A Novel Thermostable Keratinase from Deinococcus geothermalis with Potential Application in Feather Degradation
Abstract
1. Introduction
2. Materials and Methods
2.1. Strains, Plasmids and Materials for Gene Cloning and Expression
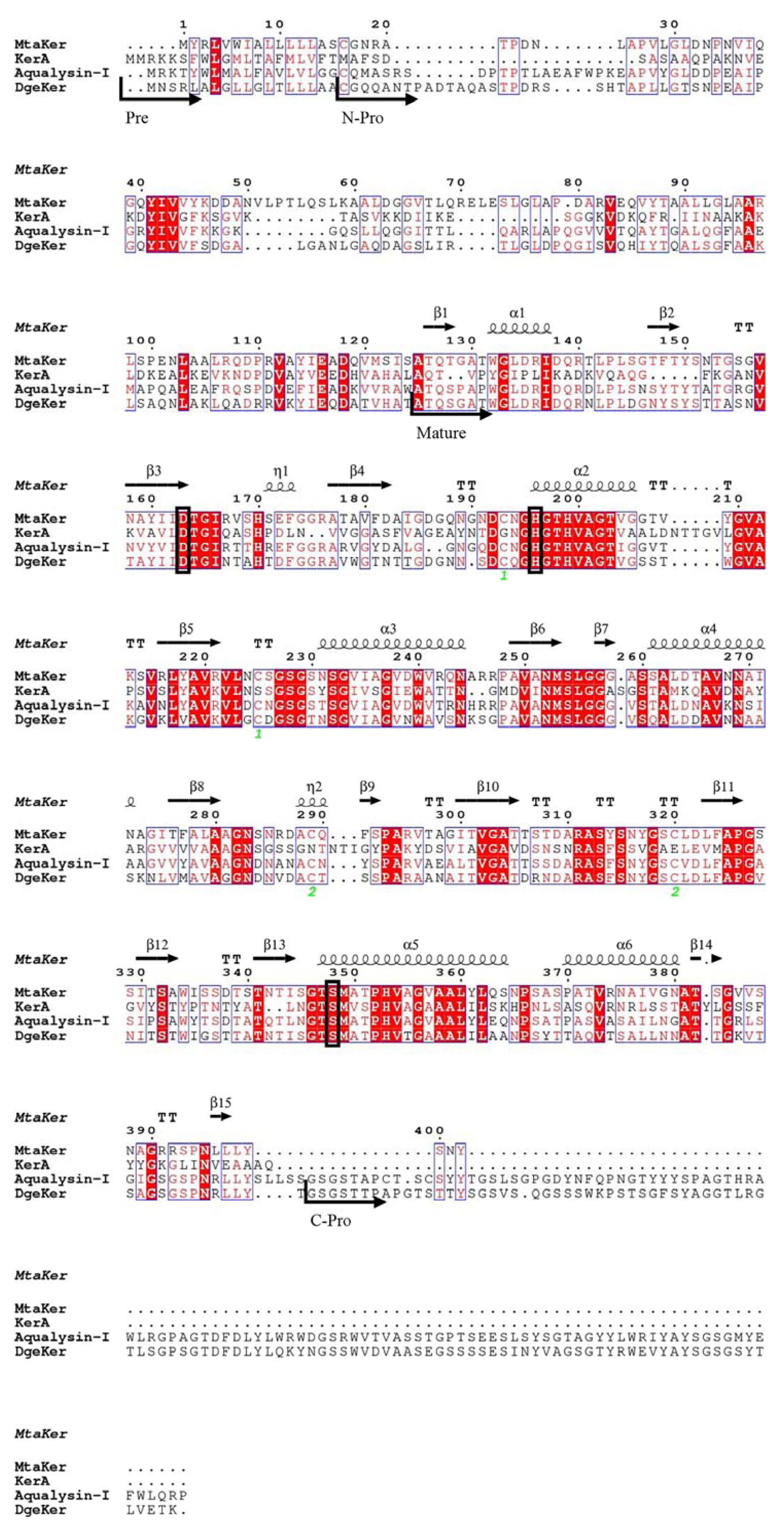
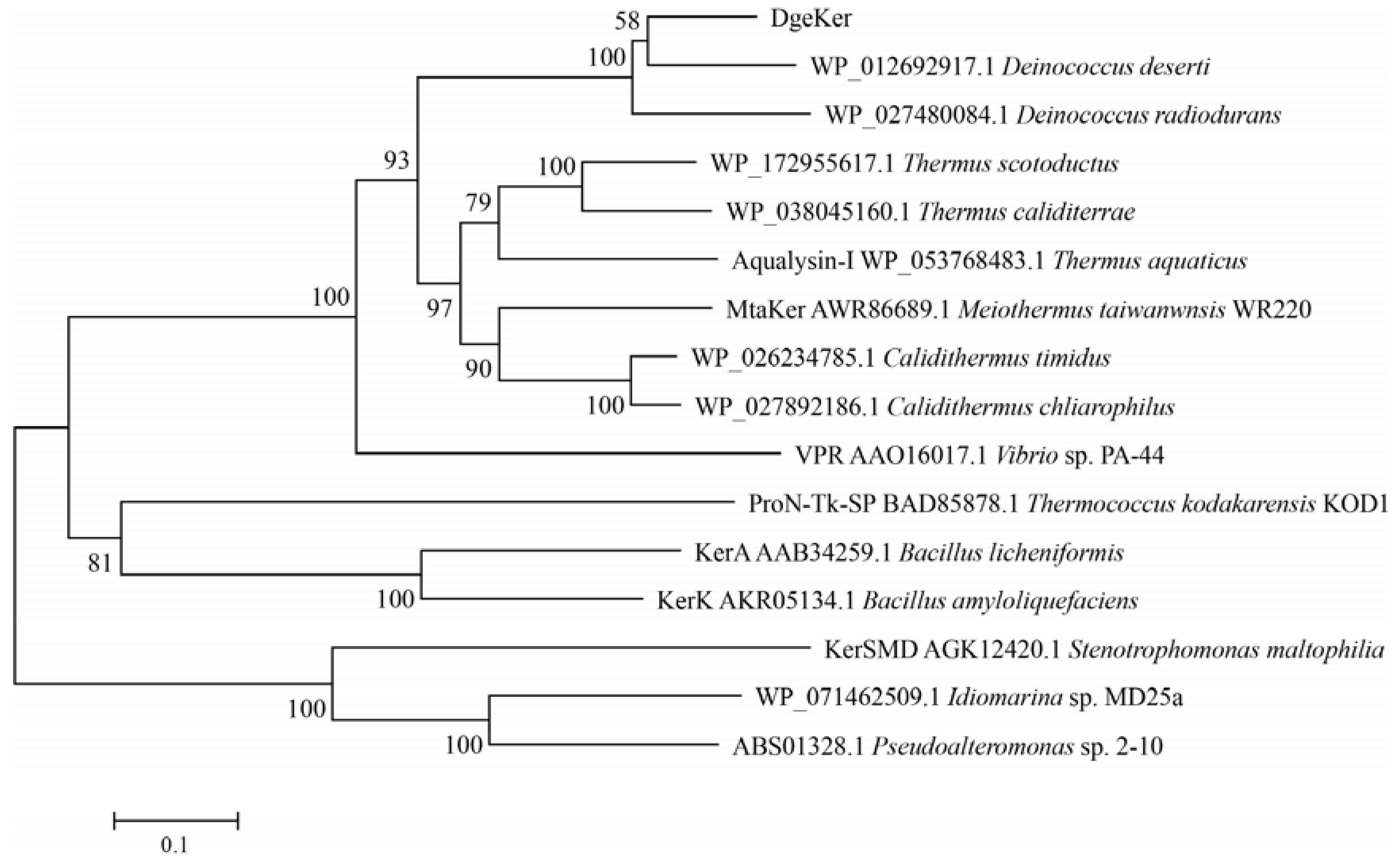
2.2. Sequence Analysis
2.3. Plasmids Construction
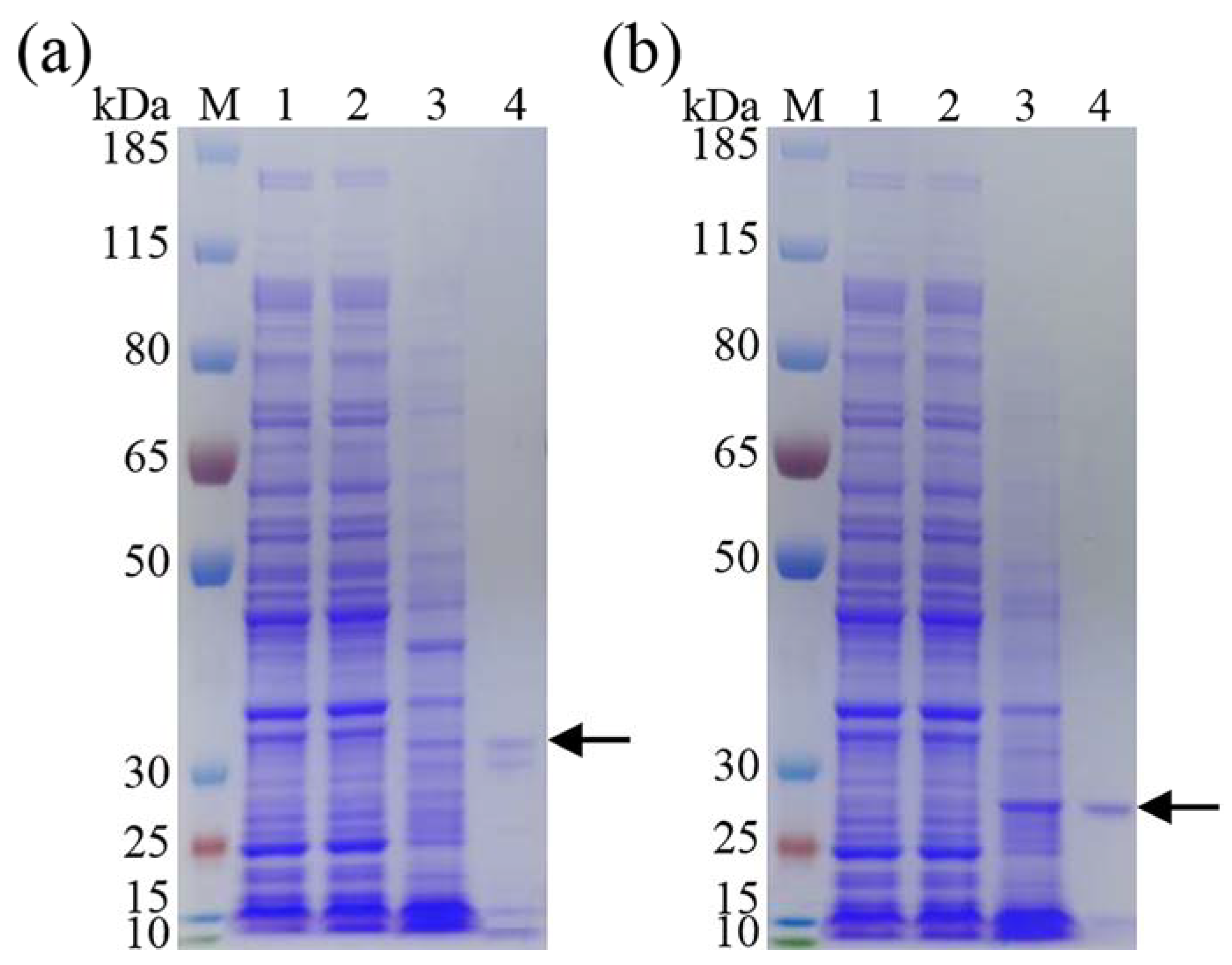
2.4. Expression and Purification of Keratinases
2.5. Keratinolytic Activity Assay
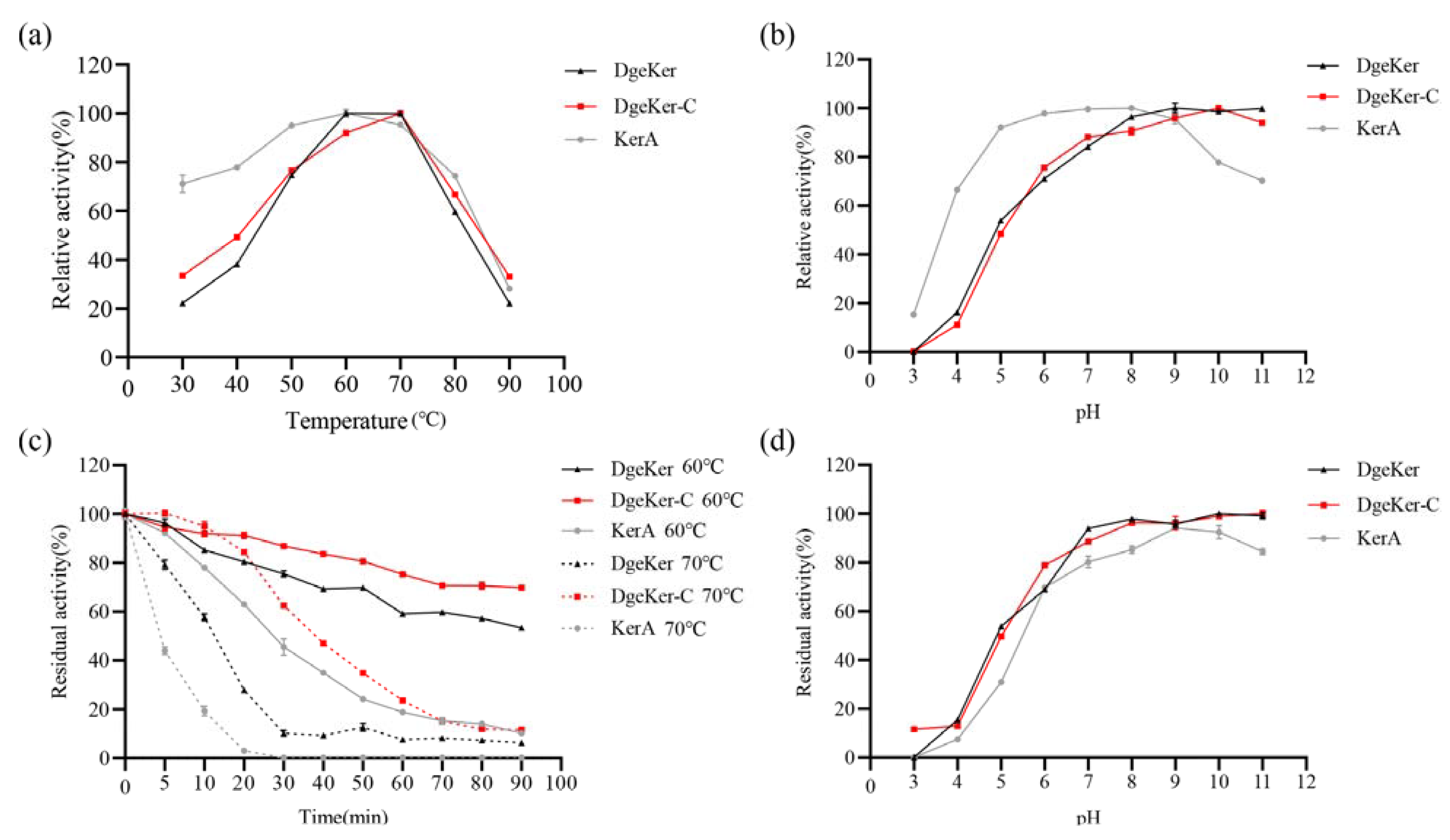
2.6. Effects of pH, Temperature, and Reagents on Enzyme Activity and Stability
2.7. Chicken Feather Degradation In Vitro
2.8. Statistical Analysis
3. Results
3.1. Sequence Analysis of DgeKer
3.2. Expression and Purification of Recombinant DgeKer and DgeKer-C
3.3. Effects of Temperature and pH on Activity and Stability
3.4. Effects of Metal Ions and Chemical Agents on Keratinases Stability
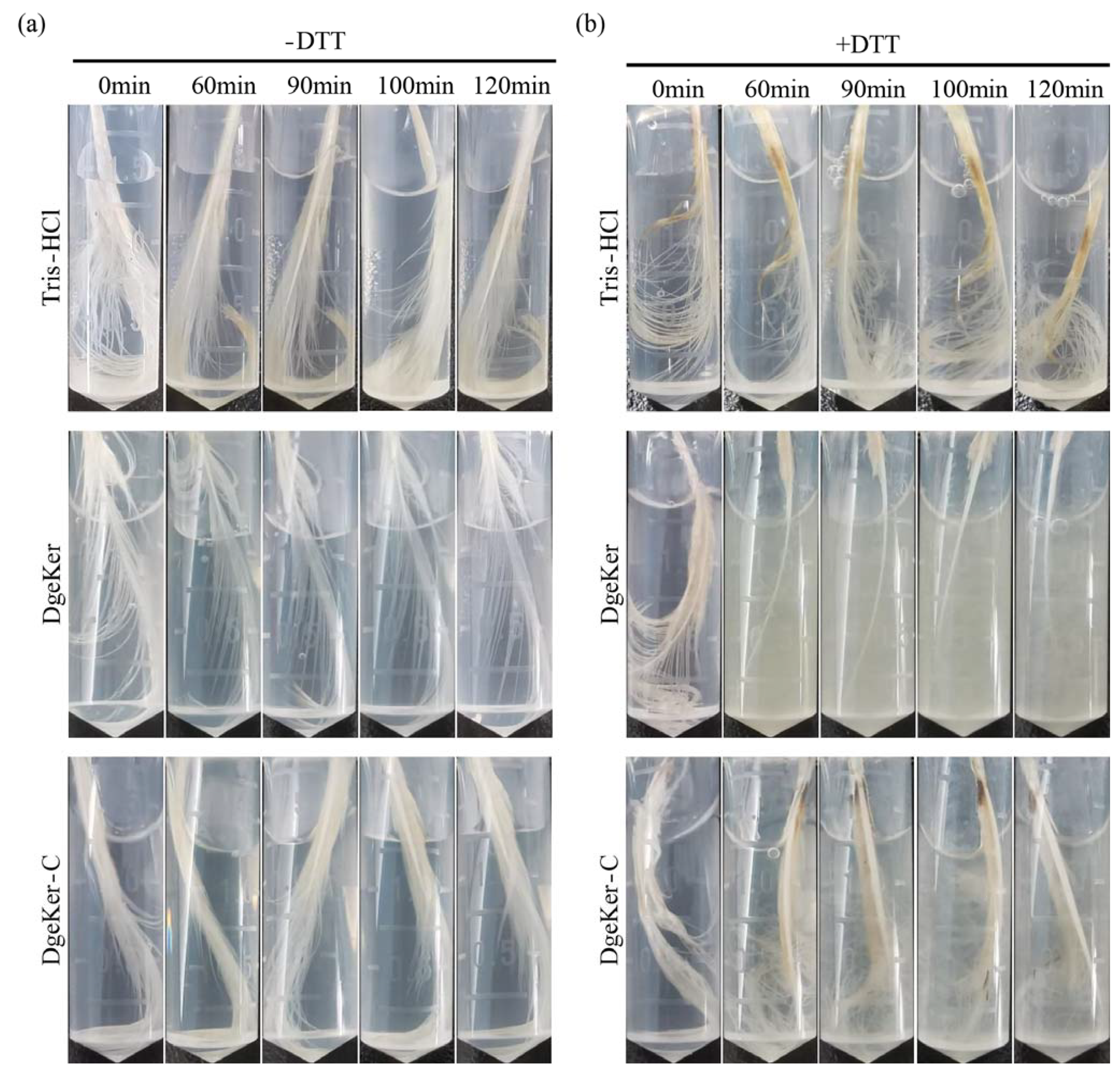
3.5. Chicken Feather Degradation of DgeKer and DgeKer-C In Vitro
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Korniłłowicz-Kowalska, T.; Bohacz, J. Biodegradation of keratin waste: Theory and practical aspects. Waste Manag. 2011, 31, 1689–1701. [Google Scholar] [CrossRef] [PubMed]
- Alba, A.C.; Strauch, T.A.; Keisler, D.H.; Wells, K.D.; Kesler, D.C. Using a keratinase to degrade chicken feathers for improved extraction of glucocorticoids. Gen. Comp. Endocrinol. 2019, 270, 35–40. [Google Scholar] [CrossRef] [PubMed]
- Hassan, M.A.; Taha, T.H.; Hamad, G.M.; Hashem, M.; Alamri, S.; Mostafa, Y.S. Biochemical characterisation and application of keratinase from Bacillus thuringiensis MT1 to enable valorisation of hair wastes through biosynthesis of vitamin B-complex. Int. J. Biol. Macromol. 2020, 153, 561–572. [Google Scholar] [CrossRef]
- Sharma, R.; Gupta, R. Coupled action of γ-glutamyl transpeptidase-glutathione and keratinase effectively degrades feather keratin and surrogate prion protein, Sup 35NM. Bioresour. Technol. 2012, 120, 314–317. [Google Scholar] [CrossRef]
