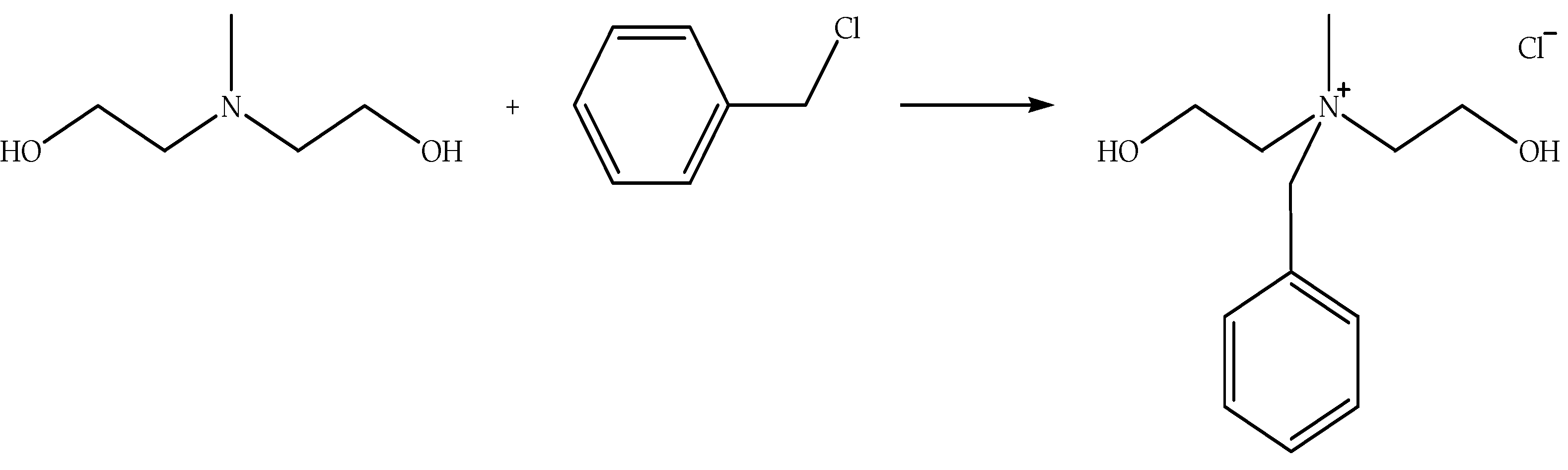
4.1. Influence of QASs and QAILs on Polymer Blends
Table 7 indicates the thermo-mechanical properties of polymer blends influenced by QASs and QAILs. QAS like HTAB (chemical structure is shown in
Figure 5) has been applied for compatibilization of Nylon-6/LNR blends [
9]. The application of HTAB influenced the degradation temperature, glass transition temperature, tensile strength, tensile modulus, tensile extension, and impact strength of the blends. The degradation temperature of the blends decreased because LNR possessed lower initial degradation temperature than nylon-6, which deteriorated the degradation temperature of the overall blends. The result also shows a decreasing trend with the blends containing high LNR content, which is also due to the low degradation temperature of LNR compared to nylon-6. Furthermore, the glass transition temperature of the blends also decreased because of the improvement of the compatibility between nylon-6 and LNR. This is caused by HTAB, which consisted of polar and non-polar groups that acted as a compatibilizer by creating interactions between them [
9]. Moreover, the mechanical properties, such as tensile strength and tensile modulus of the blends decreased because of the increase of their segmental movement. It is considered that LNR acted as a stress dilutor, which is responsible for lowering the tensile strength and the tensile modulus. This is induced by the presence of HTAB that is responsible for better homogeneous distribution of LNR in the blends. However, the tensile extension and impact strength of the blends increased by up to 100% and 35%, respectively, compared to the neat nylon-6. This is attributed to the HTAB-compatibilized Nylon-6/LNR blends, which eventually increased the elongation and toughness of the blends [
9].
