Featured Application
The electrospun mats produced may have biomaterial potentials such as therapeutic compound delivery (essential oils) and wound dressings.
Abstract
The growth of population and increase in diseases that cause an enormous demand for biomedical material consumption is a pointer to the pressing need to develop new sustainable biomaterials. Electrospun materials derived from green polymers have gained popularity in recent years for biomedical applications such as tissue engineering, wound dressings, and drug delivery. Among the various bioengineering materials used in the synthesis of a biodegradable polymer, poly(lactic acid) (PLA) has received the most attention from researchers. Hypericum perforatum oil (HPO) has antimicrobial activity against a variety of bacteria. This study aimed to investigate the development of an antibacterial sustainable material based on PLA by incorporating HPO via a simple, low-cost electrospinning method. Chemical, morphological, thermal, thickness and, air permeability properties, and in vitro antibacterial activity of the electrospun nonwoven fabric were investigated. Scanning electron microscopy (SEM) was used to examine the morphology of the electrospun nonwoven fabric, which had bead-free morphology ultrafine fibers. Antibacterial tests revealed that the Hypericum perforatum oil-loaded poly(lactic acid) nonwoven fabrics obtained had high antibacterial efficiency against Escherichia coli and Staphylococcus aureus, indicating a strong potential for use in biomedical applications.
1. Introduction
In recent years, researchers have focused on the development of new materials with a low detrimental impact on the environment, due to the increasing growth of the world’s population and pollution. Green polymers are becoming more widely used to achieve the requisite biodegradability. Poly(lactic acid) (PLA) is a biodegradable, recyclable polyester made from renewable feedstock, that is nowadays one of the most promising polymers for commercially replacing poly(styrene) (PS), poly(ethylene terephthalate) (PET), high-density poly(ethylene) (HDPE), and low-density poly(ethylene) (LDPE). Lactic acid is produced as a raw material by fermenting glucose or sucrose and is refined to high purity. PLA has been used in food packaging, textiles, and, more recently, engineering plastics and currently is a niche product with significant growth potential [1,2,3,4,5,6]. PLA production represented 10.3% of the global bioplastics production capacity in 2018, reaching almost 220,000 tons/year, with a growth rate of around 60% expected by 2023 [7].
Electrospinning is a promising method for spinning polymer solutions or melts that can be easily scaled up with the use of strong electric fields. The approach is based on the assumption that in a charged polymer solution, strong electrical forces overwhelm weak surface tension forces. A high voltage is used to eject an electrically charged polymer solution jet from the tip (Taylor cone) of a capillary tube and uniformly scatter it over the collecting substrate. Jet then flows in the direction of the external electrical field, elongating as the external and internal electrical fields interact. The jet is sprayed onto a nonwoven mat-like substrate at random. This approach works with synthetic and natural polymers that have been processed to produce fibers with diameters ranging from a few nanometers to several microns [8,9,10,11,12,13]. Electrospun fibers have sparked a lot of attention due to the ease of fabricating multifunctional materials for usage in a variety of applications such as biomedical, textile, food packaging, batteries, and filters. Especially, electrospun fibers provide a unique opportunity for biomedical applications since they combine biocompatibility with micro-/nanoscale fiber architecture. These days, researchers are more focused on the development of biomaterials that reduce the risk of disease transmission and infection spread. The demand for novel antimicrobial materials is growing [14,15,16,17,18,19,20]. Natural bioactive agents have been used in biomedical due to their significant contribution since ancient times. Lately, the use of natural compounds such as plant extracts [21,22,23], bee products [24,25,26], and oils [27,28,29] for developing electrospun fibrous materials in biomedical applications has been studied extensively. Hypericum perforatum, a member of the Hypericaceae family, has long been regarded as a valuable herbal medicine. Flavonoids, hyperforin, and hypericin are all found in this plant. Hypericum perforatum oil (HPO) has long been used topically and orally as a home remedy to treat cuts, burns, depression, hemorrhoids, diabetes, and gastrointestinal ulcers [30,31,32,33]. Various articles have been written about the extraordinary properties of electrospun materials containing Hypericum perforatum. Poly(ε-caprolactone) (PCL) nanofiber wound dressings containing alcoholic extract of Hypericum perforatum have been produced [34]. A nanofiber wound dressing containing Hypericum perforatum extract was developed using poly(lactic-co-glycolic acid) (PLGA) [35]. Hypericum perforatum oil loaded-poly(ethylene glycol) (PEG) based nanofiber material was produced [36]. The developments of thermoplastic polyurethane (TPU) electrospun mats containing Hypericum perforatum extract have been researched and its antibacterial property evaluated [37]. Electrospun PLGA/gelatin (GE) membranes containing Hypericum capitatum extract have been developed and their biocompatibility properties investigated [38]. A wound dressing was designed from zein polymer containing Hypericum perforatum oil [39]. The cellulose-based fibrous dressing material for the treatment of acute wounds was produced from carboxymethyl cellulose (CMC)/poly (ethylene oxide) (PEO)/Hypericum perforatum solutions by needle-free electrospinning method [40]. The production of PEO and chitosan (CS) based fibrous containing Hypericum perforatum for wound dressings has been investigated [41]. A new wound dressing was fabricated by electrospinning using Hypericum perforatum oil into a mixture of biodegradable PCL/GE blended polymers [42].
