Electrospun PVP/PVA Nanofiber Mat as a Novel Potential Transdermal Drug-Delivery System for Buprenorphine: A Solution Needed for Pain Management
Abstract
1. Introduction
2. Experimental
2.1. Materials
2.2. Apparatus and Characterization
2.3. The Preparation of Electrospinning Solutions and Procedure
2.3.1. The Fabrication of Poly(Vinyl Pyrrolidone) (PVP) Nanofiber Mat
2.3.2. The Fabrication of Buprenorphine Hydrochloride (Bup)/PVP Nanofiber Mat
2.3.3. The Fabrication of PVP/Poly(Vinyl Alcohol) (PVA) Nanofibers Mat
2.3.4. The Fabrication of Bup/PVP/PVA Nanofibers Mat
2.4. The Preparation of Cross-Linked Bup/PVP/PVA Nanofiber Mat
3. Dissolution Study
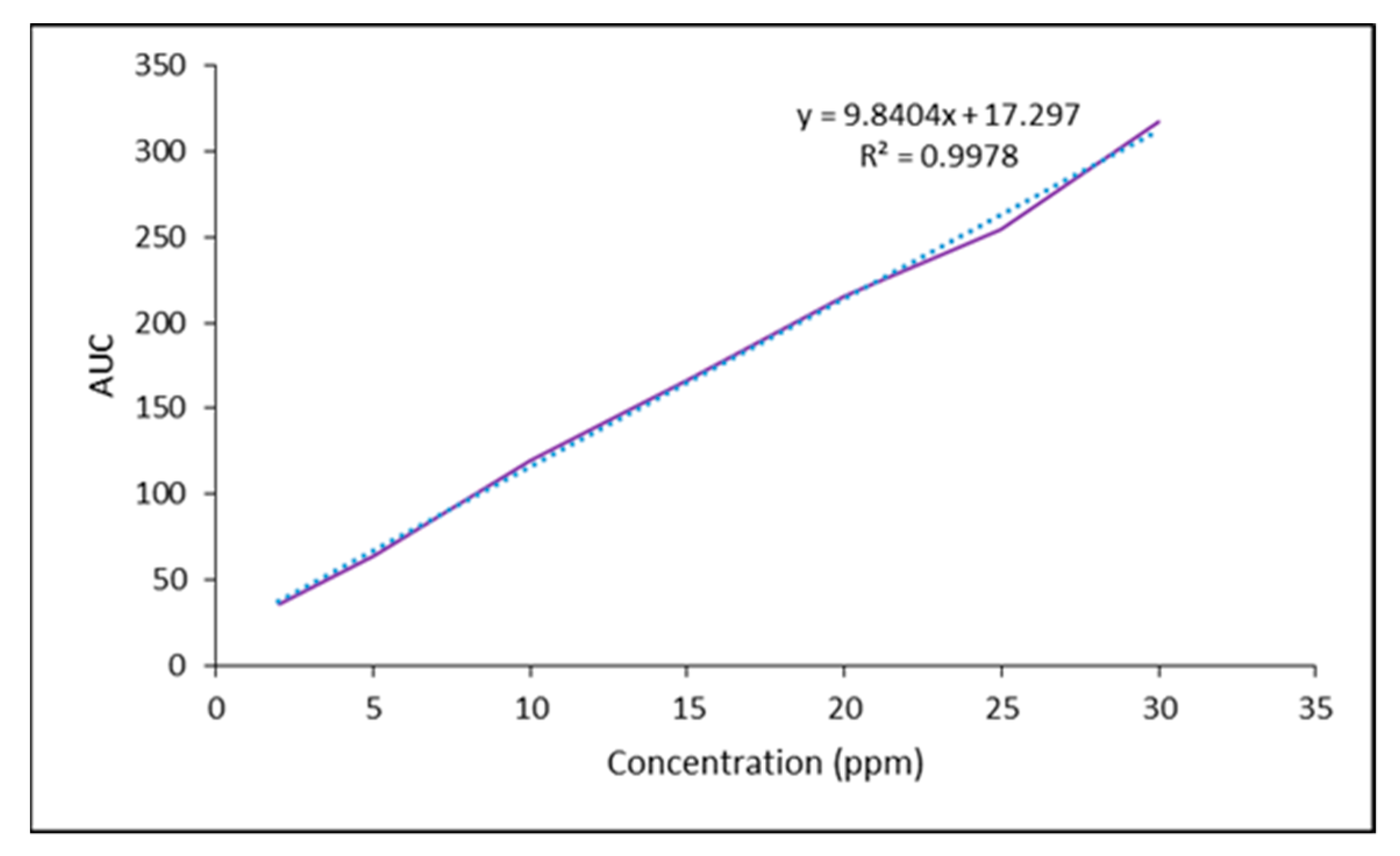
3.1. The Preparation of Bup Standard Solutions
3.2. High-Performance Liquid Chromatography
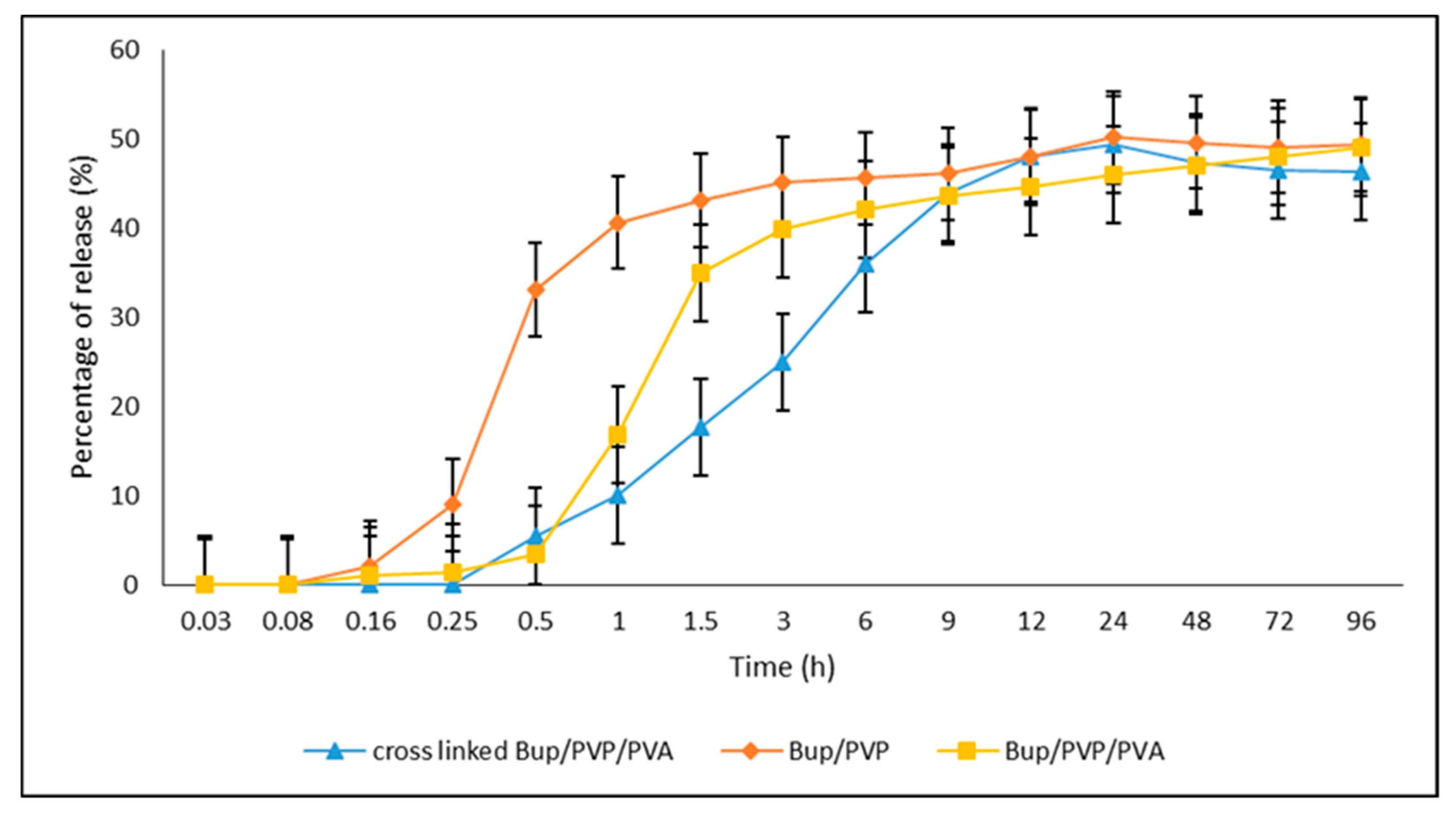
3.3. The In Vitro Dissolution Measurement of Bup/PVP, Bup/PVP/PVA and Cross-Linked Bup/PVP/PVA Nanofibers Mats
4. Results and Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Braghirolli, D.I.; Steffens, D.; Pranke, P. Electrospinning for regenerative medicine: A review of the main topics. Drug Discov. Today 2014, 19, 743–753. [Google Scholar] [CrossRef]
- Kost, J.; Langer, R. Responsive polymeric delivery systems. Adv. Drug Deliv. Rev. 2012, 64, 327–341. [Google Scholar] [CrossRef]
- Patil, S.B.; Inamdar, S.Z.; Reddy, K.R.; Raghu, A.V.; Soni, S.K.; Kulkarni, R.V. Novel biocompatible poly(acrylamide)-grafted-dextran hydrogels: Synthesis, characterization and biomedical applications. J. Microbiol. Methods 2019, 159, 200–210. [Google Scholar] [CrossRef] [PubMed]
- Patila, S.B.; Inamdara, S.Z.; Dasb, K.K.; Akamanchic, K.G.; Patild, A.V.; Inamadare, A.C.; Reddyf, K.R.; Raghug, A.V.; Kulkarnia, R.V. Tailor-made electrically-responsive poly(acrylamide)-graft-pullulan copolymer based transdermal drug delivery sys-tems: Synthesis, characterization, in-vitro and ex-vivo evaluation. J. Drug Deliv. Sci. Technol. 2020, 56, 101525. [Google Scholar] [CrossRef]
- Coelho, D.S.; Veleirinho, B.; Alberti, T.; Maestri, A.; Yunes, R.; Dias, P.F.; Maraschin, M. Electrospinning Technology: De-Signing Nanofibers toward Wound Healing Application; Intech Open: London, UK, 2018. [Google Scholar]
- Ashammakhi, N.; Ndreu, A.; Yang, Y.; Ylikauppila, H.; Nikkola, L. Nanofiber-based scaffolds for tissue engineering. Eur. J. Plast. Surg. 2012, 35, 135–149. [Google Scholar] [CrossRef]