- Ningthoujam, D.S.; Mukherjee, S.; Devi, L.J.; Singh, E.S.; Tamreihao, K.; Khunjamayum, R.; Banerjee, S.; Mukhopadhyay, D. In vitro degradation of β-amyloid fibrils by microbial keratinase. Alzheimer’s Dement. Transl. Res. Clin. Interv. 2019, 5, 154–163. [Google Scholar] [CrossRef] [PubMed]
- Ramirez, D.O.S.; Carletto, R.A.; Tonetti, C.; Giachet, F.T.; Varesano, A.; Vineis, C. Wool keratin film plasticized by citric acid for food packaging. Food Packag. Shelf Life 2017, 12, 100–106. [Google Scholar] [CrossRef]
- Li, Q. Progress in Microbial Degradation of Feather Waste. Front. Microbiol. 2019, 10, 2717. [Google Scholar] [CrossRef]
- Kanoksilapatham, W.; Intagun, W. A Review: Biodegradation and Applications of Keratin Degrading Microorganisms and Keratinolytic Enzymes, Focusing on Thermophiles and Thermostable Serine Proteases. Am. J. Appl. Sci. 2017, 14, 1016–1023. [Google Scholar] [CrossRef]
- Reis, S.V.; Beys-da-Silva, W.O.; Tirloni, L.; Santi, L.; Seixas, A.; Termignoni, C.; Silva, M.V.; Macedo, A.J. The extremophile Anoxybacillus sp. PC2 isolated from Brazilian semiarid region (Caatinga) produces a thermostable keratinase. J. Basic Microbiol. 2020, 60, 809–815. [Google Scholar]
- Foophow, T.; Tanaka, S.; Koga, Y.; Takano, K.; Kanaya, S. Subtilisin-like serine protease from hyperthermophilic archaeon Thermococcus kodakaraensis with N- and C-terminal propeptides. Protein Eng. Des. Sel. 2010, 23, 347–355. [Google Scholar] [CrossRef]
- Wu, W.-L.; Chen, M.-Y.; Tu, I.-F.; Lin, Y.-C.; EswarKumar, N.; Chen, M.-Y.; Ho, M.-C.; Wu, S.-H. The discovery of novel heat-stable keratinases from Meiothermus taiwanensis WR-220 and other extremophiles. Sci. Rep. 2017, 7, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Elleuche, S.; Schröder, C.; Sahm, K.; Antranikian, G. Extremozymes—biocatalysts with unique properties from extremophilic microorganisms. Curr. Opin. Biotechnol. 2014, 29, 116–123. [Google Scholar] [CrossRef]