PLA is an aliphatic polyester that is biocompatible and biodegradable. The US Food and Drug Administration has approved PLA for clinical applications. These properties have made PLA-based materials suitable for biomedical applications such as sutures, bone fixation implants, stents, tissue engineering scaffolds, and drug delivery carriers [6,18]. Researchers have been worked about the development antibacterial PLA-based electrospun materials using chitosan and silver [43], thymol [44], propolis [45], silver and vitamin E [46], copaiba (Copaifera sp.) oil [47], tetracycline hydrochloride and chitosan [48], cinnamon essential oil [49], thymoquinone [50], tea tree and manuka oil [51], carvacrol [52], clary sage and black pepper [53], terpinen-4-ol [54], epidermal growth factor [55], quercetin [56], Nigella sativa herbal extract [57], Eucalyptus essential oil [58], silver diclofenac complex [59], chitosan, black pepper essential oil and limonene [60], selenium and clarithromycin [61], cefazolin [62], silver nanoparticles [63], zinc oxide [64], thyme essential oil [65], zenian (Carum copticum) essential oil [66], birch bark triterpene extract [67], Capparis spinosa L. extracts [68], Plumbago europaea plant extract [69], curcumin [70], babassu oil [71], and cypress (Cupressus sempervirens L.) essential oil [72].
Despite all the efforts mentioned above, the effects of Hypericum perforatum oil on the morphology of poly(lactic acid) electrospun mats have not yet been studied. PLA is easily available, biodegradable and renewable [1,2,3,4,5,6]. Hypericum perforatum oil exhibits bioactive properties such as antioxidant, anti-inflammatory, anticarcinogenic, and antifungal [30,31,32,33]. Moreover, it is already widely used and accessible in the medical field. It has been proposed that developing an advanced material using a combination of the beneficial properties of Hypericum perforatum oil and the advantages of poly(lactic acid) might be an alternative in bioengineering applications. This study contributes to the development of new sustainable materials and opens a new door to other studies.
This research work aims to develop antimicrobial PLA-based nonwoven fabrics containing HPO using a simple, environmentally-friendly method. HPO/PLA nonwoven fabrics were fabricated by electrospinning HPO/PLA aqueous solutions. The morphology, chemical composition, thermal and physical properties of the electrospun nonwovens were investigated. The electrospun HPO/PLA nonwoven fabrics demonstrated good antibacterial activity and have the potential for use in a variety of biomedical applications, particularly wound dressing, due to the combination of the antibacterial activity of the HPO and the favorable biocompatible and biodegradable polymer due to the PLA fibers.