- Schnell, E.; Klinkhammer, K.; Balzer, S.; Brook, G.; Klee, D.; Dalton, P.; Mey, J. Guidance of glial cell migration and axonal growth on electrospun nanofibers of poly-epsilon-caprolactone and a collagen/poly-epsilon-caprolactone blend. Biomaterials 2007, 28, 3012. [Google Scholar] [CrossRef]
- Großerhode, C.; Wehlage, D.; Grothe, T.; Grimmelsmann, N.; Fuchs, S.; Hartmann, J.; Mazur, P.; Reschke, V.; Siemens, H.; Rattenholl, A.; et al. Investigation of microalgae growth on electrospun nanofiber mats. AIMS Environ. Sci. 2017, 4, 376–385. [Google Scholar] [CrossRef]
- Pawłowska, S.; Rinoldi, C.; Nakielski, P.; Ziai, Y.; Urbanek, O.; Li, X.; Kowalewski, T.A.; Ding, B.; Pierin, F. Ultraviolet Light-Assisted Electrospinning of Core–Shell Fully Cross-Linked P(NIPAAm-co-NIPMAAm) Hydrogel-Based Nanofibers for Thermal-ly Induced Drug Delivery Self-Regulation. Adv. Mater. Interfaces 2020, 7, 2000247. [Google Scholar] [CrossRef]
- Nakielski, P.; Pawłowska, S.; Rinoldi, C.; Ziai, Y.; De Sio, L.; Urbanek, O.; Zembrzycki, K.; Pruchniewski, M.; Lanzi, M.; Salatelli, E.; et al. Multifunctional Platform Based on Electrospun Nanofibers and Plasmonic Hydrogel: A Smart Nanostructured Pillow for Near-Infrared Light-Driven Biomedical Applications. ACS Appl. Mater. Interfaces 2020, 12, 54328–54342. [Google Scholar] [CrossRef] [PubMed]
- Faraji, A.H.; Wipf, P. Nanoparticles in cellular drug delivery. Bioorganic Med. Chem. 2009, 17, 2950–2962. [Google Scholar] [CrossRef]
- Vass, P.; Szabó, E.; Domokos, A.; Hirsch, E.; Galata, D.; Farkas, B.; Démuth, B.; Andersen, S.K.; Vigh, T.; Verreck, G.; et al. Scale-up of electrospinning technology: Applications in the pharmaceutical industry. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2020, 12, e1611. [Google Scholar] [CrossRef] [PubMed]
- Deepak, A.; Goyal, A.K.; Rath, G. Nanofiber in transmucosal drug delivery. J. Drug Deliv. Sci. Technol. 2018, 43, 379–387. [Google Scholar] [CrossRef]
- Pant, B.; Park, M.; Park, S.-J. Drug Delivery Applications of Core-Sheath Nanofibers Prepared by Coaxial Electrospinning: A Review. Pharmaceutics 2019, 11, 305. [Google Scholar] [CrossRef]
- Torres-Martinez, E.J.; Bravo, J.M.C.; Medina, A.S.; González, G.L.P.; Gómez, L.J.V. A Summary of Electrospun Nanofibers as Drug Delivery System: Drugs Loaded and Biopolymers Used as Matrices. Curr. Drug Deliv. 2018, 15, 1360–1374. [Google Scholar] [CrossRef] [PubMed]