- Promchai, R.; Boonchalearn, A.; Visessanguan, W.; Luxananil, P. Rapid production of extracellular thermostable alkaline halophilic protease originating from an extreme haloarchaeon, Halobacterium salinarum by recombinant Bacillus subtilis. Biocatal. Agric. Biotechnol. 2018, 15, 192–198. [Google Scholar] [CrossRef]
- Ferreira, A.C.; Nobre, M.F.; Rainey, F.A.; Silva, M.T.; Wait, R.; Burghardt, J.; Chung, A.P.; Da Costa, M.S. Deinococcus geothermalis sp. nov. and Deinococcus murrayi sp. nov., two extremely radiation-resistant and slightly thermophilic species from hot springs. Int. J. Syst. Evol. Microbiol. 1997, 47, 939–947. [Google Scholar] [CrossRef]
- Zhou, C.; Qin, H.; Chen, X.; Zhang, Y.; Xue, Y.; Ma, Y. A novel alkaline protease from alkaliphilic Idiomarina sp. C9-1 with potential application for eco-friendly enzymatic dehairing in the leather industry. Sci. Rep. 2018, 8, 1–18. [Google Scholar] [CrossRef]
- Gong, J.-S.; Ye, J.-P.; Tao, L.-Y.; Su, C.; Qin, J.; Zhang, Y.-Y.; Li, H.; Li, H.; Xu, Z.-H.; Shi, J.-S. Efficient keratinase expression via promoter engineering strategies for degradation of feather wastes. Enzym. Microb. Technol. 2020, 137, 109550. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Wang, H.; Lv, Y.; Bai, Y.; Luo, H.; Shi, P.; Huang, H.; Yao, B. Construction of a Rapid Feather-Degrading Bacterium by Overexpression of a Highly Efficient Alkaline Keratinase in Its Parent StrainBacillus amyloliquefaciensK. J. Agric. Food Chem. 2016, 64, 78–84. [Google Scholar] [CrossRef] [PubMed]
- Ren, Y.; Luo, H.; Huang, H.; Hakulinen, N.; Wang, Y.; Wang, Y.; Su, X.; Bai, Y.; Zhang, J.; Yao, B.; et al. Improving the catalytic performance of Proteinase K from Parengyodontium album for use in feather degradation. Int. J. Biol. Macromol. 2020, 154, 1586–1595. [Google Scholar] [CrossRef] [PubMed]
- Dalmaso, G.Z.L.; Ferreira, D.; Vermelho, A.B. Marine extremophiles: A source of hydrolases for biotechnological applications. Mar. Drugs 2015, 13, 1925–1965. [Google Scholar] [CrossRef]
- Bennur, T.; Kumar, A.R.; Zinjarde, S.; Javdekar, V. Nocardiopsis species as potential sources of diverse and novel extracellular enzymes. Appl. Microbiol. Biotechnol. 2014, 98, 9173–9185. [Google Scholar] [CrossRef] [PubMed]