2. Experimental Study
2.1. Materials
HPO (Aromatics of Dreams (Арoматика Мрії), Kiev, Ukraine) was purchased from the local herbal market (Lodz, Poland). Commercial PLA (a thermoplastic fiber-grade resin derived, Ingeo™ 6201D Fiber Grade PLA, the average molecular weight (Mw) 59.1 kg/mol, polydispersity index (Mw/Mn) 1.29, and an L-lactide content of isomer 1.4% D) [2] was obtained from NatureWorks LLC (Minnetonka, USA). Dichloromethane was purchased from POCH Basic (Gliwice, Poland) and acetone was Eurochem Sp. z o.o (Katowice, Poland). All chemicals were used as received, with no further purification. Escherichia coli (ATCC 10536) and Staphylococcus aureus (ATCC 6538) came from the American Type Culture Collection (ATCC) and are stored at the Department of Environmental Biotechnology, Lodz University of Technology. Cotton gauze was (Matocomp Gaze Comprey, TZMO SA, Torun, Poland) purchased from the local pharmacy (Lodz, Poland).
2.2. Methods
2.2.1. Preparation of Solutions for Electrospinning
Initially, a PLA 9% solution was prepared by dissolving in mixed solvents of dichloromethane and acetone 50:50 (v/v) at room temperature (RT) and stirring for 8 h. Subsequently, the 3HPO/PLA and 5HPO/PLA solutions (v/v) were prepared at RT temperature by dissolving in 3:97 (v/v) and 5:95 (v/v) HPO/PLA mixtures, respectively. HPO/PLA electrospun were blended and mixed to get homogenous solutions. A magnetic stirrer (Magnetic motor stirrer MS 11, WIGO, Warsaw, Poland) was used to prepare the solutions. Three solutions were obtained for electrospinning: PLA, 3HPO/PLA, and 5HPO/PLA.
2.2.2. Preparation of Electrospun Nonwoven Fabrics
Electrospun PLA and HPO/PLA nonwoven fabrics were fabricated by a homemade electrospinning device (Lodz University of Technology, Lodz, Poland). A needle with a flattened tip (inner diameter = 1.2 mm) was used for electrospinning. The solution was fed at a rate of 5 mL/h at a voltage of 22 kV. The electrospun fibers were collected on aluminum foil at a distance of 15 cm between the needle tip and the collector. Nonwoven fabrics were gathered on a cylindrical drum rotating (circumference = 1 m) at 35 rpm. All electrospinning trials were done at room conditions (temperature = 25 ± 2 °C; relative humidity = 50 ± 5%). For each electrospun nonwoven sample, 50 mL of polymer solutions were used.
2.2.3. Measurements and Characterizations
The surface structure and morphology of electrospun nonwovens fabrics were observed via scanning electron microscopy (Nova™ NanoSEM 230, FEI Company, Hillsboro, OR, USA). The diameters of the fibers were measured using imaging software (ImageJ, National Institutes of Health, Madison, WI, USA). The average fiber diameter and distribution for each experiment were estimated using micrographs of fiber morphology and 100 random measurements.
The chemical structure of the HPO, PLA, and HPO/PLA electrospun samples was analyzed by Fourier transform infrared spectroscopy (FTIR; Nicolet 6700, Thermo Electron Corp., Madison, WI, USA) with an attenuated total reflectance (ATR) accessory. All spectra were recorded in the wavelength range from 4000 to 600 cm−1. The investigation was carried out using the SpectraGryph program (Dr. Friedrich Menges, Oberstdorf, Germany).
The thermogravimetric analysis of all electrospun mats was carried out with a Perkin Elmer TGA 7 thermal analyser in a platinum measuring cell and using the Pyris program for data handling. The measurements were performed in artificial air with a 20˚C/min heating rate. Electrospun mats were heated up to 600 °C, starting from room temperature. Electrospun mats were acclimatized for at least one day in dry conditions (humidity below 5%) before measurement. The thermogravimetric analyzer is controlling sample weight before and during measurement.
The thickness of the electrospun nonwoven fabrics was measured using a digital thickness gauge for nonwovens (Checkline J-40-V, Electromatic Equipment Co., New York, NY, USA) at ten points. It was reported values were the average and standard deviations of obtained results.
The weight of the electrospun nonwoven fabrics was measured using a digital balance (Radwag WPS 510/C, Radom, Poland) at five different samples (5 × 5 cm2). It was reported values were the average and standard deviations of obtained results. The weight of the PLA, 3HPO/PLA, and 5HPO/PLA electrospun nonwoven fabrics were measured as 2.86 ± 0.37 g/m2, 3.04 ± 0.29 g/m2, and 3.13 ± 0.88 g/m2, respectively.
