Portable near Infrared Spectroscopy as a Tool for Fresh Tomato Quality Control Analysis in the Field
Abstract
1. Introduction
2. Materials and Methods
2.1. Samples
2.2. Spectra Acquirement
2.3. Chemical Analysis
2.4. Multivariate Analysis
3. Results and Discussion
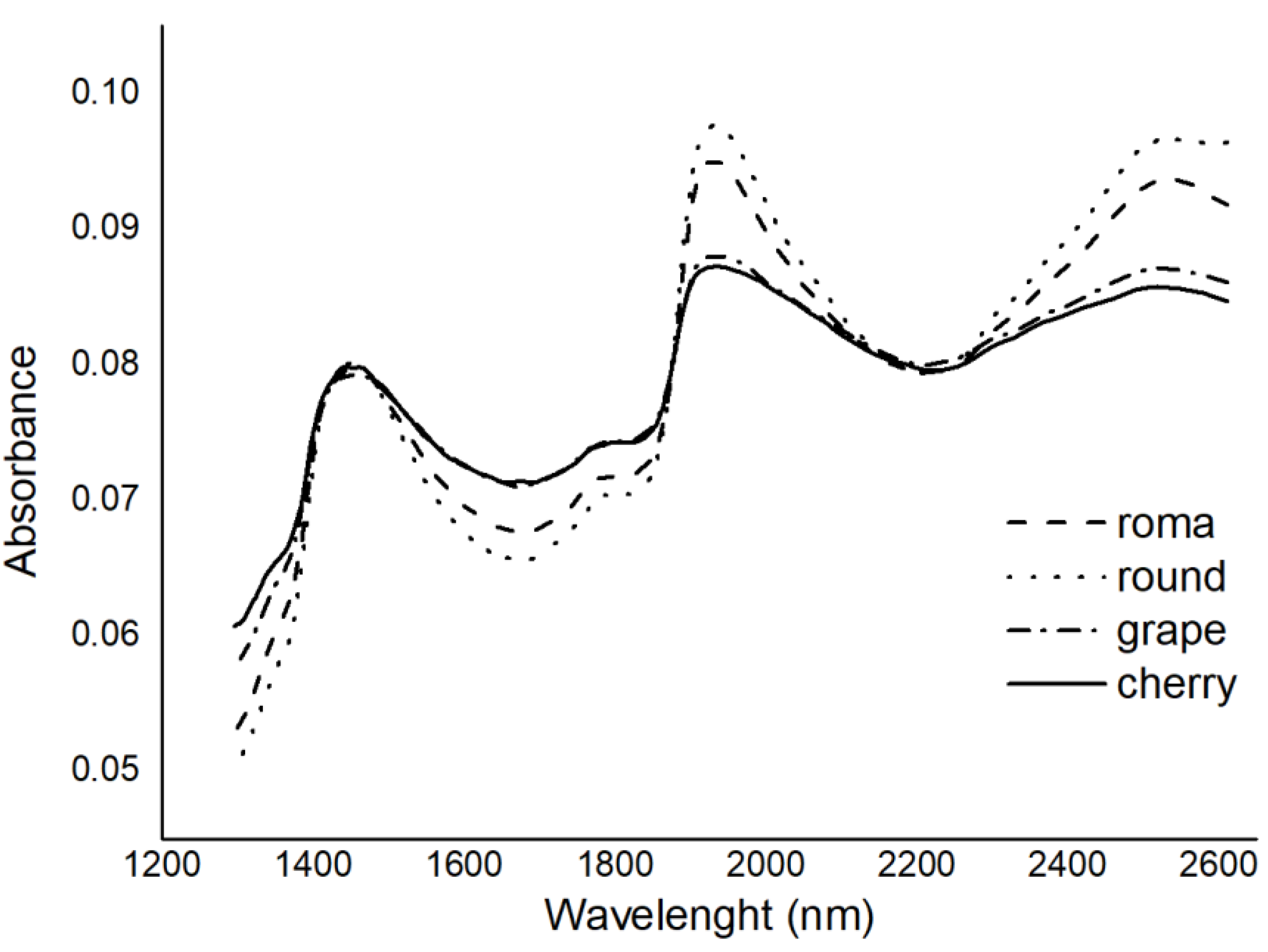
3.1. Reference Values
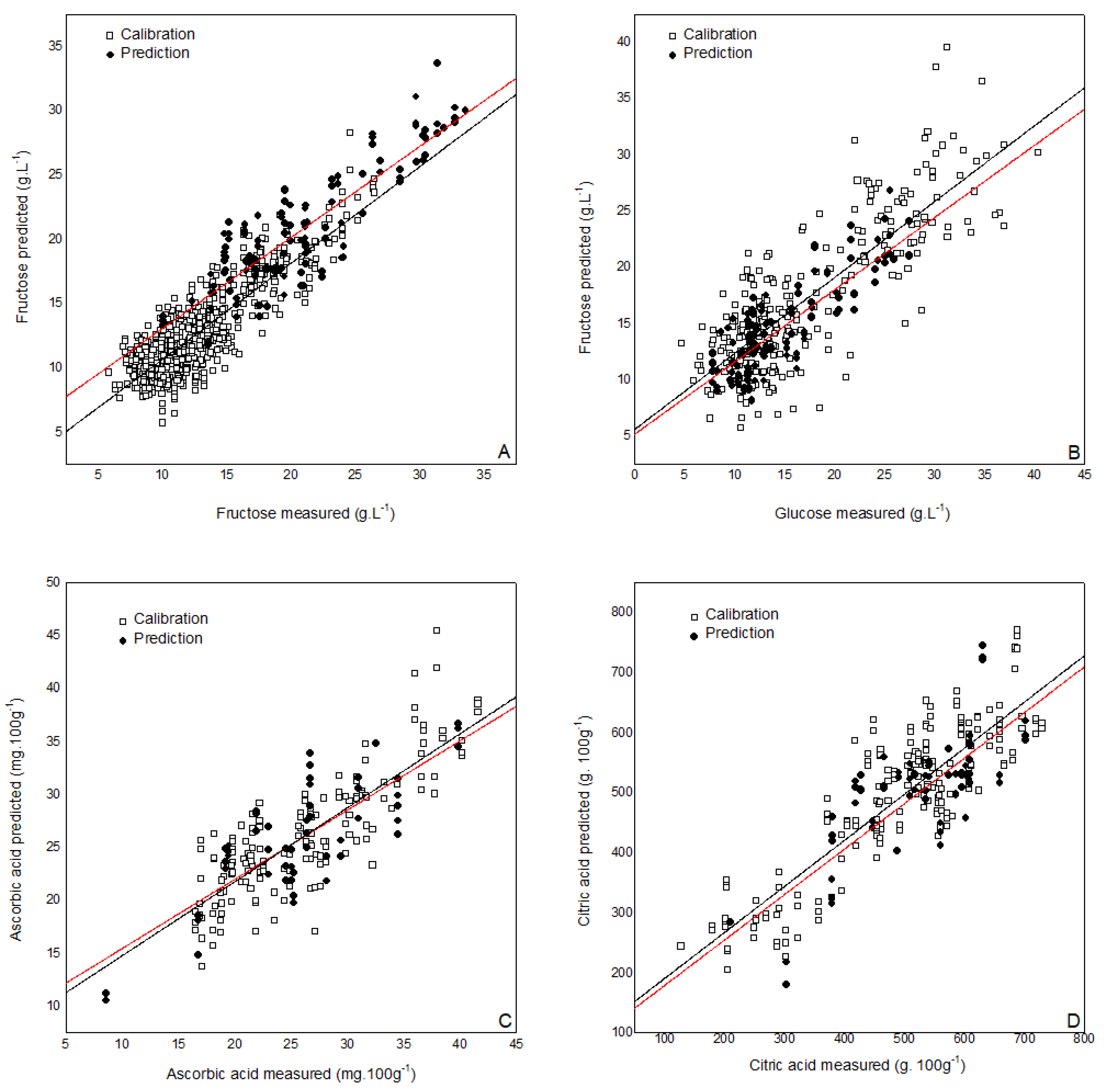
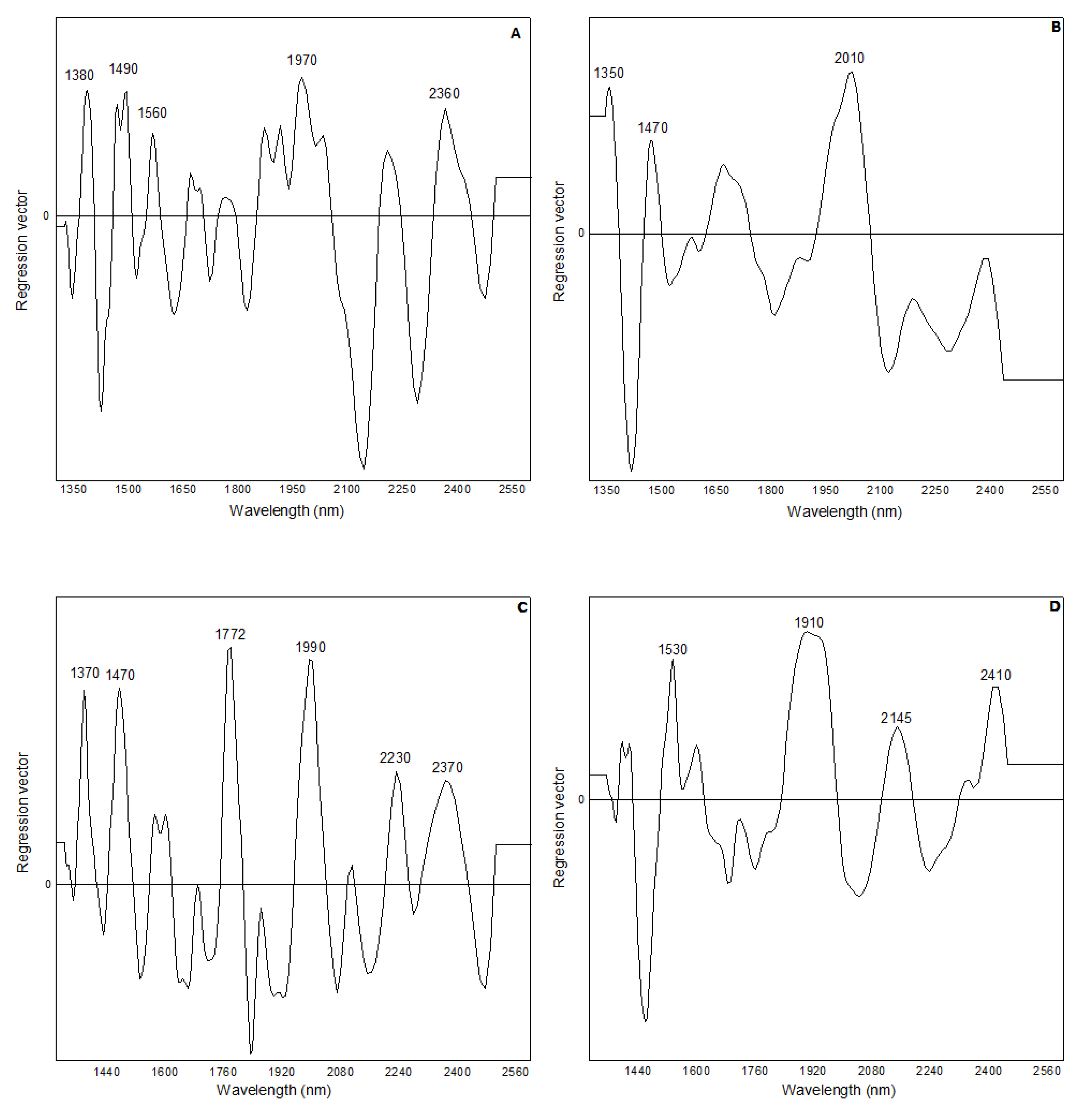
3.2. Partial Least Squares (PLS) Prediction Models Based on Near Infrared (NIR) Measurements
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- FAO. Food and Agriculture Organization of the United Nations. Available online: http://www.fao.org/home/en (accessed on 20 February 2021).
