Temperature Dependence of Density and Viscosity of Biobutanol-Gasoline Blends
Abstract
Featured Application
Abstract
1. Introduction
2. Materials and Methods
2.1. Material
2.2. Volumetric Mass Density Measuring
2.3. Viscosity Measuring
2.4. Mathematical Models for the Density, Dynamic, and Kinematic Viscosity
2.5. Statistical Data Processing
3. Results and Discussion
3.1. Volumetric Mass Density
3.2. Dynamic Viscosity
3.3. Kinematic Viscosity
3.4. Mathematical Models
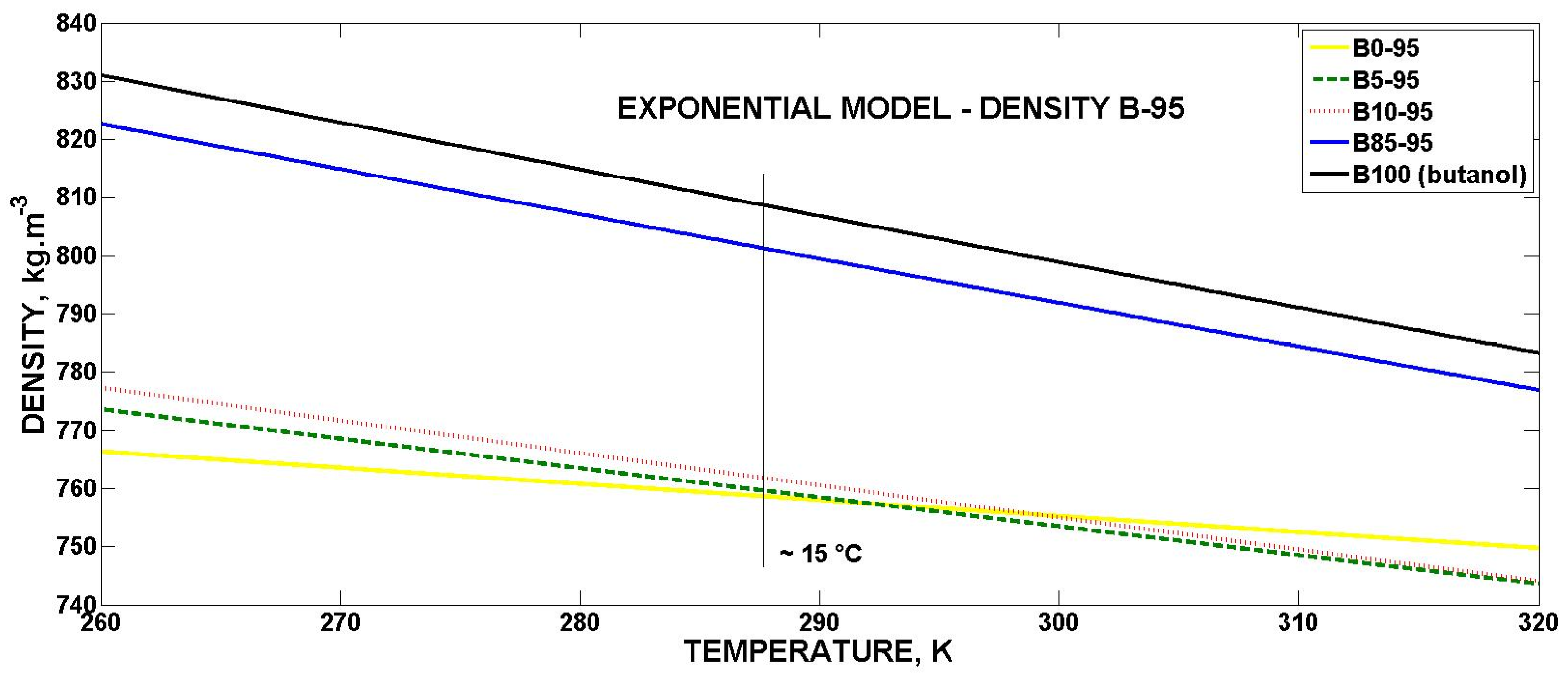
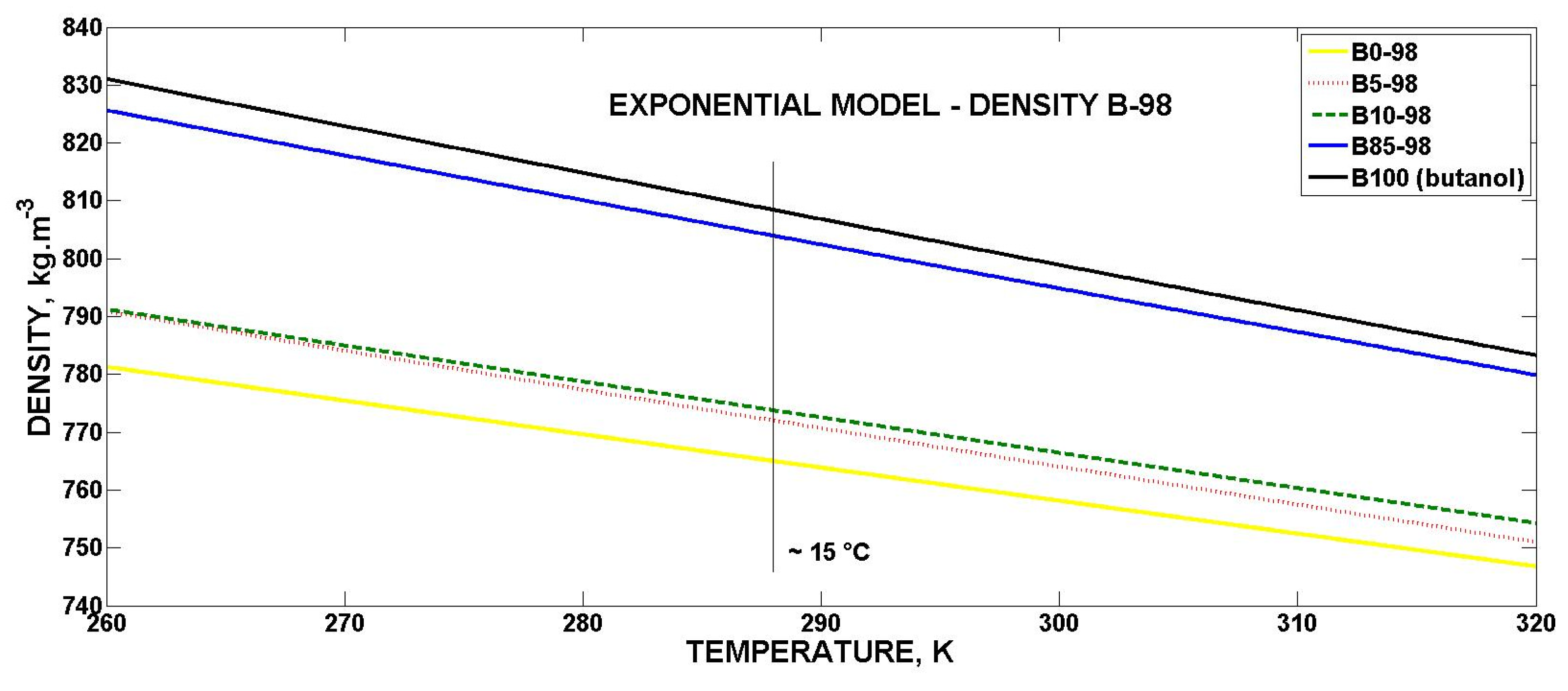
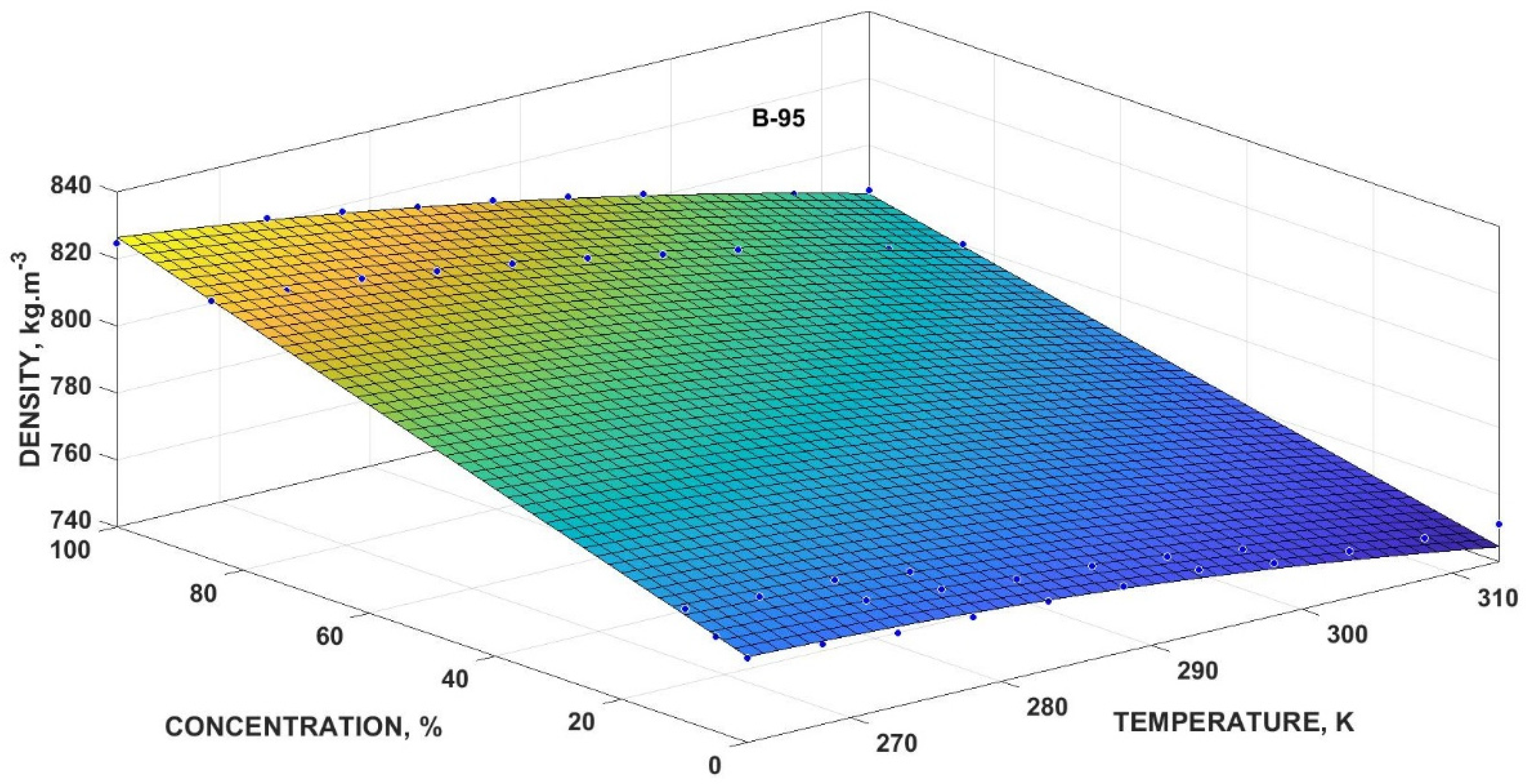
3.4.1. Mathematical Model for Volumetric Mass Density
3.4.2. Mathematical Models for Dynamic Viscosity
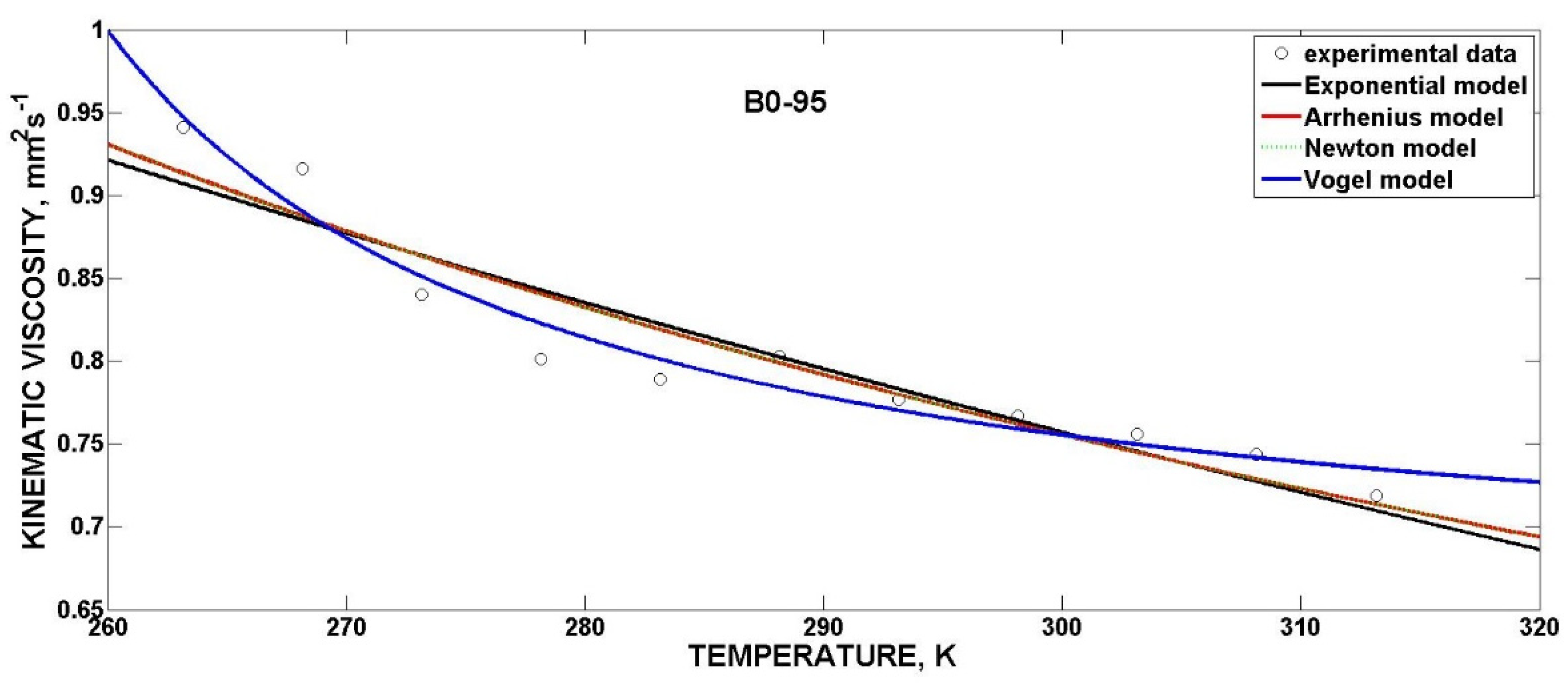
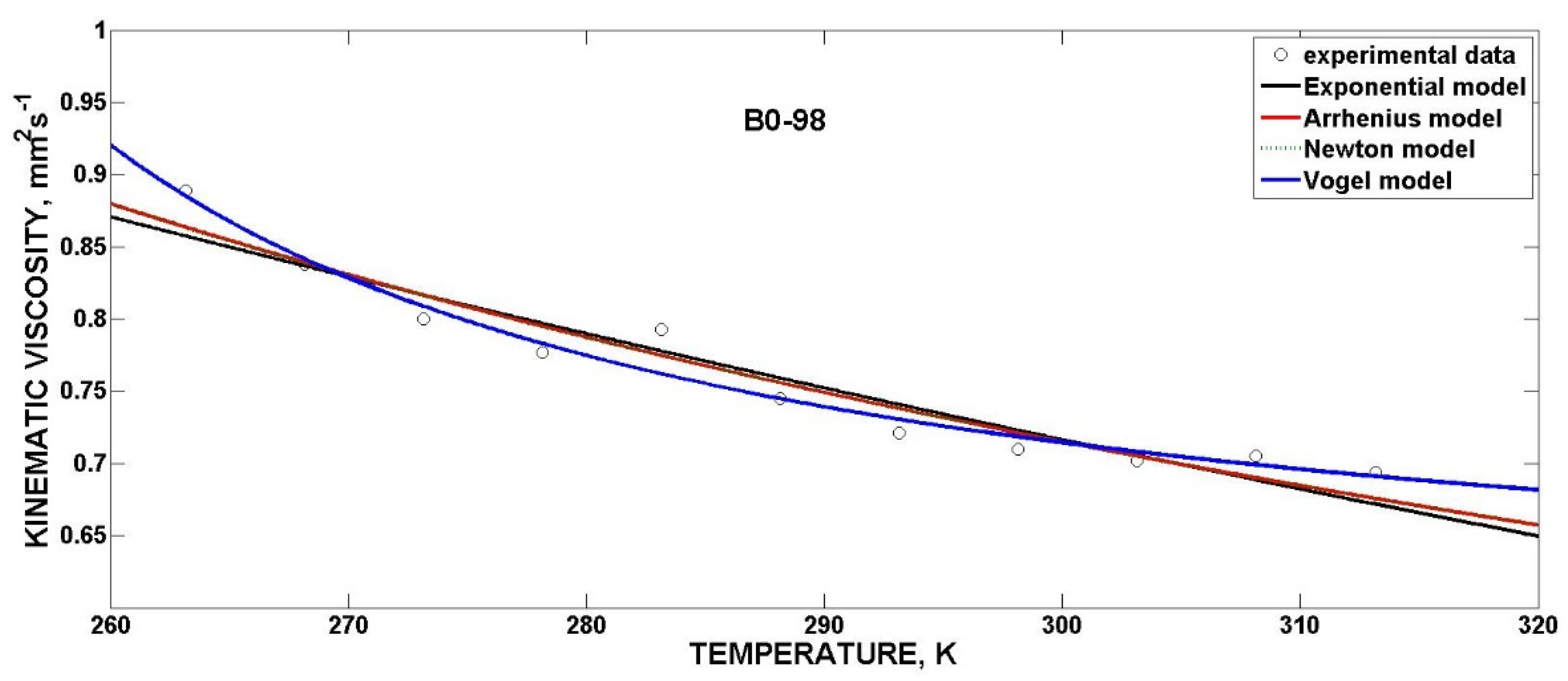
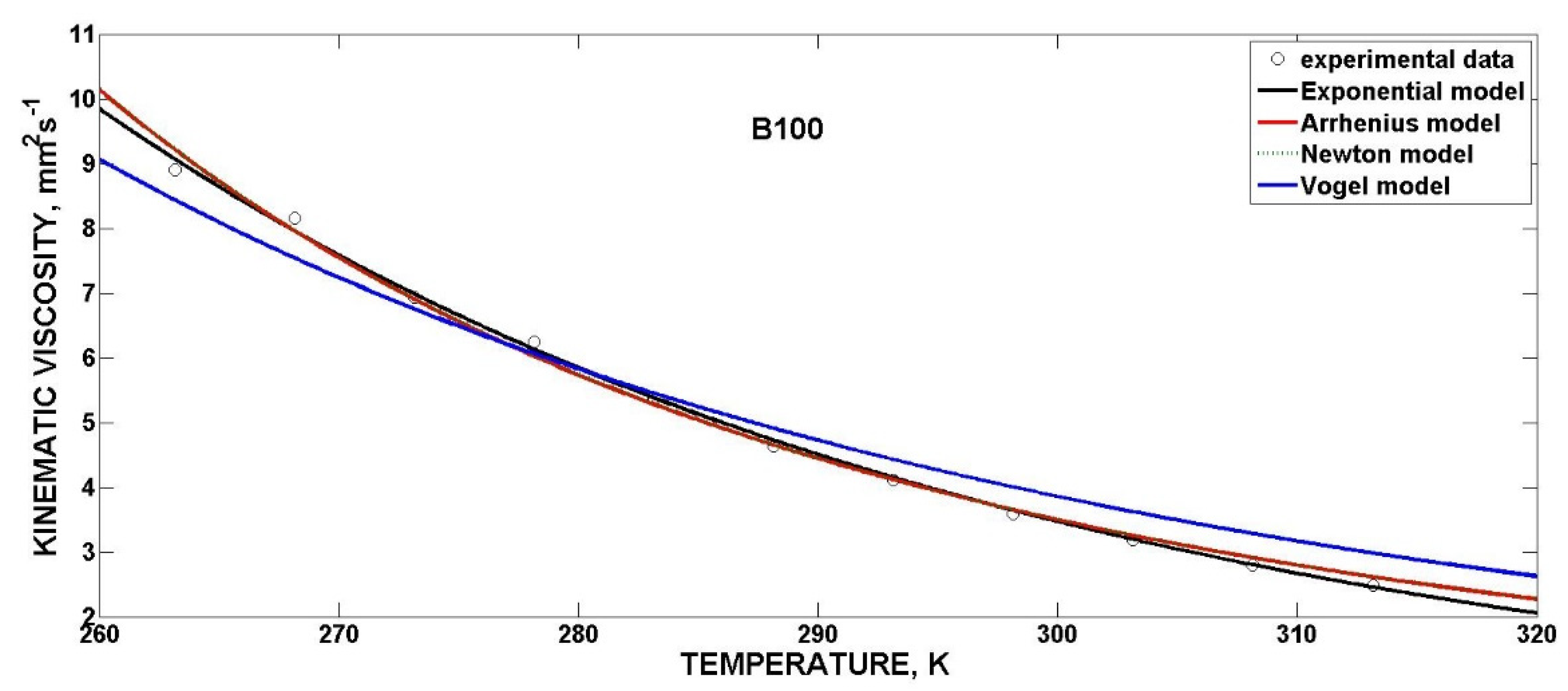
3.4.3. Mathematical Models for Kinematic Viscosity
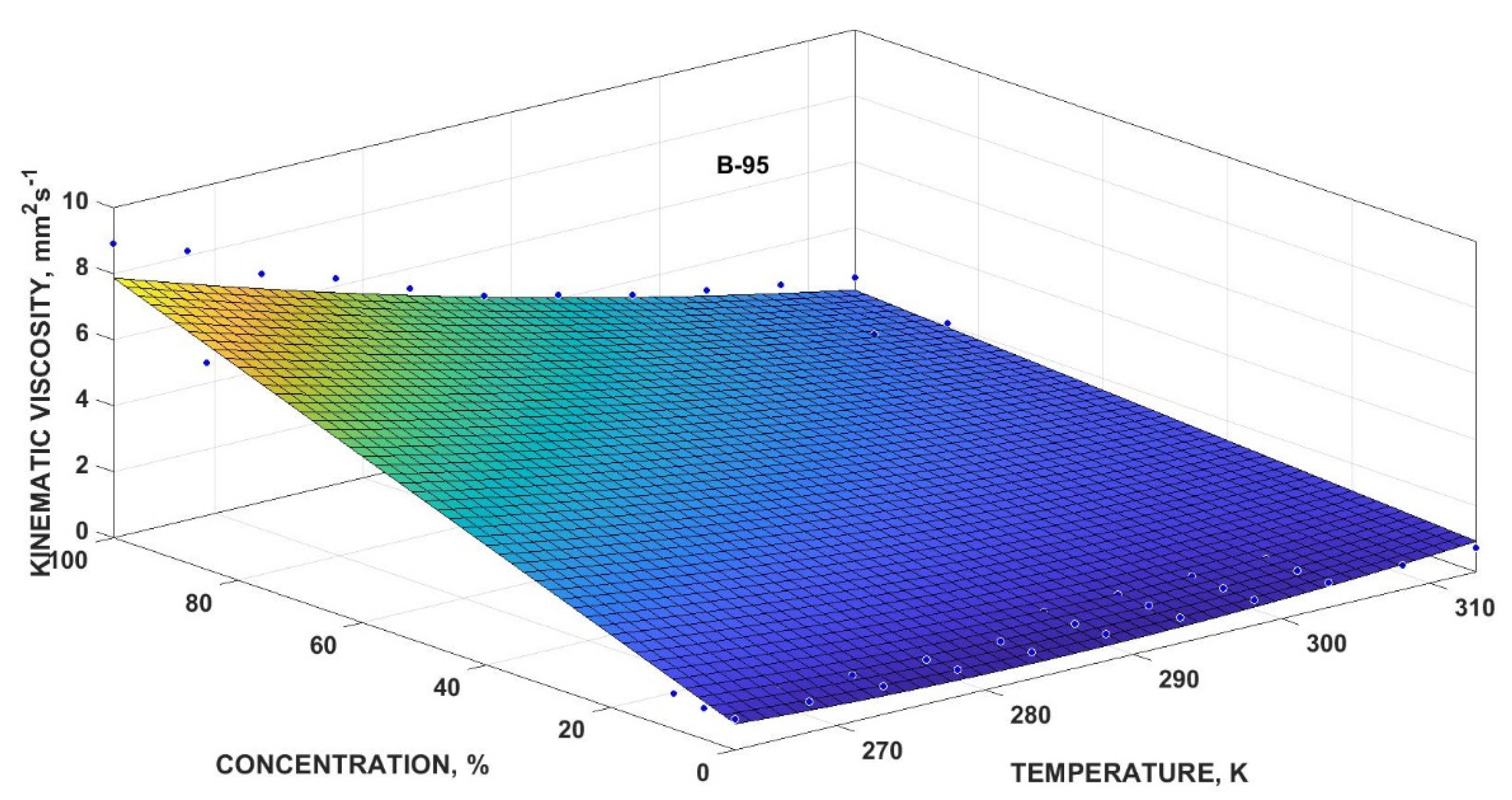
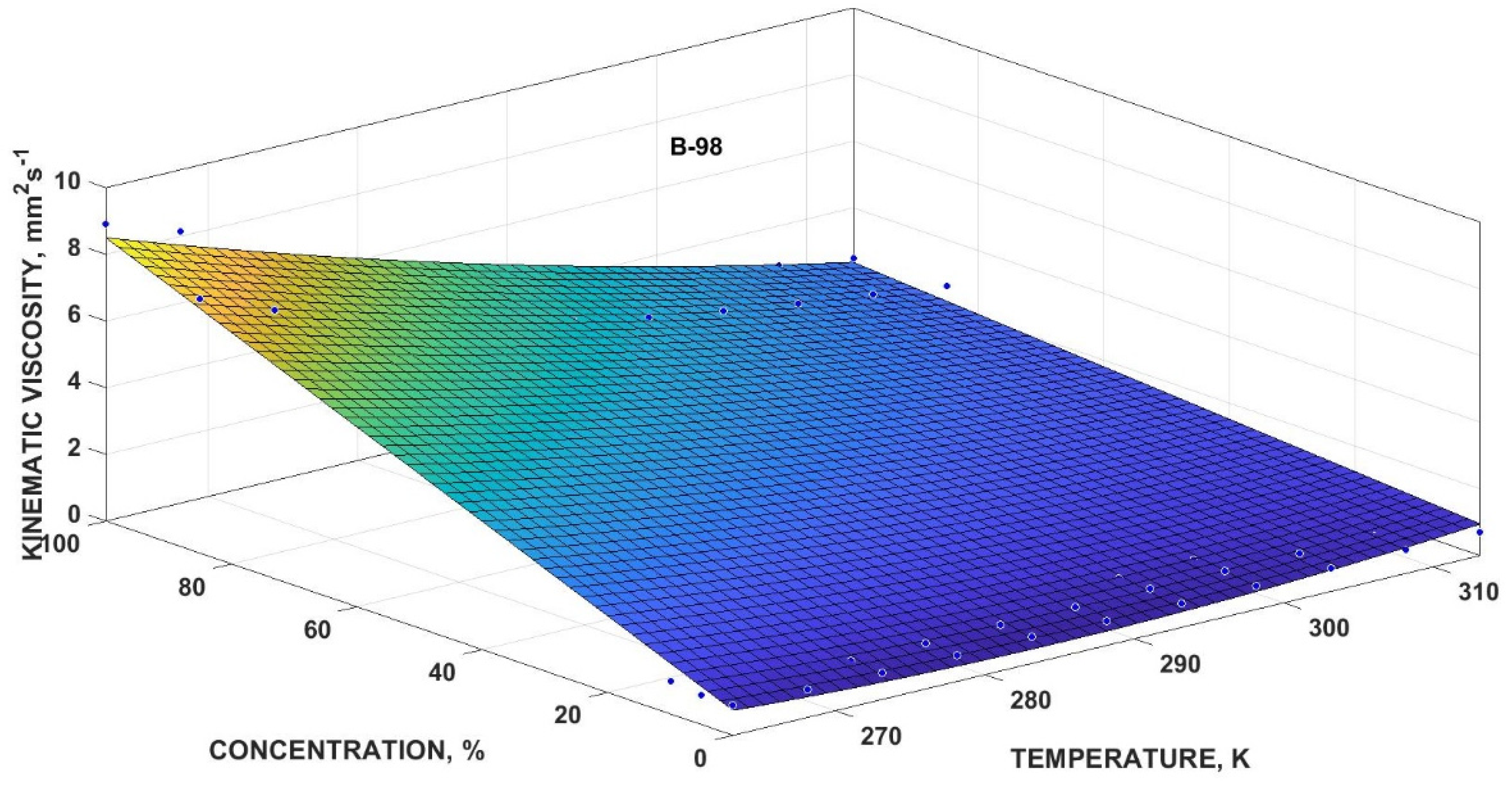
3.4.4. Mathematical Multivariate Models
4. Conclusions
- The RON 98 gasoline reaches higher values in the volumetric mass density than the RON 95 gasoline.
- The volumetric mass density of the biobutanol-gasoline blends almost linearly (coefficient of exponential model b → 0) decreases with an increasing temperature for all concentrations.
- The RON 98 gasoline reaches lower values in the dynamic and kinematic viscosity than the RON 95 gasoline.
- The dynamic and kinematic viscosity of the biobutanol-gasoline blends nonlinear decreases with an increasing temperature for all concentrations.
- The biobutanol-gasoline blends with a higher biobutanol content have higher dynamic and kinematic viscosity values.
- The dynamic and kinematic viscosity of the biobutanol-gasoline blends with a content up to 10 vol.% of biobutanol is almost the same as the dynamic and kinematic viscosity of the RON 95 and RON 98 gasolines without a biocomponent (p < 0.05).
- The dynamic and kinematic viscosity of the biobutanol-gasoline blends with a content up to 85 vol.% of biobutanol is similar to the dynamic and kinematic viscosity of the pure biobutanol (p < 0.05).
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Temperature (°C) | Temperature (K) | Volumetric Mass Density RON 95 (kg∙m−3) | ||||
|---|---|---|---|---|---|---|
| B0 | B5 | B10 | B85 | B100 | ||
| −10 | 263.15 | 765.1 ± 1.49 | 768.3 ± 1.63 | 773.5 ± 1.41 | 817.1 ± 1.62 | 824.6 ± 1.60 |
| −5 | 268.15 | 763.8 ± 1.56 | 766.7 ± 1.53 | 771.5 ± 1.49 | 814.8 ± 1.81 | 822.8 ± 1.50 |
| 0 | 273.15 | 761.9 ± 1.27 | 768.4 ± 1.40 | 771.1 ± 1.52 | 813.1 ± 1.58 | 821.3 ± 1.31 |
| 5 | 278.15 | 761.1 ± 1.42 | 766.2 ± 1.48 | 768.3 ± 1.50 | 809.7 ± 1.58 | 817.9 ± 1.60 |
| 10 | 283.15 | 760.6 ± 1.61 | 764.0 ± 1.61 | 765.2 ± 1.45 | 806.5 ± 1.81 | 813.9 ± 1.27 |
| 15 | 288.15 | 759.6 ± 1.53 | 762.4 ± 1.52 | 762.6 ± 1.39 | 803.0 ± 1.83 | 810.5 ± 1.41 |
| 20 | 293.15 | 759.0 ± 1.48 | 759.7 ± 1.38 | 760.0 ± 1.61 | 798.7 ± 1.53 | 806.3 ± 1.44 |
| 25 | 298.15 | 755.8 ± 1.42 | 756.5 ± 1.29 | 756.6 ± 1.41 | 794.6 ± 1.57 | 801.7 ± 1.47 |
