On the Set of Fellini’s Movies: Investigating and Preserving Multi-Material Stage Costumes Exploiting Spectroscopic and Mass Spectrometric Techniques
Abstract
1. Introduction
2. Materials and Methods
2.1. Samples
2.1.1. Reference Materials
2.1.2. Historical Samples
2.2. Optical Microscope
2.3. ATR-FTIR
2.4. SEM-EDX
2.5. Py-GC/MS
2.6. HPLC Analyses
2.6.1. Sample Pretreatment
2.6.2. HPLC Conditions
3. Results
3.1. “Il Casanova”
3.2. “Roma”
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Stone, R. Art of the Andes from Chavin to Inca (World of Art); Thames & H: New York, NY, USA, 2012; ISBN 0500204152. [Google Scholar]
- Grishanov, S. Structure and properties of textile materials. In Handbook of Textile and Industrial Dyeing; Clark, M., Ed.; Woodhead Publishing: Cambridge, UK, 2011; Volume 1. [Google Scholar]
- Kauffman, G.B. Rayon: The first semi-synthetic fiber product. J. Chem. Educ. 1992, 70, 887–893. [Google Scholar] [CrossRef]
- Clark, M. General aspects of dyeing. In Handbook of Textile and Industrial Dyeing; Clarke, M., Ed.; Woodhead Publishing: Cambridge, UK, 2011; Volume 1. [Google Scholar]
- Restivo, A.; Degano, I.; Ribechini, E.; Pérez-Arantegui, J.; Colombini, M.P. Field-emission scanning electron microscopy and energy-dispersive X-ray analysis to understand the role of tannin-based dyes in the degradation of historical wool textiles. Microsc. Microanal. 2014, 20, 1534–1543. [Google Scholar] [CrossRef]
- Timar-Balazsy, A.; Eastop, D. Chemical Principles of Textile Conservation; Routledge: New York, NY, USA, 1998; ISBN 9780080501048. [Google Scholar]
- Houck, M.M. Identification of Textile Fibers; Woodhead Publishing: Cambridge, UK, 2009; ISBN 9781845692667. [Google Scholar]
- Carrillo, F.; Colom, X.; Suñol, J.J.; Saurina, J. Structural FTIR analysis and thermal characterisation of lyocell and viscose-type fibres. Eur. Polym. J. 2004, 40, 2229–2234. [Google Scholar] [CrossRef]
- Pereira, A.P.D.S.; Silva, M.H.P.D.; Lima Júnior, É.P.; Paula, A.D.S.; Tommasini, F.J. Processing and Characterization of PET Composites Reinforced With Geopolymer Concrete Waste. Mater. Res. 2017, 20, 411–420. [Google Scholar] [CrossRef]
- Kakida, H.; Tashiro, K. Mechanism and kinetics of stabilization reactions of polyacrylonitrile and related copolymers III. Comparison among the various types of copolymers as viewed from isothermal DSC thermograms and FT-IR spectral changes. Polym. J. 1997, 29, 557–562. [Google Scholar] [CrossRef][Green Version]
- Charles, J.; Ramkumaar, G.R.; Azhagiri, S.; Gunasekaran, S. FTIR and thermal studies on nylon-66 and 30% glass fibre reinforced nylon. J. Chem. 2009, 6, 23–33. [Google Scholar] [CrossRef]
- Broda, J.; Przybyło, S.; Kobiela-Mendrek, K.; Biniaś, D.; Rom, M.; Grzybowska-Pietras, J.; Laszczak, R. Biodegradation of sheep wool geotextiles. Int. Biodeterior. Biodegrad. 2016, 115, 31–38. [Google Scholar] [CrossRef]