- Costa, J.M.; Heuvelink, E. The global tomato industry. In Tomatoes; Heuvelink, E., Ed.; CABI: Wallingford, UK, 2018; pp. 1–26. [Google Scholar]
- Bai, Y.; Lindhout, P. Domestication and Breeding of Tomatoes: What have We Gained and What Can We Gain in the Future? Ann. Bot. 2007, 100, 1085–1094. [Google Scholar] [CrossRef]
- Ibáñez, G.; Valcárcel, M.; Cebolla-Cornejo, J.; Roselló, S. FT-MIR determination of taste-related compounds in tomato: A high throughput phenotyping analysis for selection programs. J. Sci. Food Agric. 2019, 99, 5140–5148. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Saona, L.E.; Aykas, D.P. Tomato Chemistry, Industrial Processing and Product Development; Porretta, S., Ed.; Food Chemistry, Function and Analysis, Royal Society of Chemistry: Cambridge, UK, 2019; ISBN 978-1-78801-396-3. [Google Scholar]
- Reuhs, B.L.; Simsek, S. Nuclear Magnetic Resonance. In Food Analysis; Nielsen, S.S., Ed.; Food Science Text Series; Springer: Cham, Switzerland, 2017; ISBN 978-3-319-45774-1. [Google Scholar]
- Nicolaï, B.M.; Beullens, K.; Bobelyn, E.; Peirs, A.; Saeys, W.; Theron, K.I.; Lammertyn, J. Nondestructive measurement of fruit and vegetable quality by means of NIR spectroscopy: A review. Postharvest Biol. Technol. 2007, 46, 99–118. [Google Scholar] [CrossRef]
- Wang, L.; Sun, D.-W.; Pu, H.; Cheng, J.-H. Quality analysis, classification, and authentication of liquid foods by near-infrared spectroscopy: A review of recent research developments. Crit. Rev. Food Sci. Nutr. 2017, 57, 1524–1538. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.-H.; Nie, Y.-F.; Liu, B.; Kuai, Z.-Z.; Zhao, M.; Liu, F. Mechanical properties of AlSi10Mg lattice structures fabricated by selective laser melting. Mater. Des. 2020, 192, 108709. [Google Scholar] [CrossRef]
- Crocombe, R.A. Portable Spectroscopy. Appl. Spectrosc. 2018, 72, 1701–1751. [Google Scholar] [CrossRef]
- Erfan, M.; Sabry, Y.M.; Sakr, M.; Mortada, B.; Medhat, M.; Khalil, D. On-Chip Micro–Electro–Mechanical System Fourier Transform Infrared (MEMS FT-IR) Spectrometer-Based Gas Sensing. Appl. Spectrosc. 2016, 70, 897–904. [Google Scholar] [CrossRef]
- Pérez-marín, D.; Torres, I.; Entrenas, J.; Vega, M.; Sánchez, M. Pre-Harvest Screening on-Vine of Spinach Quality and Safety Using NIRS Technology. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2019, 207, 242–250. [Google Scholar] [CrossRef]
- Sánchez, M.-T.; De la Haba, M.-J.; Serrano, I.; Pérez-Marín, D. Application of NIRS for Nondestructive Measurement of Quality Parameters in Intact Oranges During On-Tree Ripening and at Harvest. Food Anal. Methods 2012, 6, 826–837. [Google Scholar] [CrossRef]
- Marques, E.J.N.; De Freitas, S.T.; Pimentel, M.F.; Pasquini, C. Rapid and non-destructive determination of quality parameters in the ‘Tommy Atkins’ mango using a novel handheld near infrared spectrometer. Food Chem. 2016, 197, 1207–1214. [Google Scholar] [CrossRef]
- Beghi, R.; Spinardi, A.; Bodria, L.; Mignani, I.; Guidetti, R. Apples Nutraceutic Properties Evaluation Through a Visible and Near-Infrared Portable System. Food Bioprocess Technol. 2012, 6, 2547–2554. [Google Scholar] [CrossRef]