- Khoshnevis, N.; Rezaei, S.; Forootanfar, H.; Faramarzi, M.A. Efficient Keratinolysis of Poultry Feather Waste by the Halotolerant Keratinase from Salicola Marasensis. Iran. J. Pharm. Res: IJPR 2019, 18, 1862–1870. [Google Scholar] [PubMed]
- Dalmaso, G.Z.; Lage, C.A.; Mazotto, A.M.; de Souza Dias, E.P.; Caldas, L.A.; Ferreira, D.; Vermelho, A.B. Extracellular peptidases from Deinococcus radiodurans. Extremophiles 2015, 19, 989–999. [Google Scholar] [CrossRef] [PubMed]
- Pietrow, O.; Panek, A.; Synowiecki, J. Extracellular proteolytic activity of Deinococcus geothermalis. Afr. J. Biotechnol. 2013, 12. [Google Scholar]
- Zhang, R.-Y.; Huang, Y.; Qin, W.-J.; Quan, Z.-X. The complete genome of extracellular protease-producing Deinococcus sp. D7000 isolated from the hadal region of Mariana Trench Challenger Deep. Mar. Genom. 2020, 100832. [Google Scholar] [CrossRef]
- Zeng, Y.-H.; Shen, F.-T.; Tan, C.-C.; Huang, C.-C.; Young, C.-C. The flexibility of UV-inducible mutation in Deinococcus ficus as evidenced by the existence of the imuB–dnaE2 gene cassette and generation of superior feather degrading bacteria. Microbiol. Res. 2011, 167, 40–47. [Google Scholar] [CrossRef]
- Kurosaka, K.; Ohta, T.; Matsuzawa, H. A 38 kDa precursor protein of aqualysin I (a thermophilic subtilisin-type protease) with a C-terminal extended sequence: Its purification and in vitro processing. Mol. Microbiol. 1996, 20, 385–389. [Google Scholar] [CrossRef] [PubMed]
- Tian, Y.; Xu, W.; Guang, C.; Zhang, W.; Mu, W. Thermostable Amylosucrase fromCalidithermus timidusDSM 17022: Insight into Its Characteristics and Tetrameric Conformation. J. Agric. Food Chem. 2019, 67, 9868–9876. [Google Scholar] [CrossRef] [PubMed]
- Arnórsdóttir, J.; Smáradóttir, R.B.; Magnússon, Ó.T.; Thorbjarnardóttir, S.H.; Eggertsson, G.; Kristjánsson, M.M. Characterization of a cloned subtilisin-like serine proteinase from a psychrotrophic Vibrio species. Eur. J. Biochem. 2002, 269, 5536–5546. [Google Scholar] [CrossRef]
