Development of Autonomous Robot Osteotomy for Mandibular Ramal Bone Harvest and Evaluation of Its Accuracy: A Phantom Mandible-Based Trial
Abstract
Featured Application
Abstract
1. Introduction
2. Materials and Methods
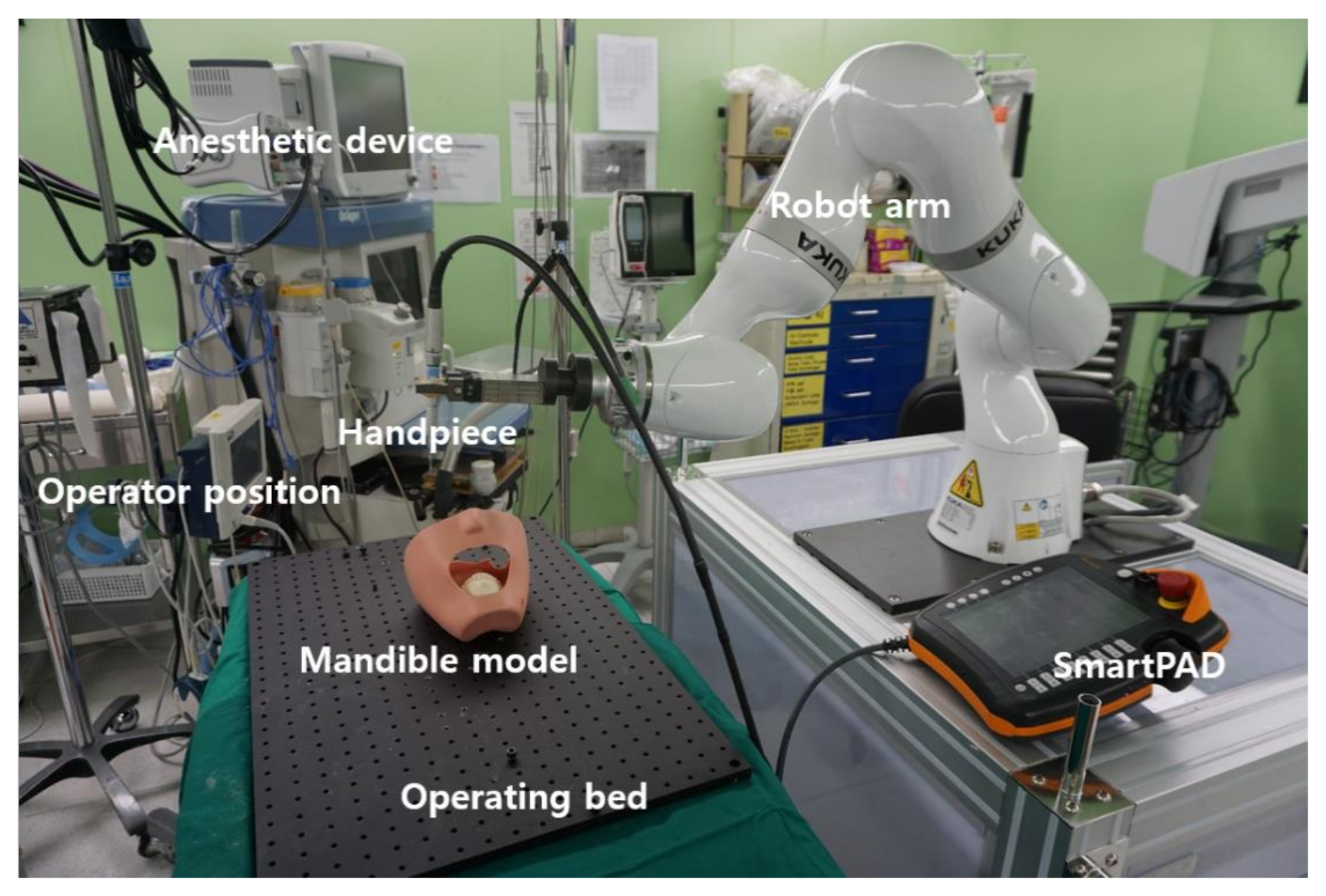
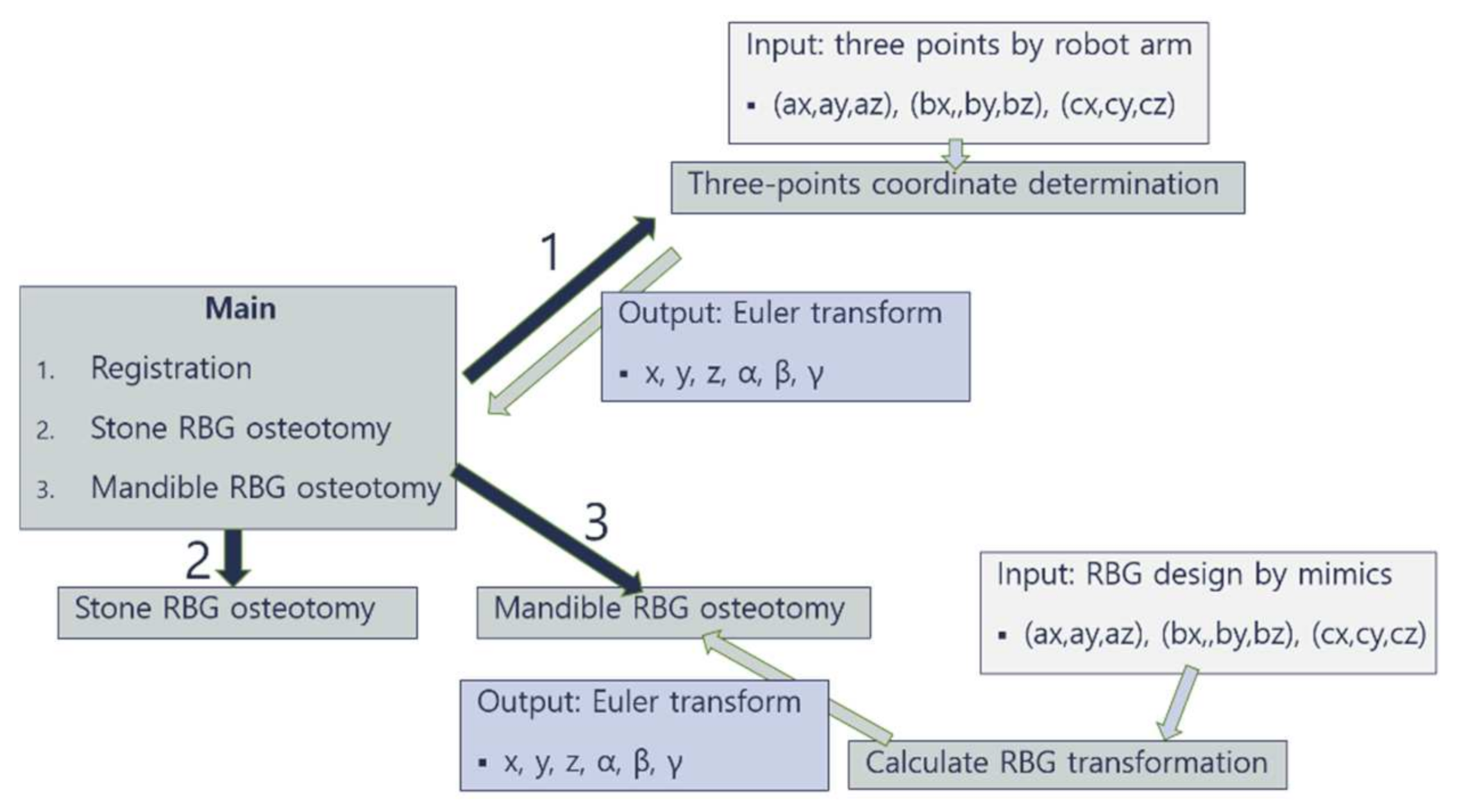
2.1. Overview of Robotic System
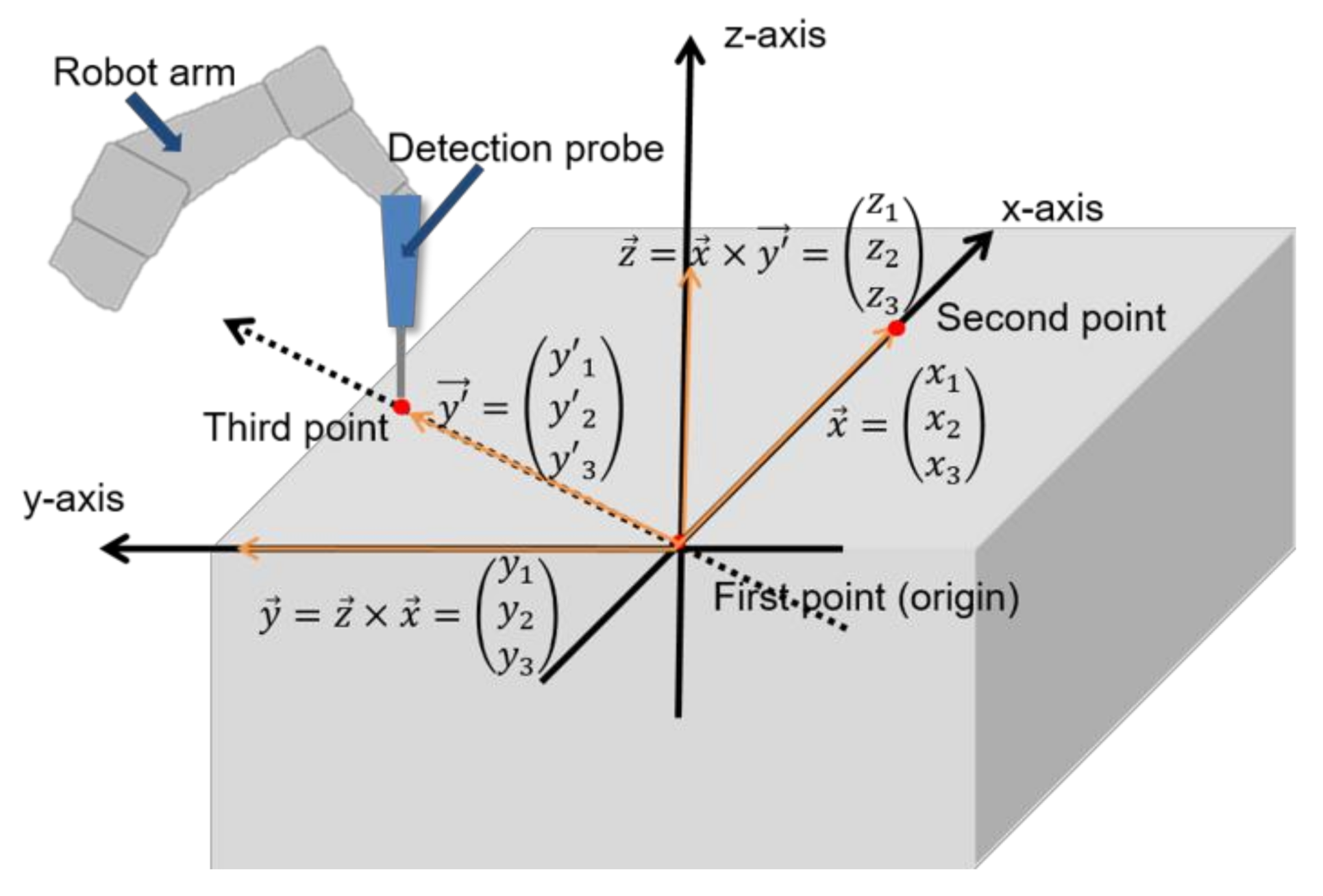
2.2. Three-Points Direct Coordinate Determination
2.3. Autonomous Robot Osteotomy on Stone Model
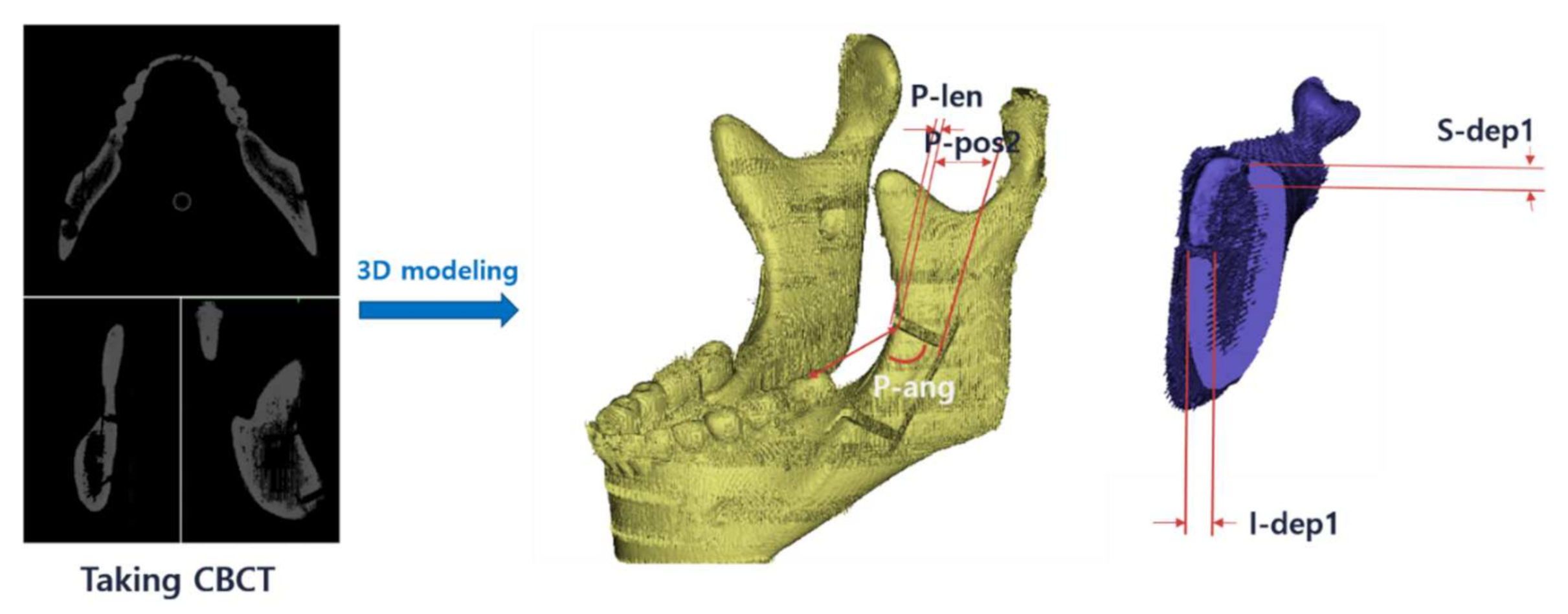
2.3.1. Study Design
2.3.2. Accuracy Evaluation
2.4. Comparison of Robot and Manual RBG Osteotomy on Mandible
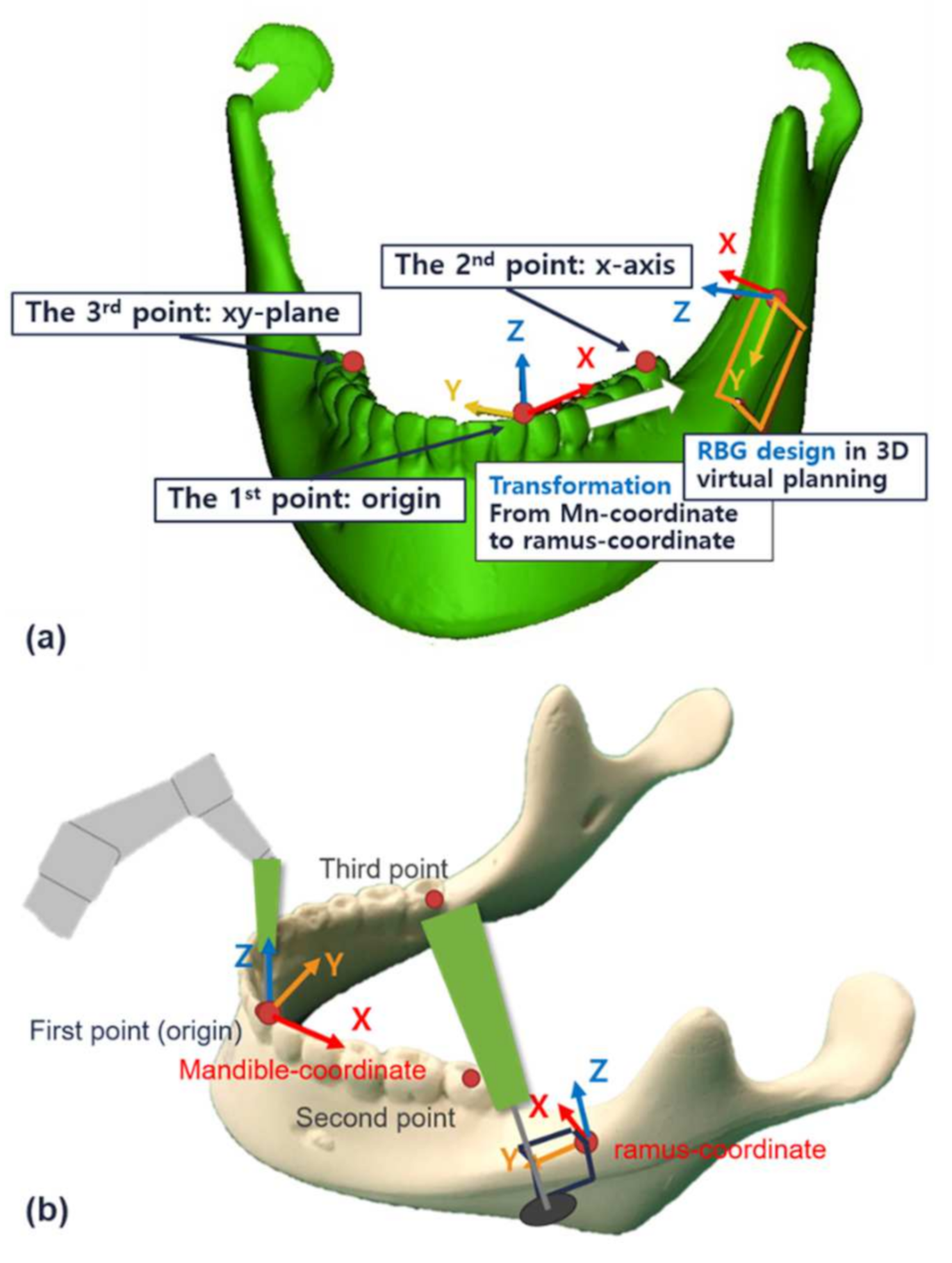
2.4.1. Design for 3D Virtual Planning
2.4.2. Comparison and Evaluation
3. Results
3.1. Flow Diagram
3.2. Autonomous Robot Osteotomy on Stone Model and Its Accuracy
3.3. Comparison between the Robotic and Manual RBG Osteotomy on Mandible
3.4. Ergonomics and Safety
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Moustris, G.P.; Hiridis, S.C.; Deliparaschos, K.M.; Konstantinidis, K.M. Evolution of autonomous and semi-autonomous robotic surgical systems: A review of the literature. Int. J. Med. Robot. 2011, 7, 375–392. [Google Scholar] [CrossRef] [PubMed]
- Korb, W.; Marmulla, R.; Raczkowsky, J.; Mühling, J.; Hassfeld, S. Robots in the operating theatre—Chances and challenges. Int. J. Oral Maxillofac. Surg. 2004, 33, 721–732. [Google Scholar] [CrossRef] [PubMed]
