Chitooligosaccharides Production
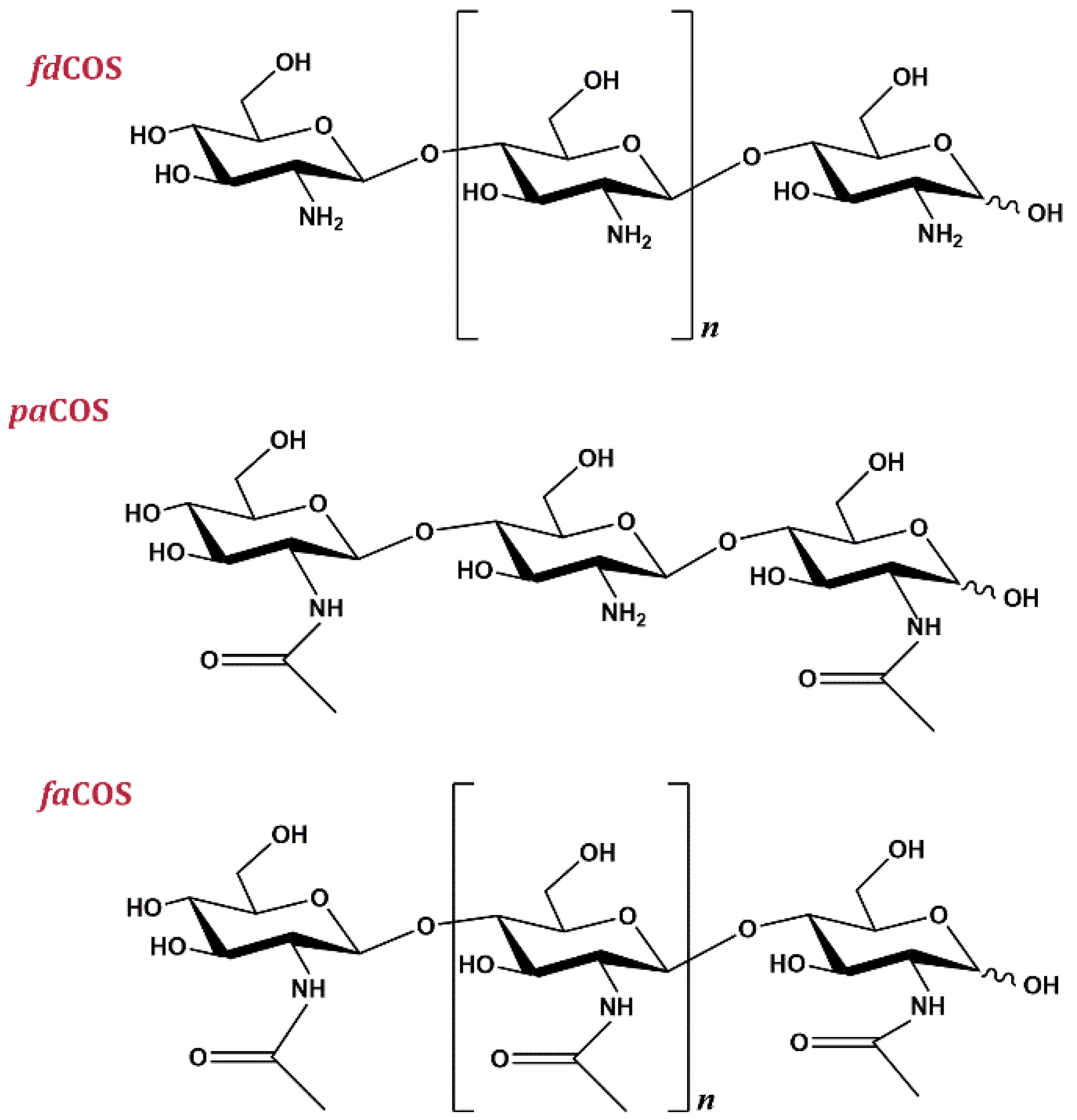
3. Types of Chitooligosaccharides
3.1. Fully Deacetylated Chitooligosaccharides (fdCOS)
3.2. Fully Acetylated Chitooligosaccharides (faCOS)
3.3. Partially Acetylated Chitooligosaccharides (paCOS)
4. Large-Scale Procedure for the Production of Chitooligosaccharides
5. Bioactive Properties of COS
Effect of COS Composition on Anti-Inflammatory Properties
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Hamed, I.; Özogul, F.; Regenstein, J.M. Industrial applications of crustacean by-products (chitin, chitosan, and chitooligosaccharides): A review. Trends Food Sci. Technol. 2016, 48, 40–50. [Google Scholar] [CrossRef]
- Hamer, S.N.; Cord-Landwehr, S.; Biarnés, X.; Planas, A.; Waegeman, H.; Moerschbacher, B.M.; Kolkenbrock, S. Enzymatic production of defined chitosan oligomers with a specific pattern of acetylation using a combination of chitin oligosaccharide deacetylases. Sci. Rep. 2015, 5, 8716. [Google Scholar] [CrossRef]
- Zhou, J.; Wen, B.; Xie, H.; Zhang, C.; Bai, Y.; Cao, H.; Che, Q.; Guo, J.; Su, Z. Advances in the preparation and assessment of the biological activities of chitosan oligosaccharides with different structural characteristics. Food Funct. 2021, 12, 926–951. [Google Scholar] [CrossRef] [PubMed]
- Grifoll-Romero, L.; Pascual, S.; Aragunde, H.; Biarnés, X.; Planas, A. Chitin deacetylases: Structures, specificities, and biotech applications. Polymers 2018, 10, 352. [Google Scholar] [CrossRef] [PubMed]
- Xia, W.; Liu, P.; Zhang, J.; Chen, J. Biological activities of chitosan and chitooligosaccharides. Food Hydrocoll. 2011, 25, 170–179. [Google Scholar] [CrossRef]
- Park, B.K.; Kim, M.M. Applications of chitin and its derivatives in biological medicine. Int. J. Mol. Sci. 2010, 11, 5152–5164. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Yu, B. Recent advances in the synthesis of chitooligosaccharides and congeners. Tetrahedron 2014, 70, 1023–1046. [Google Scholar] [CrossRef]
- Sun, H.; Gao, L.; Xue, C.; Mao, X. Marine-polysaccharide degrading enzymes: Status and prospects. Compr. Rev. Food Sci. Food Saf. 2020, 19, 2767–2796. [Google Scholar] [CrossRef]
- Chen, A.S.; Taguchi, T.; Sakai, K.; Kikuchi, K.; Wang, M.W.; Miwa, I. Antioxidant activities of chitobiose and chitotriose. Biol. Pharm. Bull. 2003, 26, 1326–1330. [Google Scholar] [CrossRef] [PubMed]
- Kumar, M.; Brar, A.; Vivekanand, V.; Pareek, N. Bioconversion of chitin to bioactive chitooligosaccharides: Amelioration and coastal pollution reduction by microbial resources. Mar. Biotechnol. 2018, 20, 269–281. [Google Scholar] [CrossRef]
- Zhao, M.; Gu, L.; Li, Y.; Chen, S.; You, J.; Fan, L.; Wang, Y.; Zhao, L. Chitooligosaccharides display anti-tumor effects against human cervical cancer cells via the apoptotic and autophagic pathways. Carbohydr. Polym. 2019, 224, 115171. [Google Scholar] [CrossRef] [PubMed]
