Applications of Graphene-Based Nanomaterials in Environmental Analysis
Abstract
1. Introduction
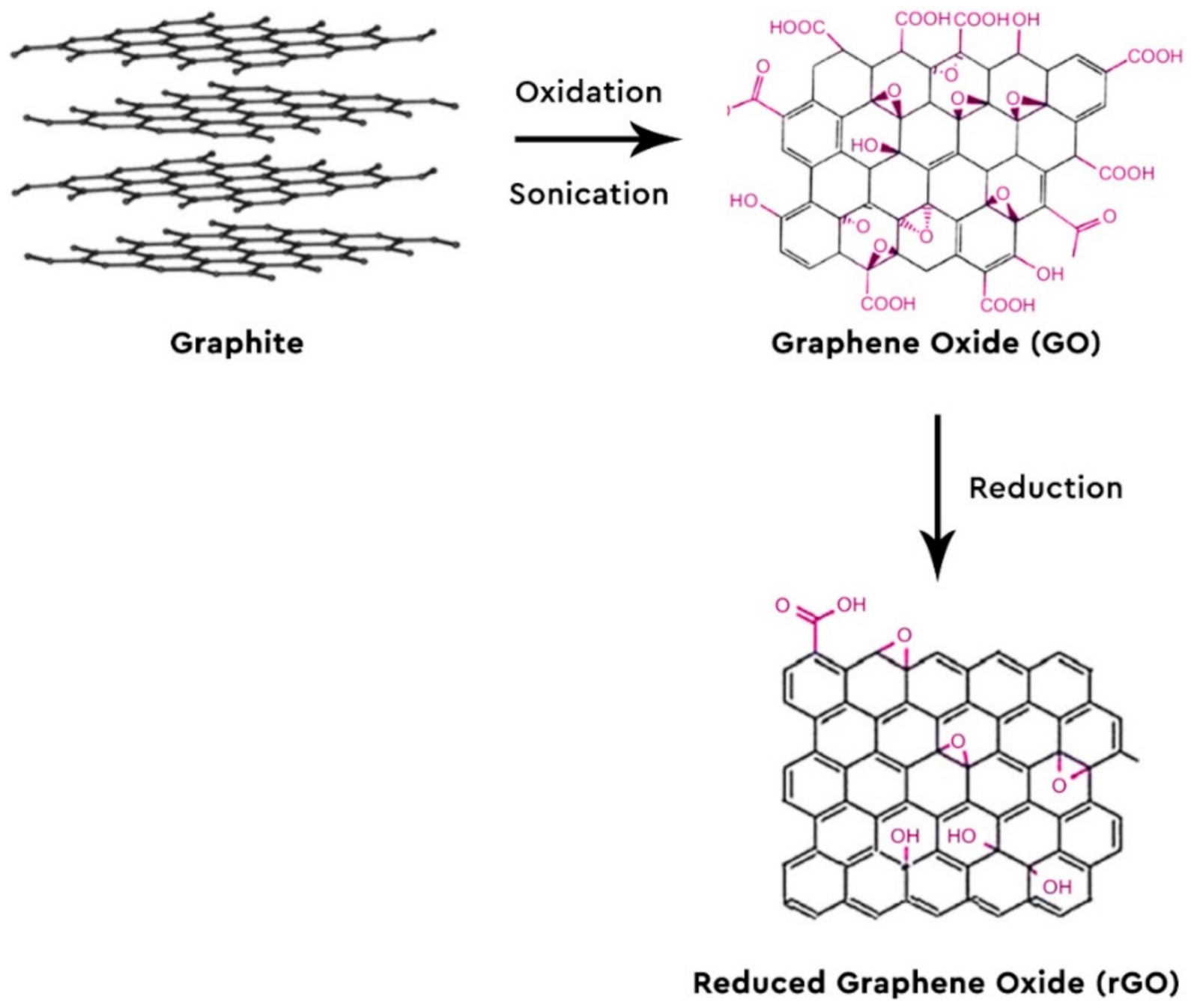
2. Graphene-Based Nanomaterials and Nanocomposites
2.1. Synthesis of Magnetic Nanoparticles
2.2. Synthesis of Fe3O4-GO Nanocomposites
2.3. Synthesis of GO Membranes Composites
2.4. Synthesis of Graphene Aerogels
2.5. Synthesis of GO Alginate Beads
3. Analytical Applications in Environmental Samples
3.1. Applications of G and GO
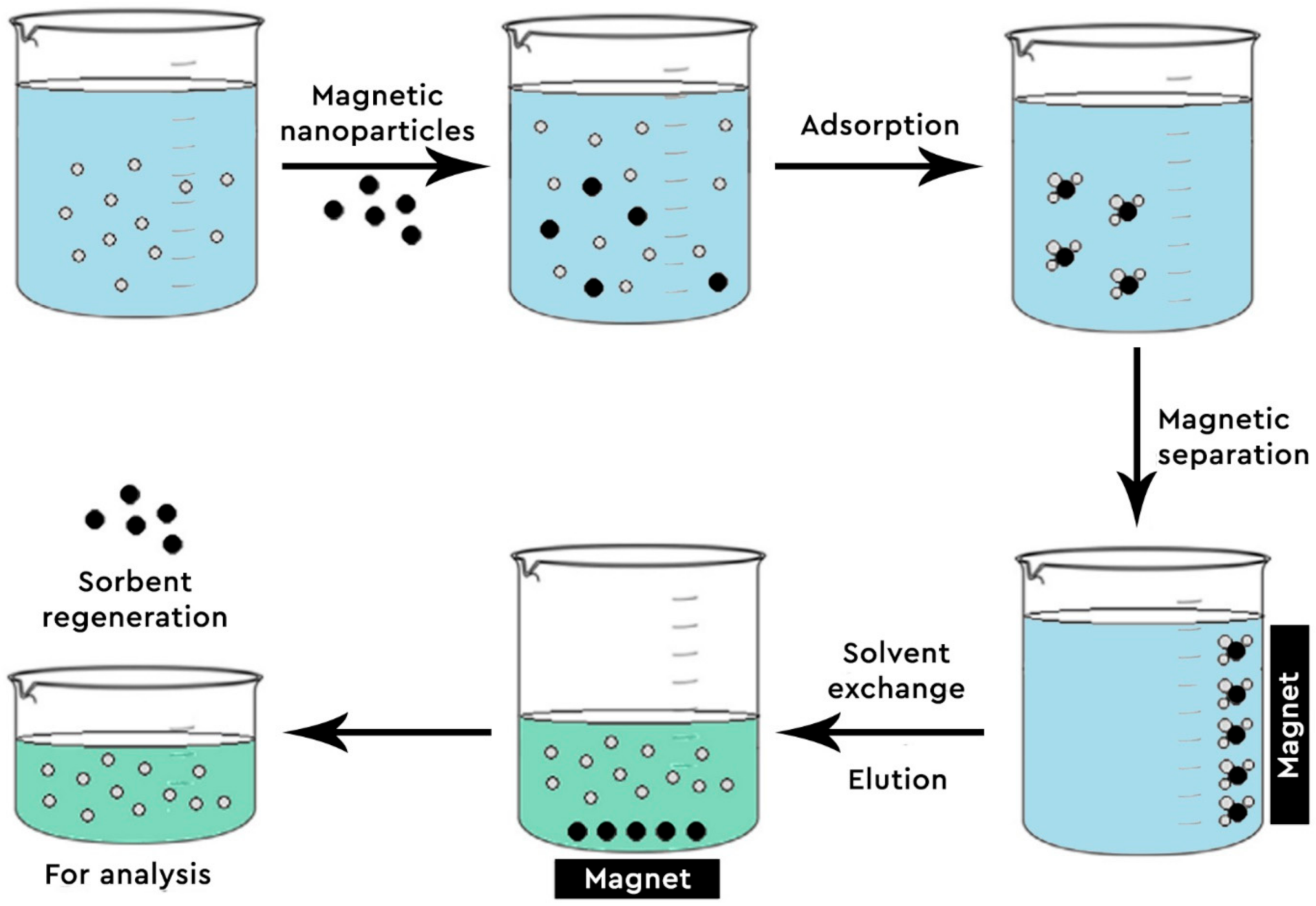
3.2. Applications of GO-Fe3O4
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| ACN | Acetonitrile |
| d-SPE | Dispersive Solid Phase Extraction |
| d-μSPE | Dispersive Micro Solid Phase Extraction |
| d-MSPE | Dispersive Magnetic Solid Phase Extraction |
| ED-XRF | Energy Dispersive X-ray Fluorescence Spectrometry |
| ETAAS | Electrothermal Atomic Absorption Spectrometry |
| FAAS | Flame Atomic Absorption Spectrometry |
| FT-IR | Fourier-Transform Infrared Spectroscopy |
| G | Graphene |
| GC-MS | Gas Chromatography—Mass Spectrometry |
| GO | Graphene Oxide |
| HF-SPME | Hollow-fiber Solid Phase Micro Extraction |
| HPLC | High Pressure Liquid Chromatography |
| HPLC-DAD | High Pressure Liquid Chromatography—Diode-Array Detector |
| ICP-OES | Inductively Coupled Plasma Optical Emission Spectrometry |
| LOD | Limit of Detection |
| mGO | Magnetic Graphene Oxide (Fe3O4/Graphene Oxide) |
| MNP | Magnetic Nanoparticles |
| MSPE | Magnetic Solid Phase Extraction |
| PAH | Polycyclic Aromatic Hydrocarbons |
| PT-SPE | Pipette-tip Solid Phase Extraction |
| rGO | Reduced Graphene Oxide |
| SEM | Scanning Electron Microscope |
| SPE | Solid Phase Extraction |
| SPME | Solid Phase Microextraction |
| UHPLC-MS | Ultra-High Pressure Liquid Chromatography–Mass Spectrometry |
References
- Chen, Y.; Xia, L.; Liang, R.; Lu, Z.; Li, L.; Huo, B.; Li, G.; Hu, Y. Advanced materials for sample preparation in recent decade. TrAC Trends Anal. Chem. 2019, 120, 115652. [Google Scholar] [CrossRef]
- Plastiras, O.-E.; Andreasidou, E.; Samanidou, V. Microextraction Techniques with Deep Eutectic Solvents. Molecules 2020, 25, 6026. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.; Sun, J.; Sun, X. Recent advances in biosensors for the detection of estrogens in the environment and food. TrAC Trends Anal. Chem. 2020, 127, 115882. [Google Scholar] [CrossRef]
- Xia, S.; Dong, J.; Chen, Y.; Wang, Y.; Chen, X. Three dimensional phytic acid-induced graphene as a solid-phase microextraction fiber coating and its analytical applications for nerolidol in tea. Chin. Chem. Lett. 2018, 29, 107–110. [Google Scholar] [CrossRef]
- Jiang, H.-L.; Li, N.; Cui, L.; Wang, X.; Zhao, R.-S. Recent application of magnetic solid phase extraction for food safety analysis. TrAC Trends Anal. Chem. 2019, 120. [Google Scholar] [CrossRef]
- Amiri, A.; Baghayeri, M.; Sedighi, M. Magnetic solid-phase extraction of polycyclic aromatic hydrocarbons using a graphene oxide/Fe3O4@polystyrene nanocomposite. Microchim. Acta 2018, 185, 1–9. [Google Scholar] [CrossRef]
- Yap, P.L.; Auyoong, Y.L.; Hassan, K.; Farivar, F.; Tran, D.N.; Ma, J.; Losic, D. Multithiol functionalized graphene bio-sponge via photoinitiated thiol-ene click chemistry for efficient heavy metal ions adsorption. Chem. Eng. J. 2020, 395, 124965. [Google Scholar] [CrossRef]
- Maciel, E.V.S.; de Toffoli, A.L.; Neto, E.S.; Nazario, C.E.D.; Lanças, F.M. New materials in sample preparation: Recent advances and future trends. TrAC Trends Anal. Chem. 2019, 119, 115633. [Google Scholar] [CrossRef]
- Shivakumar, R.; Bolker, A.; Tsang, S.H.; Atar, N.; Verker, R.; Gouzman, I.; Hala, M.; Moshe, N.; Jones, A.; Grossman, E.; et al. POSS enhanced 3D graphene—Polyimide film for atomic oxygen endurance in Low Earth Orbit space environment. Polymer 2020, 191, 122270. [Google Scholar] [CrossRef]