The air permeability of the electrospun nonwoven fabrics was measured using a Textest FX 3300 air permeability tester (Textest AG, Schwerzenbach, Switzerland) by fixing the airflow rate at 100 Pa with a test area of 20 cm2 at ten points. It was reported values were the average values and standard deviations of obtained results.
2.2.4. In Vitro Antibacterial Activity
The antibacterial efficiencies of the electrospun nonwoven fabrics were quantitatively evaluated according to the AATCC 100–1999 method “Antibacterial Finishes on Textile Materials”. Before starting the test, the microorganisms were activated in TSB medium (Tryptic Soy Broth, Merck, Darmstadt, Germany) at 37 ± °C for 24–48 h. The samples with a mass of 0.1000 ± 0.001 g were placed in sterile plastic containers with a volume of 100 mL. Then, 1 mL of inoculum was applied to the samples with the following densities: 3.5 × 106 ± 1.0 × 106 (Escherichia coli) and 1.5 × 106 ± 1.1 × 106 (Staphylococcus aureus). The inoculum density was determined by the culture method. The samples were then incubated for 24 h at a temperature of 37 ± 2 °C. The microorganisms were washed out of the samples immediately after inoculation (t = 0) and after 24 h of incubation (t = 24 h). Then it shook for 5 min in 50 mL of 0.85% sterile NaCl (Merck, Darmstadt, Germany) solution. The number of bacteria was determined by the culture method using TSA medium (Tryptic Soy Agar, Merck, Darmstadt, Germany) for 24 h at a temperature of 37 ± 2 °C. The colonies were counted after incubation, and the result was provided in a colony-forming unit (cfu)/g of samples. The tests were carried out in two separate repeats. The reduction in the number of bacteria was calculated using the following Equation:
where A is the number of bacteria from the flask containing the treated substrate after the 0 contact time and B is the number of bacteria for the addition of treated substrate at 24 h contact time.
Reduction rate (%) = [(A − B)/A] × 100
3. Results and Discussion
3.1. Morphology of the Electrospun Nonwoven Fabrics
In this research, various solutions containing different concentrations of HPO were prepared and used in the development of fibers to investigate the effect of electrospinning solution composition on the structure of the nonwoven fabrics and the electrospinning process. SEM was used to evaluate the morphologies and fiber diameter distributions of the PLA and HPO/PLA electrospun nonwoven fabrics, as shown in Figure 1. PLA electrospun fibers were estimated to have a diameter of 1.71 ± 1.02 µm. This result is in agreement with the data previously reported by Štular et al., who founded a similar average diameter for electrospun PLA fibers (~1.4 μm) [73]. Smooth cylindrical and continuous fibers were obtained from the PLA electrospinning solution. The average diameters of 3HPO/PLA and 5HPO/PLA electrospun fibers were 1.68 ± 0.58 µm, and 1.51 ± 0.64 µm, respectively. The diameter of the fibers decreased slightly after adding HPO. In addition, the fiber diameter distribution of HPO/PLA fibers was narrower than that of PLA fibers, as measured by the lower standard deviation in the graphs presented in Figure 1. This is possible since HPO is a natural surfactant, and its addition induces a reduction in surface tension of the resulting solution [74]. Lower surface tension reduces the resistance to the applied electric force, which causes the jet to stretch more, resulting in a thinner fiber diameter [75]. The fiber diameter of the HPO/PLA electrospun mats containing various amounts of HPO revealed that the fibers were randomly aligned and had thin diameters. Akşit et al. observed that increasing the amount of Hypericum capitatum extract within the PLGA/GE nanofibrous mats caused the fiber diameter to drop as the viscosity of the polymer solution decreased [38]. In addition, HPO loaded-PLA electrospun nonwoven fabrics appear to have favorable fiber diameter (1.5–1.7 µm) for cell adhesion and attachment, which is an advantage in biomedical applications, such as wound dressings and scaffolding. PLGA fibers with diameters ranging from 0.4 to 1.4 µm are suitable for cell attachment, according to researchers who have investigated the importance of the fiber diameter on the attachment of fibroblast cells [76]. To sum up, SEM images show that both PLA and HPO/PLA electrospun mats displayed a smooth and uniform morphology.