- Sánchez, Á.; Mengíbar, M.; Fernández, M.; Alemany, S.; Heras, A.; Acosta, N. Influence of preparation methods of chitooligosaccharides on their physicochemical properties and their anti-inflammatory effects in mice and in RAW264.7 macrophages. Mar. Drugs 2018, 16, 430. [Google Scholar] [CrossRef]
- Marmouzi, I.; Ezzat, S.M.; Salama, M.M.; Merghany, R.M.; Attar, A.M.; El-Desoky, A.M.; Mohamed, S.O. Recent Updates in pharmacological properties of chitooligosaccharides. BioMed. Res. Int. 2019, 2019, 4568039. [Google Scholar] [CrossRef]
- Yang, Y.; Xing, R.; Liu, S.; Qin, Y.; Li, K.; Yu, H.; Li, P. Immunostimulatory Effects of Chitooligosaccharides on RAW 264.7 Mouse Macrophages via Regulation of the MAPK and PI3K/Akt Signaling Pathways. Mar. Drugs 2019, 17, 36. [Google Scholar] [CrossRef]
- Guan, G.; Azad, M.A.K.; Lin, Y.; Kim, S.W.; Tian, Y.; Liu, G.; Wang, H. Biological effects and applications of chitosan and chito-oligosaccharides. Front. Physiol. 2019, 10, 516. [Google Scholar] [CrossRef]
- Xia, W.; Liu, P.; Liu, J. Advance in chitosan hydrolysis by non-specific cellulases. Bioresour. Technol. 2008, 99, 6751–6762. [Google Scholar] [CrossRef]
- Aktuganov, G.E.; Melentiev, A.I.; Varlamov, V.P. Biotechnological Aspects of the Enzymatic Preparation of Bioactive Chitooligosaccharides. Appl. Biochem. Microbiol. 2019, 55, 323–343. [Google Scholar] [CrossRef]
- Moon, C.; Seo, D.J.; Song, Y.S.; Hong, S.H.; Choi, S.H.; Jung, W.J. Antifungal activity and patterns of N-acetyl-chitooligosaccharide degradation via chitinase produced from Serratia marcescens PRNK-1. Microb. Pathog. 2017, 113, 218–224. [Google Scholar] [CrossRef] [PubMed]
- Ismail, S.A.; El-Sayed, H.S.; Fayed, B. Production of prebiotic chitooligosaccharide and its nano/microencapsulation for the production of functional yoghurt. Carbohydr. Polym. 2020, 234, 115941. [Google Scholar] [CrossRef] [PubMed]
- Santos-Moriano, P.; Kidibule, P.E.; Alleyne, E.; Ballesteros, A.O.; Heras, A.; Fernandez-Lobato, M.; Plou, F.J. Efficient conversion of chitosan into chitooligosaccharides by a chitosanolytic activity from Bacillus thuringiensis. Process Biochem. 2018, 73, 102–108. [Google Scholar] [CrossRef]
- Baxter, S.; Zivanovic, S.; Weiss, J. Molecular weight and degree of acetylation of high-intensity ultrasonicated chitosan. Food Hydrocoll. 2005, 19, 821–830. [Google Scholar] [CrossRef]
- Wu, Y.; Huang, Y.; Zhou, Y.; Ren, X.e.; Yang, F. Degradation of chitosan by swirling cavitation. Innov. Food Sci. Emerg. Technol. 2014, 23, 188–193. [Google Scholar] [CrossRef]
- Zainol, I.; Akil, H.M.; Mastor, A. Effect of γ-irradiation on the physical and mechanical properties of chitosan powder. Mater. Sci. Eng. C 2009, 29, 292–297. [Google Scholar] [CrossRef]