- Gopinath, S.C.; Anbu, P.; Lakshmipriya, T.; Tang, T.-H.; Chen, Y.; Hashim, U.; Ruslinda, A.R.; Arshad, M. Biotechnological aspects and perspective of microbial keratinase production. BioMed Res. Int. 2015, 2015. [Google Scholar] [CrossRef]
- Fang, Z.; Zhang, J.; Liu, B.; Jiang, L.; Du, G.; Chen, J. Cloning, heterologous expression and characterization of two keratinases from Stenotrophomonas maltophilia BBE11. Process. Biochem. 2014, 49, 647–654. [Google Scholar] [CrossRef]
- Shinde, U.; Thomas, G. Insights from Bacterial Subtilases into the Mechanisms of Intramolecular Chaperone-Mediated Activation of Furin. Proprotein Convertases; Springer: Berlin/Heidelberg, Germany, 2011; pp. 59–106. [Google Scholar]
- Su, C.; Gong, J.S.; Sun, Y.X.; Qin, J.; Zhai, S.; Li, H.; Li, H.; Lu, Z.M.; Xu, Z.H.; Shi, J.S. Combining Pro-peptide Engineering and Multisite Saturation Mutagenesis to Improve the Catalytic Potential of Keratinase. ACS Synth. Biol. 2019, 8, 425–433. [Google Scholar] [CrossRef] [PubMed]
- Foophow, T.; Tanaka, S.-I.; Angkawidjaja, C.; Koga, Y.; Takano, K.; Kanaya, S. Crystal Structure of a Subtilisin Homologue, Tk-SP, from Thermococcus kodakaraensis: Requirement of a C-terminal β-Jelly Roll Domain for Hyperstability. J. Mol. Biol. 2010, 400, 865–877. [Google Scholar] [CrossRef] [PubMed]
- Yan, B.-Q.; Chen, X.-L.; Hou, X.-Y.; He, H.; Zhou, B.-C.; Zhang, Y.-Z. Molecular analysis of the gene encoding a cold-adapted halophilic subtilase from deep-sea psychrotolerant bacterium Pseudoalteromonas sp. SM9913: Cloning, expression, characterization and function analysis of the C-terminal PPC domains. Extremophiles 2009, 13, 725–733. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.-L.; Xie, B.-B.; Lu, J.-T.; He, H.-L.; Zhang, Y. A novel type of subtilase from the psychrotolerant bacterium Pseudoalteromonas sp. SM9913: Catalytic and structural properties of deseasin MCP. Microbiology 2007, 153, 2116–2125. [Google Scholar] [CrossRef]