- Fan, M.; Dai, D.; Huang, B. Fourier transform infrared spectroscopy for natural fibres. Fourier Transform. Anal. 2012, 3, 45–68. [Google Scholar]
- La Nasa, J.; Biale, G.; Sabatini, F.; Degano, I.; Colombini, M.P.; Modugno, F. Synthetic materials in art: A new comprehensive approach for the characterization of multi-material artworks by analytical pyrolysis. Herit. Sci. 2019, 7, 8. [Google Scholar] [CrossRef]
- Sabatini, F.; Manariti, A.; di Girolamo, F.; Bonaduce, I.; Tozzi, L.; Rava, A.; Colombini, M.P.; Lluveras-Tenorio, A. Painting on polyurethane foam: “Composizione-Superficie Lunare” by Giulio Turcato. Microchem. J. 2020, 156, 104872. [Google Scholar] [CrossRef]
- Yang, Y.P.; Zhang, Y.; Lang, Y.X.; Yu, M.H. Structural {ATR}-{IR} analysis of cellulose fibers prepared from a {NaOH} complex aqueous solution. IOP Conf. Ser. Mater. Sci. Eng. 2017, 213, 12039. [Google Scholar] [CrossRef]
- Causin, V.; Casamassima, R.; Marega, C.; Maida, P.; Schiavone, S.; Marigo, A.; Villari, A. The discrimination potential of ultraviolet-visible spectrophotometry, thin layer chromatography, and Fourier transform infrared spectroscopy for the forensic analysis of black and blue ballpoint inks. J. Forensic Sci. 2008, 53, 1468–1473. [Google Scholar] [CrossRef]
- Degano, I.; Modugno, F.; Bonaduce, I.; Ribechini, E.; Colombini, M.P. Recent Advances in Analytical Pyrolysis to Investigate Organic Materials in Heritage Science. Angew. Chem. Int. Ed. 2018, 57, 7313–7323. [Google Scholar] [CrossRef] [PubMed]
- Pintus, V.; Wei, S.; Schreiner, M. Accelerated UV ageing studies of acrylic, alkyd, and polyvinyl acetate paints: Influence of inorganic pigments. Microchem. J. 2016, 124, 949–961. [Google Scholar] [CrossRef]
- La Nasa, J.; Orsini, S.; Degano, I.; Rava, A.; Modugno, F.; Colombini, M.P. A chemical study of organic materials in three murals by Keith Haring: A comparison of painting techniques. Microchem. J. 2016, 124. [Google Scholar] [CrossRef]
- La Nasa, J.; Biale, G.; Ferriani, B.; Colombini, M.P.; Modugno, F. A pyrolysis approach for characterizing and assessing degradation of polyurethane foam in cultural heritage objects. J. Anal. Appl. Pyrolysis 2018, 134, 562–572. [Google Scholar] [CrossRef]
- La Nasa, J.; Biale, G.; Ferriani, B.; Trevisan, R.; Colombini, M.P.; Modugno, F. Plastics in Heritage Science: Analytical Pyrolysis Techniques Applied to Objects of Design. Molecules 2020, 25, 1705. [Google Scholar] [CrossRef]
- Xu, Y.; Lu, Z.; Tang, R. Structure and thermal properties of bamboo viscose, Tencel and conventional viscose fiber. J. Therm. Anal. Calorim. 2006, 89, 197–201. [Google Scholar] [CrossRef]
- Muratoglu, O.K.; Argon, A.S.; Cohen, R.E.; Weinberg, M. Toughening mechanism of rubber-modified polyamides. Polymer (Guildf.) 1995, 36, 921–930. [Google Scholar] [CrossRef]
- Buschle-Diller, G.; Zeronian, S.H.; Pan, N.; Yoon, M.Y. Enzymatic Hydrolysis of Cotton, Linen, Ramie, and Viscose Rayon Fabrics. Text. Res. J. 1994, 64, 270–279. [Google Scholar] [CrossRef]