- Trombotto, S.; Ladavière, C.; Delolme, F.; Domard, A. Chemical preparation and structural characterization of a homogeneous series of chitin/chitosan oligomers. Biomacromolecules 2008, 9, 1731–1738. [Google Scholar] [CrossRef] [PubMed]
- Xia, Z.; Wu, S.; Chen, J. Preparation of water soluble chitosan by hydrolysis using hydrogen peroxide. Int. J. Biol. Macromol. 2013, 59, 242–245. [Google Scholar] [CrossRef]
- Je, J.Y.; Kim, S.K. Chitooligosaccharides as potential nutraceuticals: Production and bioactivities. Adv. Food Nutr. Res. 2012, 65, 321–336. [Google Scholar]
- Sinha, S.; Chand, S.; Tripathi, P. Recent progress in chitosanase production of monomer-free chitooligosaccharides: Bioprocess strategies and future applications. Appl. Biochem. Biotechnol. 2016, 180, 883–899. [Google Scholar] [CrossRef]
- Sheldon, R.A.; Woodley, J.M. Role of Biocatalysis in sustainable chemistry. Chem. Rev. 2018, 118, 801–838. [Google Scholar] [CrossRef]
- Oyeleye, A.; Normi, Y.M. Chitinase: Diversity, limitations, and trends in Engineering for suitable applications. Biosci. Rep. 2018, 38, BSR2018032300. [Google Scholar] [CrossRef]
- Singh, R.V.; Sambyal, K.; Negi, A.; Sonwani, S.; Mahajan, R. Chitinases production: A robust enzyme and its industrial applications. Biocatal. Biotransform. 2021, 39, 161–189. [Google Scholar] [CrossRef]
- Thadathil, N.; Velappan, S.P. Recent developments in chitosanase research and its biotechnological applications: A review. Food Chem. 2014, 150, 392–399. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Fei, Z.; Gong, J.; Yang, T.; Xu, Z.; Shi, J. Screening and characterization of a highly active chitosanase based on metagenomic technology. J. Mol. Catal. B Enzym. 2015, 111, 29–35. [Google Scholar] [CrossRef]
- Kittur, F.S.; Vishu Kumar, A.B.; Varadaraj, M.C.; Tharanathan, R.N. Chitooligosaccharides—Preparation with the aid of pectinase isozyme from Aspergillus niger and their antibacterial activity. Carbohydr. Res. 2005, 340, 1239–1245. [Google Scholar] [CrossRef]
- Montilla, A.; Ruiz-Matute, A.I.; Corzo, N.; Giacomini, C.; Irazoqui, G. Enzymatic generation of chitooligosaccharides from chitosan using soluble and immobilized glycosyltransferase (Branchzyme). J. Agric. Food Chem. 2013, 61, 10360–10367. [Google Scholar] [CrossRef] [PubMed]
- Cantarel, B.L.; Coutinho, P.M.; Rancurel, C.; Bernard, T.; Lombard, V.; Henrissat, B. The Carbohydrate-Active EnZymes database (CAZy): An expert resource for Glycogenomics. Nucleic Acids Res. 2009, 37, D233–D238. [Google Scholar] [CrossRef] [PubMed]
- Sunny, N.E.; Rajesh Kumar, S.; Venkat Kumar, S. A review on Chitinase synthesis from varied sources and its applications towards environment. Res. J. Pharm. Technol. 2018, 11, 4200–4208. [Google Scholar] [CrossRef]
- Liu, S.; Shao, S.; Li, L.; Cheng, Z.; Tian, L.; Gao, P.; Wang, L. Substrate-binding specificity of chitinase and chitosanase as revealed by active-site architecture analysis. Carbohydr. Res. 2015, 418, 50–56. [Google Scholar] [CrossRef]