| 30 | 303.15 | 753.8 ± 1.29 | 750.3 ± 1.47 | 751.3 ± 1.48 | 787.9 ± 1.55 | 794.2 ± 1.78 |
| 35 | 308.15 | 752.3 ± 1.25 | 747.2 ± 1.62 | 749.4 ± 1.37 | 784.2 ± 1.58 | 790.8 ± 1.52 |
| 40 | 313.15 | 751.1 ± 1.55 | 744.2 ± 1.60 | 747.6 ± 1.24 | 780.2 ± 1.48 | 786.6 ± 1.57 |
| Temperature (°C) | Temperature (K) | Volumetric Mass Density RON 98 (kg∙m−3) | ||||
|---|---|---|---|---|---|---|
| B0 | B5 | B10 | B85 | B100 | ||
| −10 | 263.15 | 776.1 ± 1.63 | 785.1 ± 1.40 | 786.3 ± 1.33 | 820.6 ± 1.42 | 824.6 ± 1.60 |
| −5 | 268.15 | 775.6 ± 1.41 | 784.4 ± 1.37 | 784.7 ± 1.36 | 818.3 ± 1.37 | 822.8 ± 1.50 |
| 0 | 273.15 | 775.0 ± 1.26 | 783.3 ± 1.35 | 783.9 ± 1.27 | 816.5 ± 1.39 | 821.3 ± 1.31 |
| 5 | 278.15 | 772.1 ± 1.37 | 779.9 ± 1.36 | 781.3 ± 1.38 | 812.7 ± 1.49 | 817.9 ± 1.60 |
| 10 | 283.15 | 769.6 ± 1.40 | 777.0 ± 1.57 | 778.7 ± 1.40 | 809.5 ± 1.44 | 813.9 ± 1.27 |
| 15 | 288.15 | 765.6 ± 1.29 | 773.8 ± 1.47 | 775.9 ± 1.45 | 806.0 ± 1.44 | 810.5 ± 1.41 |
| 20 | 293.15 | 763.2 ± 1.12 | 770.1 ± 1.38 | 771.9 ± 1.36 | 802.0 ± 1.48 | 806.3 ± 1.44 |
| 25 | 298.15 | 760.4 ± 1.17 | 766.6 ± 1.19 | 768.7 ± 1.27 | 797.9 ± 1.50 | 801.7 ± 1.47 |
| 30 | 303.15 | 754.9 ± 1.35 | 760.7 ± 1.29 | 763.2 ± 1.43 | 791.5 ± 1.46 | 794.2 ± 1.78 |
| 35 | 308.15 | 752.2 ± 1.40 | 757.3 ± 1.27 | 760.1 ± 1.30 | 787.6 ± 1.40 | 790.8 ± 1.52 |
| 40 | 313.15 | 749.4 ± 1.43 | 753.6 ± 1.33 | 756.8 ± 1.32 | 783.4 ± 1.42 | 786.6 ± 1.57 |
| Temperature (°C) | Temperature (K) | Dynamic Viscosity RON 95 (mPa∙s) | ||||
|---|---|---|---|---|---|---|
| B0 | B5 | B10 | B85 | B100 | ||
| −10 | 263.15 | 0.72 ± 0.025 j,k | 0.73 ± 0.021 m | 0.83 ± 0.010 o | 5.12 ± 0.026 ω | 7.34 ± 0.025 λ |
| −5 | 268.15 | 0.70 ± 0.018 d,e,f,g,h,i | 0.71 ± 0.023 l | 0.78 ± 0.010 n | 4.50 ± 0.023 τ | 6.71 ± 0.027 δ |
| 0 | 273.15 | 0.64 ± 0.035 d,e,f | 0.66 ± 0.014 k | 0.73 ± 0.017 m | 3.88 ± 0.017 ρ | 5.69 ± 0.025 η |
| 5 | 278.15 | 0.61 ± 0.035 d,e,f | 0.63 ± 0.019 d,e | 0.69 ± 0.015 l | 3.45 ± 0.017 z | 5.11 ± 0.031 ω |
| 10 | 283.15 | 0.60 ± 0.018 e,f,g,h,i,j | 0.63 ± 0.015 d,e,f,g | 0.64 ± 0.012 i,j,k | 3.05 ± 0.017 x | 4.38 ± 0.016 π |
| 15 | 288.15 | 0.61 ± 0.032 d,e,f | 0.62 ± 0.013 d,e,f | 0.64 ± 0.010 h,i,j | 2.75 ± 0.015 v | 3.76 ± 0.012 β |
| 20 | 293.15 | 0.59 ± 0.025 c,d | 0.62 ± 0.016 d,e,f,g,h | 0.64 ± 0.014 f,g,h,i,j | 2.47 ± 0.007 t | 3.32 ± 0.010 y |
| 25 | 298.15 | 0.58 ± 0.031 b,c | 0.62 ± 0.015 d,e,f | 0.64 ± 0.010 g,h,i,j | 2.21 ± 0.011 s | 2.88 ± 0.010 w |
| 30 | 303.15 | 0.57 ± 0.024 b | 0.60 ± 0.015 c,d | 0.63 ± 0.012 e,f,g,h,i,j | 1.97 ± 0.010 r | 2.53 ± 0.009 u |
| 35 | 308.15 | 0.56 ± 0.021 b | 0.58 ± 0.021 b | 0.62 ± 0.010 d,e,f | 1.79 ± 0.010 q | 2.21 ± 0.010 s |
| 40 | 313.15 | 0.54 ± 0.018 a | 0.57 ± 0.015 b | 0.61 ± 0.013 d,e | 1.61 ± 0.012 p | 1.95 ± 0.010 r |
| Temperature (°C) | Temperature (K) | Dynamic Viscosity RON 98 (mPa∙s) | ||||
|---|---|---|---|---|---|---|
| B0 | B5 | B10 | B85 | B100 | ||
| −10 | 263.15 | 0.69 ± 0.027 i | 0.70 ± 0.010 k | 0.77 ± 0.011 m | 4.89 ± 0.019 τ | 7.34 ± 0.025 λ |
