Developing an Adaptive 3D Vertebrae Model of Scoliosis Patients for Customize Garment Design
Abstract
:Featured Application
Abstract
1. Introduction
2. Related Literature Review
3. Anatomical Description and Generic Model of Different Vertebrae Morphology
4. 3D Adaptive Vertebrae Model
4.1. Generic Model
4.1.1. Lumbar Vertebrae Model
4.1.2. Dorsal Vertebrae Model
4.1.3. Cervical Vertebrae Model
5. Results and Discussion
5.1. Virtual and Real Model of Cervical Vertebrae
5.2. Developments of Adaptive Vertebra Model in a Body
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bankoff, ADP. Biomechanical Characteristics of the Bone, Human Musculoskeletal Biomechanics; Goswami, T., Ed.; InTech, 2012; pp. 61–86. ISBN 978-953-307-638-6. Available online: http://www.intechopen.com/books/human-musculoskeletal-biomechanics/biomechanical-characteristics-of-thebone (accessed on 21 January 2021).
- Li, H.; Leow, W.K.; Huang, C.H.; Howe, T.S. Modeling and Measurement of 3D Deformation of Scoliotic Spine Using 2D X-ray Images. In Computer Analysis of Images and Patterns. CAIP 2009; Jiang, X., Petkov, N., Eds.; Lecture Notes in Computer Science; Springer: Berlin/Heidelberg, Germany, 2009; Volume 5702. [Google Scholar] [CrossRef] [Green Version]
- Cobb, J.R. Outlines for the study of scoliosis. J. Bone Jt. Surg. 1948, 5, 261–275. [Google Scholar]
- Langensiepen, S.; Semler, O.; Sobottke, R.; O Fricke, O.; Franklin, J.; Schönau, E.; Eysel, P. Measuring procedures to determine the Cobb angle in idiopathic scoliosis: A systematic review. Eur. Spine J. 2013, 22, 2360–2371. [Google Scholar] [CrossRef] [Green Version]
- Albisinni, U. Radiology of the musculoskeletal system. Radiol. Med. 1995, 90, 653–657. [Google Scholar]
- Matussek, J.; Mellerowicz, H.; Klöckner, C.; Sauerlandt, B.; Nahr, K.; Neff, G. Zwei-und dreidimensionale korrektur von skoliosen durch korsettbehandlung. Optimierte konservative therapie der idiopathischen skoliose durch ein weiterentwickeltes Cheneau-korsett. Orthopade 2000, 29, 490–499. [Google Scholar] [CrossRef]
- Artusi, C.A.; Montanaro, E.; Tuttobene, S.; Romagnolo, A.; Zibetti, M.; Lopiano, L. Pisa syndrome in Parkinson’s disease is associated with specific cognitive alterations. Front. Neurol. 2019, 10, 1–7. [Google Scholar] [CrossRef]
- Barone, P.; Santangelo, G.; Amboni, M.; Pellecchia, M.T.; Vitale, C. Pisa syndrome in Parkinson’s disease and parkinsonism: Clinical features, pathophysiology, and treatment. Lancet Neurol. 2016, 15, 1063–1074. [Google Scholar] [CrossRef]
- Abtew, M.A.; Bruniaux, P.; Boussu, F. Development of adaptive bust for female soft body armour using three-dimensional (3D) warp interlock fabrics: Three-dimensional (3D) design process. IOP Conf. Ser. Mater. Sci. Eng. 2017, 254, 052001. [Google Scholar] [CrossRef] [Green Version]
- Dong, Z.; Jiang, G.; Wu, Z.; Honglian, C. 3D parametric human modeling for warp-knitted seamless garment. Int. J. Cloth. Sci. Technol. 2015, 27, 532–548. [Google Scholar] [CrossRef]
- Chu, C.-H.; Tsai, Y.-T.; Wang, C.C.L.; Kwokb, T.H. Exemplar-based statistical model for semantic parametric design of human body. Comput. Ind. 2010, 61, 541–549. [Google Scholar] [CrossRef]
- Lin, Y.; Wang, M.J. Constructing 3D human model from front and side images. Expert Syst. Appl. 2012, 39, 5012–5018. [Google Scholar] [CrossRef]
- Wang, C.C.L. Parameterization and parametric design of mannequins. Comput. Des. 2005, 37, 83–98. [Google Scholar] [CrossRef]
- Frostell, A.; Hakim, R.; Thelin, E.P.; Mattsson, P.; Svensson, M. A Review of the Segmental Diameter of the Healthy Human Spinal Cord. Front. Neurol. 2016, 7, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Cramer, J.; Quigley, E.; Hutchins, T.; Shah, L. Educational Material for 3D Visualization of Spine Procedures: Methods for Creation and Dissemination. J. Digit. Imaging 2017, 30, 296–300. [Google Scholar] [CrossRef] [PubMed]
- Saveh, A.H.; Zali, A.R.; Ashrafi, F.; Shahzadi, S.; Seddighi, S.; Momenzadeh, S.; Behnam, B.; Dehpour, O.; Chizari, M.; Gammada, K. The Spine Vertebral Bodies 3D Modeling and its Biomechanical Advantages. Int. Clin. Neurosci, J. 2015, 2, 23–25. [Google Scholar]
- Dalvit Carvalho da Silva, R.; Jenkyn, T.R.; Carranza, V.A. Convolutional Neural Networks and Geometric Moments to Identify the Bilateral Symmetric Midplane in Facial Skeletons from CT Scans. Biology 2021, 10, 182. [Google Scholar] [CrossRef]
- Kubicka, A.M.; Stefaniak, J.; Lubiatowski, P.; Długosz, J.; Dzianach, M.; Redman, M.; Piontek, J.; Romanowski, L. Reliability of measurements performed on two dimensional and three dimensional computed tomography in glenoid assessment for instability. Int. Orthop. 2016, 40, 2581–2588. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fuller, C.B.; Farnsworth, C.L.; Bomar, J.D.; Jeffords, M.E.; Murphy, J.S.; Edmonds, E.W.; Pennock, A.T.; Wenger, D.R.; Upasani, V.V. Femoral version: Comparison among advanced imaging methods. J. Orthop. Res. 2018, 36, 1536–1542. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rehm, J.; Germann, T.; Akbar, M.; Pepke, W.; Kauczor, H.U.; Weber, M.A.; Spira, D. 3D-modeling of the spine using EOS imaging system: Inter-reader reproducibility and reliability. PLoS ONE 2017, 12, e0171258. [Google Scholar] [CrossRef]
- Ilharreborde, B.; Dubousset, J.; Le Huec, J. Use of EOS imaging for the assessment of scoliosis deformities: Application to postoperative 3D quantitative analysis of the trunk. Eur. Spine J. 2014, 23, S397–S405. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hui, S.C.N.; Pialasse, J.P.; Wong, J.Y.H.; Lam, T.-P.; Ng, B.K.W.; Cheng, J.C.Y.; Chu, W.C.W. Radiation dose of digital radiography (DR) versus micro-dose x-ray (EOS) on patients with adolescent idiopathic scoliosis: 2016 SOSORT-IRSSD ‘John Sevastic Award’ Winner in Imaging Research. Scoliosis Spinal Disord. 2016, 11, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Humbert, L.; De Guise, J.A.; Aubert, B.; Godbout, B.; Skalli, W. 3D reconstruction of the spine from biplanar X-rays using parametric models based on transversal and longitudinal inferences. Med. Eng. Phys. 2009, 31, 681–687. [Google Scholar] [CrossRef]