- Huang, Q.S.; Xie, X.L.; Liang, G.; Gong, F.; Wang, Y.; Wei, X.Q.; Wang, Q.; Ji, Z.L.; Chen, Q.X. The GH18 family of chitinases: Their domain architectures, functions and evolutions. Glycobiology 2012, 22, 23–34. [Google Scholar] [CrossRef] [PubMed]
- Kidibule, P.E.; Santos-Moriano, P.; Jiménez-Ortega, E.; Ramírez-Escudero, M.; Limón, M.C.; Remacha, M.; Plou, F.J.; Sanz-Aparicio, J.; Fernández-Lobato, M. Use of chitin and chitosan to produce new chitooligosaccharides by chitinase Chit42: Enzymatic activity and structural basis of protein specificity. Microb. Cell Fact. 2018, 17, 47. [Google Scholar] [CrossRef]
- Lienemann, M.; Boer, H.; Paananen, A.; Cottaz, S.; Koivula, A. Toward understanding of carbohydrate binding and substrate specificity of a glycosyl hydrolase 18 family (GH-18) chitinase from Trichoderma harzianum. Glycobiology 2009, 19, 1116–1126. [Google Scholar] [CrossRef]
- Hoell, I.A.; Vaaje-Kolstad, G.; Eijsink, V.G.H. Structure and function of enzymes acting on chitin and chitosan. Biotechnol. Genet. Eng. Rev. 2010, 27, 331–366. [Google Scholar] [CrossRef]
- Aktuganov, G.E.; Melent’ev, A.I. Specific features of chitosan depolymerization by chitinases, chitosanases, and nonspecific enzymes in the production of bioactive chitooligosaccharides. Appl. Biochem. Microbiol. 2017, 53, 611–627. [Google Scholar] [CrossRef]
- Weikert, T.; Niehues, A.; Cord-Landwehr, S.; Hellmann, M.J.; Moerschbacher, B.M. Reassessment of chitosanase substrate specificities and classification. Nat. Commun. 2017, 8, 1698. [Google Scholar] [CrossRef]
- Olicón-Hernández, D.R.; Vázquez-Landaverde, P.A.; Cruz-Camarillo, R.; Rojas-Avelizapa, L.I. Comparison of chito-oligosaccharide production from three different colloidal chitosans using the endochitonsanolytic system of Bacillus thuringiensis. Prep. Biochem. Biotechnol. 2017, 47, 116–122. [Google Scholar] [CrossRef]
- Pantaleone, D.; Yalpani, M.; Scollar, M. Unusual susceptibility of chitosan to enzymic hydrolysis. Carbohydr. Res. 1992, 237, 325–332. [Google Scholar] [CrossRef]
- Santos-Moriano, P.; Fernandez-Arrojo, L.; Mengibar, M.; Belmonte-Reche, E.; Peñalver, P.; Acosta, F.N.; Ballesteros, A.O.; Morales, J.C.; Kidibule, P.; Fernandez-Lobato, M.; et al. Enzymatic production of fully deacetylated chitooligosaccharides and their neuroprotective and anti-inflammatory properties. Biocatal. Biotransform. 2018, 36, 57–67. [Google Scholar] [CrossRef]
- Arnold, N.D.; Brück, W.M.; Garbe, D.; Brück, T.B. Enzymatic modification of native chitin and conversion to specialty chemical products. Mar. Drugs 2020, 18, 93. [Google Scholar] [CrossRef]
- Mallakuntla, M.K.; Vaikuntapu, P.R.; Bhuvanachandra, B.; Podile, A.R. Selection and mutational analyses of the substrate interacting residues of a chitinase from Enterobacter cloacae subsp. cloacae (EcChi2) to improve transglycosylation. Int. J. Biol. Macromol. 2020, 165, 2432–2441. [Google Scholar] [CrossRef] [PubMed]