- Thornton, J.; Yeats, C.; Bentley, S.; Bateman, A. Faculty of 1000 evaluation for New knowledge from old: In silico discovery of novel protein domains in Streptomyces coelicolor. BMC Microbiol. 2003, 3, 1–20. [Google Scholar] [CrossRef]
- Kunert, J.; Kasafírek, E. Preliminary characterization of extracellular proteolytic enzymes of dermatophytes by chromogenic substrates. Med. Mycol. 1988, 26, 187–194. [Google Scholar] [CrossRef] [PubMed]
- Brandelli, A.; Daroit, D.J.; Riffel, A. Biochemical features of microbial keratinases and their production and applications. Appl. Microbiol. Biotechnol. 2010, 85, 1735–1750. [Google Scholar] [CrossRef]
- Cai, C.G.; Lou, B.G.; Zheng, X.D. Keratinase production and keratin degradation by a mutant strain of Bacillus subtilis. J. Zhejiang Univ. Sci. B 2008, 9, 60–67. [Google Scholar] [CrossRef]
- Tiwary, E.; Gupta, R. Medium optimization for a novel 58kDa dimeric keratinase from Bacillus licheniformis ER-15: Biochemical characterization and application in feather degradation and dehairing of hides. Bioresour. Technol. 2010, 101, 6103–6110. [Google Scholar] [CrossRef]
- Park, G.-T.; Son, H.-J. Keratinolytic activity of Bacillus megaterium F7-1, a feather-degrading mesophilic bacterium. Microbiol. Res. 2009, 164, 478–485. [Google Scholar] [CrossRef]
- Pereira, J.Q.; Ambrosini, A.; Passaglia, L.M.P.; Brandelli, A. A new cold-adapted serine peptidase from Antarctic Lysobacter sp. A03: Insights about enzyme activity at low temperatures. Int. J. Biol. Macromol. 2017, 103, 854–862. [Google Scholar] [CrossRef] [PubMed]
- Bouacem, K.; Bouanane-Darenfed, A.; Jaouadi, N.Z.; Joseph, M.; Hacene, H.; Ollivier, B.; Fardeau, M.-L.; Bejar, S.; Jaouadi, B. Novel serine keratinase from Caldicoprobacter algeriensis exhibiting outstanding hide dehairing abilities. Int. J. Biol. Macromol. 2016, 86, 321–328. [Google Scholar] [CrossRef] [PubMed]
- Peng, Z.; Mao, X.; Zhang, J.; Du, G.; Chen, J. Biotransformation of keratin waste to amino acids and active peptides based on cell-free catalysis. Biotechnol. Biofuels 2020, 13, 61. [Google Scholar] [CrossRef] [PubMed]