- El-Gaoudy, H.; Kourkoumelis, N.; Varella, E.; Kovala-Demertzi, D. The effect of thermal aging and color pigments on the Egyptian linen properties evaluated by physicochemical methods. Appl. Phys. A 2011, 105, 497–507. [Google Scholar] [CrossRef]
- Needles, H.L.; Nowak, K.C.J. Heat-Induced Aging of Linen. In Historic Textile and Paper Materials II; American Chemical Society: Washington, DC, USA, 1989; pp. 159–167. [Google Scholar]
- Augsten, K.; Mühlig, P.; Herrmann, C. Glycoproteins and skin-core structure in Nephila clavipes spider silk observed by light and electron microscopy. Scanning 2000, 22, 12–15. [Google Scholar] [CrossRef]
- D’orazio, L.; Martuscelli, E.; Orsello, G.; Riva, F.; Scala, G.; Taglialatela, A. Nature, Origin and Technology of Natural Fibres of Textile Artefacts Recovered in the Ancient Cities around Vesuvius. J. Archaeol. Sci. 2000, 27, 745–754. [Google Scholar] [CrossRef]
- El-Nagar, K.; Sanad, S.H.; Mohamed, A.S.; Ramadan, A. Mechanical Properties and Stability to Light Exposure for Dyed Egyptian Cotton Fabrics with Natural and Synthetic Dyes. Polym. Plast. Technol. Eng. 2005, 44, 1269–1279. [Google Scholar] [CrossRef]
- Shim, W.S.; Kim, J.P.; Lee, J.J.; Koh, J.; Kim, I.S. Probing of an environmentally friendly regenerated cellulose material having bimorphic behavior. Fibers Polym. 2008, 9, 691–697. [Google Scholar] [CrossRef]
- van Bommel, M.R.; Vanden Berghe, I.; Wallert, A.M.; Boitelle, R.; Wouters, J. High-performance liquid chromatography and non-destructive three-dimensional fluorescence analysis of early synthetic dyes. J. Chromatogr. A 2007, 1157, 260–272. [Google Scholar] [CrossRef] [PubMed]
- Caycedo, M. Identification of Fifteen First Priority Textile Dyes from the Schweppe Collection with Raman and Surface Enhanced Raman Spectroscopy (SERS); Universiteit van Amsterdam: Amsterdam, The Netherlands, 2012. [Google Scholar]
- Chen, V.J.; Smith, G.D.; Holden, A.; Paydar, N.; Kiefer, K. Chemical analysis of dyes on an Uzbek ceremonial coat: Objective evidence for artifact dating and the chemistry of early synthetic dyes. Dye Pigments 2016, 131, 320–332. [Google Scholar] [CrossRef]
- Douglas, J.G.; Kavich, G.; Mori, C.; Wallace, D.; Barden, R. Materials characterization of the Ruby Slippers from the 1939 classic film, The Wizard of Oz. Herit. Sci. 2018, 6, 49. [Google Scholar] [CrossRef]
- Liu, J.; Zhou, Y.; Zhao, P.; Peng, Z.; Wang, S. Identification of early synthetic dyes in historical Chinese textiles of the late nineteenth century by high-performance liquid chromatography coupled with diode array detection and mass spectrometry. Color. Technol. 2016, 132, 177–185. [Google Scholar] [CrossRef]
- Sabatini, F.; Giugliano, R.; Degano, I. Photo-oxidation processes of Rhodamine B: A chromatographic and mass spectrometric approach. Microchem. J. 2018, 140, 114–122. [Google Scholar] [CrossRef]