| −5 | 268.15 | 0.65 ± 0.029 d,e | 0.68 ± 0.010 j | 0.74 ± 0.010 l | 4.39 ± 0.016 π | 6.71 ± 0.027 δ |
| 0 | 273.15 | 0.62 ± 0.030 d,e,f | 0.65 ± 0.008 f,g,h,i | 0.69 ± 0.006 j,k | 3.81 ± 0.011 ρ | 5.69 ± 0.025 η |
| 5 | 278.15 | 0.60 ± 0.029 e,f,g,h,i | 0.65 ± 0.007 h,i | 0.65 ± 0.007 f,g,h,i | 3.28 ± 0.010 y | 5.11 ± 0.031 ω |
| 10 | 283.15 | 0.61 ± 0.031 d,e | 0.65 ± 0.008 h,i | 0.63 ± 0.009 d,e | 2.92 ± 0.010 x | 4.38 ± 0.016 π |
| 15 | 288.15 | 0.57 ± 0.030 e,f,g | 0.65 ± 0.004 g,h,i | 0.63 ± 0.010 d,e,f | 2.62 ± 0.013 v | 3.76 ± 0.012 β |
| 20 | 293.15 | 0.55 ± 0.022 d,e,f | 0.65 ± 0.008 h,i | 0.64 ± 0.010 e,f,g,h | 2.31 ± 0.012 t | 3.32 ± 0.010 z |
| 25 | 298.15 | 0.54 ± 0.026 d,e | 0.63 ± 0.007 d,e | 0.64 ± 0.010 e,f,g,h | 2.09 ± 0.010 r | 2.88 ± 0.010 w |
| 30 | 303.15 | 0.53 ± 0.035 a,b | 0.63 ± 0.006 d,e | 0.63 ± 0.006 d,e | 1.88 ± 0.011 p | 2.53 ± 0.009 u |
| 35 | 308.15 | 0.53 ± 0.028 a | 0.61 ± 0.008 b,c | 0.63 ± 0.004 d,e | 1.69 ± 0.010 o | 2.21 ± 0.010 s |
| 40 | 313.15 | 0.52 ± 0.018 a | 0.60 ± 0.009 a,b | 0.62 ± 0.010 c,d | 1.50 ± 0.008 n | 1.95 ± 0.010 q |
| Temperature (°C) | Temperature (K) | Kinematic Viscosity RON 95 (mm2∙s−1) | ||||
|---|---|---|---|---|---|---|
| B0 | B5 | B10 | B85 | B100 | ||
| −10 | 263.15 | 0.94 ± 0.006 l,m,n | 0.96 ± 0.024 n | 1.07 ± 0.011 p | 6.27 ± 0.019 τ | 8.90 ± 0.016 β |
| −5 | 268.15 | 0.92 ± 0.012 k,l | 0.93 ± 0.024 l,m | 1.01 ± 0.011 o | 5.52 ± 0.015 θ | 8.16 ± 0.021 ρ |
| 0 | 273.15 | 0.84 ± 0.021 i,j | 0.86 ± 0.011 j | 0.95 ± 0.020 m,n | 4.77 ± 0.012 δ | 6.93 ± 0.019 π |
| 5 | 278.15 | 0.80 ± 0.026 e,f | 0.81 ± 0.012 e,f | 0.90 ± 0.018 k | 4.26 ± 0.013 λ | 6.25 ± 0.015 τ |
| 10 | 283.15 | 0.79 ± 0.027 d,e | 0.81 ± 0.011 e,f | 0.84 ± 0.014 g,h,i,j | 3.78 ± 0.013 y | 5.38 ± 0.016 ω |
| 15 | 288.15 | 0.80 ± 0.021 e,f | 0.81 ± 0.012 e,f | 0.84 ± 0.012 h,i,j | 3.45 ± 0.011 w | 4.63 ± 0.022 η |
| 20 | 293.15 | 0.78 ± 0.025 c,d | 0.82 ± 0.014 f,g,h | 0.84 ± 0.017 g,h,i | 3.09 ± 0.003 u | 4.12 ± 0.017 z |
| 25 | 298.15 | 0.77 ± 0.020 b,c,d | 0.82 ± 0.012 f,g | 0.85 ± 0.017 i,j | 2.78 ± 0.008 t | 3.59 ± 0.020 x |
| 30 | 303.15 | 0.76 ± 0.015 b,c | 0.80 ± 0.012 e,f | 0.84 ± 0.014 g,h,i | 2.50 ± 0.008 s | 3.18 ± 0.009 v |
| 35 | 308.15 | 0.74 ± 0.016 b | 0.78 ± 0.025 c,d | 0.83 ± 0.012 f,g,h,i | 2.28 ± 0.008 r | 2.80 ± 0.015 t |
| 40 | 313.15 | 0.72 ± 0.013 a | 0.77 ± 0.012 b,c,d | 0.82 ± 0.016 f,g | 2.06 ± 0.011 q | 2.48 ± 0.021 s |
| Temperature (°C) | Temperature (K) | Kinematic Viscosity RON 98 (mm2∙s−1) | ||||
|---|---|---|---|---|---|---|
| B0 | B5 | B10 | B85 | B100 | ||
| −10 | 263.15 | 0.89 ± 0.010 j,k | 0.89 ± 0.011 n | 0.98 ± 0.012 p | 5.96 ± 0.013 τ | 8.90 ± 0.016 β |
| −5 | 268.15 | 0.84 ± 0.006 c,d,e,f | 0.87 ± 0.011 l,m | 0.94 ± 0.011 o | 5.37 ± 0.011 θ | 8.16 ± 0.021 ρ |
| 0 | 273.15 | 0.80 ± 0.008 d,e,f,g | 0.83 ± 0.009 d,e,f,g,h,i | 0.88 ± 0.006 m,n | 4.67 ± 0.006 ω | 6.93 ± 0.019 π |
| 5 | 278.15 | 0.78 ± 0.012 g,h,i,j,k | 0.83 ± 0.008 h,i,j,k | 0.83 ± 0.008 e,f,g,h,i,j | 4.04 ± 0.005 λ | 6.25 ± 0.015 τ |