- Sánchez, M.-T.; De La Haba, M.-J.; Guerrero, J.-E.; Garrido-Varo, A.; Pérez-Marín, D. Testing of a local approach for the prediction of quality parameters in intact nectarines using a portable NIRS instrument. Postharvest Biol. Technol. 2011, 60, 130–135. [Google Scholar] [CrossRef]
- Sánchez, M.-T.; De La Haba, M.J.; Benítez-López, M.; Fernández-Novales, J.; Garrido-Varo, A.; Pérez-Marín, D. Non-destructive characterization and quality control of intact strawberries based on NIR spectral data. J. Food Eng. 2012, 110, 102–108. [Google Scholar] [CrossRef]
- Pérez-Marín, D.; Paz, P.; Guerrero, J.-E.; Garrido-Varo, A.; Sánchez, M.-T. Miniature handheld NIR sensor for the on-site non-destructive assessment of post-harvest quality and refrigerated storage behavior in plums. J. Food Eng. 2010, 99, 294–302. [Google Scholar] [CrossRef]
- Torres, I.; Pérez-Marín, D.; De la Haba, M.-J.; Sánchez, M.-T. Developing universal models for the prediction of physical quality in citrus fruits analysed on-tree using portable NIRS sensors. Biosyst. Eng. 2017, 153, 140–148. [Google Scholar] [CrossRef]
- Liu, Y.; Gao, R.; Hao, Y.; Sun, X.; Ouyang, A. Improvement of Near-Infrared Spectral Calibration Models for Brix Prediction in ‘Gannan’ Navel Oranges by a Portable Near-Infrared Device. Food Bioprocess Technol. 2010, 5, 1106–1112. [Google Scholar] [CrossRef]
- Castrignanò, A.; Buttafuoco, G.; Malegori, C.; Genorini, E.; Iorio, R.; Stipic, M.; Girone, G.; Venezia, A. Assessing the Feasibility of a Miniaturized Near-Infrared Spectrometer in Determining Quality Attributes of San Marzano Tomato. Food Anal. Methods 2019, 12, 1497–1510. [Google Scholar] [CrossRef]
- Sánchez, M.-T.; Torres, I.; De La Haba, M.-J.; Chamorro, A.; Garrido-Varo, A.; Pérez-Marín, D. Rapid, simultaneous, and in situ authentication and quality assessment of intact bell peppers using near-infrared spectroscopy technology. J. Sci. Food Agric. 2019, 99, 1613–1622. [Google Scholar] [CrossRef]
- Cirilli, M.; Bellincontro, A.; Urbani, S.; Servili, M.; Esposto, S.; Mencarelli, F.; Muleo, R. On-field monitoring of fruit ripening evolution and quality parameters in olive mutants using a portable NIR-AOTF device. Food Chem. 2016, 199, 96–104. [Google Scholar] [CrossRef]
- Fernández-Espinosa, A.J. Combining PLS regression with portable NIR spectroscopy to on-line monitor quality parameters in intact olives for determining optimal harvesting time. Talanta 2016, 148, 216–228. [Google Scholar] [CrossRef]
- Blakey, R.J. Evaluation of avocado fruit maturity with a portable near-infrared spectrometer. Postharvest Biol. Technol. 2016, 121, 101–105. [Google Scholar] [CrossRef]
- Sorak, D.; Herberholz, L.; Iwascek, S.; Altinpinar, S.; Pfeifer, F.; Siesler, H.W. New Developments and Applications of Handheld Raman, Mid-Infrared and Near-Infrared Spectrometers. Appl. Spectrosc. Rev. 2012, 47, 83–115. [Google Scholar] [CrossRef]
- Kenda, A.; Frank, A.; Kraft, M.; Tortschanoff, A.; Sandner, T.; Schenk, H.; Scherf, W. Compact High-Speed Spectrometers Based on MEMS Devices with Large Amplitude In-Plane Actuators. Procedia Chem. 2009, 1, 556–559. [Google Scholar] [CrossRef]
- USDA. Tomato Grading Visual Aids. Available online: https://www.ams.usda.gov/sites/default/files/media/Tomato_Visual_Aids%5B1%5D.pdf (accessed on 10 December 2015).