- Seoud, L.; Adankon, M.M.; Labelle, H.; Dansereau, J.; Cheriet, F. Prediction of Scoliosis Curve Type Based on the Analysis of Trunk Surface Topography. In Proceedings of the IEEE International Symposium on Biomedical Imaging: From Nano to Macro, Rotterdam, The Netherlands, 14–17 April 2010; IEEE: Piscataway, NJ, USA, 2010; pp. 408–411. [Google Scholar]
- Bachmann, K.R.; Yaszay, B.; Bartley, C.E.; Bastrom, T.P.; Reighard, F.G.; Upasani, V.V.; Newton, P.O.; Harms Study Group Investigators. A three-dimensional analysis of scoliosis progression in non-idiopathic scoliosis: Is it similar to adolescent idiopathic scoliosis? Childs Nerv. Syst. 2019, 35, 1585–1590. [Google Scholar] [CrossRef]
- De Smet, A.; Tarlton, M.; Cook, L.; Berridge, A.S.; Asher, M.A. The top view for analysis of scoliosis progression. Radiology 1983, 147, 369–372. [Google Scholar] [CrossRef]
- Lim, H.; Istook, C.L.; Cassill, N.L. Advanced Mass Customization in Apparel. J. Text. Appar. Technol. Manag. 1992, 6, 1–16. [Google Scholar]
- Panero, E.; Muscolo, G.G.; Pastorelli, S.; Gastaldi, L. Model Based Analysis of Trunk Exoskeleton for Human Efforts Reduction. Adv. Intell. Syst. Comput. 2020, 980, 410–418. [Google Scholar]
- Yang, X.; Huang, T.H.; Hu, H.; Yu, S.; Zhang, S.; Zhou, X.; Carriero, A.; Yue, G.; Su, H. Spine-Inspired Continuum Soft Exoskeleton for Stoop Lifting Assistance. IEEE Robot. Autom. Lett. 2019, 4, 4547–4554. [Google Scholar] [CrossRef] [Green Version]
- Burns, L.D.; Mullet, K.K.; Bryant, N.O. The Business of Fashion: Designing, Manufacturing, and Marketing; Fairchild Books: New York, NY, USA, 2002. [Google Scholar]
- Wang, C.C.L.; Yuen, M.M.F. CAD methods in garment design. Comput. Des. 2005, 37, 583–584. [Google Scholar] [CrossRef]
- Abtew, M.A.; Bruniaux, P.; Boussu, F.; Loghin, C.; Cristian, I.; Chen, Y.; Wang, L. A systematic pattern generation system for manufacturing customized seamless multi-layer female soft body armour through dome-formation (moulding) techniques using 3D warp interlock fabrics. J. Manuf. Syst. 2018, 49, 61–74. [Google Scholar] [CrossRef]
- Abtew, M.A.; Bruniaux, P.; Boussu, F.; Loghin, M.C. Development of comfortable and well-fitted bra pattern for customized female soft body armor through 3D design process of adaptive bust on virtual mannequin. Comput. Ind. 2018, 100, 7–20. [Google Scholar] [CrossRef]
- Abtew, M.A.; Bruniaux, P.; Boussu, F.; Loghin, C.; Cristian, I.; Chen, Y.; Wang, L. Female seamless soft body armor pattern design system with innovative reverse engineering approaches. Int. J. Adv. Manuf. Technol. 2018, 98, 2271–2285. [Google Scholar] [CrossRef]
- Protopsaltou, D.; Luible, C.; Arevalo, M.; Magnenat-Thalmann, N. A Body and Garment Creation Method for an Internet Based Virtual Fitting Room. In Advances in Modelling, Animation and Rendering; Vince, J., Earnshaw, R., Eds.; Springer: London, UK, 2002; pp. 105–122. [Google Scholar]
- Cichocka, A.; Bruniaux, P.; Frydrych, I. 3D Garment Modelling—Creation of a Virtual Mannequin of the Human Body. Fibres Text. East. Eur. 2014, 22, 123–131. [Google Scholar]
- Baek, S.-Y.; Lee, K. Parametric human body shape modeling framework for human-centered product design. Comput. Des. 2012, 44, 56–67. [Google Scholar] [CrossRef]
- Liu, Y.J.; Zhang, D.L.; Yuen, M.M.F. A survey on CAD methods in 3D garment design. Comput. Ind. 2010, 61, 576–593. [Google Scholar] [CrossRef] [Green Version]
- Javadi Toghchi, M.; Bruniaux, P.; Campagne, C.; Cayla, A.; Loghin, C.; Cristian, I.; Chen, Y.; Wang, L. Virtual Mannequin Simulation for Customized Electromagnetic Shielding Maternity Garment Manufacturing. Designs 2019, 3, 53. [Google Scholar] [CrossRef] [Green Version]
- Azouz, B.; Rioux, M.; Shu, C.; Richard, L. Analysis of Human Shape Variation Using Volumetric Techniques. In Proceedings of the 17th Annual Conference on Computer Animation and Social Agents (CASA2004), Geneva, Switzerland, 7–9 July 2004. [Google Scholar]
- Cho, Y.; Okada, N.; Park, H.; Takatera, M. An interactive body model for individual pattern making. Int. J. Cloth. Sci. Technol. 2005. [CrossRef]
- Hong, Y.; Zeng, X.; Bruniaux, P.; Liu, K. Interactive virtual try-on based three-dimensional garment block design for disabled people of scoliosis type. Text. Res. J. 2016, 87, 1261–1274. [Google Scholar] [CrossRef]
- Hong, Y.; Bruniaux, P.; Zeng, X.; Antonela, C.; Liu, K.; Chen, Y. Visual-simulation-based personalized garment block design method for physically disabled people with scoliosis (PDPS). Autex Res. J. 2018, 18, 35–45. [Google Scholar] [CrossRef] [Green Version]
- Hong, Y.; Zeng, X.; Bruniaux, P.; Kaixuan, L.; Yan, C.; Xujing, Z. Collaborative 3D-To-2D Tight-Fitting Garment Pattern Design Process for Scoliotic People. Fibres Text. East. Eur. 2017, 5, 113–117. [Google Scholar] [CrossRef]
- Galbusera, F.; Bassani, T. The Spine: A Strong, Stable, and Flexible Structure with Biomimetics Potential. Biomimetics 2019, 4, 60. [Google Scholar] [CrossRef] [Green Version]
- Takao, T.; Kubota, K.; Maeda, T.; Okada, S.; Morishita, Y.; Mori, E.; Yugue, I.; Kawano, O.; Sakai, H.; Ueta, T.; et al. A radiographic evaluation of facet sagittal angle in cervical spinal cord injury without major fracture or dislocation. Spinal Cord 2017, 55, 515–517. [Google Scholar] [CrossRef] [Green Version]
- Lévy, S.; Baucher, G.; Roche, P.H.; Evin, M.; Callot, V.; Arnoux, P.J. Biomechanical comparison of spinal cord compression types occurring in Degenerative Cervical Myelopathy. Clin. Biomech. 2020, 81, 1–33. [Google Scholar] [CrossRef] [PubMed]
- Montemurro, N.; Perrini, P.; Mangini, V.; Galli, M.; Papini, A. The Y-shaped trabecular bone structure in the odontoid process of the axis: A CT scan study in 54 healthy subjects and biomechanical considerations. J. Neurosurg. Spine 2019, 30, 585–592. [Google Scholar] [CrossRef] [PubMed]
- Adams, J.E.; Lenchik, L.; Roux, C.; Genant, H. Vertebral Fracture Initiative, Part II, Radiological Assessment of Vertebral Fracture. 2010. Available online: http://www.iofbonehealthorg/what-we-do/training-and-education/educational-slide-kits/vertebral-fracture-teaching-program (accessed on 21 January 2021).