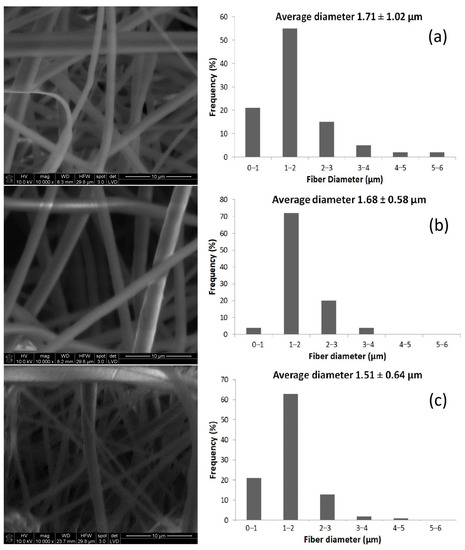
Figure 1.
SEM images and fiber diameter distributions of the electrospun nonwoven fabrics obtained from (a) PLA, (b) 3HPO/PLA and (c) 5HPO/PLA (×10,000) (average ± standard deviation).
3.2. ATR-FTIR Analysis of the Electrospun Nonwoven Fabrics
The functional groups of HPO, PLA, and HPO/PLA electrospun nonwoven fabrics were determined by ATR-FTIR spectroscopy (Figure 2).
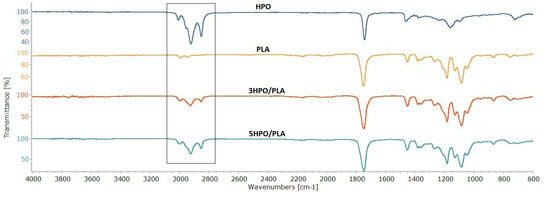
Figure 2.
ATR-FTIR spectra of the HPO, the PLA, and the HPO/PLA electrospun nonwoven fabrics.
The characteristic peaks of the HPO observed at 3308−1, 2853−1, 1743 cm−1, and 844 cm−1 were assigned to aromatic C-H stretching, aliphatic C-H stretching, C=O stretching, and aromatic rings, respectively [34,41]. The signal at 1237 cm−1 typically overlaps, most notably C–O ester, which is found in oils [77]. The characteristic peaks of the PLA electrospun fabrics observed at 2994 cm−1, 2944 cm−1,1745 cm−1, 1182 cm−1, 1085 cm−1, and 1045 cm−1 were assigned to C=O groups, asymmetric and symmetric stretching vibrations of the C-O-C group, and C-CH3 stretching, respectively [78,79]. The characteristic peaks of the 3HPO/PLA electrospun fabrics observed at 2995 cm−1, 2926 cm−1, and 2854 cm−1, assigned to –CH3 asymmetric, –CH3 symmetric, C-H stretching. The characteristic peaks of the 5HPO/PLA electrospun fabrics observed at 2997 cm−1, 2925 cm−1, and 2854 cm−1, were assigned to –CH3 asymmetric, –CH3 symmetric, C-H stretching. A small shift from 2994 cm−1 (PLA) to 2995–2997 cm−1 (3HPO/PLA-5HPO/PLA) was observed. This shift in the absorption peak shows the miscibility and interaction of HPO and PLA. With the addition of HPO to PLA fibers, the spectra of PLA electrospun nonwoven fabrics changed and the adsorption bands in the frequency range 3000–2800 cm−1 were broadened due to CH stretching vibrations of different groups in HPO. The peak intensity of the stretching of CH bands, which corresponds to the varying concentration of the HPO compound, shows that HPO was successfully incorporated into the PLA fibers.