| 10 | 283.15 | 0.79 ± 0.014 d,e,f,g | 0.84 ± 0.009 i,j,k | 0.81 ± 0.010 b,c,d,e | 3.61 ± 0.005 z | 5.38 ± 0.016 ω |
| 15 | 288.15 | 0.75 ± 0.012 g,h,i,j,k | 0.84 ± 0.004 i,j,k | 0.81 ± 0.011 d,e,f,g | 3.25 ± 0.010 y | 4.63 ± 0.022 η |
| 20 | 293.15 | 0.72 ± 0.013 e,f,g,h,i,j | 0.84 ± 0.009 k,l | 0.83 ± 0.012 f,g,h,i,j | 2.88 ± 0.010 w | 4.12 ± 0.017 z |
| 25 | 298.15 | 0.71 ± 0.013 d,e,f,g,h,i,j | 0.82 ± 0.008 d,e,f,g,h | 0.83 ± 0.011 g,h,i,j,k | 2.62 ± 0.008 u | 3.59 ± 0.020 x |
| 30 | 303.15 | 0.70 ± 0.011 a,b | 0.83 ± 0.007 e,f,g,h,i,j | 0.83 ± 0.006 d,e,f,g,h,i | 2.38 ± 0.010 s | 3.18 ± 0.009 v |
| 35 | 308.15 | 0.71 ± 0.009 a | 0.81 ± 0.009 b,c,d | 0.83 ± 0.004 e,f,g,h,i,j | 2.15 ± 0.008 r | 2.80 ± 0.015 t |
| 40 | 313.15 | 0.69 ± 0.009 a | 0.79 ± 0.011 a,b,c, | 0.81 ± 0.012 d,e,f,g | 1.92 ± 0.007 q | 2.48 ± 0.021 s |
References
- Pereira, L.G.; Chagas, M.F.; Dias, M.O.S.; Cavalett, O.; Bonomi, A. Life cycle assessment of butanol production in sugarcane biorefineries in Brazil. J. Clean. Prod. 2015, 96, 557–568. [Google Scholar] [CrossRef]
- Arshad, M.; Zia, M.A.; Shah, F.A.; Ahmad, M. An overview of biofuel. In Perspectives on Water Usage for Biofuels Production; Springer: Cham, Switzerland, 2018; pp. 1–37. [Google Scholar]
- Kokkinos, N.C.; Nikolaou, N.; Psaroudakis, N.; Mertis, K.; Mitkidou, S.; Mitropoulos, A.C. Two-step conversion of LLCN olefins to strong anti-knocking alcohol mixtures catalysed by Rh, Ru/TPPTS complexes in aqueous media. Catal. Today 2015, 247, 132–138. [Google Scholar] [CrossRef]
- Hönig, V.; Kotek, M.; Orsák, M.; Hromádko, J. Use of biobutanol in gasoline engines. Listy Cukrov. Řepařské 2015, 131, 311–315. [Google Scholar]
- Thangavelu, S.K.; Ahmed, A.S.; Ani, F.N. Review on bioethanol as alternative fuel for spark ignition engines. Renew. Sustain. Energy Rev. 2016, 56, 820–835. [Google Scholar] [CrossRef]
- Marri, V.B.; Kotha, M.M.; Gaddale, A.P.R. Butanol and pentanol: The promising biofuels for CI engines—A review. Renew. Sustain. Energy Rev. 2017, 78, 1068–1088. [Google Scholar]
- Festel, G.W. Biofuels—Economic aspects. Chem. Eng. Technol. 2008, 31, 715–720. [Google Scholar] [CrossRef]
- Ramírez-Verduzco, L.F.; Rodríguez-Rodríguez, J.E.; Jaramillo-Jacob, A.D.R. Predicting cetane number, kinematic viscosity, density and higher heating value of biodiesel from its fatty acid methyl ester composition. Fuel 2012, 91, 102–111. [Google Scholar] [CrossRef]
- Han, X.; Yang, Z.; Wang, M.; Tjong, J.; Zheng, M. Clean combustion of n -butanol as a next generation biofuel for diesel engines. Appl. Energy 2017, 198, 347–359. [Google Scholar] [CrossRef]
- Pugazhendhi, A.; Mathimani, T.; Varjani, S.; Rene, E.R.; Kumar, G.; Kim, S.-H.; Ponnusamy, V.K.; Yoon, J.-J. Biobutanol as a promising liquid fuel for the future—Recent updates and perspectives. Fuel 2019, 253, 637–646. [Google Scholar] [CrossRef]
- Qureshi, N.; Meagher, M.; Huang, J.; Hutkins, R. Acetone butanol ethanol (ABE) recovery by pervaporation using silicalite-silicone composite membrane from fed-batch reactor of Clostridium acetobutylicum. J. Membr. Sci. 2001, 187, 93–102. [Google Scholar] [CrossRef]