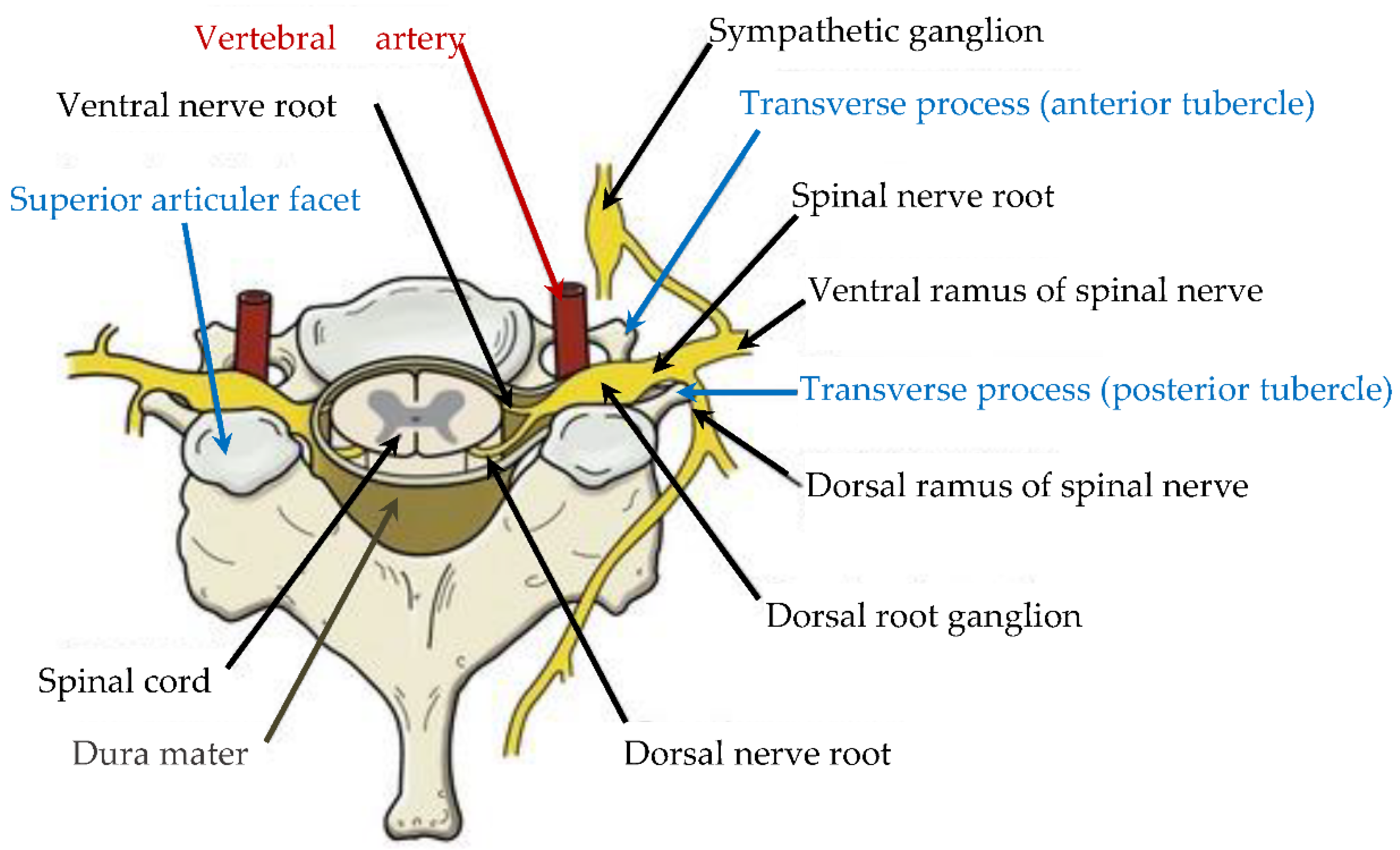

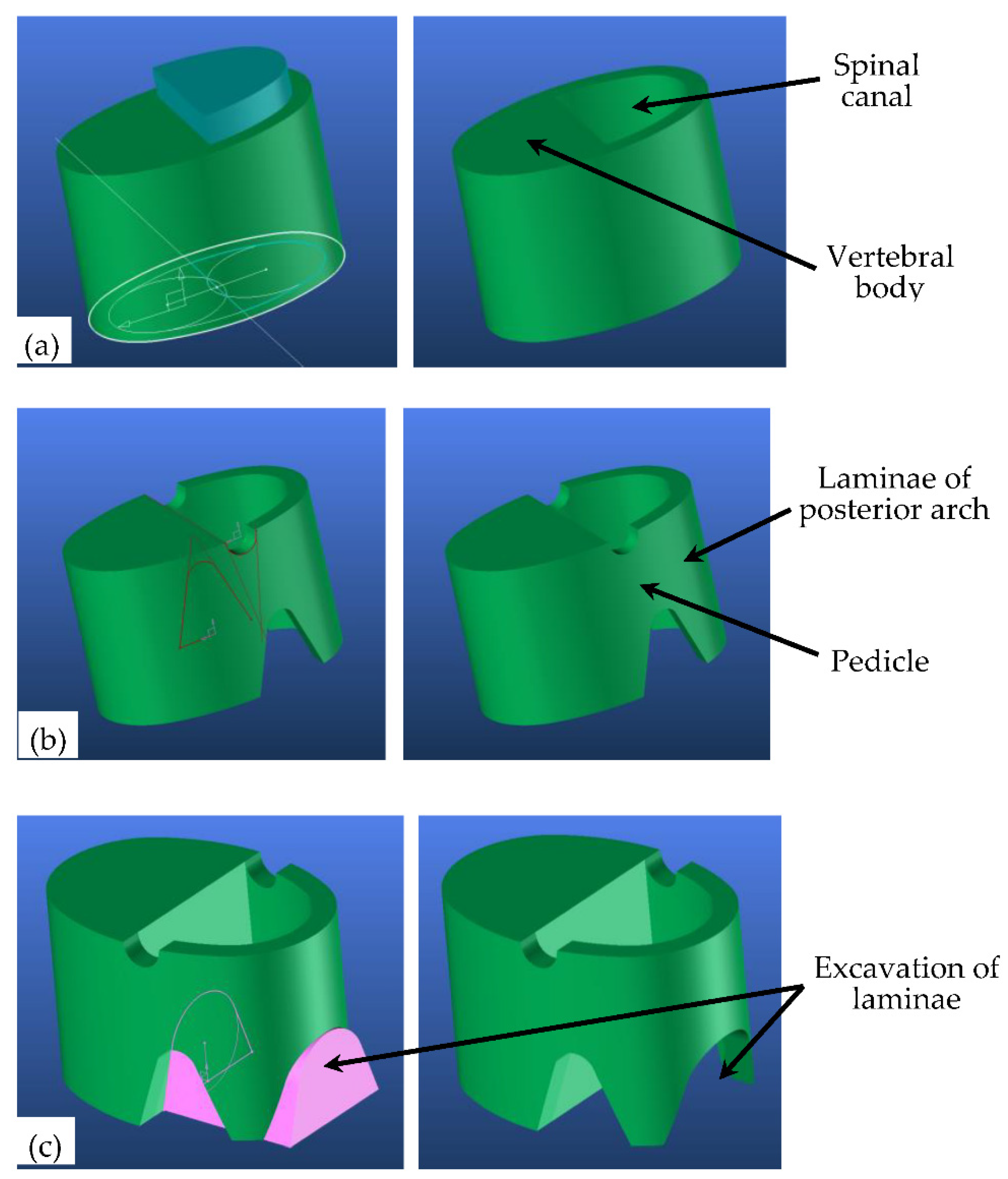
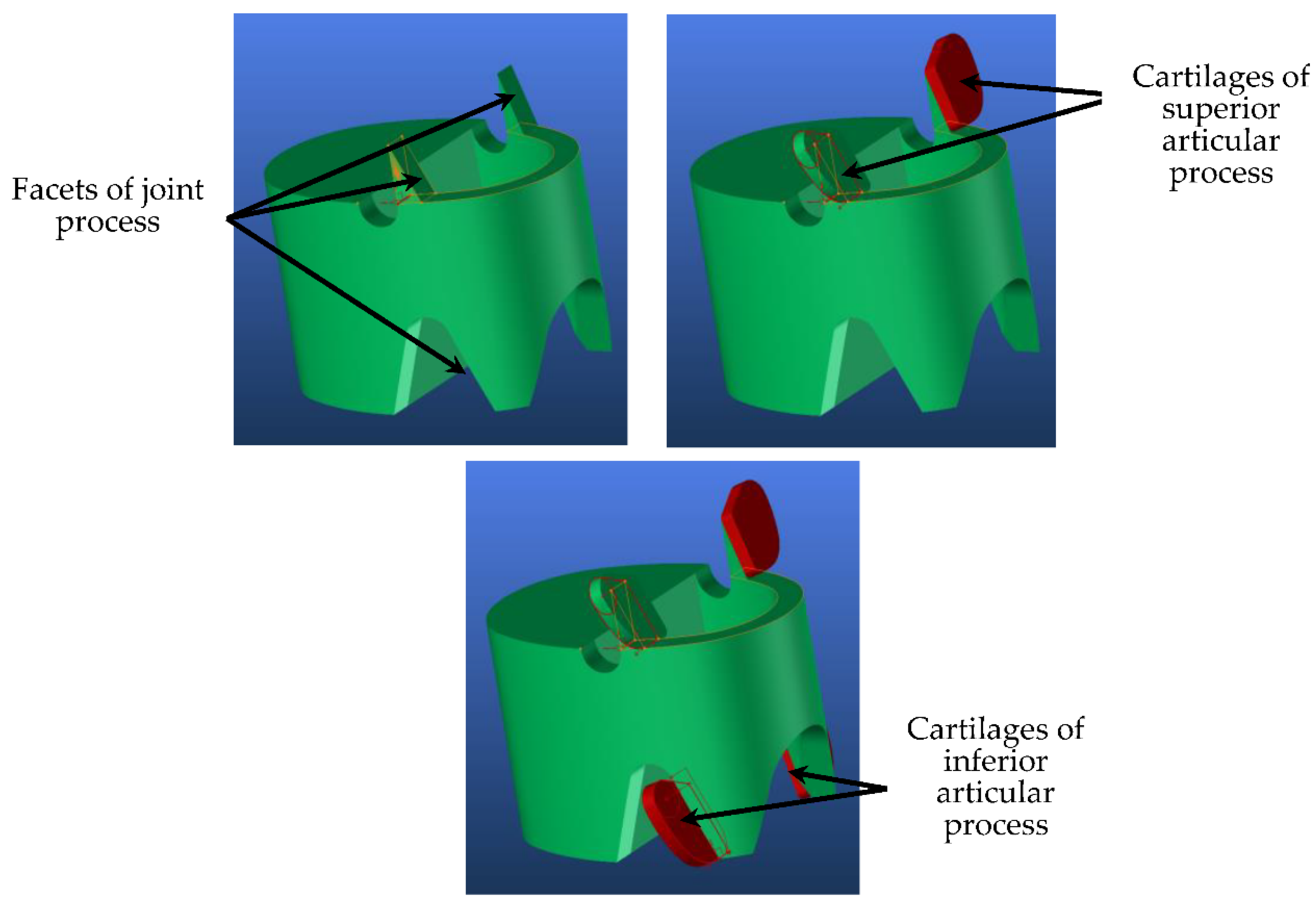
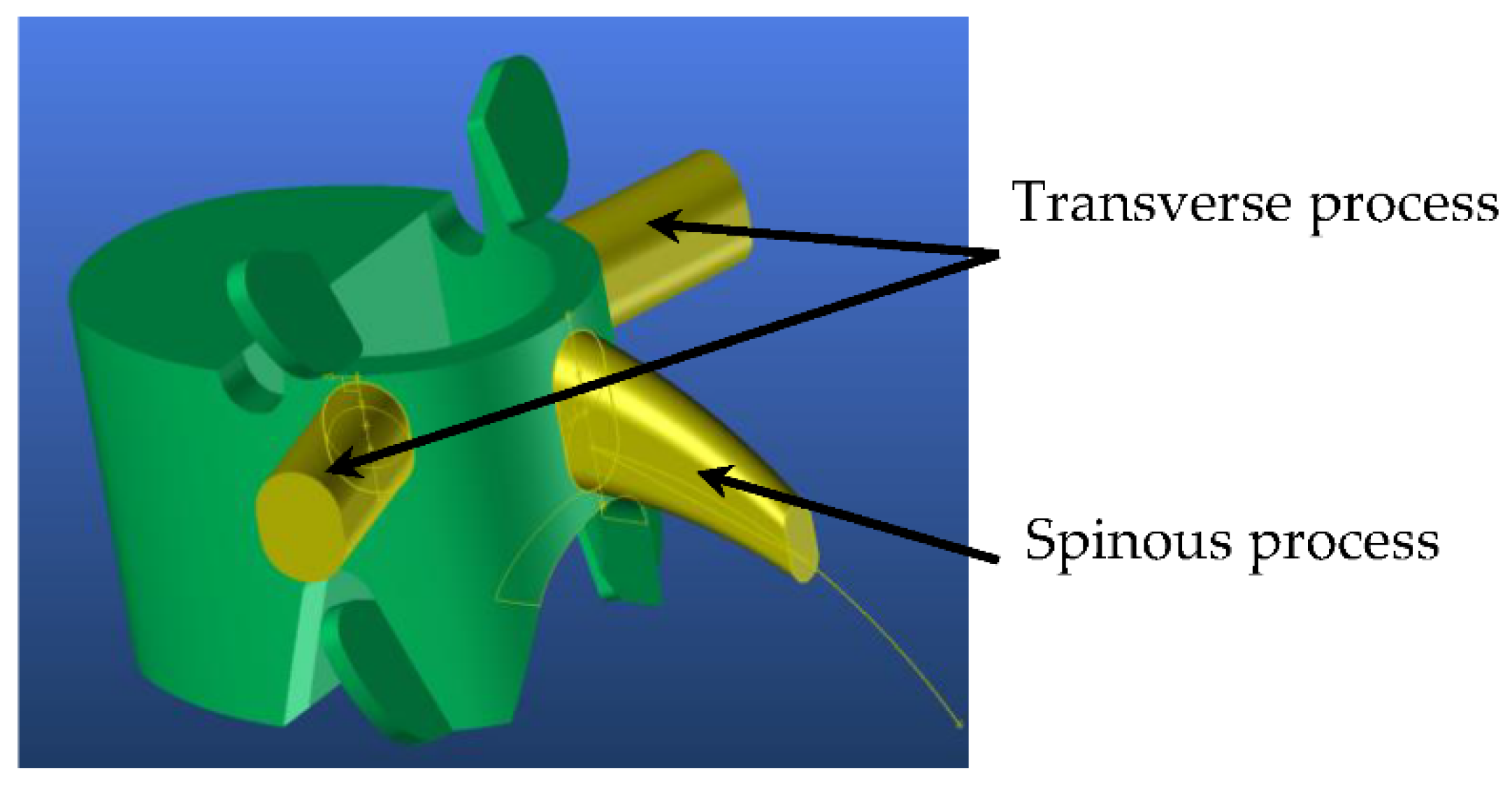
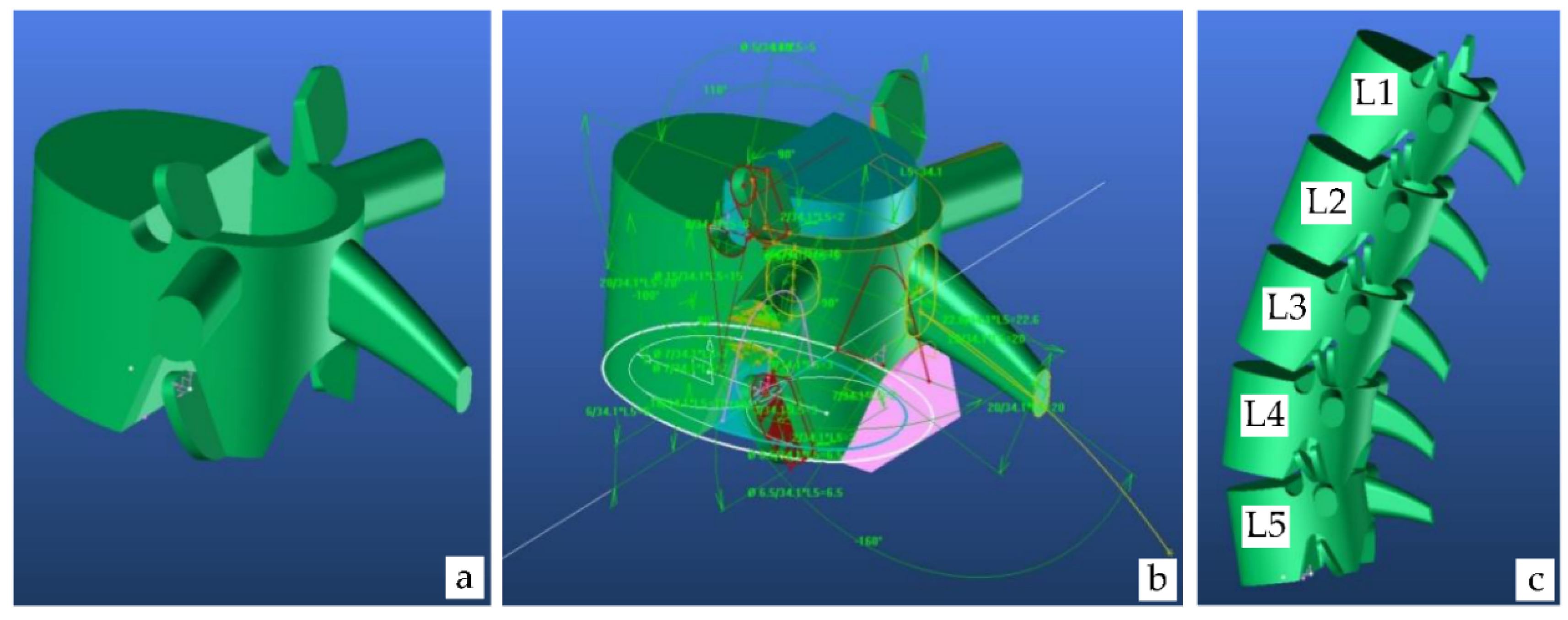
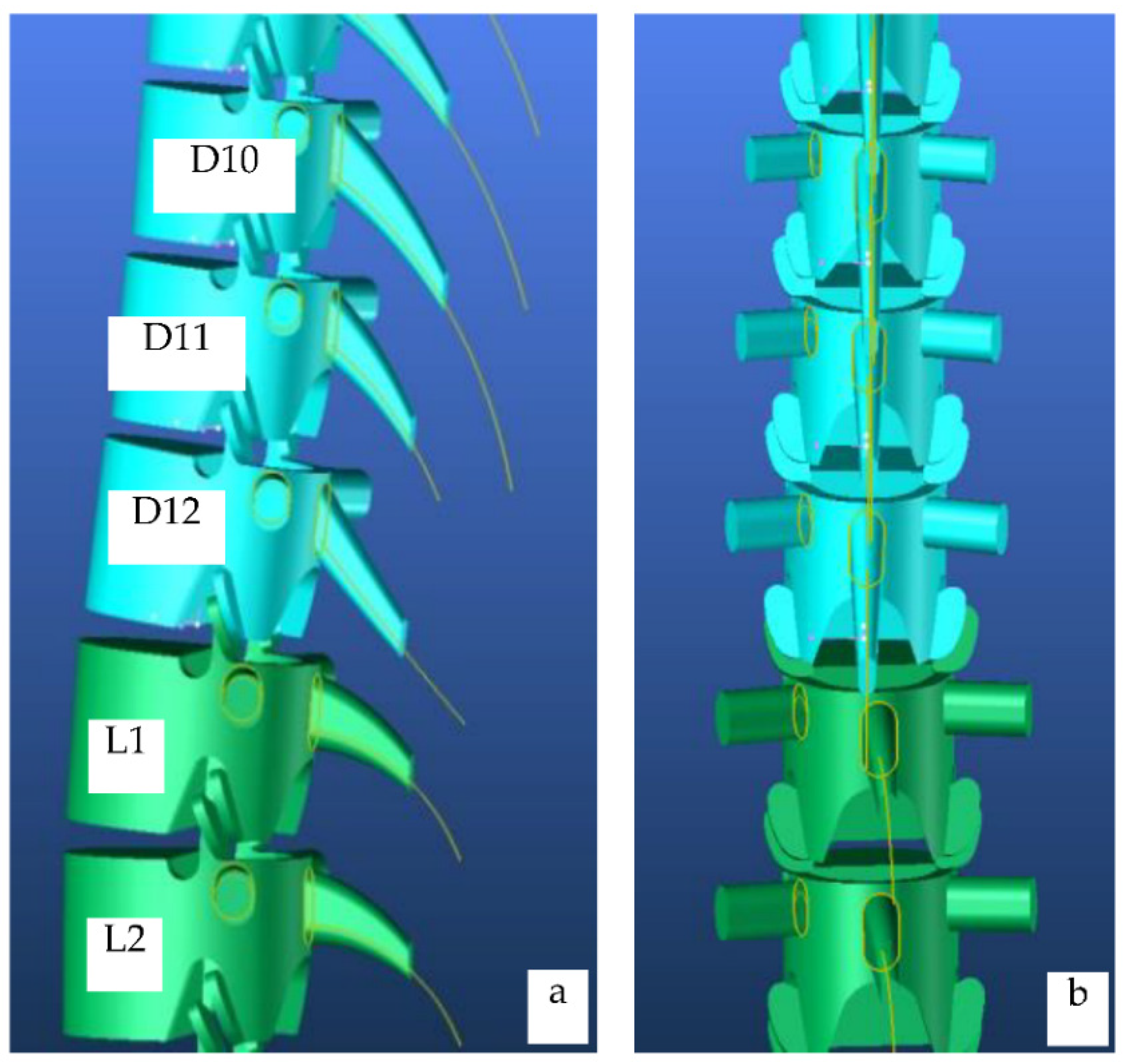

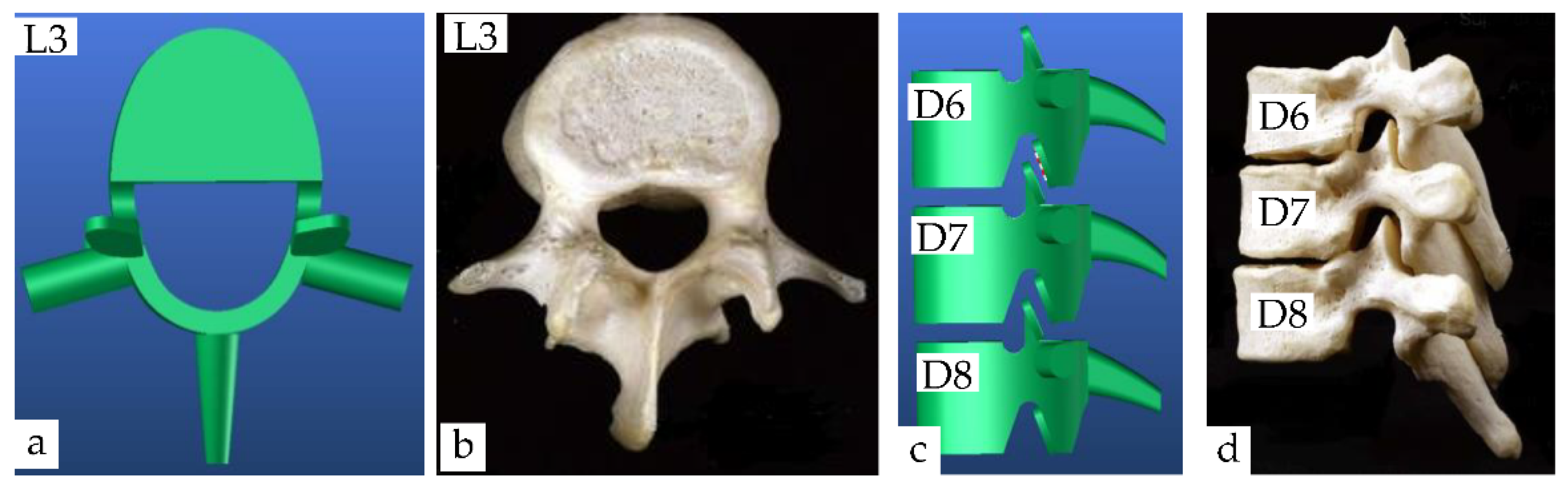
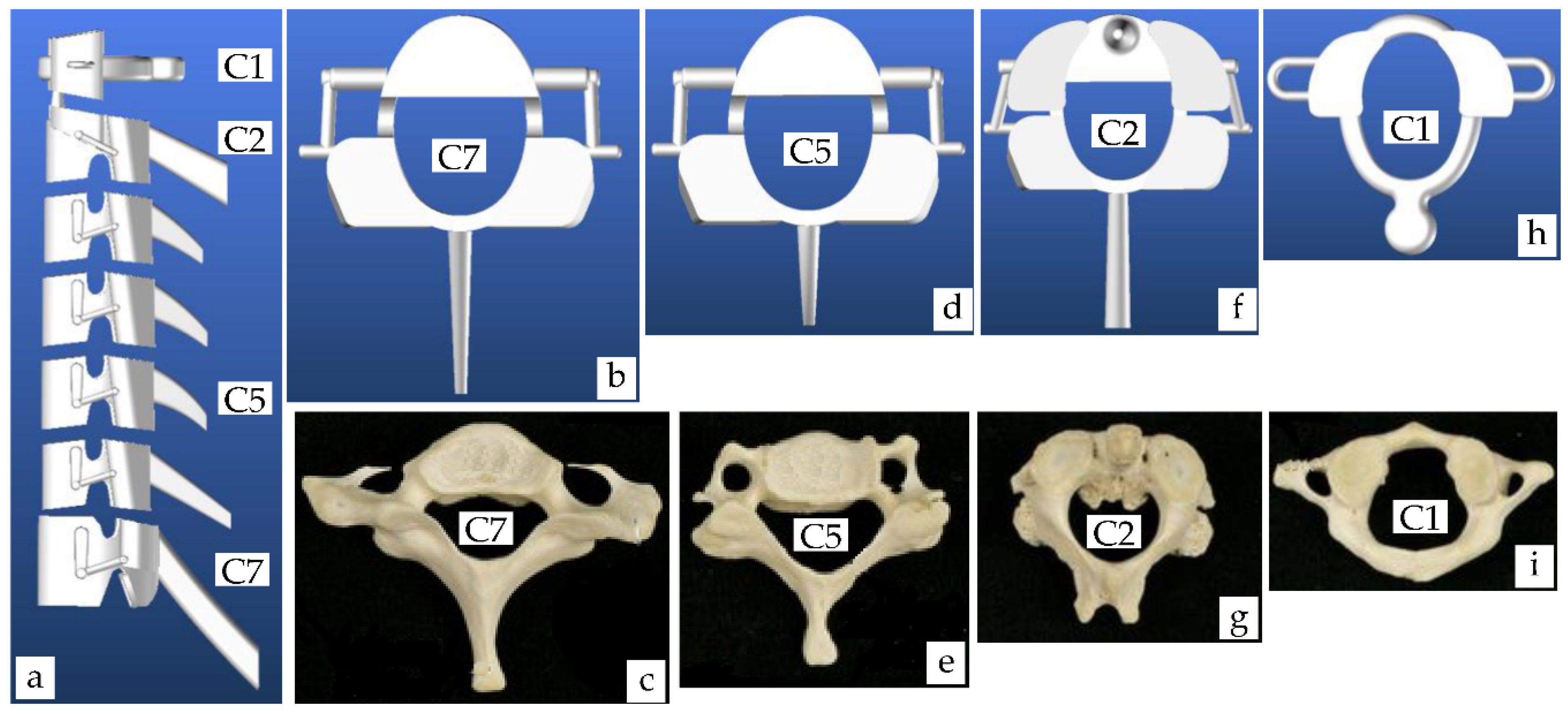

| Segment | Percentage of Spine | Cumulative Percentage of Spine |
|---|---|---|
| C1 | 3.2 | 3.2 |
| C2 | 3.2 | 6.4 |
| C3 | 2.8 | 9.1 |
| C4 | 2.7 | 11.9 |
| C5 | 2.7 | 14.6 |
| C6 | 2.6 | 17.2 |
| C7 | 3.1 | 20.2 |
| D1 | 3.4 | 23.6 |
| D2 | 3.7 | 27.3 |
| D3 | 3.4 | 31.1 |
| D4 | 3.9 | 34.9 |
| D5 | 3.9 | 38.8 |
| D6 | 4.2 | 42.9 |
| D7 | 4.3 | 47.3 |
| D8 | 4.5 | 51.7 |
| D9 | 4.6 | 56.3 |
| D10 | 4.8 | 61.2 |
| D11 | 5.1 | 66.2 |
| D12 | 5.4 | 71.7 |
| L1 | 5.7 | 77.3 |
| L2 | 5.8 | 83.1 |
| L3 | 5.7 | 88.8 |
| L4 | 5.7 | 94.6 |