- Pourjavaheri, F.; Pour, S.O.; Jones, O.A.; Smooker, P.M.; Brkljača, R.; Sherkat, F.; Blanch, E.W.; Gupta, A.; Shanks, R.A. Extraction of keratin from waste chicken feathers using sodium sulfide and L-cysteine. Process Biochem. 2019, 82, 205–214. [Google Scholar] [CrossRef]
- Gupta, R.; Sharma, R.; Beg, Q.K. Revisiting microbial keratinases: Next generation proteases for sustainable biotechnology. Crit. Rev. Biotechnol. 2012, 33, 216–228. [Google Scholar] [CrossRef] [PubMed]
- Miyoshi, S.; Wakae, H.; Tomochika, K.; Shinoda, S. Functional domains of a zinc metalloprotease from Vibrio vulnificus. J. Bacteriol. 1997, 179, 7606–7609. [Google Scholar] [CrossRef] [PubMed][Green Version]
| Genes | Primer Sequences (5′ to 3′) |
|---|---|
| dgeKer | F1: 5′tcgagctccgtcgacaagcttATGTGCGGACAGCAGGCGAAT |
| R1: 5′gtggtggtggtggtgctcgagCTTCGTCTCGACCAGGGTGTA | |
| dgeKer-C | F1: 5′tcgagctccgtcgacaagcttATGTGCGGACAGCAGGCGAAT |
| R2: 5′gtggtggtggtggtgctcgagGGTGTAGAGCAGGCGGTTGG |
| Keratinases | t1/2 (min) |
|---|---|
| DgeKer | 103.45 ± 1.26 b |
| DgeKer-C | 169.10 ± 8.20 c |
| KerA | 26.46 ± 0.22 a |
| Substrate | Activity of Different Keratinases (U/mg) | |
|---|---|---|
| DgeKer | DgeKer-C | |
| Soluble keratin | 40,192.30 ± 230.48 | 40,408.89 ± 342.73 |
| Feather powder | 1494.78 ± 25.78 | 1462.54 ± 71.19 |
| Metal Irons | Concentration (mmol/L) | Residual Activity (%) | |
|---|---|---|---|
| DgeKer | DgeKer-C | ||
| Control | 100.00 ± 0.27 m | 100.00 ± 3.11 k | |
| K+ | 1 | 91.77 ± 0.49 j,k | 87.45 ±1.41 g |
| 5 | 104.11 ± 0.14 o | 88.25 ± 0.58 g,h,* | |
| Li+ | 1 | 102.11 ± 0.59 n | 88.60 ± 0.56 g,h,* |
| 5 | 113.97 ± 0.62 p | 88.81 ± 1.72 g,h,* | |
| Mg2+ | 1 | 103.35 ± 1.07 n,o | 93.80 ± 1.12 j,* |
| 5 | 121.53 ± 0.75 q | 87.09 ± 0.71 g,* | |
| Ca2+ | 1 | 94.07 ± 0.72 k | 92.13 ± 1.18 i,j |
| 5 | 83.06 ± 0.36 g | 90.65 ± 0.88 h,i,* | |
| Co2+ | 1 | 91.20 ± 1.66 j | 99.20 ± 3.37 k,* |
| 5 | 92.82 ± 0.49 k,l | 101.07 ± 1.37 k,* | |
| Mn2+ | 1 | 85.07 ± 0.89 h | 80.94 ± 0.45 f |