It can be seen in
Table 7 that fewer studies have focused on the influence of the QASs on the thermo-mechanical properties of polymer blends. QAS like BDAC (chemical structure is shown in
Figure 5) is applied for compatibilization of PET/PE blends [
11]. The application of BDAC influenced the degradation temperature, tensile strength, tensile modulus, and tensile extension of the blends. The degradation temperature of the blends decreased because of the presence of the benzyl group and aromatic structure in BDAC that lowered the thermal stability of the blends. Therefore, BDAC acted as a thermal degradation promoter for the blends, which can catalyze the polymer degradation with a thermal dependence. This decreasing trend also occurred in the PP/Nylon-6 blends compatibilized by PP-g-MA [
64]. However, the mechanical properties, such as tensile strength and tensile modulus of the blends increased by up to 14% and 24%, respectively, compared to the neat PET/PE blend. This is attributed to the vital role of the BDAC, which acted as a compatibilization agent between PET and PE [
11]. Nevertheless, the tensile extension of the blends decreased because of their lower ductility compared to the blend without BDAC [
11]. Moreover, the polymer chains of PET/PE blends containing BDAC are stiffer in comparison with the neat blend that reduced the ductility and deformability as well. This effectively decreased the tensile extension of the BDAC-compatibilized PET/PE blends.
On the other hand, QAIL like BmimCl (chemical structure is shown in
Figure 6) has been applied for compatibilization of NW/Cellulose blends [
10]. The application of BmimCl influenced the degradation temperature, glass transition temperature, tensile strength, tensile modulus, and tensile extension of the blends (
Table 7). The thermal properties, such as degradation temperature and glass transition temperature of the blends increased by up to 25% and 33%, respectively, compared to the neat NW. This is attributed to the miscibility between NW and cellulose as well as the formation of strong interaction between these biopolymers generated by BmimCl [
10]. Moreover, the mechanical properties, for instance, tensile strength, tensile modulus, and tensile extension of the blends increased by up to 66%, 100%, and 56%, respectively, in comparison with the NW. This is due to the increase of the cellulose content in the blends and also good interfacial adhesion between them [
10].
QAIL like DmimNTf
2 (chemical structure is shown in
Figure 6) has been applied for compatibilization of PBS/RS blends [
12]. The application of DmimNTf
2 has influenced the degradation temperature, melting temperature, tensile strength, tensile modulus, tensile extension, flexural strength, and flexural extension of the blends (
Table 7). The degradation temperature of the blends increased by up to 2.3% compared to the neat PBS/RS blend. This is attributed to the high degradation temperature of DmimNTf
2 and the existence of intermolecular interactions between the blend components generated by DmimNTf
2. However, the melting temperature of the blends decreased because of the presence of DmimNTf
2, which improved the interfacial adhesion between PBS and RS [
12]. In the compatible polymer blends, they commonly have the melting temperature lower than their individual components [
14]. Thus, it can be considered that the PBS/RS blends containing DmimNTf
2 have good compatibility in comparison with the neat blend. Furthermore, the mechanical properties, such as tensile strength, tensile modulus, and flexural strength of the blends decreased since fewer loads are required for withstanding the exerted force. This is, of course, not unusual, the compatible polymer blends are not necessarily linked to the increase of the tensile and flexural strengths. Nevertheless, the tensile extension and flexural extension of the blends increased by up to 233% and 17%, respectively, in comparison with the blend without DmimNTf
2. This is caused by the improvement of the compatibility between PBS and RS since DmimNTf
2 acted as a compatibilizer [
12].