- Wechtersbach, L.; Cigić, B. Reduction of dehydroascorbic acid at low pH. J. Biochem. Biophys. Methods 2007, 70, 767–772. [Google Scholar] [CrossRef] [PubMed]
- Akpolat, H.; Barineau, M.; Jackson, K.A.; Aykas, D.P.; Rodriguez-Saona, L.E. Portable Infrared Sensing Technology for Phenotyping Chemical Traits in Fresh Market Tomatoes. Lwt 2020, 124, 109164. [Google Scholar] [CrossRef]
- Bertin, N.; Génard, M. Tomato quality as influenced by preharvest factors. Sci. Hortic. 2018, 233, 264–276. [Google Scholar] [CrossRef]
- Gilboa, I.L.N.; Shen, E.Y.S.; Schaffer, A.A. Fgr, a Major Locus That Modulates the Fructose to Glucose Ratio in Mature Tomato Fruits. Theor. Appl. Genet. 2000, 100, 256–262. [Google Scholar]
- Davies, J.; Hobson, G. The Constituints of Tomato Fruit—The Influence of Environment, Nutrition and Genotype. Food Sci. Nutr. 1981, 15, 205–208. [Google Scholar]
- Siddiqui, W.; Chakraborty, I.; Mishra, P.; Hazra, P. Bioactive attributes of tomatoes possessing dg, ogc and rin genes. Food Funct. 2014, 5, 936. [Google Scholar] [CrossRef]
- Araus, J.L.; Cairns, J.E. Field high-throughput phenotyping: The new crop breeding frontier. Trends Plant Sci. 2014, 19, 52–61. [Google Scholar] [CrossRef]
- Beć, K.B.; Grabska, J.; Huck, C.W. Near-Infrared Spectroscopy in Bio-Applications. Molecules 2020, 25, 2948. [Google Scholar] [CrossRef] [PubMed]
- Clément, A.; Dorais, M.; Vernon, M. Nondestructive Measurement of Fresh Tomato Lycopene Content and Other Physico-chemical Characteristics Using Visible—NIR Spectroscopy. J. Agric. Food Chem. 2008, 56, 9813–9818. [Google Scholar] [CrossRef] [PubMed]
- Flores, K.; Sánchez, M.-T.; Pérez-Marín, D.; Guerrero, J.-E.; Garrido-Varo, A. Feasibility in NIRS instruments for predicting internal quality in intact tomato. J. Food Eng. 2009, 91, 311–318. [Google Scholar] [CrossRef]
- Kaur, A.; Donis-Gonzalez, I.R.; Clair, D.A.S. Evaluation of a hand-held spectrophotometer as an in-field phenotyping tool for tomato and pepper fruit quality. Plant Phenome J. 2020, 3, 1–23. [Google Scholar] [CrossRef]
- Feng, L.; Zhang, M.; Adhikari, B.; Guo, Z. Nondestructive Detection of Postharvest Quality of Cherry Tomatoes Using a Portable NIR Spectrometer and Chemometric Algorithms. Food Anal. Methods 2019, 12, 914–925. [Google Scholar] [CrossRef]
- Pavia, D.L.; Kris, G.S.; Lampman, G.M.; Vyvyan, J.R. Introdução à Espectroscopia; Cengage: Boston, MA, USA, 2010; ISBN 978-8522107087. [Google Scholar]