| L5 | 5.5 | 100 |
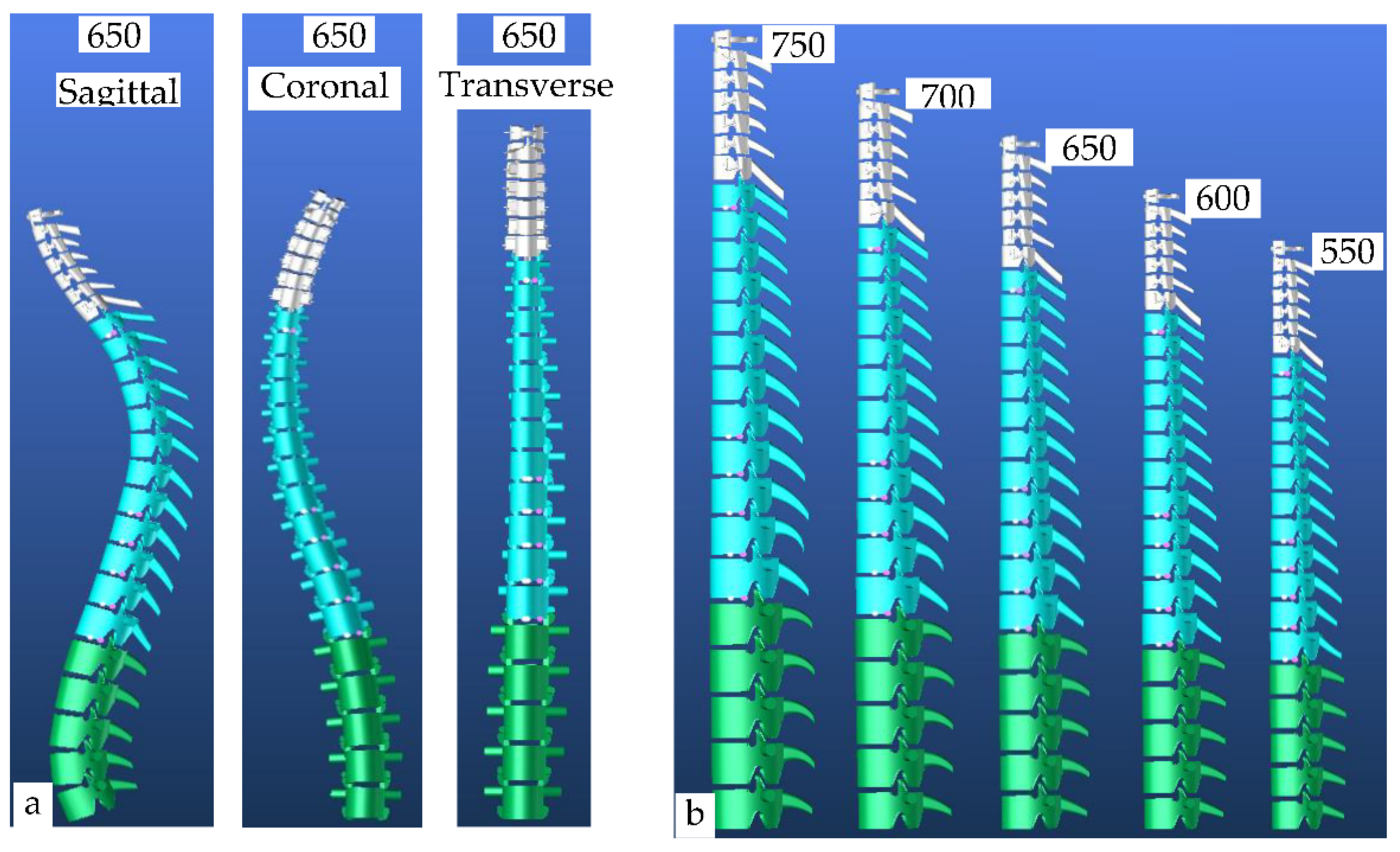
| Segment | H750 | H700 | H650 | H600 | H550 | Sagital | H650 | Coronal | H650 | Transverse | H650 |
|---|---|---|---|---|---|---|---|---|---|---|---|
| lC1 | 18 | 16.8 | 15.6 | 14.4 | 13.2 | αC1 | 10 | βC1 | −26 | γC1 | 15 |
| lC2 | 18.75 | 17.5 | 16.25 | 15 | 13.75 | αC2 | 15 | βC2 | −23 | γC2 | 12 |
| lC3 | 19.5 | 18.2 | 16.9 | 15.6 | 14.3 | αC3 | 20 | βC3 | −20 | γC3 | 9 |
| lC4 | 20.25 | 18.9 | 17.55 | 16.2 | 14.85 | αC4 | 25 | βC4 | −17 | γC4 | 6 |
| lC5 | 20.25 | 18.9 | 17.55 | 16.2 | 14.85 | αC5 | 28 | βC5 | −14 | γC5 | 3 |
| lC6 | 19.5 | 18.2 | 16.9 | 15.6 | 14.3 | αC6 | 30 | βC6 | −11 | γC6 | 0 |
| lC7 | 23.25 | 21.7 | 20.15 | 18.6 | 17.05 | αC7 | 36 | βC7 | −8 | γC7 | −3 |
| lD1 | 25.5 | 23.8 | 22.,1 | 20.4 | 18.7 | αD1 | 33 | βD1 | −6 | γD1 | −6 |
| lD2 | 27.75 | 25.9 | 24.05 | 22.2 | 20.35 | αD2 | 27 | βD2 | −3 | γD2 | −9 |