- García, V.; Päkkilä, J.; Ojamo, H.; Muurinen, E.; Keiski, R.L. Challenges in biobutanol production: How to improve the efficiency? Renew. Sustain. Energy Rev. 2011, 15, 964–980. [Google Scholar] [CrossRef]
- Jin, C.; Yao, M.; Liu, H.; Lee, C.-F.F.; Ji, J. Progress in the production and application of n-butanol as a biofuel. Renew. Sustain. Energy Rev. 2011, 15, 4080–4106. [Google Scholar] [CrossRef]
- Tredici, M.R. Photobiology of microalgae mass cultures: Understanding the tools for the next green revolution. Biofuels 2010, 1, 143–162. [Google Scholar] [CrossRef]
- Kokkinos, N.; Lazaridou, A.; Stamatis, N.; Orfanidis, S.; Mitropoulos, A.C.; Christoforidis, A.; Nikolaou, N. Biodiesel production from selected microalgae strains and determina tion of its properties and combustion specific characteristics. J. Eng. Sci. Technol. Rev. 2015, 8, 1–6. [Google Scholar] [CrossRef]
- Hestekin, J.; Lopez, A.; Clausen, E.; Potts, T. Biobutanol. In Applications of Microbial Engineering, 1st ed.; Gupta, V.K., Schmoll, M., Maki, M., Tuohy, M., Mazutti, M.A., Eds.; CRC Press: Boca Raton, FL, USA, 2013; pp. 450–487. [Google Scholar]
- Rakopoulos, D.; Rakopoulos, C.; Giakoumis, E.; Dimaratos, A.; Kyritsis, D. Effects of butanol–diesel fuel blends on the performance and emissions of a high-speed DI diesel engine. Energy Convers. Manag. 2010, 51, 1989–1997. [Google Scholar] [CrossRef]
- Dimian, A.C.; Bildea, C.S.; Kiss, A.A. Bioethanol and biobutanol. In Applications in Design and Simulation of Sustainable Chemical Processes; Elsevier: Amsterdam, The Netherlands, 2019; pp. 285–327. [Google Scholar]
- Kumbár, V.; Dostál, P. Temperature dependence density and kinematic viscosity of petrol, bioethanol and their blends. Pak. J. Agric. Sci. 2014, 51, 175–179. [Google Scholar]
- Yilmaz, N. Temperature-dependent viscosity correlations of vegetable oils and biofuel–diesel mixtures. Biomass Bioenergy 2011, 35, 2936–2938. [Google Scholar] [CrossRef]
- Peterka, B.; Pexa, M.; Čedík, J.; Aleš, Z. The influence of biobutanol on performance parameters of mobile generator. Agron. Res. 2016, 14, 167–173. [Google Scholar]
- Rakopoulos, C.D.; Rakopoulos, D.C.; Giakoumis, E.G.; Kyritsis, D.C. The combustion ofn-butanol/diesel fuel blends and its cyclic variability in a direct injection diesel engine. Proc. Inst. Mech. Eng. Part A J. Power Energy 2011, 225, 289–308. [Google Scholar] [CrossRef]
- Qi, D.; Lee, C. Combustion and emissions behaviour for ethanol–gasoline-blended fuels in a multipoint electronic fuel injection engine. Int. J. Sustain. Energy 2014, 35, 1–16. [Google Scholar] [CrossRef]
- Hönig, V.; Pexa, M.; Mařík, J.; Linhart, Z.; Zeman, P. Biobutanol standardizing waste cooking oil as a biofuel. Pol. J. Environ. Stud. 2017, 26, 69–78. [Google Scholar] [CrossRef]
- Kumbár, V.; Polcar, A.; Votava, J. Physical and mechanical properties of bioethanol and gasoline blends. Listy Cukrov. Řepařské 2015, 131, 112–116. [Google Scholar]