- Souto, C.S.C.N. Analysis of Early Synthetic Dyes with HPLC-DAD-MS; Universidade de Lisboa: Lisboa, Portugal, 2010. [Google Scholar]
- Ursino, F. Il Costume da Vescovo Ideato da Danilo Donati per il Film Roma (1972) di Federico Fellini: Dal Comune di Rimini-Archivio Federico Fellini: Il Restauro di Un’opera Polimaterica Contemporanea; Opificio delle Pietre Dure: Florence, Italy, 2019. [Google Scholar]
- Gennaioli, R.; Triolo, L. Il restauro di due costumi creati da Danilo Donati per i film Roma (1972) e Il Casanova di Federico Fellini (1976). Kermes 2019, 116, 4–5. [Google Scholar]
- Guinot, P.; Andary, C. Molecules involved in the dyeing process with flavonoids. Dye Hist. Archaeol. 2006, 25, 21–22. [Google Scholar]
- Vasko, P.D.; Blackwell, J.; Koenig, J.L. Infrared and raman spectroscopy of carbohydrates: Part I: Identification of O-H and C-H-related vibrational modes for D-glucose, maltose, cellobiose, and dextran by deuterium-substitution methods. Carbohydr. Res. 1971, 19, 297–310. [Google Scholar] [CrossRef]
- Wang, S.; Hu, Y.; Uzoejinwa, B.B.; Cao, B.; He, Z.; Wang, Q.; Xu, S. Pyrolysis mechanisms of typical seaweed polysaccharides. J. Anal. Appl. Pyrolysis 2017, 124, 373–383. [Google Scholar] [CrossRef]
- Herbst, W.; Hunger, K.; Wilker, G.; Ohleier, H.; Winter, R. Industrial Organic Pigments, 3rd ed.; Herbst, W., Hunger, K., Wilker, G., Ohleier, H., Eds.; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2004; ISBN 9783527602421. [Google Scholar]
- Teramoto, H.; Miyazawa, M. Molecular Orientation Behavior of Silk Sericin Film as Revealed by ATR Infrared Spectroscopy. Biomacromolecules 2005, 6, 2049–2057. [Google Scholar] [CrossRef]
- Orsini, S.; Parlanti, F.; Bonaduce, I. Analytical pyrolysis of proteins in samples from artistic and archaeological objects. J. Anal. Appl. Pyrolysis 2017, 124, 643–657. [Google Scholar] [CrossRef]
- Degano, I.; Sabatini, F.; Braccini, C.; Colombini, M.P. Triarylmethine dyes: Characterization of isomers using integrated mass spectrometry. Dye Pigments 2019, 160, 587–596. [Google Scholar] [CrossRef]
- Gessner, T.; Mayer, U. Triarylmethane and Diarylmethane Dyes. In Ullmann’s Encyclopedia of Industrial Chemistry; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2000. [Google Scholar]
- Tsuge, S.; Ohtani, H.; Watanabe, C. Pyrolysis-GC/MS Data Book of Synthetic Polymers; Elsevier: Amsterdam, The Netherlands, 2011; ISBN 9780444538925. [Google Scholar]
- Duran, A.; Perez-Maqueda, R.; Perez-Rodriguez, J.L. Degradation processes of historic metal threads used in some Spanish and Portuguese ornamentation pieces. J. Cult. Herit. 2019, 36, 135–142. [Google Scholar] [CrossRef]
- Láró, M.; Gál, T.; Tóth, A. The Characterization and Deterioration of Modern Metallic Threads. Stud. Conserv. 2000, 45, 95–105. [Google Scholar] [CrossRef]