- Rujitanaroj, P.-O.; Pimpha, N.; Supaphol, P. Wound-dressing materials with antibacterial activity from electrospun gelatin fiber mats containing silver nanoparticles. Polymer 2008, 49, 4723–4732. [Google Scholar] [CrossRef]
- Rieger, K.A.; Birch, N.P.; Schiffman, J.D. Designing electrospun nanofiber mats to promote wound healing—A review. J. Mater. Chem. B 2013, 1, 4531–4541. [Google Scholar] [CrossRef] [PubMed]
- Sebe, I.; Szabó, P.; Kállai-Szabó, B.; Zelkó, R. Incorporating small molecules or biologics into nanofibers for optimized drug re-lease: A review. Int. J. Pharm. 2015, 494, 516–530. [Google Scholar] [CrossRef] [PubMed]
- Joshi, D.; Garg, T.; Goyal, A.K.; Rath, G. Development and Characterization of Novel Medicated Nanofibers Against Periodonti-tis. Curr. Drug Deliv. 2015, 12, 564–577. [Google Scholar] [CrossRef] [PubMed]
- Schiffman, J.D.; Schauer, C.L. A Review: Electrospinning of Biopolymer Nanofibers and their Applications. Polym. Rev. 2008, 48, 317–352. [Google Scholar] [CrossRef]
- Thenmozhi, S.; Dharmaraj, N.; Kadirvelu, K.; Kim, H.Y. Electrospun nanofibers: New generation materials for advanced applications. Mater. Sci. Eng. B 2017, 217, 36. [Google Scholar] [CrossRef]
- Wang, C.; Ma, C.; Wu, Z.; Liang, H.; Yan, P.; Song, J.; Ma, N.; Zhao, Q. Enhanced Bioavailability and Anticancer Effect of Cur-cumin-Loaded Electrospun Nanofiber: In Vitro and In Vivo Study. Nanoscale Res. Lett. 2015, 10, 439. [Google Scholar] [CrossRef]
- Yoo, H.J.; Kim, H.D. Characteristics of waterborne Polyurethane/ Poly(N-vinyl pyrrolidone) composite films for wound-healing dressings. J. Appl. Polym. Sci. 2008, 107, 331–338. [Google Scholar] [CrossRef]
- Yu, D.G.; Zhang, X.F.; Shen, X.X.; Brandford-White, C.; Zhu, L.M. Ultrafine ibuprofen-loaded polyvinyl pyrrolidone fiber mats using electrospinning. Polym. Int. 2009, 58, 1010–1013. [Google Scholar] [CrossRef]
- Yu, D.-G.; Branford-White, C.; White, K.; Li, X.-L.; Zhu, L.-M. Dissolution Improvement of Electrospun Nanofiber-Based Solid Dispersions for Acetaminophen. AAPS Pharm. Sci. Tech. 2010, 11, 809–817. [Google Scholar] [CrossRef] [PubMed]