- Peleg, M. Temperature–viscosity models reassessed. Crit. Rev. Food Sci. Nutr. 2018, 58, 2663–2672. [Google Scholar] [CrossRef]
- Peleg, M.; Normand, M.D.; Corradini, M.G. The arrhenius equation revisited. Crit. Rev. Food Sci. Nutr. 2012, 52, 830–851. [Google Scholar] [CrossRef] [PubMed]
- Trávníček, P.; Valach, M.; Hlaváčová, Z.; Mareček, J.; Vítěz, T.; Junga, P. Selected physical properties of liquid biofuels. Res. Agric. Eng. 2013, 59, 121–127. [Google Scholar] [CrossRef]
- Hlavac, P.; Božiková, M.; Cviklovič, V. Dynamic viscosity and activation energy of wort during fermentation and storing. Acta Technol. Agric. 2016, 19, 6–9. [Google Scholar]
- Hlaváčová, Z.; Božiková, M.; Hlaváč, P.; Regrut, T.; Ardonová, V. Selected physical properties of various diesel blends. Int. Agrophys. 2018, 32, 93–100. [Google Scholar] [CrossRef]
- Barabás, I. Predicting the temperature dependent density of biodiesel–diesel–bioethanol blends. Fuel 2013, 109, 563–574. [Google Scholar] [CrossRef]
- Poling, B.E.; Prausnitz, J.M.; O’Connell, J.P. Properties of Gases and Liquids, 5th ed.; McGraw-Hill Education: New York, NY, USA, 2001; pp. 154–196. [Google Scholar]
- Tesfa, B.; Mishra, R.; Gu, F.; Powles, N. Prediction models for density and viscosity of biodiesel and their effects on fuel supply system in CI engines. Renew. Energy 2010, 35, 2752–2760. [Google Scholar] [CrossRef]
- Olanrewaju, A.A.; Hasan, S.W.; Abu-Zahra, M.R.M. Rheological and physicochemical characterization of UAE crude oil. Pet. Sci. Technol. 2016, 34, 659–664. [Google Scholar] [CrossRef]
- Kumbár, V.; Votava, J. Differences in engine oil degradation in spark-ignition and compres-sion-ignition engine. Eksploat. Niezawodn. Maint. Reliab. 2014, 16, 622–628. [Google Scholar]
- Brandão, L.F.P.; Suarez, P.A.Z. Determination of the alternative butanol/gasoline and butanol/diesel fuel blends heats of combustion by a heat-loss compensated semi-microcalorimeter. J. Therm. Anal. Calorim. 2018, 132, 1953–1960. [Google Scholar] [CrossRef]



| Sample | Volume of Biobutanol | Volume of Gasoline |
|---|---|---|
| B0 | 0% | 100% |
| B5 | 5% | 95% |
| B10 | 10% | 90% |
| B85 | 85% | 15% |
| B100 | 100% | 0% |
| Property | Unit | Gasoline | Bioethanol | Biobutanol |
|---|---|---|---|---|
| Chemical formula | C4 to C12 | C2H5OH | C4H9OH | |
| Molecular mass | kg·mol−1 | 109 | 46 | 74 |
| Volumetric mass density (15 °C) | kg·m−3 | 720–775 | 789 | 814 |
| Calorific value | MJ·kg−1 | 43.3 | 29 | 33 |
| Heat of vaporization | kJ·kg−1 | 349 | 918 | 583 |
| Octane number ROM | - | 91–99 | 107 | 96 |
| Boiling point | °C | 27–225 | 78 | 104 |
| Flash point | °C | −45 | 13 | 35 |
| Auto ignition Temp. | °C | 258 | 420 | 415 |
| Stoichiometric AFR | - | 14.7 | 9.01 | 11.12 |
| Exponential | ρ0 (kg·m−3) | b (K−1) | R2 | SSE |
|---|---|---|---|---|
| B0-95 | 842.7 | −0.0003653 | 0.9675 | 7.094 |
| B5-95 | 918.5 | −0.0006602 | 0.9108 | 67.90 |
| B10-95 | 939.7 | −0.0007296 | 0.9800 | 17.37 |
| B85-95 | 1054 | −0.0009530 | 0.9793 | 33.96 |
| B0-98 | 950.2 | −0.0007528 | 0.9705 | 27.75 |