- Rodriguez-saona, L.E.; Ayvaz, H.; Wehling, R.L. Infrared and Raman Spectroscopy. In Food Analysis; Nielsen, S.S., Ed.; Springer: Berlin/Heidelberg, Germany, 2017; pp. 107–127. ISBN 978-3-319-45774-1. [Google Scholar]
- Osborne, B.G. Near-Infrared Spectroscopy in Food Analysis. Encycl. Anal. Chem. 2000, 1–14. [Google Scholar] [CrossRef]
- Bureau, S.; Cozzolino, D.; Clark, C.J. Contributions of Fourier-transform mid infrared (FT-MIR) spectroscopy to the study of fruit and vegetables: A review. Postharvest Biol. Technol. 2019, 148, 1–14. [Google Scholar] [CrossRef]
- Wilkerson, E.D.; Anthon, G.E.; Barrett, D.M.; Sayajon, G.F.G.; Santos, A.M.; Rodriguez-Saona, L.E. Rapid Assessment of Quality Parameters in Processing Tomatoes using Hand-Held and Benchtop Infrared Spectrometers and Multivariate Analysis. J. Agric. Food Chem. 2013, 61, 2088–2095. [Google Scholar] [CrossRef]


| Parameters | Tomato Type | N | Average | SD | Range | CV (%) |
|---|---|---|---|---|---|---|
| Soluble solids (° Brix) | all | 319 | 5.72 | 1.68 | 2.92–11.22 | 29.47 |
| Roma | 106 | 4.45 | 0.82 | 2.92–9.93 | 11.52 | |
| round | 47 | 4.59 | 0.53 | 3.49–5.95 | 11.54 | |
| grape | 93 | 6.81 | 1.24 | 4.29–9.36 | 18.25 | |
| cherry | 73 | 6.91 | 1.8 | 3.96–11.22 | 26.13 | |
| Glucose (g. L−1) | all | 319 | 16.61 | 7.37 | 4.68–39.12 | 44.44 |
| Roma | 106 | 12.92 | 4.29 | 7.57–32.87 | 33.18 | |
| round | 47 | 12.06 | 3.31 | 4.68–21.74 | 27.46 | |
| grape | 93 | 20.66 | 7.14 | 7.94–35.79 | 34.58 | |
| cherry | 73 | 20.26 | 8.34 | 8.65–39.12 | 40.47 | |
| Fructose (g. L−1) | all | 319 | 22.25 | 8.71 | 5.33–47.01 | 39.17 |
| Roma | 106 | 16.18 | 4.34 | 10.57–36.04 | 26.84 | |
| round | 47 | 17.10 | 5.48 | 5.33–42.67 | 32.05 | |
| grape | 93 | 26.39 | 8.13 | 11.44–45.18 | 30.83 | |
| cherry | 73 | 28.41 | 8.40 | 15.31–47.01 | 29.56 | |
| TA (g citric acid. 100 g−1) | all | 204 | 0.41 | 0.2 | 0.12–1.40 | 49.31 |
| Roma | 84 | 0.40 | 0.21 | 0.13–1.40 | 51.96 | |
| round | 45 | 0.38 | 0.2 | 0.15–1.29 | 53.59 | |
| grape | 43 | 0.49 | 0.22 | 0.15–1.09 | 45.68 | |
| cherry | 32 | 0.40 | 0.14 | 0.12–1.77 | 36.91 | |
| Ascorbic acid (mg. 100 g−1) | all | 149 | 29.32 | 14.71 | 3.77–77.91 | 44.84 |
| Roma | 63 | 27.58 | 11.42 | 12.09–56.91 | 41.39 | |
| round | 35 | 24.6 | 9.13 | 11.30–41.65 | 37.12 | |
| grape | 27 | 33.85 | 22.59 | 3.78–77.91 | 52.10 | |