- Sebe, I.; Bodai, Z.; Eke, Z.; Kállai-Szabó, B.; Szabó, P.; Zelkó, R. Comparison of directly compressed vitamin B12 tablets pre-pared from micronized rotary-spun microfibers and cast films. Drug Dev. Ind. Pharm. 2015, 41, 1438–1442. [Google Scholar] [CrossRef]
- Yu, D.-G.; Yang, J.-M.; Branford-White, C.; Lu, P.; Zhang, L.; Zhu, L.-M. Third generation solid dispersions of ferulic acid in electrospun composite nanofibers. Int. J. Pharm. 2010, 400, 158–164. [Google Scholar] [CrossRef] [PubMed]
- Tonglairoum, P.; Ngawhirunpat, T.; Rojanarata, T.; Kaomongkolgit, R.; Opanasopit, P. Fast-acting clotrimazole composited PVP/HPbCD nanofibers for oral candidiasis application. Pharm. Res. 2014, 31, 1893–1906. [Google Scholar] [CrossRef] [PubMed]
- Vigh, T.; Horváthová, T.; Balogh, A.; Sóti, P.L.; Drávavölgyi, G.; Nagy, Z.K.; Marosi, G. Polymer-free and polyvinyl pirrolidone-based electrospun solid dosage forms for drug dissolution enhancement. Eur. J. Pharm. Sci. 2013, 49, 595–602. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.-Y.; Branford-White, C.; Shen, X.-X.; Yu, D.-G.; Zhu, L.-M. Time-engineeringed biphasic drug release by electrospun nanofiber meshes. Int. J. Pharm. 2012, 436, 88–96. [Google Scholar] [CrossRef]
- Jiang, Y.-N.; Mo, H.-Y.; Yu, D.-G. Electrospun drug-loaded core–sheath PVP/zein nanofibers for biphasic drug release. Int. J. Pharm. 2012, 438, 232–239. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Jiao, Y.; Shao, X.; Zhou, C. Controlled dual release of hydrophobic and hydrophilic drugs from electrospun poly(l-lactic acid) fiber mats loaded with chitosan microspheres. Mater. Lett. 2011, 65, 2800–2803. [Google Scholar] [CrossRef]
- Ganesh, M.; Aziz, A.S.; Ubaidulla, U.; Hemalatha, P.; Saravanakumar, A.; Ravikumar, R.; Peng, M.M.; Choi, E.Y.; Jang, H.T. Sulfanilamide and silver nanoparticles-loaded polyvinyl alcohol-chitosan composite electrospun nanofibers: Synthesis and evalu-ation on synergism in wound healing. J. Ind. Eng. Chem. 2016, 39, 127. [Google Scholar] [CrossRef]
- Jannesari, M.; Varshosaz, J.; Morshed, M.; Zamani, M. Composite poly (vinyl alcohol)/poly (vinyl acetate) electrospun nano-fibrous mats as a novel wound dressing matrix for controlled release of drugs. Int. J. Nanomed. 2011, 6, 993. [Google Scholar]
- Mabrouk, M.; Mostafa, A.; Oudadesse, H.; Mahmoud, A.; El-Gohary, M. Effect of ciprofloxacin incorporation in PVA and PVA bioactive glass composite scaffolds. Ceram. Int. 2014, 40, 4833–4845. [Google Scholar] [CrossRef]