- Kyzas, G.Z.; Travlou, N.A.; Deliyanni, E.A. The role of chitosan as nanofiller of graphite oxide for the removal of toxic mercury ions. Colloids Surf. B Biointerfaces 2014, 113, 467–476. [Google Scholar] [CrossRef]
- Kyzas, G.Z.; Deliyanni, E.A.; Matis, K.A. Graphene oxide and its application as an adsorbent for wastewater treatment. J. Chem. Technol. Biotechnol. 2014, 89, 196–205. [Google Scholar] [CrossRef]
- Sengupta, J.; Hussain, C.M. Graphene and its derivatives for Analytical Lab on Chip platforms. TrAC Trends Anal. Chem. 2019, 114, 326–337. [Google Scholar] [CrossRef]
- Nayl, A.; Abd-Elhamid, A.; El-Shanshory, A.A.; Soliman, H.M.; Kenawy, E.-R.; Aly, H. Development of sponge/graphene oxide composite as eco-friendly filter to remove methylene blue from aqueous media. Appl. Surf. Sci. 2019, 496, 143676. [Google Scholar] [CrossRef]
- Gazzari, S.; Cortés-Arriagada, D. Interaction of trivalent arsenic on different topologies of Fe-doped graphene nanosheets at water environments: A computational study. J. Mol. Liq. 2019, 289, 1–7. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, Y.; Tang, Y.; Li, X.; Zhang, X.; Li, C.; Xu, S. Magnetic solid-phase extraction based on Fe3O4/graphene oxide nanoparticles for the determination of malachite green and crystal violet in environmental water samples by HPLC. Int. J. Environ. Anal. Chem. 2018, 98, 215–228. [Google Scholar] [CrossRef]
- Martín, A.; Escarpa, A. Graphene: The cutting–edge interaction between chemistry and electrochemistry. TrAC Trends Anal. Chem. 2014, 56, 13–26. [Google Scholar] [CrossRef]
- Mateos, R.; Vera, S.; Díez-Pascual, A.M.; Andrés, M.P.S. Graphene solid phase extraction (SPE) of synthetic antioxidants in complex food matrices. J. Food Compos. Anal. 2017, 62, 223–230. [Google Scholar] [CrossRef]
- Magesa, F.; Wu, Y.; Tian, Y.; Vianney, J.-M.; Buza, J.; He, Q.; Tan, Y. Graphene and graphene like 2D graphitic carbon nitride: Electrochemical detection of food colorants and toxic substances in environment. Trends Environ. Anal. Chem. 2019, 23, e00064. [Google Scholar] [CrossRef]
- Yang, M.; Tian, H.; Zhu, J.; He, J. Graphene Nanomaterials in Energy and Environment Applications. Handb. Graphene 2019, 5, 1–25. [Google Scholar] [CrossRef]
- Qi, C.; Zhao, L.; Lin, Y.; Wu, D. Graphene oxide/chitosan sponge as a novel filtering material for the removal of dye from water. J. Colloid Interface Sci. 2018, 517, 18–27. [Google Scholar] [CrossRef]
- Zhou, S.; Yao, W.; Wang, Z.; Ma, L.; Lu, Z.; Hou, C. The first-principles calculations to explore the mechanism of oxygen diffusion on vacancy defective graphene in marine environment. Appl. Surf. Sci. 2020, 525, 146585. [Google Scholar] [CrossRef]
- Zaaba, N.; Foo, K.; Hashim, U.; Tan, S.; Liu, W.-W.; Voon, C. Synthesis of Graphene Oxide using Modified Hummers Method: Solvent Influence. Proc. Eng. 2017, 184, 469–477. [Google Scholar] [CrossRef]
- Maggira, M.; Deliyanni, E.A.; Samanidou, V.F. Synthesis of Graphene Oxide Based Sponges and Their Study as Sorbents for Sample Preparation of Cow Milk Prior to HPLC Determination of Sulfonamides. Molecules 2019, 24, 2086. [Google Scholar] [CrossRef] [PubMed]
- García-Mesa, J.; Leal, P.M.; Guerrero, M.L.; Alonso, E.V. Simultaneous determination of noble metals, Sb and Hg by magnetic solid phase extraction on line ICP OES based on a new functionalized magnetic graphene oxide. Microchem. J. 2019, 150, 104141. [Google Scholar] [CrossRef]
- Shahabi, M.; Raissi, H. Comprehensive theoretical prediction of the dynamics and stability properties of Tegafur pharmaceutical agent on the Graphene based nanostructures in aqueous environment. Appl. Surf. Sci. 2018, 455, 32–36. [Google Scholar] [CrossRef]