3.3. Thermal Properties of the Electrospun Nonwoven Fabrics
Weight loss as a function of temperature due to degradation is measured by thermogravimetric analysis (TGA). In the TGA curves, the onset temperature, where the deterioration process began, and the end temperature, where the combustion process ended, were both evaluated. The TGA profiles of PLA and HPO/PLA electrospun nonwoven fabrics are shown in Figure 3. Thermal degradation of PLA occurs in a single weight loss phase, as evidenced by the TGA curves. PLA has higher onset degradation temperatures than PLA/HPO blends. The profile for PLA degradation peak was at ~368 °C [79] and it was completely decomposed at ~444 °C, associated with the loss of ester groups by unzipping depolymerization [80]. On the other hand, the HPO/PLA samples of both the 3HPO/PLA and the 5HPO/PLA electrospun nonwoven fabrics showed nearly two steps of degradation, as shown in Figure 4. These degradations occurred at temperatures ranging from ~360–354 °C to ~500–550 °C, respectively.
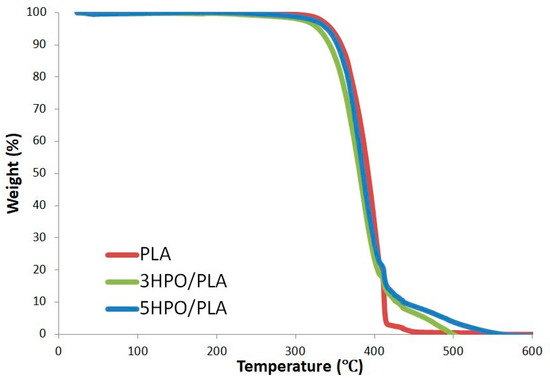
Figure 3.
TGA thermograms of the PLA, and HPO-loaded PLA electrospun nonwoven fabrics.
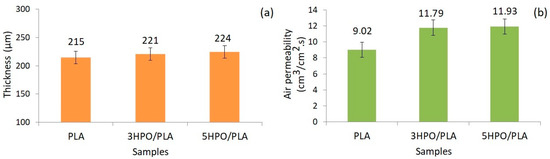
Figure 4.
The thickness (a) and, air permeability (b) test results of PLA and HPO/PLA electrospun nonwoven fabrics.
The thermal properties of the two HPO/PLA electrospun nonwoven fabrics showed almost identical trends. The addition of HPO resulted in a slight decrease in onset temperature (~10–15 °C), indicating that blended samples have low thermal stability, accounted for by the presence of HPO dispersed in the polymer. When the HPO content is increased from 3% to 5%, the temperature of decomposition increased remarkably, while the temperature of onset thermal degradation decreased insignificantly. The decomposition temperature of the 5HPO/PLA blend is ~50 °C higher than that of the 3HPO/PLA due to the presence of HPO in the matrix. The large difference in decomposition temperatures between the two starting materials may contribute to the degradation mechanism. This reassembly produces a physical barrier on the material’s surface, reducing the permeability of volatile degradation products out of the blend and, as a result, delaying the blend’s breakdown [77,81]. This result is similar to results in the literature, which reported that the thermal behavior of epoxidized soybean oil (a simultaneous compatibilizer and plasticizer) modified PLA natural rubber-based compound [81]. The thermal properties indicate that the HPO/PLA electrospun fabrics have higher decomposition temperatures than PLA.
3.4. Thickness and Air Permeability of the Electrospun Nonwoven Fabrics
Figure 4 shows the thickness and air permeability test results of PLA and HPO/PLA electrospun nonwoven fabrics. Thickness is an essential characteristic of textile materials since it has a direct influence on performance attributes such as permeability. The thicknesses of the PLA, 3HPO/PLA, and 5HPO/PLA electrospun nonwoven fabrics were measured as 215 ± 12 μm, 221 ± 8 μm, and 224 ± 6 μm, respectively. The volume of the electrospinning solutions was used to regulate the thickness. Although all electrospun nonwoven fabrics were made in the same way, the slightly higher thickness value of the HPO/PLA blended fabrics can be attributed to the existence of lower surface tension that reduces resistance to applied electric force, causing fibers to accumulate in a narrower area on the collector. Thus, the presence of HPO into PLA electrospinning solution increased nonwoven mat thickness.