- Asensio, R.C.; Moya, M.S.A.; de la Roja, J.M.; Gómez, M. Analytical characterization of polymers used in conservation and restoration by ATR-FTIR spectroscopy. Anal. Bioanal. Chem. 2009, 395, 2081–2096. [Google Scholar] [CrossRef] [PubMed]
- Vijayakumar, S.; Rajakumar, P.R. Infrared spectral analysis of waste pet samples. Int. Lett. Chem. Phys. Astron. 2012, 4, 58–65. [Google Scholar] [CrossRef]
- Dunn, T.B.T.-F.P. (Ed.) 22-Oriented Plastic Films; William Andrew Publishing: Oxford, UK, 2015; pp. 177–185. ISBN 978-0-323-26436-5. [Google Scholar]
- Tebelius, L.K.; Stetz, E.M.; Urban, M.W. Surface and interfacial fourier transform infrared spectroscopic studies of latexes. XIV. Surface phase separation in polystyrene/poly-n-butyl acrylate latex films. J. Appl. Polym. Sci. 1996, 62, 1887–1892. [Google Scholar] [CrossRef]
- Disperse Yellow 3. In Iarc Monographs of the Evaluation of Carcinogenic Risks to Humans; IARC: Lyon, France, 1990; Volume 48, pp. 149–159.
- Confortin, D.; Neevel, H.; Van Bommel, M.; Reissland, B. Crystal Violert: Study of the Degradation of an Early Synthetic Dye, Crystal Violet. J. Phys. Conf. Ser. 2010, 231, 197–201. [Google Scholar]
- Bonadio, S.; Cimò, M.; Triolo, L.; Ursino, F. Il restauro dell’effimero. Due costumi di Danilo Donati per i film Roma (1972) e Il Casanova di Federico Fellini (1976). Kermes 2020, 31, 216–227. [Google Scholar]

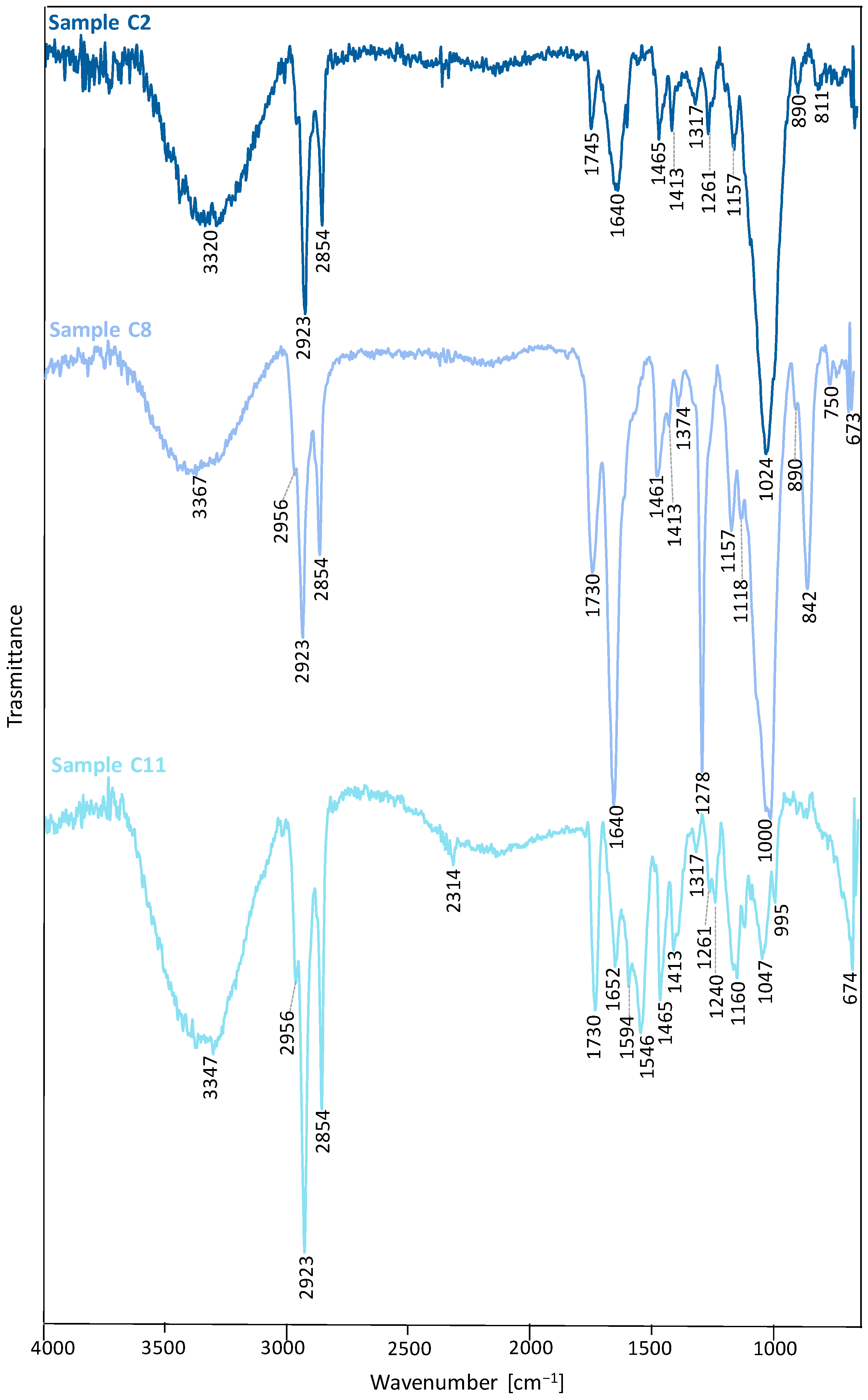
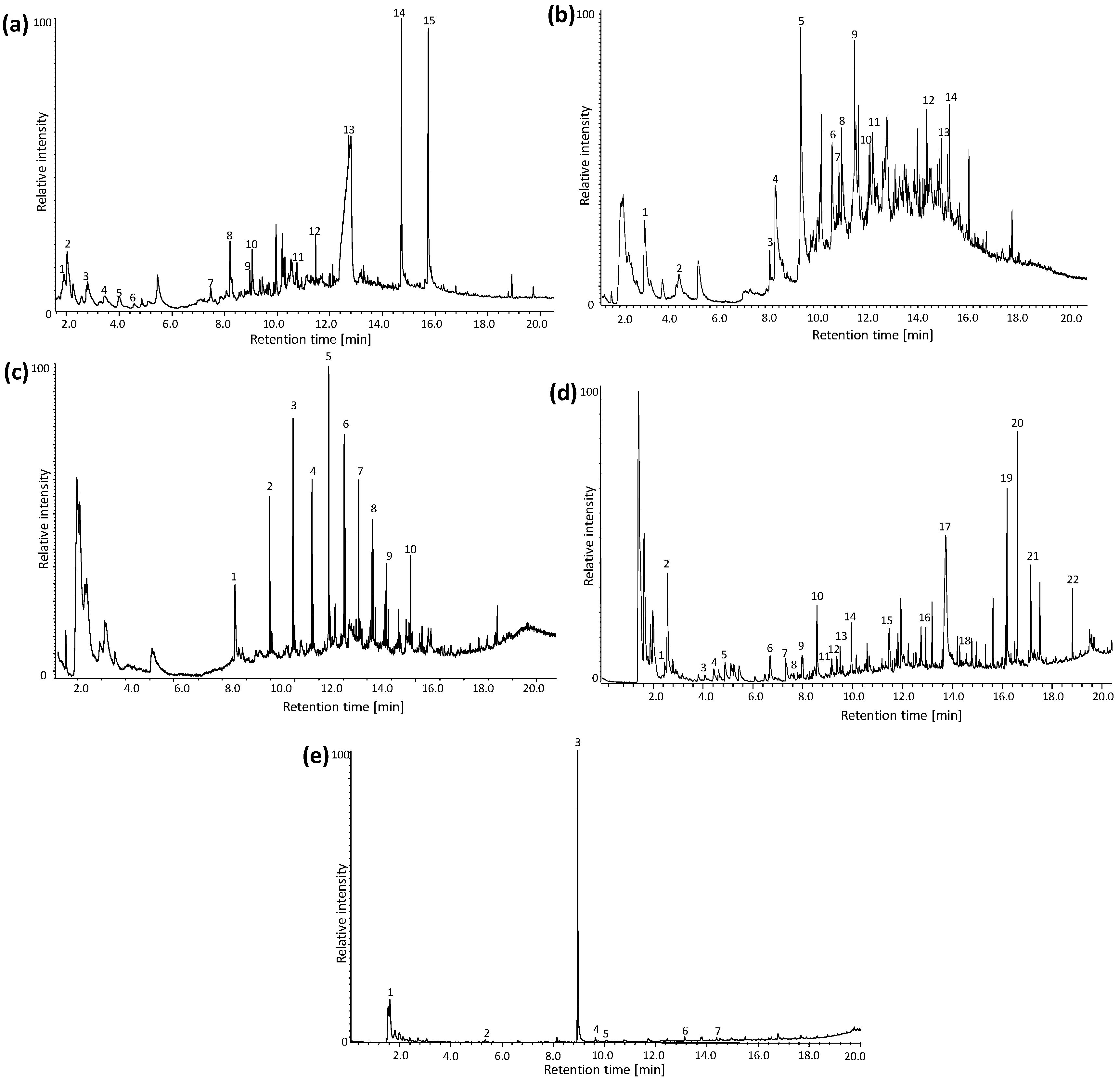
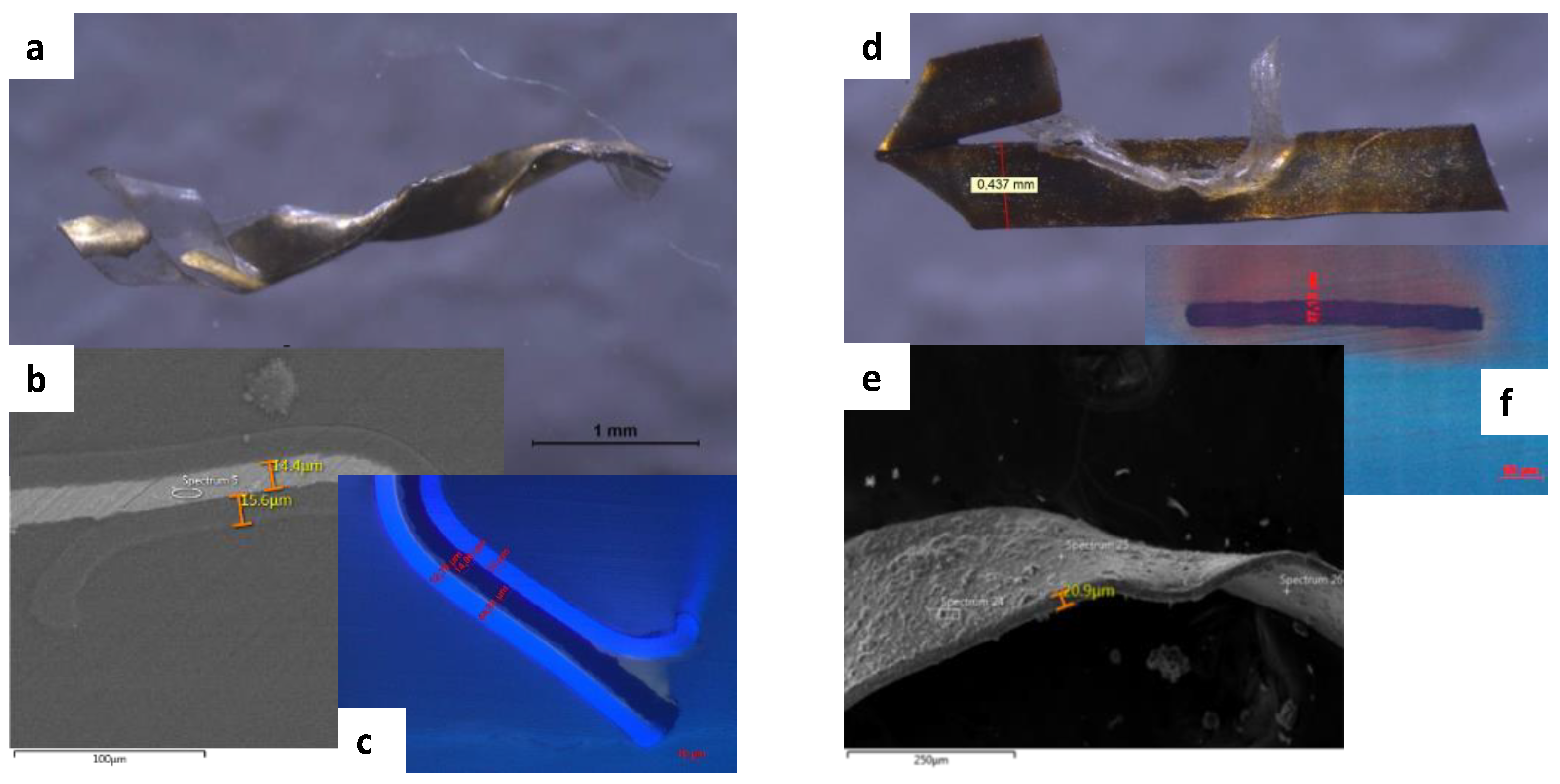

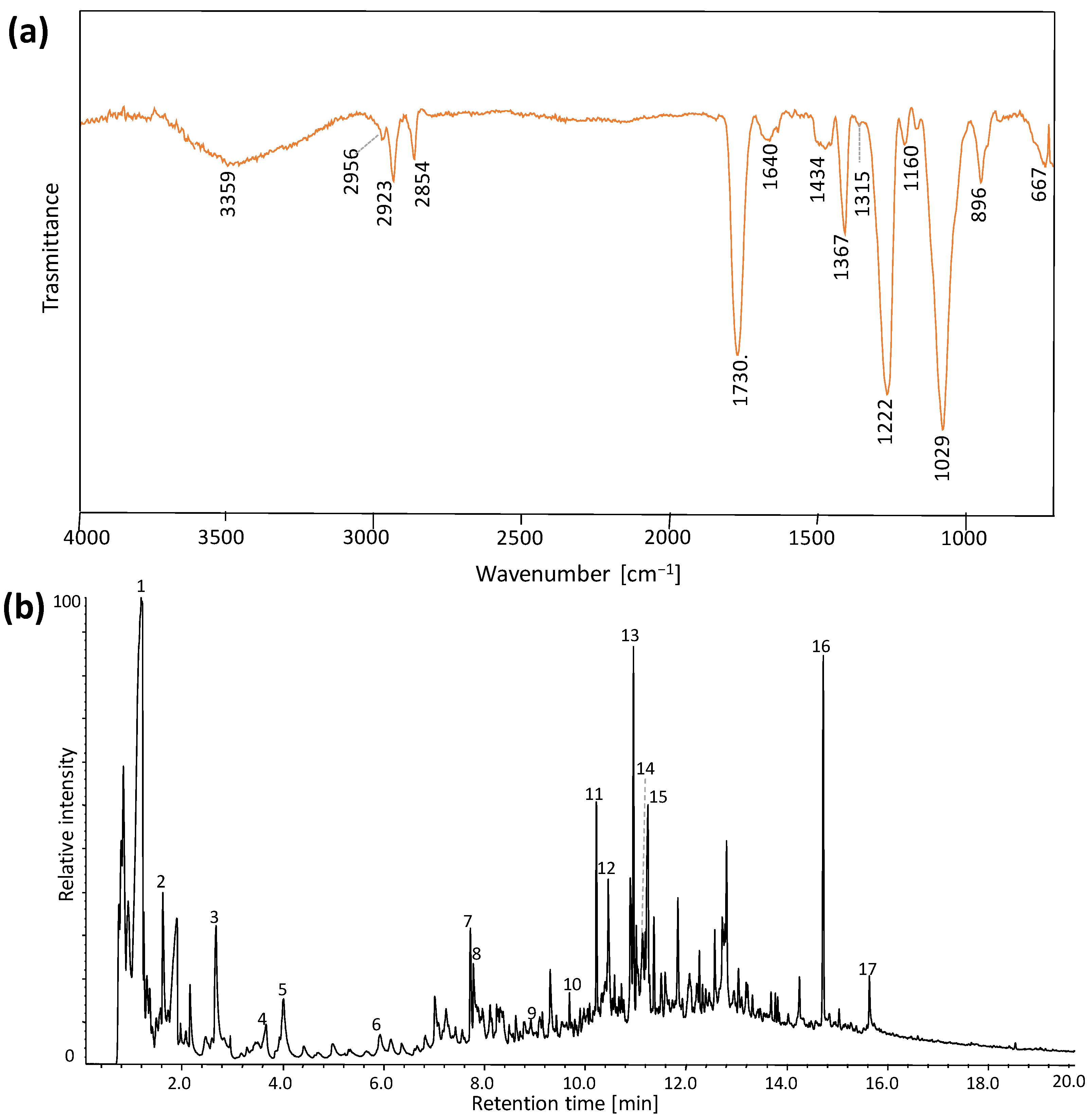
| Wavenumber (cm−1) | Vibrational Modes | Wavenumber (cm−1) | Vibrational Modes |
|---|---|---|---|
| Sample C2 | Sample C11 | ||
| 3320 | ν OH | 3347 | νOH |
| 2923 | νa CH2 | 2956, 2923 | νa CH2 |
| 2854 | νs CH2 | 2854 | νs CH2 |
| 1745 | ν C=O | 2314 | superior harmonic of NH |
| 1640 | δ OH (bound water) | 1730 | ν C=O |
| 1465, 1317 | ν C–C (aromatic ring) | 1652 | δ OH (bound water) |
| 1413 | δa CH2 (in-plane), δa C-O-H | 1594, 1546, 1465 | ν C–C (aromatic ring) |
| 1261, 1157 | ν C-O | 1413 | δ CH2 (in-plane, aromatic ring) |
| 1027 | δ CH (in-plane, aromatic ring) | 1317 | ν C-C |
| 890, 811 | δ CH (out-of-plane, aromatic ring) | 1261 | ν C-O |
| Sample C8 | 1240 | νa C-C-O | |
| 3367 | ν OH | 1160 | νa C-O-C |
| 2956, 2923 | νa CH2 | 1047 | ν C-O |
| 2854 | νs CH2 | 995 | δ CH (in-plane, aromatic ring) |
| 1730 | ν C=O | 674 | δ CH (out-of-plane, aromatic ring) |
| 1640 | δ OH (bound water) | ||
| 1461, 1413 | δa CH2 (in-plane) | ||
| 1374 | δ CH | ||
| 1278, 1157, 1118 | ν C-O | ||
| 995, 890, 842 | δ CH (out-of-plane, aromatic ring) | ||
| 750, 673 | ω C-H (aromatic ring) | ||
| 842 | δ C-O-H | ||
| 723 | ν C-O | ||
| # | tr (min) | Compound | Mass Spectra | |
|---|---|---|---|---|
| Sample C2 | 1 | 1.5 | 2,5-dimethylfuran | 96, 91, 81, 67, 53, 43, 39 |
| 2 | 1.9 | 5-methyl-2,3-dihydrofuran | 84, 69, 56, 41 | |
| 3 | 2.7 | 2-cyclopentenone | 82, 78, 54, 39, 28 | |