- Li, W.-K.; Shi, Y.-P. Recent advances and applications of carbon nanotubes based composites in magnetic solid-phase extraction. TrAC Trends Anal. Chem. 2019, 118, 652–665. [Google Scholar] [CrossRef]
- Jon, C.-S.; Meng, L.-Y.; Li, D. Recent review on carbon nanomaterials functionalized with ionic liquids in sample pretreatment application. TrAC Trends Anal. Chem. 2019, 120, 115641. [Google Scholar] [CrossRef]
- Sun, C.; Wang, Z.; Chen, L.; Li, F. Fabrication of robust and compressive chitin and graphene oxide sponges for removal of microplastics with different functional groups. Chem. Eng. J. 2020, 393, 124796. [Google Scholar] [CrossRef]
- Yao, Y.; Ping, J. Recent advances in graphene-based freestanding paper-like materials for sensing applications. TrAC Trends Anal. Chem. 2018, 105, 75–88. [Google Scholar] [CrossRef]
- Wang, Z.; Han, Q.; Xia, J.; Xia, L.; Ding, M.; Tang, J. Graphene-based solid-phase extraction disk for fast separation and preconcentration of trace polycyclic aromatic hydrocarbons from environmental water samples. J. Sep. Sci. 2013, 36, 1834–1842. [Google Scholar] [CrossRef]
- Núñez, C.; Triviño, J.J.; Segura, R.; Arancibia, V. Development of a fast and sensitive method for the determination of As(III) at trace levels in urine by differential pulse anodic voltammetry using a simple graphene screen–printed electrode. Microchem. J. 2020, 159, 105393. [Google Scholar] [CrossRef]
- Wang, Z.; Yao, M.; Wang, X.; Li, S.; Liu, Y.; Yang, G. Influence of reaction media on synthesis of dialdehyde cellulose/GO composites and their adsorption performances on heavy metals. Carbohydr. Polym. 2019, 232, 115781. [Google Scholar] [CrossRef]
- Zhang, Y.; Guan, J.; Wu, J.; Ding, S.; Yang, J.; Zhang, J.; Dong, A.; Deng, L. N-alkylated chitosan/graphene oxide porous sponge for rapid and effective hemostasis in emergency situations. Carbohydr. Polym. 2019, 219, 405–413. [Google Scholar] [CrossRef]
- Wu, J.; Chen, L.; Mao, P.; Lu, Y.; Wang, H. Determination of chloramphenicol in aquatic products by graphene-based SPE coupled with HPLC-MS/MS. J. Sep. Sci. 2012, 35, 3586–3592. [Google Scholar] [CrossRef]
- Service, R.F. Carbon Sheets an Atom Thick Give Rise to Graphene Dreams. Science 2009, 324, 875–877. [Google Scholar] [CrossRef]
- Hou, X.; Tang, S.; Wang, J. Recent advances and applications of graphene-based extraction materials in food safety. TrAC Trends Anal. Chem. 2019, 119, 115603. [Google Scholar] [CrossRef]
- Sadegh, H. Development of graphene oxide from graphite: A review on synthesis, characterization and its application in wastewater treatment. Rev. Adv. Mater. Sci. 2017, 49, 38–43. [Google Scholar]
- Alhwaige, A.A.; Agag, T.; Ishida, H.; Qutubuddin, S. Biobased chitosan hybrid aerogels with superior adsorption: Role of graphene oxide in CO2 capture. RSC Adv. 2013, 3, 16011–16020. [Google Scholar] [CrossRef]
- Nidheesh, P.V. Graphene-based materials supported advanced oxidation processes for water and wastewater treatment: A review. Environ. Sci. Pollut. Res. 2017, 24, 27047–27069. [Google Scholar] [CrossRef]
- Wei, Y.; Zhang, Y.; Gao, X.; Ma, Z.; Wang, X.; Gao, C. Multilayered graphene oxide membranes for water treatment: A review. Carbon 2018, 139, 964–981. [Google Scholar] [CrossRef]
- Sitko, R.; Zawisza, B.; Malicka, E. Graphene as a new sorbent in analytical chemistry. TrAC Trends Anal. Chem. 2013, 51, 33–43. [Google Scholar] [CrossRef]
- Hummers, W.S.; Offeman, R.E. Preparation of Graphitic Oxide. J. Am. Chem. Soc. 1958, 80, 1339. [Google Scholar] [CrossRef]
- Wang, X.; Liu, B.; Lu, Q.; Qu, Q. Graphene-based materials: Fabrication and application for adsorption in analytical chemistry. J. Chromatogr. A 2014, 1362, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Li, Y.; Zhang, L.; Huang, H.; Hu, J.; Shah, S.M.; Su, X. Adsorption and removal of tetracycline antibiotics from aqueous solution by graphene oxide. J. Colloid Interface Sci. 2012, 368, 540–546. [Google Scholar] [CrossRef] [PubMed]