- Li, X.; Kanjwal, M.A.; Lin, L.; Chronakis, I.S. Electrospun polyvinyl-alcohol nanofibers as oral fast-dissolving delivery system of caffeine and riboflavin. Colloids Surf. B Biointerfaces 2013, 103, 182–188. [Google Scholar] [CrossRef] [PubMed]
- Nagy, Z.K.; Nyul, K.; Wagner, I.; Molnar, K.; Marosi, G. Electrospun water soluble polymer mat for ultrafast release of Donepezil HCl. Express Polym. Lett. 2010, 4, 763–772. [Google Scholar] [CrossRef]
- Fathi-Azarbayjani, A.; Chan, S.Y. Single and multi-layered nanofibers for rapid and controlled drug delivery. Chem. Pharm. Bull. 2010, 58, 143–146. [Google Scholar] [CrossRef]
- Sun, X.-Z.; Williams, G.R.; Hou, X.-X.; Zhu, L.-M. Electrospun curcumin-loaded fibers with potential biomedical applications. Carbohydr. Polym. 2013, 94, 147–153. [Google Scholar] [CrossRef] [PubMed]
- Kenawy, E.-R.; Abdel-Hay, F.I.; El-Newehy, M.H.; Wnek, G.E. Controlled release of ketoprofen from electrospun poly (vinyl al-cohol) nanofibers. Mater. Sci. Eng. A 2007, 459, 390–396. [Google Scholar] [CrossRef]
- Kataria, K.; Sharma, A.; Garg, T.; Goyal, A.K.; Rath, G. Novel Technology to Improve Drug Loading in Polymeric Nanofibers. Drug Deliv. Lett. 2014, 4, 79–86. [Google Scholar] [CrossRef]
- Zeng, J.; Aigner, A.; Czubayko, F.; Kissel, T.; Wendorff, J.H.; Greiner, A. Poly (vinyl alcohol) Nanofibers by Electrospinning as a Protein Delivery System and the Retardation of Enzyme Release by Additional Polymer Coatings. Biomacromolecules 2005, 6, 1484–1488. [Google Scholar] [CrossRef] [PubMed]
- Risdian, C.; Nasir, M.; Rahma, A.; Rachmawati, H. The Influence of Formula and Process on Physical Properties and the Release Profile of PVA/BSA Nanofibers Formed by Electrospinning Technique. J. Nano Res. 2015, 31, 103–116. [Google Scholar] [CrossRef]
- Charernsriwilaiwat, N.; Opanasopit, P.; Rojanarata, T.; Ngawhirunpat, T. Lysozyme-loaded, electrospun chitosan-based nanofiber mats for wound healing. Int. J. Pharm. 2012, 427, 379–384. [Google Scholar] [CrossRef] [PubMed]
- El-Newehy, M.H.; Al-Deyab, S.S.; Kenawy, E.R.; Abdel-Megeed, A. Fabrication of Electrospun Antimicrobial Nanofibers Con-taining Metronidazole Using Nanospider Technology. Fibers Polym. 2012, 13, 709–717. [Google Scholar] [CrossRef]