BmimCl has also been applied for compatibilization of PHBV/Cellulose blends [
15]. The application of BmimCl influenced the degradation temperature, glass transition temperature, melting temperature, tensile strength, tensile modulus, and tensile extension of the blends (
Table 7). The thermal properties, such as degradation temperature and glass transition temperature of the blends increased by up to 20% and 700%, respectively, compared to the neat PHBV. This is attributed to the partial miscibility between PHBV and cellulose in their regenerated form. However, the melting temperature of the blends decreased because of the formation of hydrogen bonding interaction between the blend components regenerated from BmimCl [
15]. The lower melting temperature of the polymer blends than their individual components can also be considered as an indicator to show the compatibility between the blend components; this can take place when they have intermolecular interactions. Nonetheless, the mechanical properties, for instance, tensile strength, tensile modulus, and tensile extension of the blends increased by up to 429%, 190%, and 44%, respectively, in comparison with the PHBV. This is because of the presence of interaction between PHBV and cellulose, which improved the compatibility of the blends [
15].
4.2. Influence of QASs on Polymer Composites
Table 8 shows the thermo-mechanical properties of polymer composites influenced by QASs. QAS like HTAB has been applied for compatibilization of HDPE/LDPE/Cellulose composites [
16]. The application of HTAB has influenced the degradation temperature, melting temperature, tensile strength, tensile modulus, tensile extension, and impact strength of the composites. The degradation temperature of the composites decreased because of the lower initial degradation temperature of HTAB than other composite components, which affected the degradation temperature of the overall composites. Nevertheless, the melting temperature of the composites remains unchanged because of the presence of crystal lattice of HTAB in the composites [
16]. Moreover, the mechanical properties, such as tensile strength and tensile modulus of the composites increased by up to 25% and 25%, respectively, compared to the neat HDPE/LDPE/Cellulose composite. This is attributed to the existence of HTAB, which enhanced the interfacial adhesion between HDPE/LDPE matrix and cellulose filler, as a result, improved the stress transfer from HDPE/LDPE to cellulose. However, the tensile extension and impact strength of the composites decreased caused by the excess of the crystal lattice of HTAB in the composites, which increased their brittleness [
16]. This is related to the increase of the stiffness property of the composites. It is a well-known fact that the crystal lattice with higher content in the matrix can decrease the tensile extension and impact strength of the composites as a rule of mixture.
QAS like OTAB (chemical structure is shown in
Figure 5) has been applied for compatibilization of PBE/BN composites [
18]. The application of OTAB has influenced the thermal conductivity, flexural strength, flexural modulus, and impact strength of the composites (
Table 8). The thermal conductivity of the composites increased by up to 79%, compared to the neat PBE/BN composite. This is attributed to the improvement of interfacial adhesion between PBE matrix and BN filler, which induced heat transfer across the interface efficiently [
18]. Moreover, the mechanical properties, such as flexural strength, flexural modulus, and impact strength of the composites increased by up to 38%, 8.5%, and 11%, respectively, in comparison with the neat composite. This is because of the long alkyl chain length of OTAB, which effectively adsorbed on the surface of the composites [
18]. Therefore, the compatibilization by applying OTAB not only increased the thermal conductivity of the composites but also improved their flexural and impact properties as well.
HTAB has also been applied for compatibilization of HDPE/Agar composites [
22]. The application of HTAB has influenced the degradation temperature, melting temperature, tensile strength, tensile modulus, tensile extension, and impact strength of the composites (
Table 8). The thermal properties, such as degradation temperature and melting temperature of the composites decreased compared to the neat HDPE/Agar composite. This is due to the presence of interactions between HDPE matrix and agar filler generated by HTAB [
22]. Moreover, the mechanical properties, for instance, tensile strength and tensile modulus of the composites decreased because of their ductile behavior with the presence of HTAB. The existence of ductile behavior leads to the decrease of brittleness of the composites, which also reduced the stiffness property of the composites. This is induced by HTAB that improved the interfacial adhesion between HDPE and agar. Nevertheless, the tensile extension and impact strength of the composites increased by up to 72% and 24%, respectively, in comparison with the neat composite. This is attributed to the compatibilization effect of HTAB, which improved the compatibility between the composite components, as a result, increased the ductility of the HDPE/Agar composites [
22].