- Ballantyne, G.H. Robotic surgery, telerobotic surgery, telepresence, and telementoring. Surg. Endosc. Other Interv. Tech. 2002, 16, 1389–1402. [Google Scholar] [CrossRef] [PubMed]
- Clavero, J.; Lundgren, S. Ramus or Chin Grafts for Maxillary Sinus Inlay and Local Onlay Augmentation: Comparison of Donor Site Morbidity and Complications. Clin. Implant Dent. Relat. Res. 2003, 5, 154–160. [Google Scholar] [CrossRef] [PubMed]
- Kwon, I.J.; Lee, B.H.; Eo, M.Y.; Kim, S.M.; Lee, J.H.; Lee, S.K. Pathologic mandibular fracture after biting crab shells following ramal bone graft. Dent. Traumatol. 2016, 32. [Google Scholar] [CrossRef]
- Zhu, J.H.; Deng, J.; Liu, X.J.; Wang, J.; Guo, Y.X.; Guo, C.B. Prospects of Robot-Assisted Mandibular Reconstruction with Fibula Flap: Comparison with a Computer-Assisted Navigation System and Freehand Technique. J. Reconstr. Microsurg. 2016, 32, 661–669. [Google Scholar] [CrossRef]
- Gui, H.; Zhang, S.; Luan, N.; Lin, Y.; Shen, S.G.F.; Bautista, J.S. A novel system for navigation-and robot-assisted craniofacial surgery: Establishment of the principle prototype. J. Craniofac. Surg. 2015, 26, e746–e749. [Google Scholar] [CrossRef]