| lD3 | 27.75 | 25.9 | 24.05 | 22.2 | 20.35 | αD3 | 17 | βD3 | 0 | γD3 | −12 |
| lD4 | 29.25 | 27.3 | 25.35 | 23.4 | 21.45 | αD4 | 14 | βD4 | 3 | γD4 | −15 |
| lD5 | 29.25 | 27.3 | 25.35 | 23.4 | 21.45 | αD5 | 8 | βD5 | 6 | γD5 | −18 |
| lD6 | 31.5 | 29.4 | 27.3 | 25.2 | 23.1 | αD6 | 1 | βD6 | 9 | γD6 | −21 |
| lD7 | 32.25 | 30.1 | 27.95 | 25.8 | 23.65 | αD7 | −10 | βD7 | 12 | γD7 | −24 |
| lD8 | 33.75 | 31.5 | 29.25 | 27 | 24.75 | αD8 | −13 | βD8 | 15 | γD8 | −27 |
| lD9 | 34.5 | 32.2 | 29.9 | 27.6 | 25.3 | αD9 | −14 | βD9 | 18 | γD9 | −24 |
| lD10 | 36 | 33.6 | 31.2 | 28.8 | 26.4 | αD10 | −15 | βD10 | 21 | γD10 | −21 |
| lD11 | 38.25 | 35.7 | 33.15 | 30.6 | 28.05 | αD11 | −16 | βD11 | 18 | γD11 | −18 |
| lD12 | 40.5 | 37.8 | 35.1 | 32.4 | 29.7 | αD12 | −18 | βD12 | 15 | γD12 | −15 |
| lL1 | 42.75 | 39.9 | 37.05 | 34.2 | 31.35 | αL1 | −15 | βL1 | 12 | γL1 | −12 |