Besides, QAS like IDAB (chemical structure is shown in
Figure 5) has been applied for compatibilization of SBR/Bentonite composites [
20]. The application of IDAB has influenced the degradation temperature, tensile strength, tensile modulus, and tensile extension of the composites (
Table 8). The degradation temperature of the composites is expected to be lower compared to the neat SBR/Bentonite composite. This is because of the low degradation temperature of IDAB compared to other components in the composites [
20]. However, the mechanical properties, such as tensile strength and tensile modulus of the composites increased by up to 20% and 34%, respectively, in comparison with the neat composite. This is attributed to the increase of the cross-linking density of the SBR, which is caused by the presence of interactions between SBR matrix and bentonite filler, as a result, improving the compatibility between them [
20]. Nonetheless, the tensile extension of the composites decreased because of the increase of their stiffness property with the presence of IDAB. The result indicated that the compatibilization of the composites by IDAB has allowed bentonite to restrict the movement of SBR chains. Consequently, it increased the rigidity property, which reduced the strain behavior of the composites and led to the lower tensile extension.
The compatibilization by applying QASs is not only limited to conventional polymer composites, but they can also be applied in polymer nanocomposites [
43]. HTAB is applied for compatibilization of Nylon-6/LNR/MMT nanocomposites [
24]. The application of HTAB influenced the degradation temperature, glass transition temperature, tensile strength, tensile modulus, tensile extension, and impact strength of the nanocomposites (
Table 8). The thermal properties, such as degradation temperature and glass transition temperature of the nanocomposites increased by up to 19% and 81%, respectively, compared to the neat Nylon-6/LNR matrix. This is attributed to the formation of intermolecular interactions between Nylon-6/LNR matrix and MMT filler generated by HTAB [
24]. Moreover, the mechanical properties, for instance, tensile strength, tensile modulus, and impact strength of the nanocomposites increased by up to 48%, 149%, and 6.5%, respectively, in comparison with the neat matrix. This is due to the compatibilization by HTAB that has effectively provided a reinforcing effect on the nanocomposites [
24]. Nevertheless, the tensile extension of the nanocomposites decreased because of the increase of their stiffness property. This typical phenomenon can occur not only in the conventional polymer composites but also in the polymer nanocomposites due to the compatibilization effect of HTAB.
4.3. Influence of QAILs on Polymer Composites
Table 9 displays the thermo-mechanical properties of polymer composites influenced by QAILs. QAIL like HmimPF
6 (chemical structure is shown in
Figure 6) has been applied for compatibilization of XSBR/RBC composites [
17]. The application of HmimPF
6 influenced the degradation temperature, glass transition temperature, tensile strength, tensile modulus, and tensile extension of the composites. The degradation temperature of the composites decreased because of the hydrolyzation of HmimPF
6 induced by water that is released from RBC at a temperature above 100 °C. This is due to HmimPF
6 being sensitive to hydrolyzation, especially at elevated temperature. Therefore, the polymer composites heated above 100 °C released water from RBC, and it hydrolyzed HmimPF
6. As a result, it decreased the degradation temperature of the polymer composites. However, the glass transition temperature of the composites increased by up to 3.4% compared to the neat XSBR/RBC composite. This is attributed to the mobility of XSBR macromolecular chains which was restricted with the presence of HmimPF
6 [
17]. Moreover, the mechanical properties, such as tensile strength, tensile modulus, and tensile extension of the composites increased by up to 31%, 13%, and 7.0%, respectively, in comparison with the neat composite. This is due to the formation of interactions between XSBR matrix and RBC filler generated by HmimPF
6 [
17].
QAIL like BmimCl has been applied for compatibilization of Agarose/Tc composites [
19], it is blended with urea to increase its efficacy. The application of BmimCl has influenced the degradation temperature, glass transition temperature, tensile strength, tensile modulus, and tensile extension of the composites (
Table 9). The degradation temperature of the composites decreased because of the lower thermal resistance of urea than other composite components, which affected the degradation temperature of the overall composites. Nonetheless, the glass transition temperature of the composites increased by up to 23% in comparison with the neat Agarose/Tc composite. This is attributed to the formation of strong interactions between agarose matrix and the talc filler with the presence of BmimCl [
19]. Furthermore, the mechanical properties, such as tensile strength and tensile modulus of the composites increased by up to 26% and 62%, respectively, compared to the neat composite. This is because of the composites having strong and stiff properties, as well as BmimCl acted as a coupling agent or compatibilizer for the composites. However, the tensile extension of the composites decreased because the motion of agarose molecular chains was restricted by the talc filler [
19]. The result also confirmed that the stiffness property of the composites increased with the presence of compatibilizer, which enhanced the rigidity of the composites, thus moving the tensile extension to lower values.