- Augello, M.; Deibel, W.; Nuss, K.; Cattin, P.; Jürgens, P. Comparative microstructural analysis of bone osteotomies after cutting by computer-assisted robot-guided laser osteotome and piezoelectric osteotome: An in vivo animal study. Lasers Med. Sci. 2018, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Suero, E.M.; Westphal, R.; Zaremba, D.; Citak, M.; Hawi, N.; Citak, M.; Stuebig, T.; Krettek, C.; Liodakis, E. Robotic guided waterjet cutting technique for high tibial dome osteotomy: A pilot study. Int. J. Med. Robot. Comput. Assist. Surg. 2017, 13, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Baek, K.W.; Deibel, W.; Marinov, D.; Griessen, M.; Bruno, A.; Zeilhofer, H.F.; Cattin, P.; Juergens, P. Clinical applicability of robot-guided contact-free laser osteotomy in cranio-maxillo-facial surgery: In-vitro simulation and in-vivo surgery in minipig mandibles. Br. J. Oral Maxillofac. Surg. 2015, 53, 976–981. [Google Scholar] [CrossRef] [PubMed]
- Shoemake, K. Euler Angle Conversion. In Graphics Gems IV; Academic Press: Cambridge, MA, USA, 1994; pp. 222–229. [Google Scholar]
- Lee, S.J.; Woo, S.Y.; Huh, K.H.; Lee, S.S.; Heo, M.S.; Choi, S.C.; Han, J.J.; Yang, H.J.; Hwang, S.J.; Yi, W.J. Virtual skeletal complex model- and landmark-guided orthognathic surgery system. J. Cranio Maxillofac. Surg. 2016, 44, 557–568. [Google Scholar] [CrossRef]