| 4 | 3.5 | Furfuryl alcohol | 98, 8170, 53, 41 | |
| 5 | 4.0 | 2-methylfuran | 96, 81, 67, 53, 39 | |
| 6 | 4.2 | 2-ethylfuran | 82, 53, 39, 27 | |
| 7 | 7.7 | Furfuryl acetate | 140, 98, 81, 52, 43 | |
| 8 | 8.4 | 2,4-dimethylfuran | 96, 81, 67, 53, 41, 39 | |
| 9 | 9.3 | 5-methylfurfural | 110, 95, 81, 53, 43, 39 | |
| 10 | 9.5 | 5-methyl-2(5H)-furanone | 98, 83, 69, 53, 41 | |
| 11 | 10.9 | 5-acetoxymethyl-2-furaldehyde | 168, 126, 109, 97, 79, 69, 53, 43 | |
| 12 | 11.7 | 2,5-dihydrofuran | 70, 51, 41, 39 | |
| 13 | 12.7 | Levoglucosan | 162, 98, 85, 73, 60, 57, 43, 31, 29 | |
| 14 | 14.7 | Palmitic acid | 256, 213, 129, 73, 60, 43 | |
| 15 | 15.7 | Stearic acid | 284, 241, 185, 129, 111, 97, 85, 73, 57, 43 | |
| Sample C5 | 1 | 2.7 | Benzene | 78, 63, 52, 39 |
| 2 | 4.6 | Toluene | 92, 65, 51, 39 | |
| 3 | 7.8 | Ethylbenzene | 106, 91, 77, 65, 51, 39 | |
| 4 | 8.1 | Styrene | 104, 89, 78, 63, 51, 39 | |
| 5 | 9.6 | Phenol | 94, 66, 55, 39 | |
| 6 | 10.4 | 2-methylphenol | 108, 90, 79, 63, 51, 40 | |
| 7 | 10.6 | 4-methylphenol | 108, 90, 77, 63, 51, 39 | |
| 8 | 10.7 | 3-methylphenol | 108, 90, 77, 63, 53, 41 | |
| 9 | 11.4 | 4-ethylphenol | 122, 107, 91, 77, 65, 51, 39 | |
| 10 | 12.3 | 3-vinylbenzene | 192, 177, 161, 151, 91, 73, 65 | |
| 11 | 12.4 | Indole | 117, 90, 63, 50, 39 | |
| 12 | 15.5 | DPK-1 | 154, 125, 86, 70, 55, 41 | |
| 13 | 15.2 | DPK-2 | 154, 125, 73, 60, 43 | |
| 14 | 15.4 | DPK-3 | 221, 194, 154, 125, 70, 41 | |
| Sample C7 | 1 | 7.5 | 1-decene | 140, 111, 97, 83, 70, 56, 41 |
| 2 | 9.0 | 1-undecene | 154, 139, 111, 97, 83, 70, 55, 41 | |
| 3 | 9.9 | 1-dodecene | 168, 153, 111, 97, 83, 69, 55 | |
| 4 | 10,8 | 1-tridecene | 182, 167, 154, 111, 97, 83, 69, 55, 41 | |
| 5 | 11.5 | 1-tetradecene | 196, 182, 167, 154, 111, 97, 83, 69, 55, 41, | |
| 6 | 12.1 | 1-hexadecene | 224, 210, 196, 182, 167, 154, 111, 97, 83, 69 55 41 | |
| 7 | 12.7 | 1-eptadecene | 238, 196, 182, 167, 154, 111, 97, 83, 69, 55, 41 | |
| 8 | 13.3 | 1-octadecene | 252, 224, 196, 182, 167, 154, 111, 97, 83, 69, 55, 41 | |
| 9 | 13.8 | 1-nonadecene | 266, 238, 196, 182, 167, 154, 111, 97, 83, 69, 55, 41 | |
| 10 | 14.9 | 1-undecene | 280, 252, 196, 182, 167, 154, 111, 97, 83, 69, 55, 41 | |
| Sample C8 | 1 | 2.7 | Benzene | 78, 63, 52, 39 |
| 2 | 2.8 | Hydroxyacetone | 74, 58, 43 | |
| 3 | 4.2 | 2-methylfuran | 82, 53, 39, 27 | |
| 4 | 4.6 | Toluene | 92, 65, 51, 39 | |
| 5 | 5.0 | Allyl propryl ether | 100, 71, 58, 41, 27 | |
| 6 | 6.8 | Furfural | 96, 67, 39 | |
| 7 | 7.7 | 1,2-dimethylbenzene | 106, 91, 77, 65, 51, 41 | |
| 8 | 7.8 | Furfuryl methyl ester | 112, 95, 81, 53, 39 | |
| 9 | 8.1 | Styrene | 104, 89, 78, 63, 51, 39 | |
| 10 | 8.4 | 2-methyl-2-cyclopentenone | 96, 81, 67, 53, 39 | |
| 11 | 8.7 | Furfuryl alcohol | 98, 81, 69, 53, 41, 38 | |
| 12 | 9.3 | 5-methylfurfural | 110, 95, 81, 53, 43, 39 | |