- Valcárcel, M.; Cárdenas, S.; Simonet, B.; Moliner-Martínez, Y.; Lucena, R. Carbon nanostructures as sorbent materials in analytical processes. TrAC Trends Anal. Chem. 2008, 27, 34–43. [Google Scholar] [CrossRef]
- Zhang, J.; Lin, S.; Han, M.; Su, Q.; Xia, L.; Hui, Z. Adsorption Properties of Magnetic Magnetite Nanoparticle for Coexistent Cr(VI) and Cu(II) in Mixed Solution. Water 2020, 12, 446. [Google Scholar] [CrossRef]
- Jędrzak, A.; Grześkowiak, B.F.; Coy, E.; Wojnarowicz, J.; Szutkowski, K.; Jurga, S.; Jesionowski, T.; Mrówczyński, R. Dendrimer based theranostic nanostructures for combined chemo- and photothermal therapy of liver cancer cells in vitro. Colloids Surf. B Biointerfaces 2019, 173, 698–708. [Google Scholar] [CrossRef]
- Wierucka, M.; Biziuk, M. Application of magnetic nanoparticles for magnetic solid-phase extraction in preparing biological, environmental and food samples. TrAC Trends Anal. Chem. 2014, 59, 50–58. [Google Scholar] [CrossRef]
- Massart, R. Preparation of aqueous magnetic liquids in alkaline and acidic media. IEEE Trans. Magn. 1981, 17, 1247–1248. [Google Scholar] [CrossRef]
- Kyzas, G.Z.; Travlou, N.A.; Kalogirou, O.; Deliyanni, E.A. Magnetic graphene oxide: Effect of preparation route on reactive black 5 adsorption. Materials 2013, 6, 1360–1376. [Google Scholar] [CrossRef]
- Kluchova, K.; Zboril, R.; Tucek, J.; Pecova, M.; Zajoncova, L.; Safarik, I.; Mashlan, M.; Markova, I.; Jancik, D.; Sebela, M.; et al. Superparamagnetic maghemite nanoparticles from solid-state synthesis—Their functionalization towards peroral MRI contrast agent and magnetic carrier for trypsin immobilization. Biomaterials 2009, 30, 2855–2863. [Google Scholar] [CrossRef]
- Zheng, Y.H.; Cheng, Y.; Bao, F.; Wang, Y.S. Synthesis and magnetic properties of Fe3O4 nanoparticles. Mater. Res. Bull. 2006, 41, 525–529. [Google Scholar] [CrossRef]
- Li, C.; Wei, Y.; Liivat, A.; Zhu, Y.; Zhu, J. Microwave-solvothermal synthesis of Fe3O4 magnetic nanoparticles. Mater. Lett. 2013, 107, 23–26. [Google Scholar] [CrossRef]
- Deng, H.; Huang, J.; Qin, C.; Xu, T.; Ni, H.; Ye, P. Preparation of high-performance nanocomposite membranes with hydroxylated graphene and graphene oxide. J. Water Process. Eng. 2021, 40, 101945. [Google Scholar] [CrossRef]
- Zhi, D.; Li, T.; Li, J.; Ren, H.; Meng, F. A review of three-dimensional graphene-based aerogels: Synthesis, structure and application for microwave absorption. Compos. Part B Eng. 2021, 211. [Google Scholar] [CrossRef]
- Arshad, F.; Selvaraj, M.; Banat, F.; Abu Haija, M. Removal of metal ions and organics from real refinery wastewater using double- functionalized graphene oxide in alginate beads. J. Water Process. Eng. 2020, 38, 101635. [Google Scholar] [CrossRef]
- Manousi, N.; Rosenberg, E.; Deliyanni, E.A.; Zachariadis, G.A. Sample Preparation Using Graphene-Oxide-Derived Nanomaterials for the Extraction of Metals. Molecules 2020, 25, 2411. [Google Scholar] [CrossRef]
- Manousi, N.; Rosenberg, E.; Deliyanni, E.; Zachariadis, G.A.; Samanidou, V. Magnetic Solid-Phase Extraction of Organic Compounds Based on Graphene Oxide Nanocomposites. Molecules 2020, 25, 1148. [Google Scholar] [CrossRef]
- Wang, Y.-K.; Gao, S.-T.; Ma, J.-J.; Li, J.-C. Application of Graphene as a Sorbent for Simultaneous Preconcentration and Determination of Trace Amounts of Cobalt and Nickel in Environmental Water and Vegetable Samples. J. Chin. Chem. Soc. 2012, 59, 1468–1477. [Google Scholar] [CrossRef]