| B5-98 | 989.9 | −0.0008632 | 0.9720 | 35.31 |
| B10-98 | 973.6 | −0.0007975 | 0.9710 | 31.33 |
| B85-98 | 1057 | −0.0009501 | 0.9824 | 28.75 |
| B100 | 1074 | −0.0009863 | 0.9745 | 45.80 |
| Exponential | η0 (mPa·s) | b (K−1) | R2 | SSE |
|---|---|---|---|---|
| B0-95 | 2.785 | −0.005277 | 0.8904 | 0.003453 |
| B5-95 | 2.291 | −0.004474 | 0.8800 | 0.002957 |
| B10-95 | 3.753 | −0.005958 | 0.8234 | 0.009255 |
| B85-95 | 2.785 | −0.005277 | 0.8904 | 0.003453 |
| B0-98 | 2.855 | −0.005526 | 0.9412 | 0.001755 |
| B5-98 | 1.318 | −0.002481 | 0.8729 | 0.001026 |
| B10-98 | 2.040 | −0.003918 | 0.7008 | 0.007626 |
| B85-98 | 2926 | −0.024320 | 0.9974 | 0.033080 |
| B100 | 1.141 × 104 | −0.028090 | 0.9971 | 0.088950 |
| Arrhenius | η0 (mPa·s) | Ea (J·mol−1) | R2 | SSE |
|---|---|---|---|---|
| B0-95 | 0.1329 | 3634 | 0.9101 | 0.002831 |
| B5-95 | 0.1735 | 3085 | 0.8994 | 0.002481 |
| B10-95 | 0.1194 | 4134 | 0.8563 | 0.007531 |
| B85-95 | 0.003794 | 1.576 × 104 | 0.9996 | 0.005149 |
| B0-98 | 0.118700 | 3793 | 0.9566 | 0.001295 |
| B5-98 | 0.316800 | 1698 | 0.8742 | 0.001015 |
| B10-98 | 0.208800 | 2747 | 0.7397 | 0.006637 |
| B85-98 | 0.003084 | 1.615 × 104 | 0.9989 | 0.013940 |
| B100 | 0.001479 | 1.856 × 104 | 0.9997 | 0.010480 |
| Newton | η0 (mPa·s) | b (K−1) | R2 | SSE |
|---|---|---|---|---|
| B0-95 | 0.132900 | 437.1 | 0.9101 | 0.002831 |
| B5-95 | 0.173500 | 371 | 0.8994 | 0.002481 |
| B10-95 | 0.119400 | 497.3 | 0.8563 | 0.007531 |
| B85-95 | 0.003794 | 1896 | 0.9996 | 0.005149 |
| B0-98 | 0.118700 | 456.2 | 0.9566 | 0.001295 |
| B5-98 | 0.316800 | 204.3 | 0.8742 | 0.001015 |
| B10-98 | 0.208800 | 330.4 | 0.7397 | 0.006637 |
| B85-98 | 0.003084 | 1942 | 0.9989 | 0.013940 |
| B100 | 0.001479 | 2233 | 0.9997 | 0.010480 |
| Vogel | η0 (mPa·s) | b (K) | c (K) | R2 | SSE |
|---|---|---|---|---|---|
| B0-95 | 0.47170000 | 12.59 * | −233.8 | 0.9572 | 0.0013480 |
| B5-95 | 0.51300000 | 9.758 * | −235.9 | 0.9493 | 0.0012490 |
| B10-95 | 0.54120000 | 8.194 | −244.4 | 0.9700 | 0.0015740 |
| B85-95 | 0.0079680 * | 1500 | −31.16 * | 0.9996 | 0.0047340 |
| B0-98 | 0.40190000 | 25.06 * | −216.5 | 0.9782 | 0.0006507 |
| B5-98 | 0.0008957 * | 2703 * | 50.67 * | 0.9989 | 0.0133100 |
| B10-98 | 0.58500000 | 3.513 * | −250.8 | 0.9321 | 0.0147100 |
| B85-98 | 0.0008959 * | 2703 * | 50.66 * | 0.9989 | 0.0133100 |
| B100 | 0.0037680 * | 1737 | −33.16 * | 0.9997 | 0.0093950 |
| Exponential | ν0 (mm2·s−1) | b (K−1) | R2 | SSE |
|---|---|---|---|---|
| B0-95 | 3.2970 | −0.004905 | 0.8745 | 0.006032 |
| B5-95 | 0.8766 | −0.003729 | 0.7472 | 0.008742 |
| B10-95 | 4.1030 | −0.005324 | 0.7760 | 0.017080 |
| B85-95 | 2465 | −0.022790 | 0.9961 | 0.072730 |
| B0-98 | 3.0930 | −0.004876 | 0.9157 | 0.003430 |
| B5-98 | 1.3070 | −0.001549 | 0.7127 | 0.001855 |
| B10-98 | 2.0960 | −0.003125 | 0.5800 | 0.013700 |
| B85-98 | 2356 | −0.021870 | 0.9950 | 0.131700 |
| B100 (butanol) | 8691 | −0.026090 | 0.9980 | 0.093450 |
| Arrhenius | ν0 (mm2·s−1) | Ea (J·mol−1) | R2 | SSE |
|---|---|---|---|---|
| B0-95 | 0.194500 | 3384 | 0.8962 | 0.004991 |