| 5 | 64.69 ± 0.27 d | 60.39 ± 0.96 b,* | |
| Ni2+ | 1 | 88.33 ± 0.82 i | 86.67 ± 0.87 g |
| 5 | 91.29 ± 0.23 j | 77.51 ± 0.66 e,* | |
| Cd2+ | 1 | 74.93 ± 0.62 e | 86.34 ± 0.83 g,* |
| 5 | 63.16 ± 1.02 c | 65.32 ± 1.23 c | |
| Cu2+ | 1 | 79.33 ± 0.14 f | 86.36 ± 0.09 g,* |
| 5 | 57.42 ± 0.41 b | 67.21 ± 0.71 c,* | |
| Cr3+ | 1 | 86.41 ± 0.85 h | 73.24 ± 0.31 d,* |
| 5 | 0 ± 0 a | 0 ± 0 a | |
| Chemical Reagents | Concentration (v/v) | Residual Activity (%) | |
|---|---|---|---|
| DgeKer | DgeKer-C | ||
| Control | 100.00 ± 0.27 d | 100.00 ± 3.11 d | |
| SDS | 1% | 83.16 ± 0.54 b | 93.85 ± 0.56 c,d,* |
| Tween-80 | 1% | 86.70 ± 0.62 c | 64.59 ± 4.98 b,* |
| Tween-20 | 1% | 82.68 ± 0.81 b | 71.54 ±1.00 b,* |
| DMSO | 1% | 87.75 ± 0.95 c | 92.63 ± 0.56 c,* |
| β-mercaptoethanol | 1% | 0 ± 0 a | 15.36 ± 5.14 a,* |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tang, Y.; Guo, L.; Zhao, M.; Gui, Y.; Han, J.; Lu, W.; Dai, Q.; Jiang, S.; Lin, M.; Zhou, Z.; et al. A Novel Thermostable Keratinase from Deinococcus geothermalis with Potential Application in Feather Degradation. Appl. Sci. 2021, 11, 3136. https://doi.org/10.3390/app11073136
Tang Y, Guo L, Zhao M, Gui Y, Han J, Lu W, Dai Q, Jiang S, Lin M, Zhou Z, et al. A Novel Thermostable Keratinase from Deinococcus geothermalis with Potential Application in Feather Degradation. Applied Sciences. 2021; 11(7):3136. https://doi.org/10.3390/app11073136
Chicago/Turabian StyleTang, Yin, Leizhou Guo, Mingming Zhao, Yuan Gui, Jiahui Han, Wei Lu, Qilin Dai, Shijie Jiang, Min Lin, Zhengfu Zhou, and et al. 2021. "A Novel Thermostable Keratinase from Deinococcus geothermalis with Potential Application in Feather Degradation" Applied Sciences 11, no. 7: 3136. https://doi.org/10.3390/app11073136
APA StyleTang, Y., Guo, L., Zhao, M., Gui, Y., Han, J., Lu, W., Dai, Q., Jiang, S., Lin, M., Zhou, Z., & Wang, J. (2021). A Novel Thermostable Keratinase from Deinococcus geothermalis with Potential Application in Feather Degradation. Applied Sciences, 11(7), 3136. https://doi.org/10.3390/app11073136