| 13 | 9.5 | 5-methyl-2(5H)-furanone | 98, 83, 69, 53, 41 | |
| 14 | 10.1 | 2-hydroxy-3-methyl-2-cyclopentenone | 112, 97, 83, 69, 55, 41 | |
| 15 | 11.7 | 2,5-dihydrofuran | 70, 51, 41, 39 | |
| 16 | 14.7 | Phthalic anhydride | 148, 104, 76, 61, 50, 38 | |
| 17 | 14.1 | Levoglucosan | 162, 98, 85, 73, 60, 57, 43, 31, 29 | |
| 18 | 14.7 | Diethyl phthalate | 222, 177, 149, 132, 121, 105, 93, 76, 65, 50, 45 | |
| 19 | 16.3 | Palmitic acid | 256, 213, 129, 73, 60, 43 | |
| 20 | 16.4 | Didodecyl phthalate | 502, 167, 149, 133, 123, 104, 97, 83, 76, 69, 57 | |
| 21 | 17.3 | Stearic acid | 284, 241, 185, 129, 111, 97, 85, 73, 57, 43 | |
| 22 | 14.7 | Bis(2-ethylhexyl) phthalate | 390, 279, 167, 149, 132, 113, 104, 83, 71, 57, 43 | |
| Sample C11 | 1 | 2.1 | 1-hexene | 84, 69, 56, 41 |
| 2 | 5.3 | 1-octene | 112, 83, 70, 55, 43 | |
| 3 | 9.0 | n-butyl methacrylate | 142, 127, 113, 99, 87, 69, 56 | |
| 4 | 9.6 | Decene | 168, 153, 140, 126, 111, 97, 83, 69 | |
| 5 | 9.9 | 1-dodecene | 140, 111, 97, 83, 70, 56, 41 | |
| 6 | 13.1 | 1-pentadecene | 210, 182, 154, 140, 125, 111, 97, 83, 69, 55, 41 | |
| 7 | 14.4 | 1-hexadecene | 224, 210, 196, 182, 167, 154, 111, 97, 83, 69 |
| Wavenumber (cm−1) | Vibrational Modes |
|---|---|
| 3359 | ν OH |
| 2956, 2923 | νa CH2 |
| 2852 | νs CH2 |
| 1730 | ν C=O |
| 1640 | δ OH (bound water) |
| 1434 | δ CH2 |
| 1367 | δ CH (in-plane, aromatic ring) |
| 1315 | ω CH2 |
| 1222, 1160 | νa C-O-C |
| 1029 | ν C-O, ν C-C and C-H (ring and side group) |
| 896 | ν C-C-H (deformation of aromatic ring) |
| 667 | δ C-OH (out-of-plane) |
| # | tr (min) | Compound | Mass Spectra |
|---|---|---|---|
| 1 | 1.2 | Acetic acid | 60, 45, 43 |
| 2 | 1.7 | 2-ciclopenten-1-one | 82, 78, 54, 39, 28 |
| 3 | 2.7 | 2-methylfuran | 82, 53, 39, 27 |
| 4 | 3.6 | Acetoxy-2-propanone | 116, 86, 73, 57 |
| 5 | 4.0 | 4-cyclopentene-1,3-dione | 96, 68, 54 |
| 6 | 5.9 | 1,3-cyclopentenedione | 93, 69, 55 |
| 7 | 7.7 | 2-Furfuryl-acetate | 140, 98, 81, 52 |
| 8 | 7.8 | Phenol | 94, 66, 55, 39 |
| 9 | 9.3 | 5-methylfurfural | 110, 95, 81, 53, 43, 39 |
| 10 | 9.7 | 1H-imidazole-2-methanol | 98, 81, 69, 53 |
| 11 | 10.2 | 2,5-dimethylfuran | 96, 81, 67, 53 |
| 12 | 10.5 | 1,2-benzenediol | 110, 92, 64 |
| 13 | 10.9 | 5-acetoxymethyl-2-furaldehyde | 168, 126, 109, 97, 79, 69, 53, 43 |
| 14 | 11.1 | Phthalic anhydride | 148, 104, 76, 50 |
| 15 | 11.3 | Dihydro-5-propyl-furanone | 110, 85 |
| 16 | 14.2 | Dibutyl phthalate | 278, 149, 132, 120, 106, 91, 77, 65, 56, 50, 41 |
| 17 | 16.0 | Stearic acid | 284, 241, 185, 129, 111, 97, 85, 73, 57, 43 |
| Sample | Color | Sampling Location | Fabrics or Varnish/Support for Metal Thread | Identified Dyes or Metal Foil Constituents | |
|---|---|---|---|---|---|
| Il Casanova | C1 | Yellow | Warp | Viscose | DY28, Crystal Violet (traces) |
| C2 | Black | Lace applied on the neckline | Viscose | Dioxazine family | |