- Ho, A.L.; Muftuoglu, Y.; Pendharkar, A.V.; Sussman, E.S.; Porter, B.E.; Halpern, C.H.; Grant, G.A. Robot-guided pediatric stereoelectroencephalography: Single-institution experience. J. Neurosurg. Pediatr. 2018, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Taylor, R.H.; Mittelstadt, B.D.; Paul, H.A.; Hanson, W.; Kazanzides, P.; Zuhars, J.F.; Williamson, B.; Musits, B.L.; Glassman, E.; Bargar, W.L. An image-directed robotic system for precise orthopaedic surgery. IEEE Trans. Robot. Autom. 1994, 10, 261–275. [Google Scholar] [CrossRef]
- Kavanagh, K.T. Applications of image-directed robotics in otolaryngologic surgery. Laryngoscope 1994, 104, 283–293. [Google Scholar] [CrossRef]
- Lueth, T.C.; Hein, A.; Albrecht, J.; Demirtas, M.; Zachow, S.; Heissler, E.; Klein, M.; Menneking, H.; Hommel, G.; Bier, J. A surgical robot system for maxillofacial surgery. In Proceedings of the IECON’98 24th Annual Conference of the IEEE Industrial Electronics Society, Aachen, Germany, 31 August–4 September 1998; Volume 4, pp. 2470–2475. [Google Scholar]
- Yang, G.-Z.; Cambias, J.; Cleary, K.; Daimler, E.; Drake, J.; Dupont, P.E.; Hata, N.; Kazanzides, P.; Martel, S.; Patel, R.V.; et al. Medical robotics—Regulatory, ethical, and legal considerations for increasing levels of autonomy. Sci. Robot. 2017, 2, eaam8638. [Google Scholar] [CrossRef] [PubMed]