| B5-95 | 0.279800 | 2594 | 0.7751 | 0.007778 |
| B10-95 | 0.187100 | 3711 | 0.8129 | 0.014270 |
| B85-95 | 0.006053 | 1.518 × 104 | 0.9995 | 0.010040 |
| B0-98 | 0.186200 | 3356 | 0.9345 | 0.002662 |
| B5-98 | 0.534300 | 1070 | 0.7247 | 0.001777 |
| B10-98 | 0.337500 | 2211 | 0.6217 | 0.012340 |
| B85-98 | 0.009614 | 1.459 × 104 | 0.9991 | 0.022710 |
| B100 | 0.003469 | 1.725 × 104 | 0.9950 | 0.240900 |
| Newton | ν0 (mm2·s−1) | b (K−1) | R2 | SSE |
|---|---|---|---|---|
| B0-95 | 0.194500 | 407.1 | 0.8962 | 0.004991 |
| B5-95 | 0.279800 | 371 | 0.7751 | 0.007778 |
| B10-95 | 0.187100 | 446.3 | 0.8129 | 0.014270 |
| B85-95 | 0.006053 | 1826 | 0.9995 | 0.010040 |
| B0-98 | 0.186200 | 403.6 | 0.9345 | 0.002662 |
| B5-98 | 0.534300 | 128.7 | 0.7247 | 0.001777 |
| B10-98 | 0.337500 | 265.9 | 0.6217 | 0.012340 |
| B85-98 | 0.009614 | 1755 | 0.9991 | 0.022710 |
| B100 | 0.003469 | 2075 | 0.9950 | 0.240900 |
| Vogel | ν0 (mm2·s−1) | b (K) | c (K) | R2 | SSE |
|---|---|---|---|---|---|
| B0-95 | 0.644000 | 10.02 * | −237.2 | 0.9542 | 0.002200 |
| B5-95 | 0.736600 | 3.685 * | −249.3 | 0.9069 | 0.003220 |
| B10-95 | 0.740600 | 5.904 | −247.8 | 0.9618 | 0.002911 |
| B85-95 | 0.02609 * | 1093 | −63.79 | 0.9996 | 0.006728 |
| B0-98 | 0.571300 | 16.83 * | −224.7 | 0.9670 | 0.001343 |
| B5-98 | 0.799100 | 1.329 * | −250.8 | 0.7937 | 0.001332 |
| B10-98 | 0.782300 | 2.076 * | −254.3 | 0.8879 | 0.003658 |
| B85-98 | 0.0695 * | 814.2 | −89.9 | 0.9996 | 0.01064 |
| B100 | 1.511 × 105 * | 324 * | 7770 * | 0.9645 | 1.697 |
| Volumetric Mass Density | x0 (kg·m−3) | x1 (K−1·kg·m−3) | x2 (kg·m−3) | x3 (K−2·kg·m−3) | x4 (K−1·kg·m−3) | R2 | |
| Bx-95 | 358.4 | 3.202 | 1.649 | −0.006287 | −0.003953 | 0.9944 | 178.1 |
| Bx-98 | 375.2 | 3.350 | 0.9477 | −0.006874 | −0.001859 | 0.9902 | 239.7 |
| Kinematic Viscosity | y0 (mm2·s−1) | y1 (K−1·mm2·s−1) | y2 (mm2·s−1) | y3 (K−2·mm2·s−1) | y4 (K−1·mm2·s−1) | R2 | |
| Bx-95 | 52.91 | −0.3668 | 0.3809 | 0.0006412 | −0.001178 | 0.9715 | 7.021 |
| Bx-98 | 54.61 | −0.3801 | 0.4105 | 0.0006663 | −0.001265 | 0.9897 | 2.999 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Trost, D.; Polcar, A.; Boldor, D.; Nde, D.B.; Wolak, A.; Kumbár, V. Temperature Dependence of Density and Viscosity of Biobutanol-Gasoline Blends. Appl. Sci. 2021, 11, 3172. https://doi.org/10.3390/app11073172
Trost D, Polcar A, Boldor D, Nde DB, Wolak A, Kumbár V. Temperature Dependence of Density and Viscosity of Biobutanol-Gasoline Blends. Applied Sciences. 2021; 11(7):3172. https://doi.org/10.3390/app11073172
Chicago/Turabian StyleTrost, Daniel, Adam Polcar, Dorin Boldor, Divine Bup Nde, Artur Wolak, and Vojtěch Kumbár. 2021. "Temperature Dependence of Density and Viscosity of Biobutanol-Gasoline Blends" Applied Sciences 11, no. 7: 3172. https://doi.org/10.3390/app11073172
APA StyleTrost, D., Polcar, A., Boldor, D., Nde, D. B., Wolak, A., & Kumbár, V. (2021). Temperature Dependence of Density and Viscosity of Biobutanol-Gasoline Blends. Applied Sciences, 11(7), 3172. https://doi.org/10.3390/app11073172