- Shankhwar, N.; Kumar, M.; Mandal, B.B.; Robi, P.S.; Srinivasan, A. Electrospun Polyvinyl alcohol-Polyvinyl pyrrolidone Nano-fibrous Membranes for Interactive Wound Dressing Application. J. Biomater. Sci. Polym. Ed. 2015, 27, 247–262. [Google Scholar] [CrossRef]
- National Center for Biotechnology Information. PubChem Database. CID=3033050, Buprenorphine-Hydrochloride. 2020. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Buprenorphine-hydrochloride (accessed on 14 March 2021).
- Margetts, L.; Sawyer, R. Transdermal drug delivery: Principles and opioid therapy. Contin. Educ. Anaesth. Crit. Care Pain 2007, 7, 171. [Google Scholar] [CrossRef]
- Lutfy, K.; Eitan, S.; Bryant, C.D.; Yang, Y.C.; Saliminejad, N.; Walwyn, W.; Kieffer, B.L.; Takeshima, H.; Carroll, F.I.; Maid-ment, N.T.; et al. Buprenorphine-induced antinociception is mediated by mu-opioid receptors and compromised by con-comitant activation of opioid receptor-like receptors. J. Neurosci. 2003, 23, 10331–10337. [Google Scholar] [CrossRef]
- Clark, M.D.; Krugner-Higby, L.; Smith, L.J.; Heath, T.D.; Clark, K.L.; Olson, D. Evaluation of liposome-encapsulated oxymorphone hydrochloride in mice after splenectomy. Comp. Med. 2004, 54, 558–563. [Google Scholar]
- Stokes, E.L.; Flecknell, P.A.; Richardson, C.A. Reported analgesic and anaesthetic administration to rodents undergoing experi-mental surgical procedures. Lab. Anim. 2009, 43, 149–154. [Google Scholar] [CrossRef] [PubMed]
- Likar, R. Transdermal buprenorphine in the management of persistent pain—Safety aspects. Ther. Clin. Risk Manag. 2006, 2, 115–125. [Google Scholar] [PubMed]
- Gordon, A.; Rashiq, S.; Moulin, D.E.; Clark, A.J.; Beaulieu, A.D.; Eisenhoffer, J.; Piraino, P.S.; Quigley, P.; Harsanyi, Z.; Darke, A.C. Buprenorphine Transdermal System for Opioid Therapy in Patients with Chronic Low Back Pain. Pain Res. Manag. 2010, 15, 169–178. [Google Scholar] [CrossRef] [PubMed]
- Ramakrishna, S.; Fujihara, K.; Teo, W.E.; Lim, T.C.; Ma, Z. An Introduction to Electrospinning and Nanofibers; World Scientific Publishing Co.: Hackensack, NJ, USA, 2005. [Google Scholar]
- Amariei, N.; Manea, L.R.; Bertea, A.P.; Popa, A. The Influence of Polymer Solution on the Properties of Electrospun 3D Nanostructures. IOP Conf. Ser. Mater. Sci. Eng. 2017, 209, e012092. [Google Scholar] [CrossRef]