- Tatullo, M.; Marrelli, M.; Falisi, G.; Rastelli, C.; Palmieri, F.; Gargari, M.; Zavan, B.; Paduano, F.; Benagiano, V. Mechanical influence of tissue culture plates and extracellular matrix on mesenchymal stem cell behavior: A topical review. Int. J. Immunopathol. Pharmacol. 2016, 29, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Inchingolo, F.; Tatullo, M.; Abenavoli, F.M.; Marrelli, M.; Inchingolo, A.D.; Gentile, M.; Inchingolo, A.M.; Dipalma, G. Non-syndromic multiple supernumerary teeth in a family unit with a normal karyotype: Case report. Int. J. Med. Sci. 2010, 7, 378–384. [Google Scholar] [CrossRef] [PubMed]
- Jo, H.-J.; Kim, H.-Y.; Kang, D.-C.; Leem, D.-H.; Baek, J.-A.; Ko, S.-O. A clinical study of inferior alveolar nerve damage caused by Carnoy’s solution used as a complementary therapeutic agent in a cystic lesion. Maxillofac. Plast. Reconstr. Surg. 2020, 42. [Google Scholar] [CrossRef] [PubMed]


| Model RBG Osteotomy (n = 20) | Error (Mean ± SD) | ||
|---|---|---|---|
| Posterior cut | Position (mm) | RMS | 0.85 ± 0.28 |
| Angle (Degree) | 1.10 ± 1.24 | ||
| Length (mm) | Top | 0.71 ± 0.27 | |
| Bottom | 0.93 ± 0.35 | ||
| Anterior cut | Position (mm) | RMS | 1.16 ± 0.55 |
| Angle (Degree) | 1.54 ± 1.60 | ||
| Length (mm) | Top | 0.74 ± 0.39 | |
| Bottom | 0.86 ± 0.33 | ||
| Inferior disc cut | Position (mm) | RMS | 1.14 ± 0.48 |
| Angle (Degree) | Anterior | 1.00 ± 0.96 | |
| Posterior | 0.67 ± 0.74 | ||
| Depth (mm) | Anterior | 0.75 ± 0.30 | |