| lL2 | 43.,5 | 40.6 | 37.7 | 34.8 | 31.9 | αL2 | −11 | βL2 | 9 | γL2 | −9 |
| lL3 | 42.75 | 39.9 | 37.05 | 34.2 | 31.35 | αL3 | −8 | βL3 | 6 | γL3 | −6 |
| lL4 | 42.75 | 39.9 | 37.05 | 34.2 | 31.35 | αL4 | 4 | βL4 | 3 | γL4 | −3 |
| lL5 | 41.25 | 38.5 | 35.75 | 33 | 30.25 | αL5 | 20 | βL5 | 0 | γL5 | 0 |
| Segment | H620 | Sagital | H620 | Coronal | H650 | Transverse | H620 |
|---|---|---|---|---|---|---|---|
| lC1 | 14.88 | αC1 | 10 | βC1 | 1 | γC1 | 0 |
| lC2 | 15.5 | αC2 | 15 | βC2 | 1.5 | γC2 | 0 |
| lC3 | 16.12 | αC3 | 20 | βC3 | 2 | γC3 | 0 |
| lC4 | 16.74 | αC4 | 25 | βC4 | 2.5 | γC4 | 0 |
| lC5 | 16.74 | αC5 | 28 | βC5 | 3 | γC5 | 0 |
| lC6 | 16.12 | αC6 | 30 | βC6 | 3.5 | γC6 | 0 |
| lC7 | 19.22 | αC7 | 36 | βC7 | 4 | γC7 | 0 |
| lD1 | 21.08 | αD1 | 33 | βD1 | 0 | γD1 | 0 |
| lD2 | 22.94 | αD2 | 27 | βD2 | 2.5 | γD2 | 0 |