- Su, S.; Chen, B.; He, M.; Hu, B. Graphene oxide–silica composite coating hollow fiber solid phase microextraction online coupled with inductively coupled plasma mass spectrometry for the determination of trace heavy metals in environmental water samples. Talanta 2014, 123, 1–9. [Google Scholar] [CrossRef]
- Li, C.; Lu, A.; Wang, J.; Li, J.; Ping, H.; Luan, Y.; Chen, J.; Ha, X. Determination of five sulfonylurea herbicides in environmental waters and soil by ultra- high performance liquid chromatography with tandem mass spectrometry after extraction using graphene. J. Sep. Sci. 2014, 37, 3714–3721. [Google Scholar] [CrossRef] [PubMed]
- Pourjavid, M.R.; Sehat, A.A.; Arabieh, M.; Yousefi, S.R.; Hosseini, M.H.; Rezaee, M. Column solid phase extraction and flame atomic absorption spectrometric determination of manganese(II) and iron(III) ions in water, food and biological samples using 3-(1-methyl-1H-pyrrol-2-yl)-1H-pyrazole-5-carboxylic acid on synthesized graphene oxide. Mater. Sci. Eng. C 2014, 35, 370–378. [Google Scholar] [CrossRef] [PubMed]
- Sitko, R.; Zawisza, B.; Talik, E.; Janik, P.; Osoba, G.; Feist, B.; Malicka, E. Spherical silica particles decorated with graphene oxide nanosheets as a new sorbent in inorganic trace analysis. Anal. Chim. Acta 2014, 834, 22–29. [Google Scholar] [CrossRef] [PubMed]
- Zawisza, B.; Baranik, A.; Malicka, E.; Talik, E.; Sitko, R. Preconcentration of Fe(III), Co(II), Ni(II), Cu(II), Zn(II) and Pb(II) with ethylenediamine-modified graphene oxide. Microchim. Acta 2016, 183, 231–240. [Google Scholar] [CrossRef]
- Zawisza, B.; Sitko, R.; Malicka, E.; Talik, E. Graphene oxide as a solid sorbent for the preconcentration of cobalt, nickel, copper, zinc and lead prior to determination by energy- dispersive X-ray fluorescence spectrometry. Anal. Methods 2013. [Google Scholar] [CrossRef]
- Pourjavid, M.R.; Arabieh, M.; Yousefi, S.R.; Jamali, M.R.; Rezaee, M.; Hosseini, M.H.; Sehat, A.A. Study on column SPE with synthesized graphene oxide and FAAS for determination of trace amount of Co(II) and Ni(II) ions in real samples. Mater. Sci. Eng. C 2015, 47, 114–122. [Google Scholar] [CrossRef]
- Pytlakowska, K. Dispersive micro solid-phase extraction of heavy metals as their complexes with 2-(5-bromo-2-pyridylazo)-5-diethylaminophenol using graphene oxide nanoparticles. Microchim. Acta 2016, 183, 91–99. [Google Scholar] [CrossRef]
- Pytlakowska, K.; Kozik, V.; Matussek, M.; Pilch, M.; Hachuła, B.; Kocot, K. Glycine modified graphene oxide as a novel sorbent for preconcentration of chromium, copper, and zinc ions from water samples prior to energy dispersive X-ray fluorescence spectrometric determination. RSC Adv. 2016, 6, 42836–42844. [Google Scholar] [CrossRef]
- Pytlakowska, K.; Pilch, M.; Hachuła, B.; Nycz, J.E.; Kornaus, K.; Pisarski, W.A. Energy dispersive X-ray fluorescence spectrometric determination of copper, zinc, lead and chromium species after preconcentration on graphene oxide chemically modified with mercapto-groups. J. Anal. At. Spectrom. 2019, 34, 1416–1425. [Google Scholar] [CrossRef]
- Ghazaghi, M.; Mousavi, H.Z.; Rashidi, A.M.; Shirkhanloo, H.; Rahighi, R. Innovative separation and preconcentration technique of coagulating homogenous dispersive micro solid phase extraction exploiting graphene oxide nanosheets. Anal. Chim. Acta 2016, 902, 33–42. [Google Scholar] [CrossRef]
- Zhu, X.; Cui, Y.; Chang, X.; Wang, H. Selective solid-phase extraction and analysis of trace-level Cr(III), Fe(III), Pb(II), and Mn(II) Ions in wastewater using diethylenetriamine-functionalized carbon nanotubes dispersed in graphene oxide colloids. Talanta 2016, 146, 358–363. [Google Scholar] [CrossRef]
- Chatzimitakos, T.; Samanidou, V.; Stalikas, C.D. Graphene-functionalized melamine sponges for microextraction of sulfonamides from food and environmental samples. J. Chromatogr. A 2017, 1522, 1–8. [Google Scholar] [CrossRef]