- Tanabe, T.; Morinaga, K.; Fukamizo, T.; Mitsutomi, M. Novel chitosanase from Streptomyces griseus HUT 6037 with transglycosylation activity. Biosci. Biotechnol. Biochem. 2003, 67, 354–364. [Google Scholar] [CrossRef] [PubMed]
- Nidheesh, T.; Pal, G.K.; Suresh, P.V. Chitooligomers preparation by chitosanase produced under solid state fermentation using shrimp by-products as substrate. Carbohydr. Polym. 2015, 121, 1–9. [Google Scholar] [CrossRef]
- Santos-Moriano, P.; Woodley, J.M.; Plou, F.J. Continuous production of chitooligosaccharides by an immobilized enzyme in a dual-reactor system. J. Mol. Catal. B Enzym. 2016, 133, 211–217. [Google Scholar] [CrossRef]
- Kumar, A.B.V.; Tharanathan, R.N. A comparative study on depolymerization of chitosan by proteolytic enzymes. Carbohydr. Polym. 2004, 58, 275–283. [Google Scholar]
- Pan, A.D.; Zeng, H.-Y.; Foua, G.B.; Alain, C.; Li, Y.-Q. Enzymolysis of chitosan by papain and its kinetics. Carbohydr. Polym. 2016, 135, 199–206. [Google Scholar] [CrossRef]
- Mengíbar, M.; Mateos-Aparicio, I.; Miralles, B.; Heras, A. Influence of the physico-chemical characteristics of chito-oligosaccharides (COS) on antioxidant activity. Carbohydr. Polym. 2013, 97, 776–782. [Google Scholar] [CrossRef] [PubMed]
- Ortiz-Rodríguez, T.; De La Fuente-Salcido, N.; Bideshi, D.K.; Salcedo-Hernández, R.; Barboza-Corona, J.E. Generation of chitin-derived oligosaccharides toxic to pathogenic bacteria using ChiA74, an endochitinase native to Bacillus thuringiensis. Lett. Appl. Microbiol. 2010, 51, 184–190. [Google Scholar] [CrossRef] [PubMed]
- Kidibule, P.E.; Santos-Moriano, P.; Plou, F.J.; Fernández-Lobato, M. Endo-chitinase Chit33 specificity on different chitinolytic materials allows the production of unexplored chitooligosaccharides with antioxidant activity. Biotechnol. Rep. 2020, 27, e00500. [Google Scholar] [CrossRef] [PubMed]
- Kazami, N.; Sakaguchi, M.; Mizutani, D.; Masuda, T.; Wakita, S.; Oyama, F.; Kawakita, M.; Sugahara, Y. A simple procedure for preparing chitin oligomers through acetone precipitation after hydrolysis in concentrated hydrochloric acid. Carbohydr. Polym. 2015, 132, 304–310. [Google Scholar] [CrossRef] [PubMed]
- Hao, W.; Li, K.; Li, P. Review: Advances in preparation of chitooligosaccharides with heterogeneous sequences and their bioactivity. Carbohydr. Polym. 2021, 252, 117206. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Xing, R.; Liu, S.; Li, P. Advances in preparation, analysis and biological activities of single chitooligosaccharides. Carbohydr. Polym. 2016, 139, 178–190. [Google Scholar] [CrossRef]
- Torres-Salas, P.; Del Monte-Martinez, A.; Cutiño-Avila, B.; Rodriguez-Colinas, B.; Alcalde, M.; Ballesteros, A.O.; Plou, F.J. Immobilized biocatalysts: Novel approaches and tools for binding enzymes to supports. Adv. Mater. 2011, 23, 5275–5282. [Google Scholar] [CrossRef]