| middle | 0.69 ± 0.42 | ||
| Posterior | 0.76 ± 0.30 | ||
| Superior disc cut | Position (mm) | RMS | 0.68 ± 0.36 |
| Angle (Degree) | Anterior | 1.95 ± 1.61 | |
| Posterior | 1.32 ± 1.49 | ||
| Depth (mm) | Anterior | 1.81 ± 0.68 | |
| middle | 1.71 ± 0.66 | ||
| Posterior | 1.45 ± 0.82 | ||
| Category | Error (Mean ± SD) | |
|---|---|---|
| Position (mm) | RMS | 0.93 ± 0.45 |
| Length (mm) | 0.81 ± 0.34 | |
| Angle (Degree) | 1.26 ± 1.35 | |
| Depth (mm) | 1.19 ± 0.73 | |
| p value | 0.000 | |
| Post hoc (p < 0.05) | Position-Angle Position-Depth Length-Angle Length-Depth |
| Errors of Robot Surgery | Errors of Hand Surgery | |||||||
|---|---|---|---|---|---|---|---|---|
| Cut | Category | 6 mm Disc | 20 mm Disc | p Value | 6 mm Disc | 20 mm Disc | p Value | |
| Post. cut | Position (mm) | RMS | 0.65 ± 0.17 | 0.59 ± 0.22 | 0.602 | 1.93 ± 0.37 | 1.60 ± 0.51 | 0.347 |
| variance | 0.03 | 0.05 | 0.13 | 0.26 | ||||
| Angle (°) | Mean ± SD | 0.72 ± 0.50 | 1.81 ± 1.29 | 0.754 | 6.13 ± 3.75 | 8.10 ± 2.67 | 0.057 | |
| Variance | 0.25 | 1.67 | 14.07 | 7.11 | ||||
| Length (mm) | Mean ± SD | 0.40 ± 0.14 | 0.33 ± 0.20 | 0.599 | 0.71 ± 0.26 | 0.91 ± 0.44 | 0.602 | |
| Variance | 0.02 | 0.04 | 0.07 | 0.19 | ||||
| Ant. cut | Position (mm) | RMS | 1.00 ± 0.24 | 1.28 ± 0.22 | 0.251 | 2.03 ± 0.54 | 2.53 ± 0.42 | 0.347 |
| Variance | 0.06 | 0.05 | 0.29 | 0.17 | ||||