- Mosavi, S.S.; Ghanemi, K.; Nickpour, Y. Graphene oxide nanosheets modified with trithiocyanuric acid for extraction and enrichment of Pb (II) and Cu (II) ions in seawater. Water Environ. J. 2018, 32, 377–383. [Google Scholar] [CrossRef]
- Sun, Y.; Chen, L.; Yu, J.; Yoon, B.; Lee, S.K.; Nam, J.-D.; Ci, L.; Suhr, J. Lightweight graphene oxide-based sponges with high compressibility and durability for dye adsorption. Carbon 2020, 160, 54–63. [Google Scholar] [CrossRef]
- Tao, E.; Ma, D.; Yang, S.; Hao, X. Graphene oxide-montmorillonite/sodium alginate aerogel beads for selective adsorption of methylene blue in wastewater. J. Alloys Compd. 2020, 832, 154833. [Google Scholar] [CrossRef]
- Han, Q.; Wang, Z.; Xia, J.; Chen, S.; Zhang, X.; Ding, M. Facile and tunable fabrication of Fe3O4/graphene oxide nanocomposites and their application in the magnetic solid-phase extraction of polycyclic aromatic hydrocarbons from environmental water samples. Talanta 2012, 101, 388–395. [Google Scholar] [CrossRef]
- Zeng, S.; Gan, N.; Weideman-Mera, R.; Cao, Y.; Li, T.; Sang, W. Enrichment of polychlorinated biphenyl 28 from aqueous solutions using Fe3O4 grafted graphene oxide. Chem. Eng. J. 2013, 218, 108–115. [Google Scholar] [CrossRef]
- Shi, P.; Ye, N. Magnetite–graphene oxide composites as a magnetic solid-phase extraction adsorbent for the determination of trace sulfonamides in water samples. Anal. Methods 2014, 6, 9725–9730. [Google Scholar] [CrossRef]
- Shi, P.; Ye, N. Investigation of the adsorption mechanism and preconcentration of sulfonamides using a porphyrin-functionalized Fe3O4-graphene oxide nanocomposite. Talanta 2015, 143, 219–225. [Google Scholar] [CrossRef]
- Ziaei, E.; Mehdinia, A.; Jabbari, A. A novel hierarchical nanobiocomposite of graphene oxide–magnetic chitosan grafted with mercapto as a solid phase extraction sorbent for the determination of mercury ions in environmental water samples. Anal. Chim. Acta 2014, 850, 49–56. [Google Scholar] [CrossRef]
- Aliyari, E.; Alvand, M.; Shemirani, F. Simultaneous separation and preconcentration of lead and cadmium from water and vegetable samples using a diethylenetriamine-modified magnetic graphene oxide nanocomposite. Anal. Methods 2015, 7, 7582–7589. [Google Scholar] [CrossRef]
- Kazemi, E.; Dadfarnia, S.; Shabani, A.M.H. Dispersive solid phase microextraction with magnetic graphene oxide as the sorbent for separation and preconcentration of ultra-trace amounts of gold ions. Talanta 2015, 141, 273–278. [Google Scholar] [CrossRef] [PubMed]
- Kazemi, E.; Shabani, A.M.H.; Dadfarnia, S.; Izadi, F. Speciation and determination of chromium ions by dispersive micro solid phase extraction using magnetic graphene oxide followed by flame atomic absorption spectrometry. Int. J. Environ. Anal. Chem. 2017, 97, 1080–1093. [Google Scholar] [CrossRef]
- Ahmad, H.; Jalil, A.A.; Triwahyono, S. Dispersive solid phase extraction of gold with magnetite-graphene oxide prior to its determination via microwave plasma-atomic emission spectrometry. RSC Adv. 2016, 6, 88110–88116. [Google Scholar] [CrossRef]
- Chen, X.-H.; Pan, S.-D.; Ye, M.-J.; Li, X.-P.; Zhao, Y.-G.; Jin, M.-C. Magnetic solid-phase extraction based on a triethylenetetramine-functionalized magnetic graphene oxide composite for the detection of ten trace phenolic environmental estrogens in environmental water. J. Sep. Sci. 2016, 39, 762–768. [Google Scholar] [CrossRef]
- Khan, M.; Yilmaz, E.; Sevinc, B.; Sahmetlioglu, E.; Shah, J.; Jan, M.R.; Soylak, M. Preparation and characterization of magnetic allylamine modified graphene oxide-poly(vinyl acetate-co-divinylbenzene) nanocomposite for vortex assisted magnetic solid phase extraction of some metal ions. Talanta 2016, 146, 130–137. [Google Scholar] [CrossRef]