- Míguez, N.; Gimeno-Pérez, M.; Fernández-Polo, D.; Cervantes, F.; Ballesteros, A.; Fernández-Lobato, M.; Ribeiro, M.; Plou, F. Immobilization of the β-fructofuranosidase from Xanthophyllomyces dendrorhous by entrapment in polyvinyl alcohol and its application to neo-fructooligosaccharides production. Catalysts 2018, 8, 201. [Google Scholar] [CrossRef]
- Sheldon, R.A. Enzyme immobilization: The quest for optimum performance. Adv. Synth. Catal. 2007, 349, 1289–1307. [Google Scholar] [CrossRef]
- Kidibule, P.E.; Costa, J.; Atrei, A.; Plou, F.J.; Fernandez-Lobato, M.; Pogni, R. Production and characterization of chitooligosaccharides by the fungal chitinase Chit42 immobilized on magnetic nanoparticles and chitosan beads: Selectivity, specificity and improved operational utility. RSC Adv. 2021, 11, 5529–5536. [Google Scholar] [CrossRef]
- Oh, S.-H.; Vo, T.-S.; Ngo, D.-H.; Kim, S.-Y.; Ngo, D.-N.; Kim, S.-K. Prevention of H2O2-induced oxidative stress in murine microglial BV-2 cells by chitin-oligomers. Process Biochem. 2016, 51, 2170–2175. [Google Scholar] [CrossRef]
- Luo, Z.; Dong, X.; Ke, Q.; Duan, Q.; Shen, L. Chitooligosaccharides inhibit ethanol-induced oxidative stress via activation of Nrf2 and reduction of MAPK phosphorylation. Oncol. Rep. 2014, 32, 2215–2222. [Google Scholar] [CrossRef] [PubMed]
- Kaczmarek, M.B.; Struszczyk-Swita, K.; Li, X.; Szczęsna-Antczak, M.; Daroch, M. Enzymatic modifications of chitin, chitosan, and chitooligosaccharides. Front. Bioeng. Biotechnol. 2019, 7, 243. [Google Scholar] [CrossRef]
- Jiang, M.; Guo, Z.; Wang, C.; Yang, Y.; Liang, X.; Ding, F. Neural activity analysis of pure chito-oligomer components separated from a mixture of chitooligosaccharides. Neurosci. Lett. 2014, 581, 32–36. [Google Scholar] [CrossRef] [PubMed]
- Huang, R.; Mendis, E.; Kim, S.K. Improvement of ACE inhibitory activity of chitooligosaccharides (COS) by carboxyl modification. Bioorganic Med. Chem. 2005, 13, 3649–3655. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.-J.; Pan, S.-K.; Wang, H.-B.; Wu, J.-H. Preparation of chitooligosaccharides from cicada slough and their antibacterial activity. Int. J. Biol. Macromol. 2013, 62, 348–351. [Google Scholar] [CrossRef]
- Artan, M.; Karadeniz, F.; Karagozlu, M.Z.; Kim, M.M.; Kim, S.K. Anti-HIV-1 activity of low molecular weight sulfated chitooligosaccharides. Carbohydr. Res. 2010, 345, 656–662. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Aam, B.B.; Wang, W.; Norberg, A.L.; Sørlie, M.; Eijsink, V.G.H.; Du, Y. Inhibition of angiogenesis by chitooligosaccharides with specific degrees of acetylation and polymerization. Carbohydr. Polym. 2012, 89, 511–518. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.K.; Je, J.Y.; Lee, S.J.; Kim, Y.S.; Hwang, J.W.; Sung, S.H.; Moon, S.H.; Jeon, B.T.; Kim, S.K.; Jeon, Y.J.; et al. Chitooligosaccharides induce apoptosis in human myeloid leukemia HL-60 cells. Bioorganic Med. Chem. Lett. 2012, 22, 6136–6138. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, J.C.; Eaton, P.; Franco, I.; Ramos, Ó.S.; Sousa, S.; Nascimento, H.; Gomes, A.; Santos-Silva, A.; Xavier Malcata, F.; Pintado, M.E. Evaluation of chitoligosaccharides effect upon probiotic bacteria. Int. J. Biol. Macromol. 2012, 50, 148–152. [Google Scholar] [CrossRef] [PubMed]
- Simunek, J.; Koppova, I.; Filip, L.; Tishchenko, G.; Belzecki, G. The antimicrobial action of low-molar-mass chitosan, chitosan derivatives and chitooligosaccharides on bifidobacteria. Folia Microbiol. 2010, 55, 379–382. [Google Scholar] [CrossRef] [PubMed]
- Vishu Kumar, A.B.; Varadaraj, M.C.; Gowda, L.R.; Tharanathan, R.N. Low molecular weight chitosans—Preparation with the aid of pronase, characterization and their bactericidal activity towards Bacillus cereus and Escherichia coli. Biochim. Biophys. Acta (BBA)—Gen. Subj. 2007, 1770, 495–505. [Google Scholar] [CrossRef]
- Sánchez, Á.; Mengíbar, M.; Rivera-Rodríguez, G.; Moerchbacher, B.; Acosta, N.; Heras, A. The effect of preparation processes on the physicochemical characteristics and antibacterial activity of chitooligosaccharides. Carbohydr. Polym. 2017, 157, 251–257. [Google Scholar] [CrossRef]
- Naveed, M.; Phil, L.; Sohail, M.; Hasnat, M.; Baig, M.M.F.A.; Ihsan, A.U.; Shumzaid, M.; Kakar, M.U.; Mehmood Khan, T.; Akabar, M.D.; et al. Chitosan oligosaccharide (COS): An overview. Int. J. Biol. Macromol. 2019, 129, 827–843. [Google Scholar] [CrossRef]
- Huang, R.; Mendis, E.; Rajapakse, N.; Kim, S.K. Strong electronic charge as an important factor for anticancer activity of chitooligosaccharides (COS). Life Sci. 2006, 78, 2399–2408. [Google Scholar] [CrossRef]
- Zheng, B.; Wen, Z.S.; Huang, Y.J.; Xia, M.S.; Xiang, X.W.; Qu, Y.L. Molecular weight-dependent immunostimulative activity of low molecular weight chitosan via regulating NF-KB and AP-1 Signaling Pathways in RAW264.7 macrophages. Mar. Drugs 2016, 14. [Google Scholar] [CrossRef]
- Ji, X.-G.; Chang, K.-L.; Chen, M.; Zhu, L.-L.; Osman, A.; Yin, H.; Zhao, L.-M. In vitro fermentation of chitooligosaccharides and their effects on human fecal microbial community structure and metabolites. LWT 2021, 144, 111224. [Google Scholar] [CrossRef]
- Ibrahim, H.M.; Farid, O.A.; Samir, A.; Mosaad, R.M. Preparation of chitosan antioxidant nanoparticles as drug delivery system for enhancing of anti-cancer drug. Key Eng. Mater. 2018, 759, 92–97. [Google Scholar] [CrossRef]
- Lee, H.J.; Park, J.M.; Han, Y.M.; Gil, H.K.; Kim, J.; Chang, J.Y.; Jeong, M.; Go, E.J.; Hahm, K.B. The role of chronic inflammation in the development of gastrointestinal cancers: Reviewing cancer prevention with natural anti-inflammatory intervention. Expert Rev. Gastroenterol. Hepatol. 2016, 10, 129–139. [Google Scholar] [CrossRef] [PubMed]