| Angle (°) | Mean ± SD | 0.93 ± 0.62 | 1.17 ± 0.69 | 0.117 | 7.15 ± 3.15 | 1.74 ± 1.46 | 0.602 | |
| Variance | 0.38 | 0.47 | 9.90 | 2.14 | ||||
| Length (mm) | Mean ± SD | 0.40 ± 0.24 | 0.29 ± 0.13 | 0.347 | 0.39 ± 0.27 | 0.48 ± 0.24 | 0.463 | |
| Variance | 0.06 | 0.02 | 0.07 | 0.06 | ||||
| Inf. cut | Position (mm) | RMS | 0.36 ± 0.32 | 0.52 ± 0.18 | 0.602 | 2.29 ± 0.51 | 2.65 ± 0.62 | 0.754 |
| Variance | 0.10 | 0.03 | 0.26 | 0.38 | ||||
| Angle (°) | Mean ± SD | 0.29 ± 0.65 | 1.58 ± 0.96 | 0.753 | 7.58 ± 2.99 | 7.23 ± 3.55 | 0.754 | |
| Variance | 0.42 | 0.92 | 8.94 | 12.63 | ||||
| Depth (mm) | Mean ± SD | 0.36 ± 0.35 | 0.45 ± 0.30 | 0.530 | 0.32 ± 0.24 | 0.51 ± 0.33 | 0.251 | |
| Variance | 0.12 | 0.09 | 0.06 | 0.11 | ||||
| Sup. cut | Position (mm) | RMS | 0.86 ± 0.45 | 0.53 ± 0.31 | 0.602 | 0.79 ± 0.20 | 1.09 ± 0.41 | 0.465 |
| Variance | 0.20 | 0.09 | 0.04 | 0.16 | ||||
| Angle (°) | Mean ± SD | 1.45 ± 1.08 | 1.30 ± 1.10 | 0.917 | 4.09 ± 2.80 | 5.29 ± 2.52 | 0.602 | |
| Variance | 1.17 | 1.20 | 7.83 | 6.35 | ||||
| Depth (mm) | Mean ± SD | 0.44 ± 0.21 | 1.13 ± 0.44 | 0.009 | 0.30 ± 0.25 | 0.46 ± 0.36 | 0.173 | |
| Variance | 0.05 | 0.19 | 0.06 | 0.13 | ||||
| Robot Surgery | Hand Surgery | p Value | ||
|---|---|---|---|---|
| Position | RMS | 0.70 ± 0.34 | 1.83 ± 0.65 | <0.001 |
| variance | 0.11 | 0.43 | <0.001 | |