- Islam, A.; Ahmad, H.; Zaidi, N.; Kumar, S. A graphene oxide decorated with triethylenetetramine-modified magnetite for separation of chromium species prior to their sequential speciation and determination via FAAS. Microchim. Acta 2016, 183, 289–296. [Google Scholar] [CrossRef]
- Arvand, M.; Masouleh, A.N. Magnetic solid-phase extraction of imatinib and doxorubicin as cytostatic drugs by Fe3O4/graphene oxide nanocomposite. J. Iran. Chem. Soc. 2017, 14, 1673–1682. [Google Scholar] [CrossRef]
- Dos Reis, L.C.; Vidal, L.; Canals, A. Graphene oxide/Fe3O4 as sorbent for magnetic solid-phase extraction coupled with liquid chromatography to determine 2,4,6-trinitrotoluene in water samples. Anal. Bioanal. Chem. 2017, 409, 2665–2674. [Google Scholar] [CrossRef]
- Dahaghin, Z.; Mousavi, H.Z.; Sajjadi, S.M. Trace amounts of Cd(II), Cu(II) and Pb(II) ions monitoring using Fe3O4 @graphene oxide nanocomposite modified via 2-mercaptobenzothiazole as a novel and efficient nanosorbent. J. Mol. Liq. 2017, 231, 386–395. [Google Scholar] [CrossRef]
- Dahaghin, Z.; Mousavi, H.Z.; Sajjadi, S.M. Synthesis and Application of Magnetic Graphene Oxide Modified with 8-Hydroxyquinoline for Extraction and Preconcentration of Trace Heavy Metal Ions. ChemistrySelect 2017, 2, 1282–1289. [Google Scholar] [CrossRef]
- Molaei, K.; Bagheri, H.; Asgharinezhad, A.A.; Ebrahimzadeh, H.; Shamsipur, M. SiO2-coated magnetic graphene oxide modified with polypyrrole–polythiophene: A novel and efficient nanocomposite for solid phase extraction of trace amounts of heavy metals. Talanta 2017, 167, 607–616. [Google Scholar] [CrossRef]
- Neyestani, M.R.; Shemirani, F.; Mozaffari, S.; Alvand, M. A magnetized graphene oxide modified with 2-mercaptobenzothiazole as a selective nanosorbent for magnetic solid phase extraction of gold(III), palladium(II) and silver(I). Microchim. Acta 2017, 184, 2871–2879. [Google Scholar] [CrossRef]
- Sarikhani, Z.; Manoochehri, M. Determination of Ultra Trace Cr(III) and Cr(VI) Species by Electrothermal Atomic Absorption Spectrometry after Simultaneous Magnetic Solid Phase Extraction with the Aid of a Novel Imidazolium-Functionalized Magnetite Graphene Oxide Nanocomposite. Bull. Chem. Soc. Jpn. 2017, 90, 746–753. [Google Scholar] [CrossRef]
- Ma, L.; Huang, J.; Zhou, M. Magnetic graphene oxide for efficient solid phase extraction of DEHP. IOP Conf. Ser. Mater. Sci. Eng. 2019, 544. [Google Scholar] [CrossRef]
| Adsorbent | Analyte(s) | Applications | Sample Preparation | Analytical Technique | LODs | EF 1 | Reference |
|---|---|---|---|---|---|---|---|
| G | Co(II), Ni(II) | Tap, river and sea water | SPE | FAAS | 0.36, 0.51 μg/L | 200 | [59] |
| GO-silica | Mn(II), Co(II), Ni(II), Cu(II), Cd(II), Pb(II) | Well, pond and lake water | HF-SPME | ICP-MS | 7.5, 0.39, 20, 23, 6.7, 28 ng/L | 10 | [60] |
| G | Sulfonylurea herbicides | Environmental water samples | SPE | UHPLC-MS | 0.28–0.53 ng/L | N/R 2 | [61] |
| GO | Mn(II), Fe(III) | Tap, mineral, river water | SPE | FAAS | 0.145, 0.162 μg/L | 325 | [62] |
| GO-silica | Cu(II), Pb(II) | Mineral, waste and sea water | SPE | FAAS | 0.084, 0.27 μg/L | 200–250 | [63] |
| GO-EDA | Fe(III), Co(II), Ni(II), Cu(II), Zn(II), Pb(II) | Waste water from industry | d-μSPE | ED-XRF | 0.07, 0.10, 0.07, 0.08, 0.06, 0.10 μg/L | N/R | [64] |
| GO | Co(II), Ni(II), Cu(II), Zn(II), Pb(II) | Waste water from industry | d-μSPE | ED-XRF | 0.5, 0.7, 1.8, 1.5, 1.4 μg/L | N/R | [65] |
| GO | Co(II), Ni(II) | Mineral and spring water | SPE | FAAS | 0.25, 0.18 μg/L | 250 | [66] |
| GO | Cr(III), Co(II), Ni(II), Cu(II), Zn(II), Pb(II) | Environmental water samples | d-μSPE | ED-XRF | 0.07–0.25 μg/L | N/R | [67] |