- Esnaashari, S.S.; Rezaei, S.; Mirzaei, E.; Afshari, H.; Rezayat, S.M.; Faridi-Majidi, R. Preparation and characterization of kefiran electrospun nanofibers. Int. J. Biol. Macromol. 2014, 70, 50–56. [Google Scholar] [CrossRef] [PubMed]
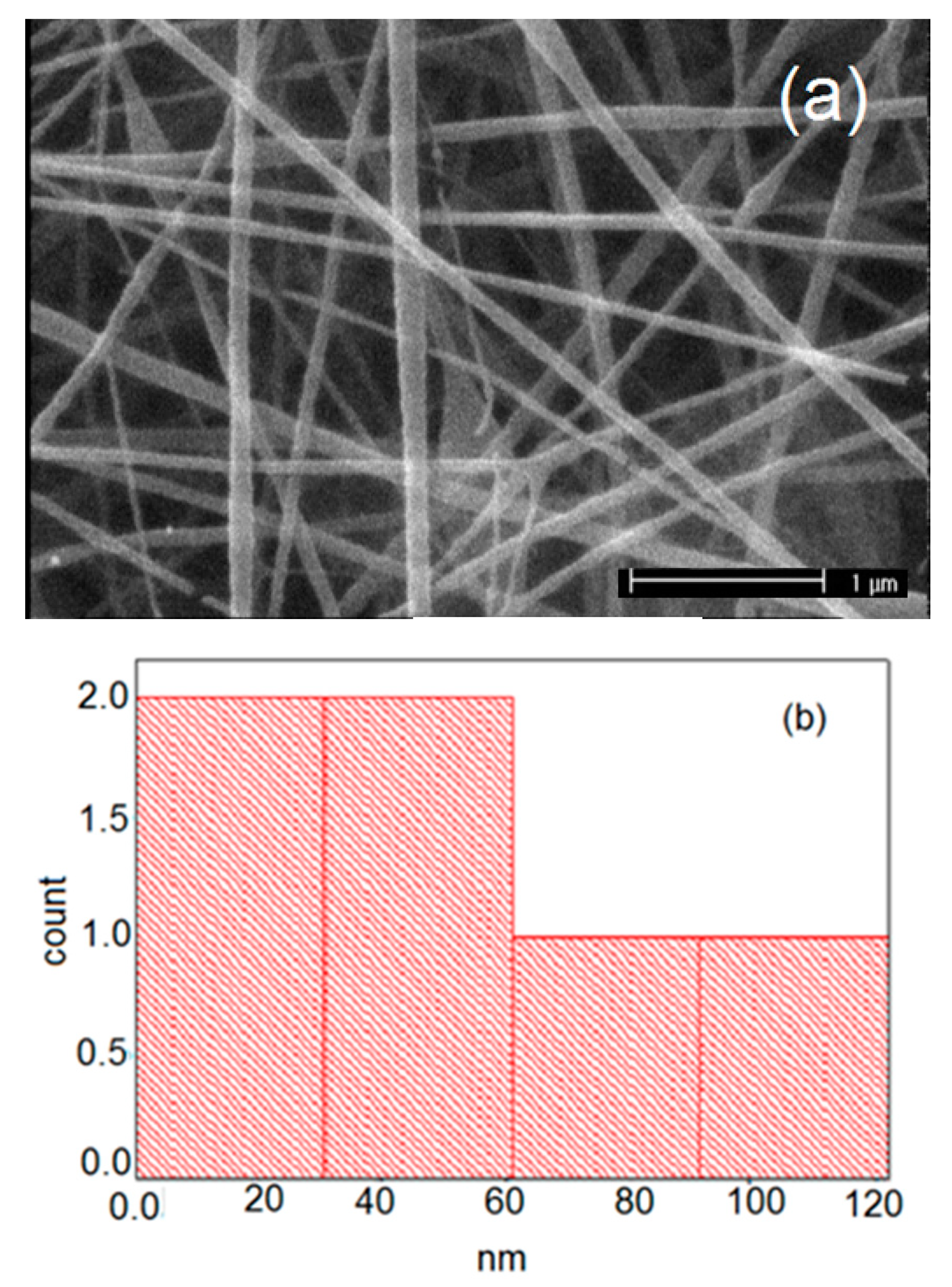
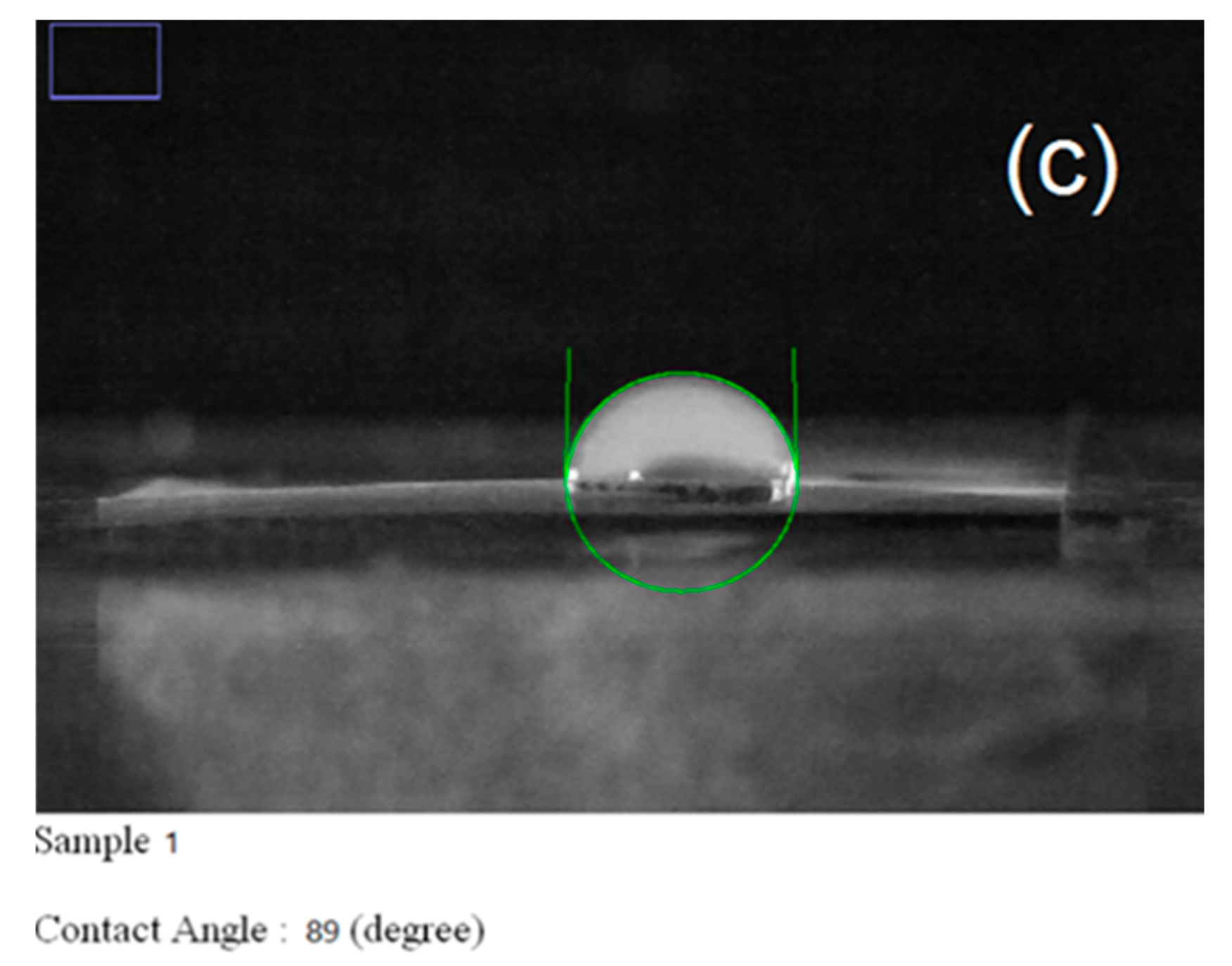
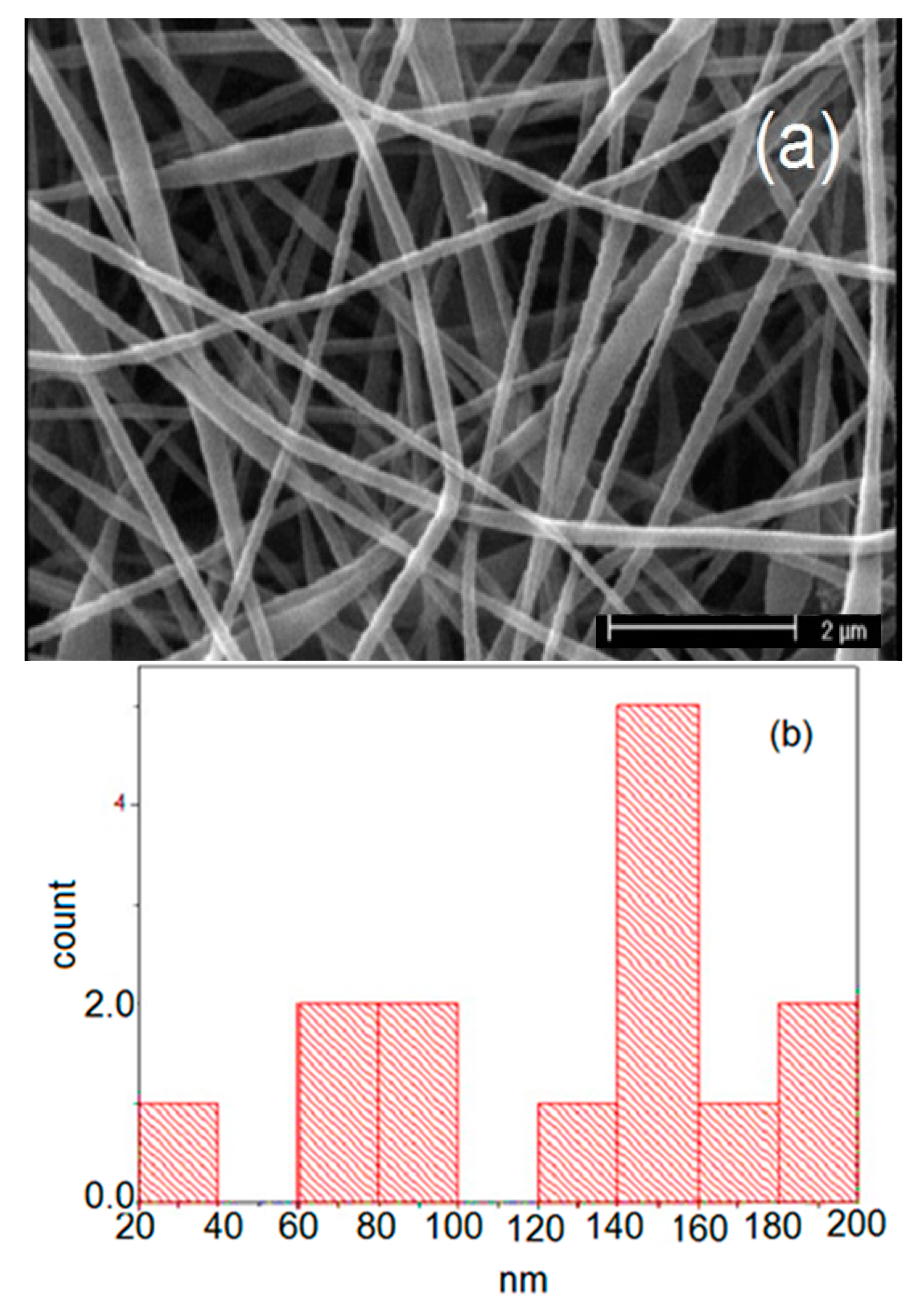
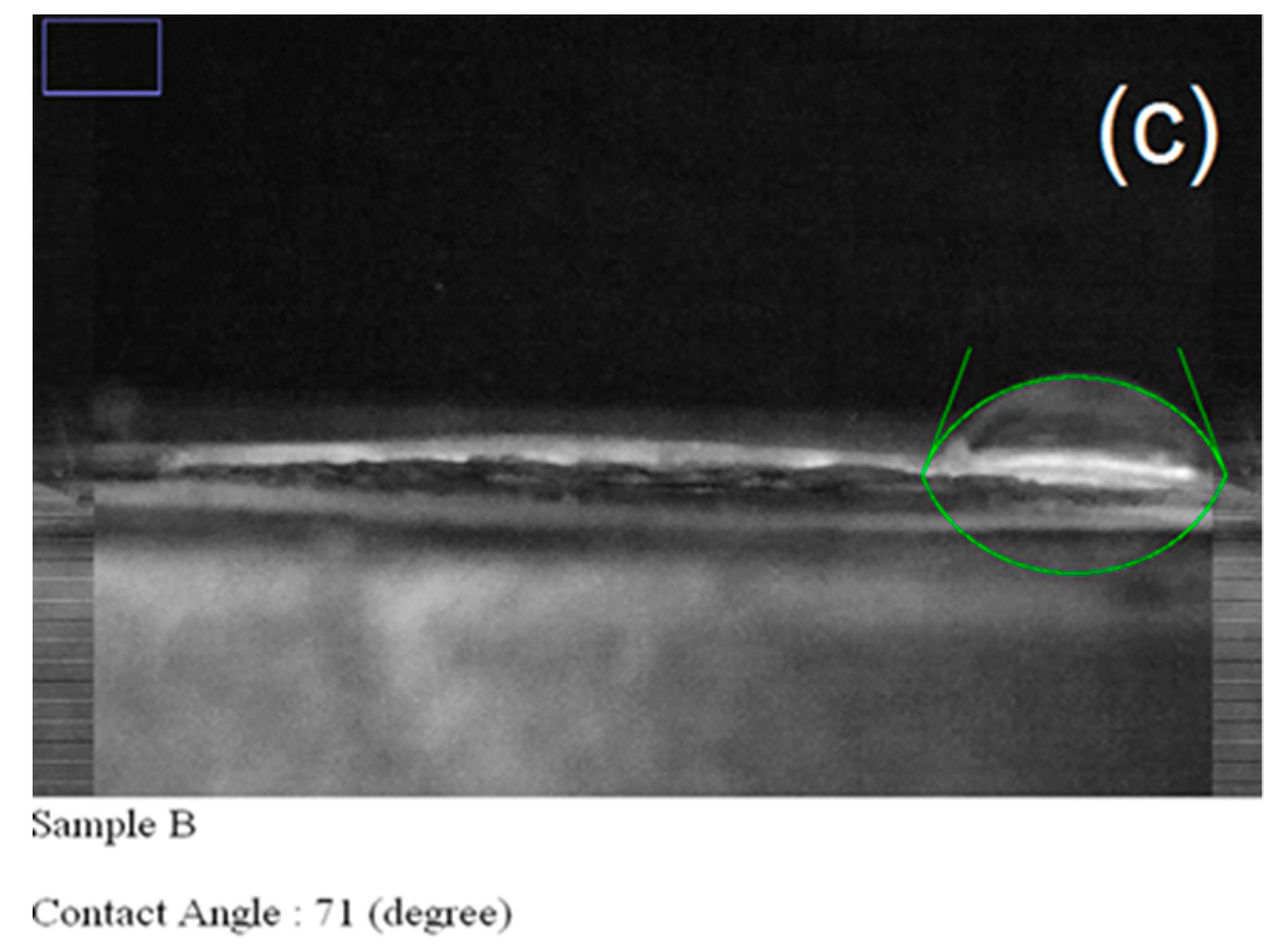
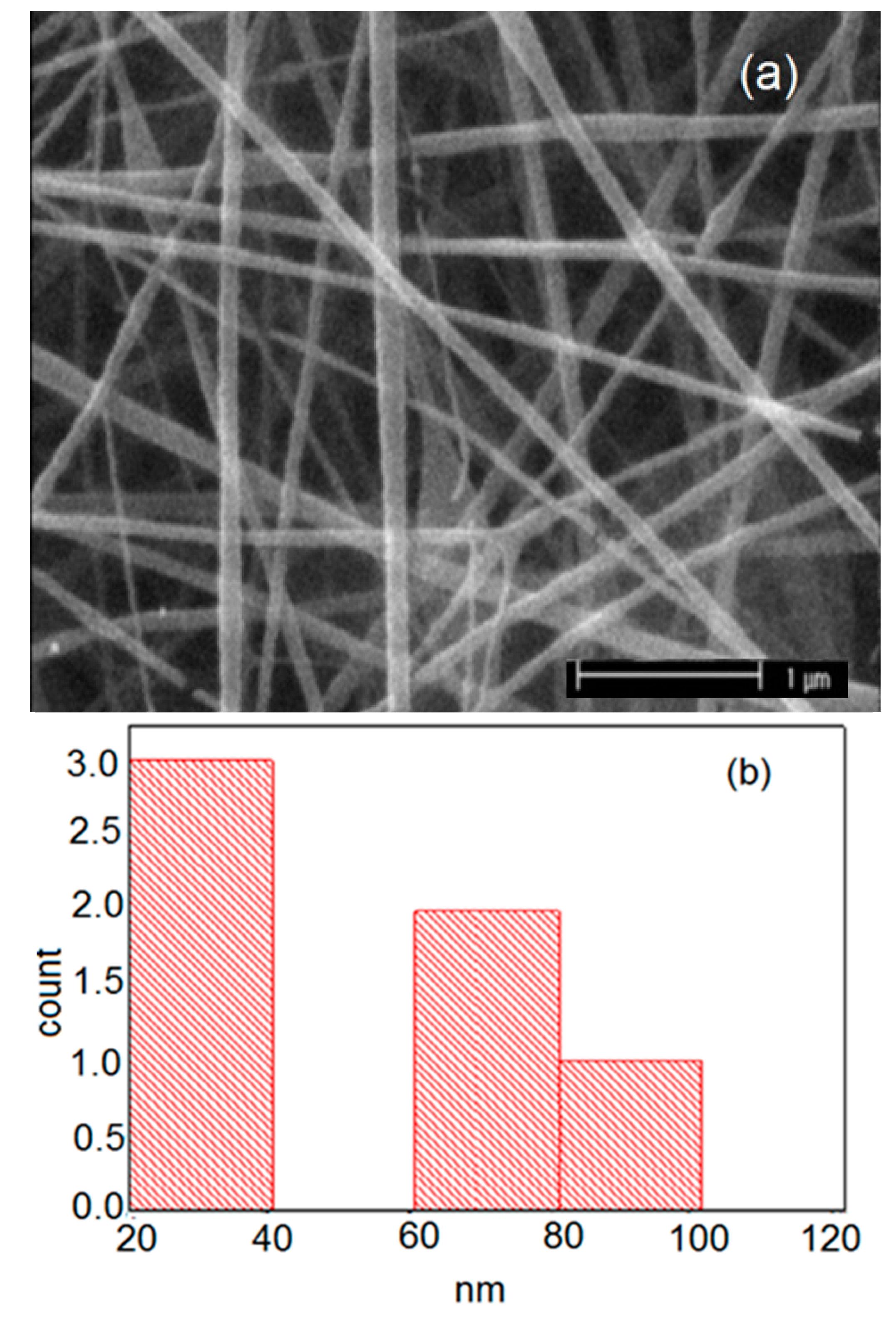
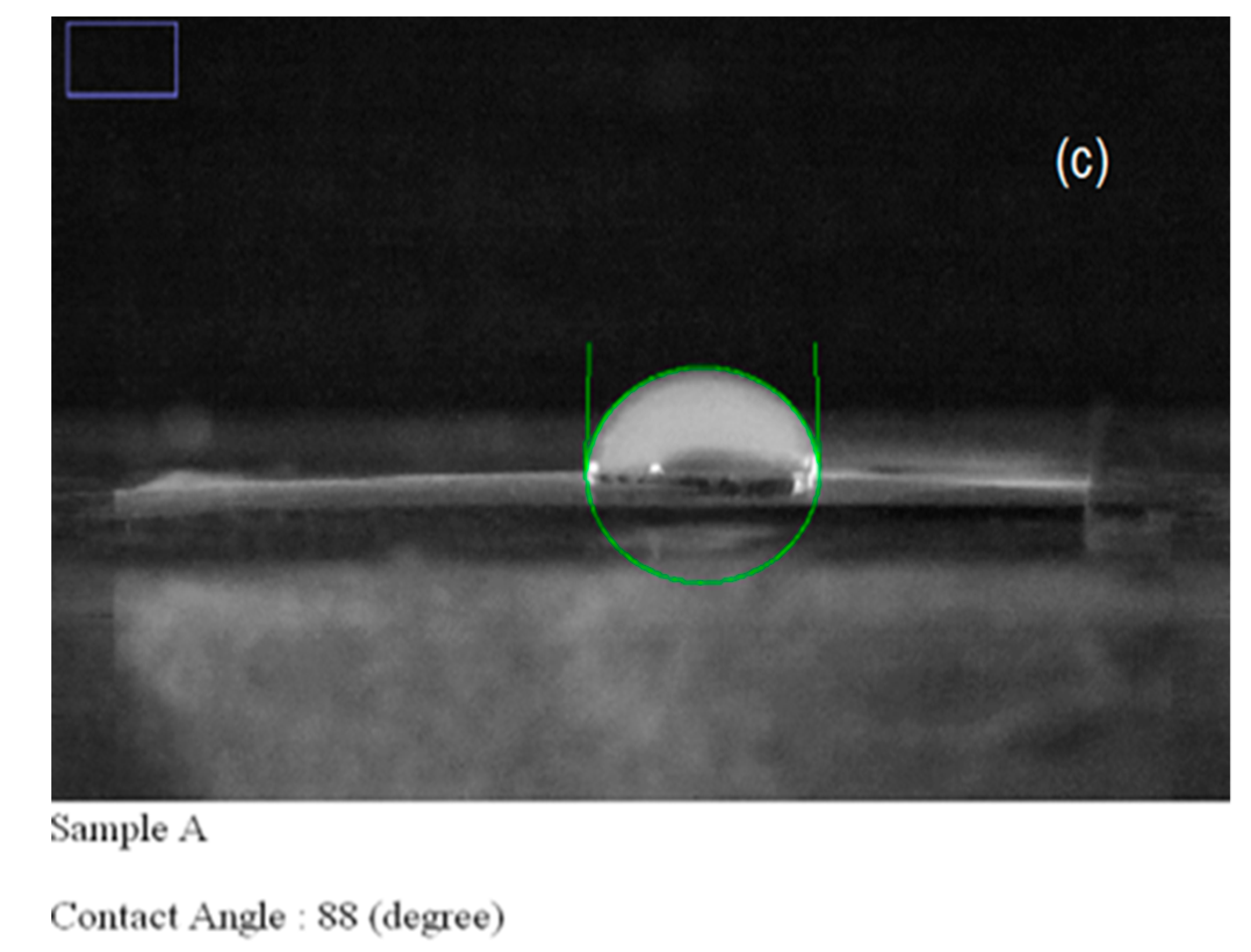
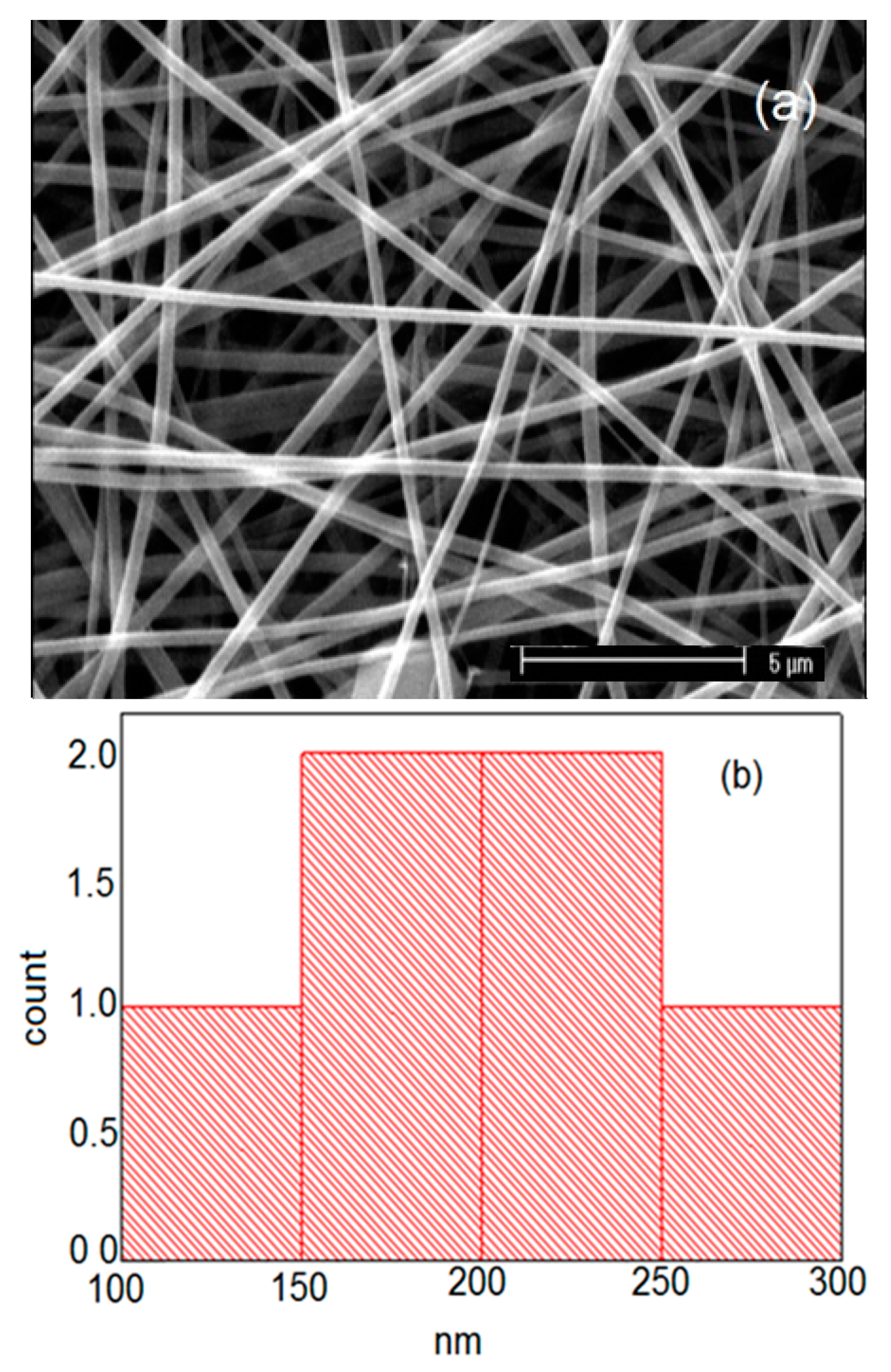
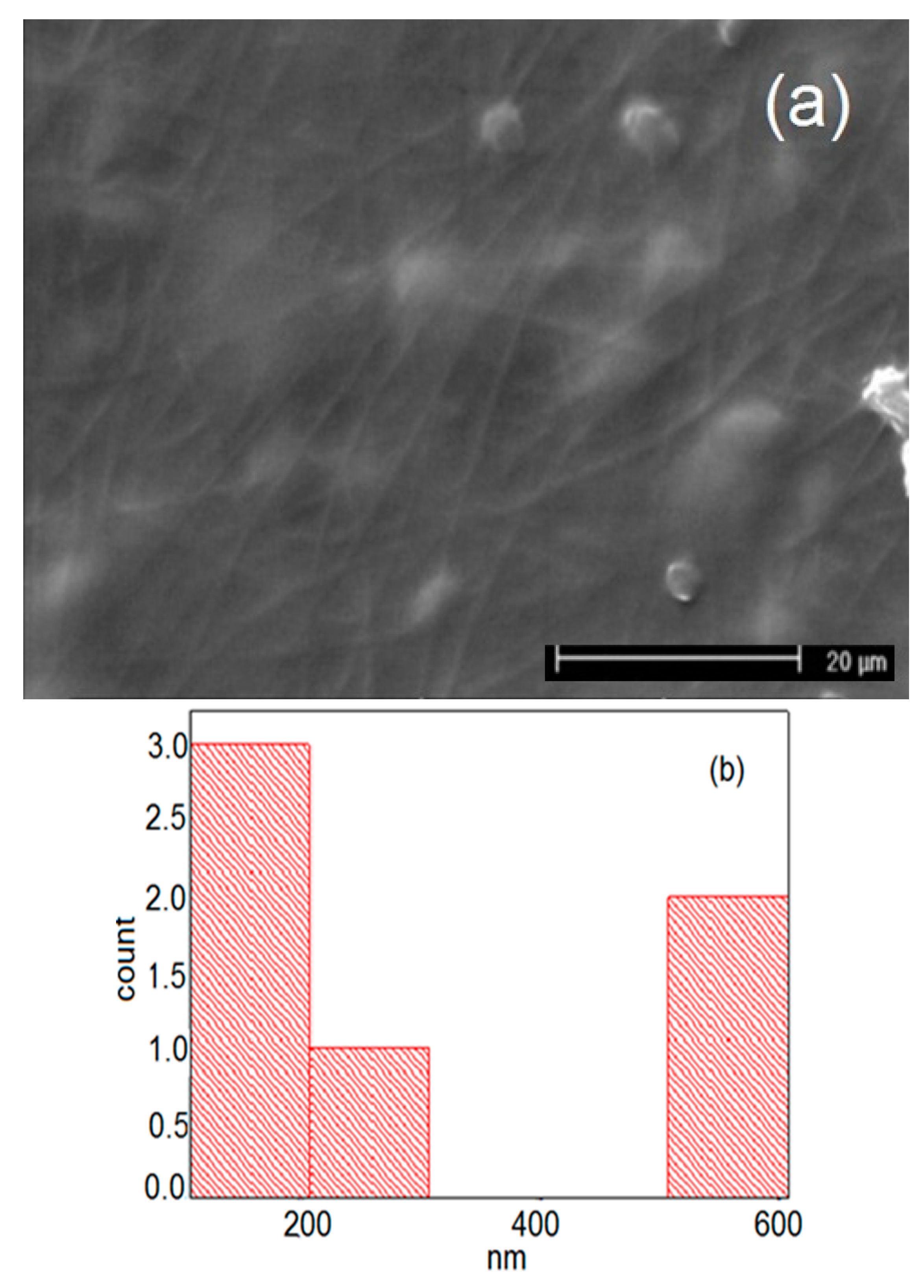

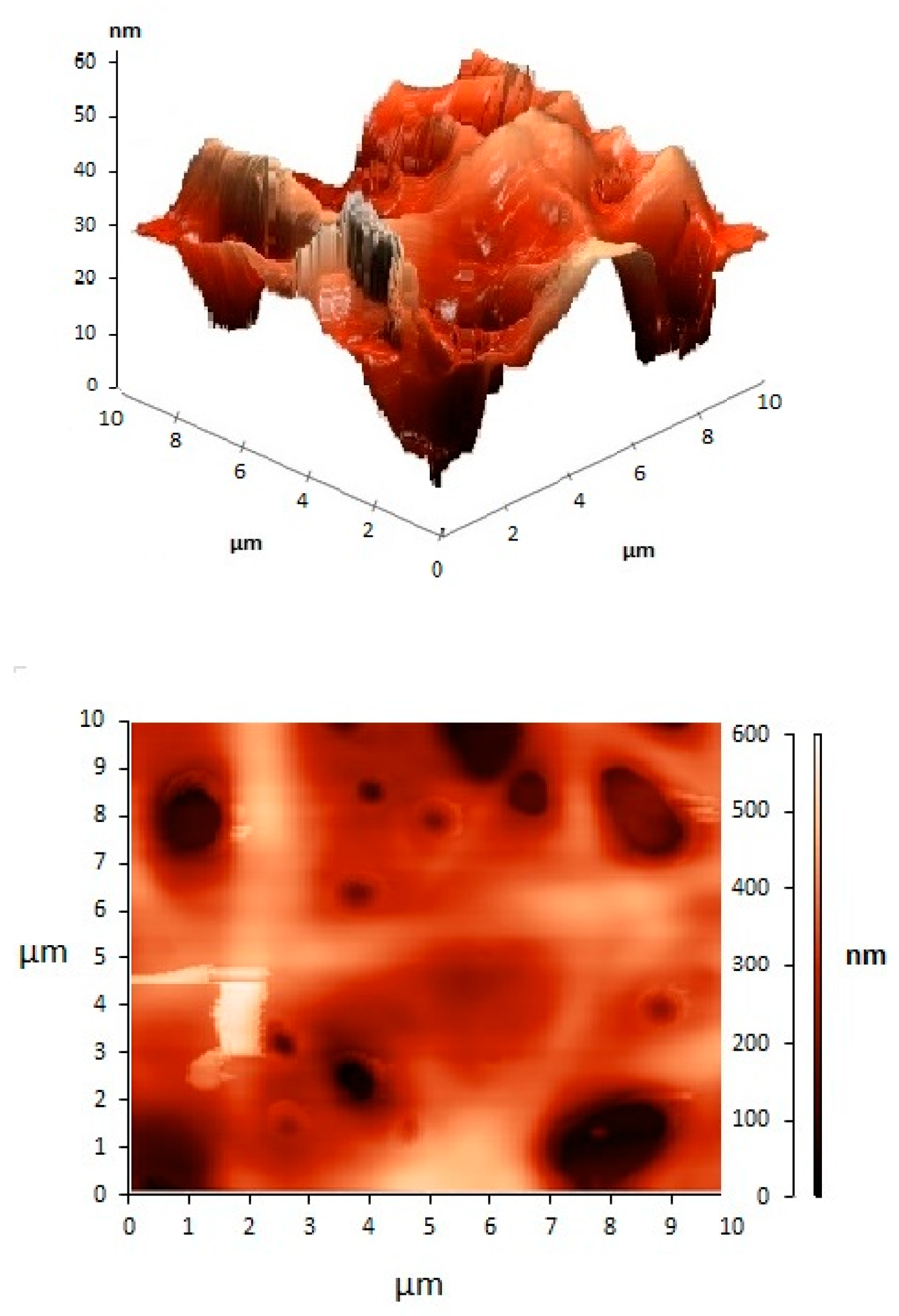
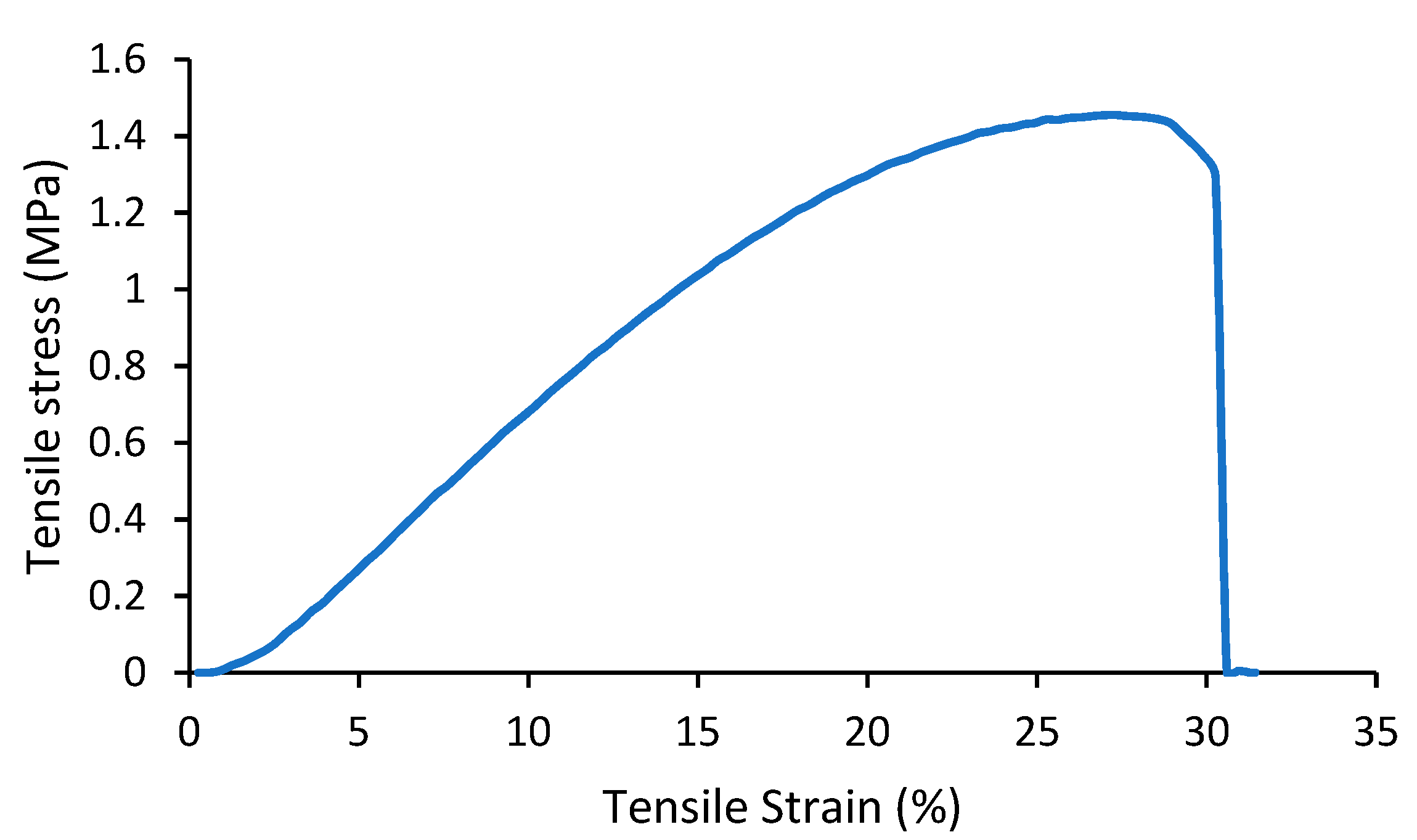
| Carrier | Other Ingredient | Drug | Remarks | Ref |
|---|---|---|---|---|
| PVP | Ibuprofen | Immediate release (dissolution in 10 s) | [24] | |
| PVP | Acetaminophen | Better dissolution-improving effects of solid dispersions nanofibers for poorly water-soluble drug model | [25] | |
| PVP | Vitamin B12 | Directly compressed micronized rotary-spun fiber-based tablet showed uniform drug release of low variations | [26] | |
| PVP | sodium dodecyl sulfate | Ferulic acid | Nanofiber-based SDs (solid dispersions) could release all the contained FA within 1 min and had a 13-fold higher permeation rate across sublingual mucosa compared to crude FA particles. | [27] |
| PVP | Hydroxypropyl-β-cyclodextrin (CD) | Clotrimazole | Complete drug release in contrast to CZ powder and lozenges containing CZ, CZ-loaded nanofibers killed the Candida significantly faster than the CZ powder and lozenges with low cytotoxicity | [28] |
| PVP | EC | Ketoprofen | Diffusion controlled release due to addition of water-insoluble polymer EC | [30] |
| PVP | zein | Ketoprofen | Sustained and prolonged release over 12 h without burst release | [31] |
| PVP | PLA | Benzoin | Controlled release for 30 day | [32] |
| PVA | Caffeine, Riofelavin | Drugs can be released in a burst manner (caffeine to an extent of 100% and riboflavin to an extent of 40% within 60 s | [36] | |
| PVA | Donepezil | [37] | ||
| PVA | CD | Haloperidol | Decrease initial disintegration time and increasing the release rate through the addition of randomly methylated b- CD | [38] |
| PVA | b-CD | Curcumin | A rate improvement at lower levels of drug content | [39] |
| PVA | Ketoprofen | A decrease in the rate of release from in the range of 1–24 h after the treatment of PVA mats with methanol | [40] | |