- Santos-Moriano, P.; Kidibule, P.; Míguez, N.; Fernández-Arrojo, L.; Ballesteros, A.O.; Fernández-Lobato, M.; Plou, F.J. Tailored enzymatic synthesis of chitooligosaccharides with different deacetylation degrees and their anti-inflammatory activity. Catalysts 2019, 9, 405. [Google Scholar] [CrossRef]
- Yoon, H.J.; Moon, M.E.; Park, H.S.; Im, S.Y.; Kim, Y.H. Chitosan oligosaccharide (COS) inhibits LPS-induced inflammatory effects in RAW 264.7 macrophage cells. Biochem. Biophys. Res. Commun. 2007, 358, 954–959. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, J.C.; Spindola, H.; de Sousa, V.; Santos-Silva, A.; Pintado, M.E.; Malcata, F.X.; Carvalho, J.E. Anti-inflammatory activity of chitooligosaccharides in vivo. Mar. Drugs 2010, 8, 1763–1768. [Google Scholar] [CrossRef]
- Pangestuti, R.; Bak, S.S.; Kim, S.K. Attenuation of pro-inflammatory mediators in LPS-stimulated BV2 microglia by chitooligosaccharides via the MAPK signaling pathway. Int. J. Biol. Macromol. 2011, 49, 599–606. [Google Scholar] [CrossRef]
- Lee, S.-H.; Senevirathne, M.; Ahn, C.-B.; Kim, S.-K.; Je, J.-Y. Factors affecting anti-inflammatory effect of chitooligosaccharides in lipopolysaccharides-induced RAW264.7 macrophage cells. Bioorganic Med. Chem. Lett. 2009, 19, 6655–6658. [Google Scholar] [CrossRef]
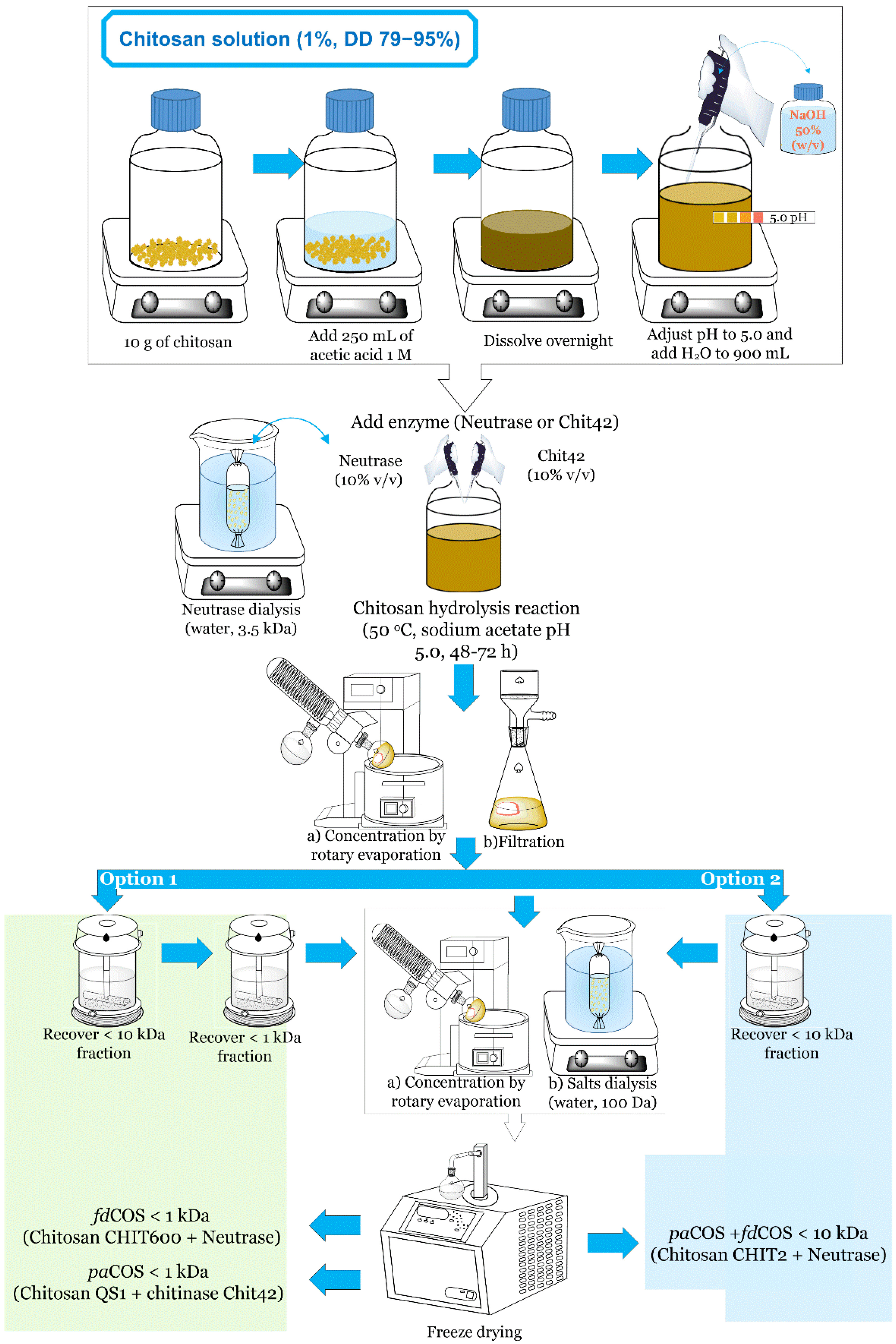

| Chitosan | Molecular Mass (kDa) 1 | Deacetylation Degree (%) 1 |
|---|---|---|
| CHIT2 | 56 | 79 |
| QS1 | 98 | 81 |
| CHIT600 | 600–100 | >90 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Miguez, N.; Kidibule, P.; Santos-Moriano, P.; Ballesteros, A.O.; Fernandez-Lobato, M.; Plou, F.J. Enzymatic Synthesis and Characterization of Different Families of Chitooligosaccharides and Their Bioactive Properties. Appl. Sci. 2021, 11, 3212. https://doi.org/10.3390/app11073212
Miguez N, Kidibule P, Santos-Moriano P, Ballesteros AO, Fernandez-Lobato M, Plou FJ. Enzymatic Synthesis and Characterization of Different Families of Chitooligosaccharides and Their Bioactive Properties. Applied Sciences. 2021; 11(7):3212. https://doi.org/10.3390/app11073212
Chicago/Turabian StyleMiguez, Noa, Peter Kidibule, Paloma Santos-Moriano, Antonio O. Ballesteros, Maria Fernandez-Lobato, and Francisco J. Plou. 2021. "Enzymatic Synthesis and Characterization of Different Families of Chitooligosaccharides and Their Bioactive Properties" Applied Sciences 11, no. 7: 3212. https://doi.org/10.3390/app11073212
APA StyleMiguez, N., Kidibule, P., Santos-Moriano, P., Ballesteros, A. O., Fernandez-Lobato, M., & Plou, F. J. (2021). Enzymatic Synthesis and Characterization of Different Families of Chitooligosaccharides and Their Bioactive Properties. Applied Sciences, 11(7), 3212. https://doi.org/10.3390/app11073212