On the other hand, QAIL like EmimAc (chemical structure is shown in
Figure 6) has been applied for compatibilization of DGEBA epoxy/Chitin composites [
21]. The application of EmimAc influenced the degradation temperature, glass transition temperature, tensile strength, tensile modulus, tensile extension, and impact strength of the composites (
Table 9). The degradation temperature of the composites remains unchanged because of the small content of chitin in the composites; thus, the overall thermal degradation behavior is similar to the neat DGEBA epoxy matrix. Nonetheless, the glass transition temperature of the composites increased by up to 9.0% compared to the neat matrix. This is attributed to the presence of chitin, which restricted the mobility of DGEBA epoxy chains prompted by intermolecular hydrogen bonding [
21]. Moreover, the mechanical properties, such as tensile strength, tensile modulus, tensile extension, and impact strength of the composites increased by up to 34%, 8.4%, 80%, and 91%, respectively, in comparison with the neat matrix. This is because of the better dispersion of chitin filler within the DGEBA epoxy matrix with the presence of EmimAc [
21].
Besides, QAIL like BmimPF
6 (chemical structure is shown in
Figure 6) has been applied for compatibilization of PU/SiO
2 composites [
23]. The application of BmimPF
6 influenced the degradation temperature, glass transition temperature, thermal conductivity, flexural strength, and flexural extension of the composites (
Table 9). The thermal properties, such as degradation temperature and glass transition temperature of the composites increased by up to 10% and 13%, respectively, compared to the neat PU/SiO
2 composite. This is attributed to the presence of BmimPF
6 that has significantly enhanced the thermal stability of the composites. Nevertheless, the thermal conductivity of the composites decreased because of the low thermal conductivity of SiO
2 filler, which is well dispersed in the cell walls of the PU matrix [
23]. In addition, the BmimPF
6-compatibilized composites improved the interfacial adhesion between PU and SiO
2, which reduced the heat transfer on the matrix, thus decreasing the thermal conductivity of the composites. The flexural strength of the composites increased by up to 15% in comparison with the neat composite. This is because of the reinforcing effect of SiO
2 and compatibilization effect of BmimPF
6. However, the flexural extension of the composites decreased because of the existence of SiO
2 aggregates that acted as defects within the PU matrix [
23]. The aggregates normally exist at high content of SiO
2 filler in the matrix. Hence, the formation of aggregates can be avoided or minimized during the preparation of the composites.
The compatibilization by applying QAILs is not only limited to conventional polymer composites, but they can also be applied in polymer nanocomposites as QASs. BmimCl is applied for compatibilization of RC/MMT nanocomposites [
25]. The application of BmimCl influenced the degradation temperature, tensile strength, tensile modulus, and tensile extension of the nanocomposites (
Table 9). The degradation temperature of the nanocomposites increased by up to 154% compared to the neat RC matrix. This is attributed to the good dispersion and exfoliation of MMT filler within the RC matrix regenerated from BmimCl [
25]. Furthermore, the mechanical properties, such as tensile strength and tensile modulus of the nanocomposites increased by up to 12% and 47%, respectively, in comparison with the neat matrix. This is caused by the presence of MMT that provided high rigidity property to the nanocomposites. Nonetheless, the tensile extension of the nanocomposites decreased because the slippage movement of RC chains was restrained by MMT filler [
25]. This increased the stiffness property of the composites, which reduced the strain behavior and led to the lower tensile extension.