| Length | Mean ± SD | 0.35 ± 0.19 | 0.62 ± 0.37 | 0.009 |
| variance | 0.03 | 0.14 | 0.004 | |
| Angle | Mean ± SD | 1.32 ± 0.96 | 5.96 ± 3.47 | <0.001 |
| variance | 0.93 | 12.08 | <0.001 | |
| Depth | Mean ± SD | 0.59 ± 0.46 | 0.40 ± 0.31 | 0.030 |
| variance | 0.21 | 0.10 | 0.014 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kwon, I.J.; Kim, S.M.; Hwang, S.J. Development of Autonomous Robot Osteotomy for Mandibular Ramal Bone Harvest and Evaluation of Its Accuracy: A Phantom Mandible-Based Trial. Appl. Sci. 2021, 11, 2885. https://doi.org/10.3390/app11062885
Kwon IJ, Kim SM, Hwang SJ. Development of Autonomous Robot Osteotomy for Mandibular Ramal Bone Harvest and Evaluation of Its Accuracy: A Phantom Mandible-Based Trial. Applied Sciences. 2021; 11(6):2885. https://doi.org/10.3390/app11062885
Chicago/Turabian StyleKwon, Ik Jae, Soung Min Kim, and Soon Jung Hwang. 2021. "Development of Autonomous Robot Osteotomy for Mandibular Ramal Bone Harvest and Evaluation of Its Accuracy: A Phantom Mandible-Based Trial" Applied Sciences 11, no. 6: 2885. https://doi.org/10.3390/app11062885
APA StyleKwon, I. J., Kim, S. M., & Hwang, S. J. (2021). Development of Autonomous Robot Osteotomy for Mandibular Ramal Bone Harvest and Evaluation of Its Accuracy: A Phantom Mandible-Based Trial. Applied Sciences, 11(6), 2885. https://doi.org/10.3390/app11062885