| cherry | 24 | 38.83 | 12.32 | 14.70–62.29 | 31.73 | |
| Citric acid (g. 100 g−1) | all | 149 | 0.54 | 0.21 | 0.11–1.10 | 39.28 |
| Roma | 63 | 0.54 | 0.22 | 0.11–0.97 | 41.99 | |
| round | 35 | 0.42 | 0.18 | 0.18–0.99 | 43.52 | |
| grape | 27 | 0.56 | 0.19 | 0.15–1.10 | 34.49 | |
| cherry | 24 | 0.64 | 0.14 | 0.42–1.04 | 21.99 |
| Parameter | Type of Tomatoes | Preprocessing | N cal | F | SECV | rCV | N pred | SEP | rpre |
|---|---|---|---|---|---|---|---|---|---|
| SSC (°Brix) | Roma-Round | Mean center + Smooth (15) + 2nd deriv (15) | 152 | 5 | 0.23 | 0.88 | 38 | 0.22 | 0.87 |
| Cherry-Grape | 166 | 6 | 0.64 | 0.89 | 42 | 0.52 | 0.88 | ||
| Glucose (g. 100 g−1) | All types | Norm + 2nd deriv (15) | 250 | 4 | 3.01 | 0.87 | 58 | 2.91 | 0.83 |
| Fructose (g. 100 g−1) | All types | Norm + 2nd deriv (15) | 250 | 5 | 3.48 | 0.87 | 58 | 2.83 | 0.87 |
| TA (g citric acid. 100 g−1) | Roma-Round | Mean center + Smooth (21) + 2nd deriv (21) | 98 | 6 | 0.03 | 0.87 | 25 | 0.02 | 0.89 |
| Cherry-Grape | 39 | 7 | 0.05 | 0.92 | 10 | 0.04 | 0.94 | ||
| Ascorbic acid (mg. 100 g−1) | All types | Norm + 2ndderiv (15) | 100 | 6 | 3.78 | 0.82 | 46 | 4.09 | 0.81 |
| Citric acid (g. 100 g−1) | All types | Norm + 2nd deriv (25) | 100 | 6 | 0.06 | 0.87 | 46 | 0.07 | 0.86 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Borba, K.R.; Aykas, D.P.; Milani, M.I.; Colnago, L.A.; Ferreira, M.D.; Rodriguez-Saona, L.E. Portable near Infrared Spectroscopy as a Tool for Fresh Tomato Quality Control Analysis in the Field. Appl. Sci. 2021, 11, 3209. https://doi.org/10.3390/app11073209
Borba KR, Aykas DP, Milani MI, Colnago LA, Ferreira MD, Rodriguez-Saona LE. Portable near Infrared Spectroscopy as a Tool for Fresh Tomato Quality Control Analysis in the Field. Applied Sciences. 2021; 11(7):3209. https://doi.org/10.3390/app11073209
Chicago/Turabian StyleBorba, Karla R., Didem P. Aykas, Maria I. Milani, Luiz A. Colnago, Marcos D. Ferreira, and Luis E. Rodriguez-Saona. 2021. "Portable near Infrared Spectroscopy as a Tool for Fresh Tomato Quality Control Analysis in the Field" Applied Sciences 11, no. 7: 3209. https://doi.org/10.3390/app11073209
APA StyleBorba, K. R., Aykas, D. P., Milani, M. I., Colnago, L. A., Ferreira, M. D., & Rodriguez-Saona, L. E. (2021). Portable near Infrared Spectroscopy as a Tool for Fresh Tomato Quality Control Analysis in the Field. Applied Sciences, 11(7), 3209. https://doi.org/10.3390/app11073209