| C3 | Yellow | Volant applied on the sleeve | Cotton | DY28, Crystal Violet (traces) | |
| C4 | Yellow | Corset | Cotton | DY28, Crystal Violet (traces) | |
| C5 | Black | Decoration of the hat | Silk | Cristal Violet | |
| C7 | Yellow | Lace applied on the sleeve | Polyethylene | DY28 | |
| C8 | Golden lamé | Right sleeve of the corset | Polyester (around the metallic foil) + viscose (background) | Al | |
| C9 | Golden foil wrapped around a black thread (C2) | Lace applied on the right side of the neckline | Polyester (around the metallic foil) + viscose (core) | n.a. | |
| C10 | Golden thread interwoven with a yellow thread | Volant applied on the left sleeve | Polyester (around the metallic foil) | Al | |
| C11 | Golden lamé | Corset decoration on the right side | Acrylic (on top of the metallic foil) | Zn, Cu (Cl) | |
| C12 | Lace made of golden threads sewn by viscose | Trimming on the left side of the neckline | Polyester (around the metallic foil) + viscose thread | Al | |
| C13 | Golden foil thread interwoven with a yellow thread (C7) | Lace applied on the sleeve | Polyester (around the metallic foil) | n.a. | |
| C14 | Golden foil interwoven with black thread (C5) | Decoration of the top part of the hat | Acrylic (on top of the metallic foil) | Ag, Cu (Cl) | |
| Roma | T | Yellow | Lining of the tunic | Viscose | DY28, C28H20N4O7S4, C28H18N4O6S4 |
| P | Yellow | Inside trimming of the chasuble | Bemberg | DY28, PY1, DY3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sabatini, F.; La Nasa, J.; Guerrini, C.; Modugno, F.; Bonadio, S.; Ursino, F.; Tosini, I.; Colombini, M.P.; Degano, I. On the Set of Fellini’s Movies: Investigating and Preserving Multi-Material Stage Costumes Exploiting Spectroscopic and Mass Spectrometric Techniques. Appl. Sci. 2021, 11, 2954. https://doi.org/10.3390/app11072954
Sabatini F, La Nasa J, Guerrini C, Modugno F, Bonadio S, Ursino F, Tosini I, Colombini MP, Degano I. On the Set of Fellini’s Movies: Investigating and Preserving Multi-Material Stage Costumes Exploiting Spectroscopic and Mass Spectrometric Techniques. Applied Sciences. 2021; 11(7):2954. https://doi.org/10.3390/app11072954
Chicago/Turabian StyleSabatini, Francesca, Jacopo La Nasa, Camilla Guerrini, Francesca Modugno, Sara Bonadio, Federica Ursino, Isetta Tosini, Maria Perla Colombini, and Ilaria Degano. 2021. "On the Set of Fellini’s Movies: Investigating and Preserving Multi-Material Stage Costumes Exploiting Spectroscopic and Mass Spectrometric Techniques" Applied Sciences 11, no. 7: 2954. https://doi.org/10.3390/app11072954
APA StyleSabatini, F., La Nasa, J., Guerrini, C., Modugno, F., Bonadio, S., Ursino, F., Tosini, I., Colombini, M. P., & Degano, I. (2021). On the Set of Fellini’s Movies: Investigating and Preserving Multi-Material Stage Costumes Exploiting Spectroscopic and Mass Spectrometric Techniques. Applied Sciences, 11(7), 2954. https://doi.org/10.3390/app11072954