| GO-Gly | Cr(III), Cu(II), Zn(II) | Tap, river, estuarine and lake water | d-μSPE | ED-XRF | 0.15, 0.07, 0.08 μg/L | 1575, 890, 810 | [68] |
| GO-S | Cu(II), Zn(II), Pb(II), Cr(III) | Lake, river, mineral, spring and sea water | d-μSPE | ED-XRF | 0.06–0.10 μg/L | 520–3120 | [69] |
| GO | Pb(II), Cd(II), Cr(III) | River water | CHd-μSPE | ETAAS | 0.035, 0.005, 0.012 μg/L | 14.7, 16.1, 15.4 | [70] |
| GO-MWCNT | Cr(III), Fe(III), Pb(II), Mn(II) | Wastewater | SPE | ICP-OES | 0.16, 0.50, 0.24, 0.38 μg/L | 75 | [71] |
| GMeS | Sulfonamides | Lake water | SPE | HPLC | 0.10–0.29 μg/kg 3 | 96–99 | [72] |
| GO-TTC | Pb(II), Cu(II) | Sea water | SPE | FAAS | 0.32, 0.13 μg/L | 83.3 | [73] |
| GO-sponges | Methylene blue | Wastewater | N/R | UV-vis | N/R | N/R | [74] |
| GO-MMT/SA | Methylene blue | Wastewater | N/R | UV-vis | N/R | N/R | [75] |
| Adsorbent | Analyte(s) | Applications | Sample Preparation | Analytical Technique | LODs | EF | Reference |
|---|---|---|---|---|---|---|---|
| mGO | PAHs | Tap, river, sea water | MSPE | HPLC-UV | 0.09–0.19 μg/L | 25 | [76] |
| mGO | PCB 28 | School sewage, river water | MSPE | GC-MS | 0.027–0.059 μg/L | 200 | [77] |
| mGO | Sulfonamides | Tap, river water | MSPE | HPLC-DAD | 0.05–0.10 mg/L | N/R | [78] |
| mGO-porphyrin | Sulfonamides | Tap, river water | MSPE | HPLC-DAD | 0.2 mg/L | N/R | [79] |
| GO-MC-MTPS | Hg(II) | Tap, sea water | MSPE | CV-AAS 1 | 0.06 μg/L | 80 | [80] |
| mGO-DETA | Cd(II), Pb(II) | Sea, river, well water | MSPE | FAAS | 0.40, 0.38 μg/L | 150, 167 | [81] |
| mGO | Cr(III), Cr(VI), Au(III) | Drinking, river, spring, sea, waste water | d-μSPE | FAAS | 0.1, 0.1, 0.004 μg/L | 200, 200, 500 | [82,83] |
| mGO | Au(III) | Tap, lake, sea water | dSPE | MP-AES | 5 ng/L | 60 | [84] |
| TETA-mGO | Phenolic estrogens | Tap, river, well water | MSPE | UFLC-MS/MS | 0.15–1.5 ng/L | 10,000 | [85] |
| mGO-DVB-VA | Pb(II), Cd(II), Cu(II), Ni(II), Co(II) | Waste water | MSPE | FAAS | 0.37–2.39 μg/L | 40 | [86] |
| mf-GO | Cr(III), Cr(VI) | River, tannery and electroplating waste water | d-MSPE | FAAS | 5.2, 1.6 μg/L | 10 | [87] |
| mGO | Imatinib, Doxorubicin | Well, waste water | MSPE | HPLC-UV | 1.9, 1.8 μg/L | N/R | [88] |
| mGO | 2,4,6-trinitrotoluene | Reservoir, drinking, waste water | MSPE | HPLC-UV | 0.3 μg/L | 153 | [89] |
| mGO-MBT 2 | Cd(II), Cu(II), Pb(II) | Tap, lake, sea water | MSPE | FAAS | 0.19, 0.35, 0.24 μg/L | 400 | [90] |
| mGO-HQ 3 | Cd(II), Pb(II) | Water samples | MSPE | FAAS | 0.09, 0.27 μg/L | 130.43 | [91] |
| mGO/SiO2@coPPy-Th | Cu(II), Cr(III), Zn(II), Cd(II), Pb(II) | Well, river, bottled mineral water | MSPE | FAAS | 0.15–0.65 μg/L | 36–44 | [92] |
| mGO-MBT | Au(III), Pd(II), Ag(I) | Waste water | MSPE | FI-ICP-OES | 45–76 ng/L | 160, 160, 140 | [93] |
| mGO-imidazolium | Cr(III), Cr(VI) | Waste water | MSPE | ETAAS | 1.9 ng/L | 357 | [94] |
| mGO | di-2-ethylhexyl phthalate | Water samples | MSPE | HPLC-DAD | 0.35 μg/L | 100 | [95] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Plastiras, O.-E.; Deliyanni, E.; Samanidou, V. Applications of Graphene-Based Nanomaterials in Environmental Analysis. Appl. Sci. 2021, 11, 3028. https://doi.org/10.3390/app11073028
Plastiras O-E, Deliyanni E, Samanidou V. Applications of Graphene-Based Nanomaterials in Environmental Analysis. Applied Sciences. 2021; 11(7):3028. https://doi.org/10.3390/app11073028
Chicago/Turabian StylePlastiras, Orfeas-Evangelos, Eleni Deliyanni, and Victoria Samanidou. 2021. "Applications of Graphene-Based Nanomaterials in Environmental Analysis" Applied Sciences 11, no. 7: 3028. https://doi.org/10.3390/app11073028
APA StylePlastiras, O.-E., Deliyanni, E., & Samanidou, V. (2021). Applications of Graphene-Based Nanomaterials in Environmental Analysis. Applied Sciences, 11(7), 3028. https://doi.org/10.3390/app11073028