| lD3 | 22.94 | αD3 | 17 | βD3 | 7.5 | γD3 | 0 |
| lD4 | 24.18 | αD4 | 14 | βD4 | 12.5 | γD4 | 0 |
| lD5 | 24.18 | αD5 | 8 | βD5 | 10 | γD5 | 0 |
| lD6 | 26.04 | αD6 | 1 | βD6 | 7.5 | γD6 | 0 |
| lD7 | 26.66 | αD7 | −10 | βD7 | 5 | γD7 | 0 |
| lD8 | 27.9 | αD8 | −13 | βD8 | 2.5 | γD8 | 0 |
| lD9 | 28.52 | αD9 | −14 | βD9 | 0 | γD9 | 0 |
| lD10 | 29.76 | αD10 | −15 | βD10 | −2.5 | γD10 | 0 |
| lD11 | 31.62 | αD11 | −16 | βD11 | −2.5 | γD11 | 0 |
| lD12 | 33.48 | αD12 | −18 | βD12 | −2.5 | γD12 | 0 |
| lL1 | 35.34 | αL1 | −15 | βL1 | −2.5 | γL1 | 0 |
| lL2 | 35.96 | αL2 | −11 | βL2 | 0 | γL2 | 0 |
| lL3 | 35.34 | αL3 | −8 | βL3 | 2.5 | γL3 | 0 |
| lL4 | 35.34 | αL4 | 4 | βL4 | 2.5 | γL4 | 0 |
| lL5 | 34.1 | αL5 | 20 | βL5 | 2.5 | γL5 | 0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mosleh, S.; Abtew, M.A.; Bruniaux, P.; Tartare, G.; Chen, Y. Developing an Adaptive 3D Vertebrae Model of Scoliosis Patients for Customize Garment Design. Appl. Sci. 2021, 11, 3171. https://doi.org/10.3390/app11073171
Mosleh S, Abtew MA, Bruniaux P, Tartare G, Chen Y. Developing an Adaptive 3D Vertebrae Model of Scoliosis Patients for Customize Garment Design. Applied Sciences. 2021; 11(7):3171. https://doi.org/10.3390/app11073171
Chicago/Turabian StyleMosleh, Sara, Mulat Alubel Abtew, Pascal Bruniaux, Guillaume Tartare, and Yan Chen. 2021. "Developing an Adaptive 3D Vertebrae Model of Scoliosis Patients for Customize Garment Design" Applied Sciences 11, no. 7: 3171. https://doi.org/10.3390/app11073171
APA StyleMosleh, S., Abtew, M. A., Bruniaux, P., Tartare, G., & Chen, Y. (2021). Developing an Adaptive 3D Vertebrae Model of Scoliosis Patients for Customize Garment Design. Applied Sciences, 11(7), 3171. https://doi.org/10.3390/app11073171