| PVA | Na | Ketoprofen | A significant decrease in the dissolution rate | [41] |
| PVA | poly(p-xylylene) (PPX) | BSA | diminishment of burst release and the significant retardation of protein release with applying PPX coating | [42] |
| PVA | EUDRAGIT1 L-100 | BSA | Modified: pH-dependent release | [43] |
| PVA | CS-EDTA | Lysozyme | Rapid release depending on loading amount | [44] |
| PVA | PEO | Metronidazole | Antimicrobial activity against Escherichia coli, Pseudomonas aeruginosa, Aspergillus niger, Penicillium notatum and Aspergillus flavus | [45] |
| PVP | PVA | Ciprofloxacin | Sustained release of the antibiotic protective action against external pathogenic microbes | [46] |
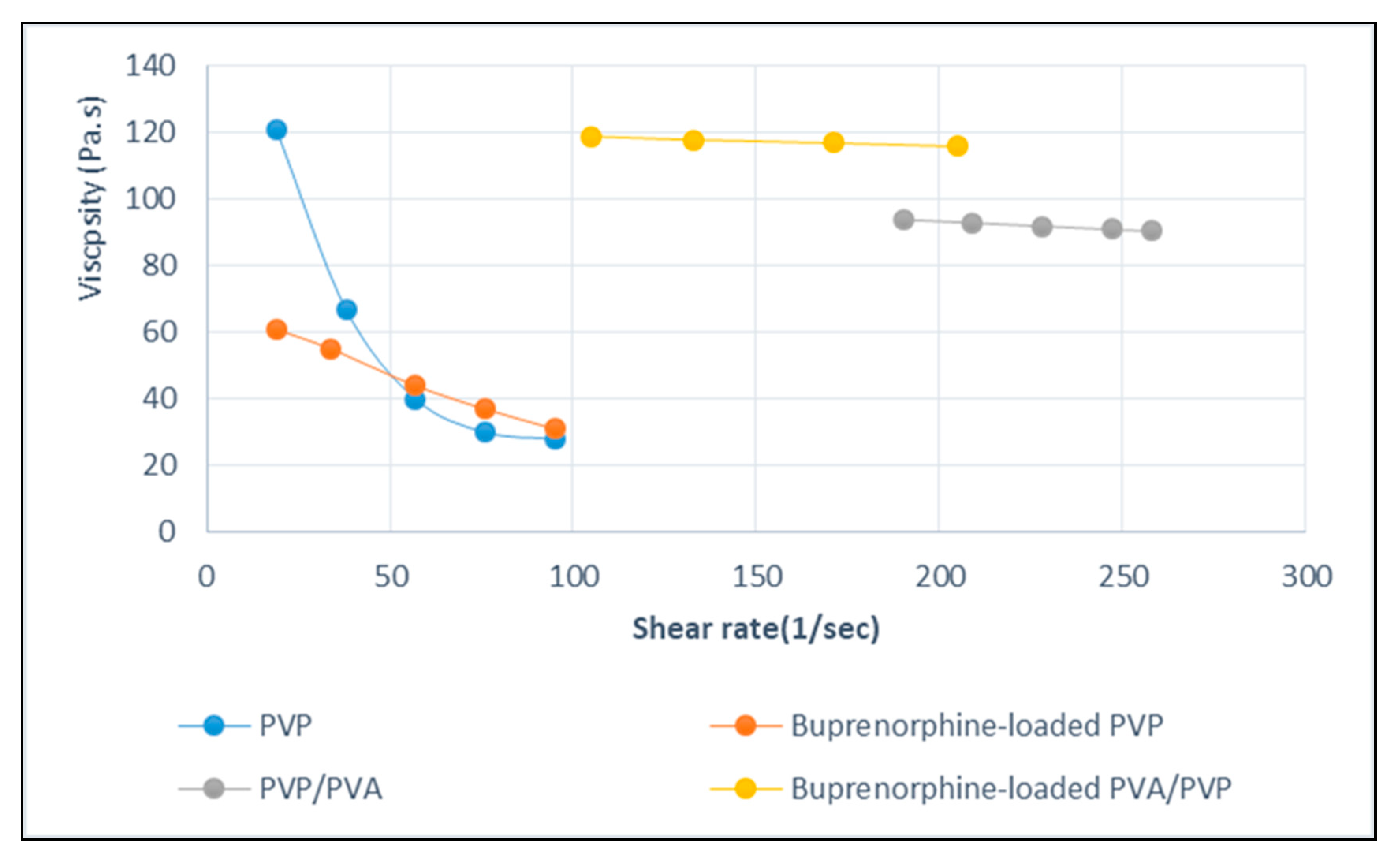
| Sample | Solution Constitution | Viscosity (Pa.s) | Shear Rate (1/s) | Electrical Conductivity of Solution (μs) | Cotact Angle of Nanofibers | Average Diameter of Nanofibers (nm) |
|---|---|---|---|---|---|---|
| 1 | Pure PVP | 121 | 19 | 226 | 89 | 49 |
| 2 | PVP/PVA | 94 | 190 | 673 | 71 | 104 |
| 3 | Bup(Buprenorphine)/PVP | 61 | 19 | 1628 | 88 | 55 |
| 4 | Bup/PVP/PVA | 119 | 105 | 1261 | 70 | 186 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rahmani, F.; Ziyadi, H.; Baghali, M.; Luo, H.; Ramakrishna, S. Electrospun PVP/PVA Nanofiber Mat as a Novel Potential Transdermal Drug-Delivery System for Buprenorphine: A Solution Needed for Pain Management. Appl. Sci. 2021, 11, 2779. https://doi.org/10.3390/app11062779
Rahmani F, Ziyadi H, Baghali M, Luo H, Ramakrishna S. Electrospun PVP/PVA Nanofiber Mat as a Novel Potential Transdermal Drug-Delivery System for Buprenorphine: A Solution Needed for Pain Management. Applied Sciences. 2021; 11(6):2779. https://doi.org/10.3390/app11062779
Chicago/Turabian StyleRahmani, Fatemeh, Hakimeh Ziyadi, Mitra Baghali, Hongrong Luo, and Seeram Ramakrishna. 2021. "Electrospun PVP/PVA Nanofiber Mat as a Novel Potential Transdermal Drug-Delivery System for Buprenorphine: A Solution Needed for Pain Management" Applied Sciences 11, no. 6: 2779. https://doi.org/10.3390/app11062779
APA StyleRahmani, F., Ziyadi, H., Baghali, M., Luo, H., & Ramakrishna, S. (2021). Electrospun PVP/PVA Nanofiber Mat as a Novel Potential Transdermal Drug-Delivery System for Buprenorphine: A Solution Needed for Pain Management. Applied Sciences, 11(6), 2779. https://doi.org/10.3390/app11062779