Air permeability is important for wound dressing, to allow oxygen in which plays a vital role in wound healing [82]. The role of oxygen in wound healing can be summarized as follows; the oxidative killing of bacteria, re-epithelialization, angiogenesis, and collagen synthesis [83]. Parameters that may affect the air permeability of materials include fiber diameter, thickness, and porosity [84]. The air permeability of the PLA, 3HPO/PLA, and 5HPO/PLA electrospun nonwoven fabrics were measured as 9.02 ± 0.55 cm3/cm2.s, 11.79 ± 1.23 cm3/cm2.s and, 11.93 ± 0.77 cm3/cm2.s, respectively. Only a minor difference in air permeability was observed after the addition of HPO in PLA electrospun nonwoven fabric. The air permeability values indicated that the addition of HPO to the PLA electrospun fabrics caused an increase. Air permeability is still a complicated phenomenon for electrospun mats because airflow may affect the geometry of nanofibers due to the mats’ lightness and thinness [85]. This increase in air permeability can be explained by the fact that the fine fibers are more affected by the air flow since the intermolecular interaction changes with the addition of HPO to the structure. Avci and Gergeroglu reported that the air permeability values of herbal extract loaded-thermoplastic polyurethane electrospun mats were increased after the addition of extract into a polymer [37]. The measurement results showed that the presence of HPO in the PLA electrospun nonwoven fabric had a minor influence on the thickness and air permeability of samples. The fact that all samples are breathable is promising for the development of PLA-based electrospun biomedical materials such as wound dressings.
3.5. In Vitro Antibacterial Activity of the Electrospun Nonwoven Fabrics
The antibacterial efficiency of the cotton gauze, PLA, and 5HPO/PLA electrospun nonwoven fabrics against Staphylococcus aureus and Escherichia coli were tested. The results of the test are given in Table 1. The images of the petri dishes demonstrating antibacterial activity of samples have been given as Supplementary Materials (Figure S1).

Table 1.
Antibacterial activity of the electrospun nonwoven fabrics.
The cotton gauze and PLA electrospun nonwoven did not exhibit a growth inhibitory effect against Staphylococcus aureus and Escherichia coli. However, the HPO/PLA electrospun nonwoven fabric exhibited a good growth inhibitory effect against Staphylococcus aureus (>99.99) and Escherichia coli (>99.99). The outcome is consistent with the literature that reported that the antimicrobial activity of the Hypericum perforatum oil-loaded electrospun PEG-PCL membranes has good antibacterial activity against Staphylococcus aureus and Escherichia coli. This result can be explained by the intrinsic antimicrobial properties of HPO. HPO contains naphthodianthrones, hypericin, hyperforin, and flavonoids, which are responsible for their antibacterial properties [36,38,40,86]. HPO is a lipophilic compound that, has been demonstrated to disrupt cellular membranes in bacteria and fungi, hence inhibiting cellular respiration and ionic transport [87]. These findings support the contention that HPO/PLA electrospun nonwoven fabrics can be used as an antibacterial material in potential biomedical fields.
Hypericum perforatum oil has long been used as a traditional treatment method for wound treatment [30,31,32,33,86,87]. Staphylococcus aureus and Escherichia coli are predominant pathogens in wounds, and the source of most pathogenic contamination has been reported to be associated with exogenous contamination from contaminated wound dressing devices from the hospital setting or with the patient's normal flora [88,89,90]. At this point, the antibacterial activity against Staphylococcus aureus and Escherichia coli of the HPO-loaded PLA electrospun nonwoven may be beneficial in the healing process. For example, in comparison with conventional dressings (cotton gauze), PLA electrospun nonwoven fabrics loaded with HPO eliminate the disadvantages of the lack of antibacterial properties. The HPO-loaded PLA electrospun nonwoven fabric is simple and low-cost to produce, using commercially available technologies. In our effort to develop a biomaterial for biomedical engineering, we prepared a green fibrous material based on PLA loaded with HPO, which was assessed for the first time for bactericidal properties. This study shows that more studies should be done to emphasize the positive effects of HPO and PLA on human health and the environment and to address their possible impact.
Preliminary measurements of Hypericum perforatum oil-load poly(lactic acid) electrospun mat as an antibacterial material was performed in vitro analysis and this study investigated some performance properties required for its use as a textile material. Further research should include characterization of the Hypericum perforatum oil-load poly(lactic acid) electrospun in a clinical report to establish its practical application, and assessment of fiber-cell interaction and wound healing.
4. Conclusions
In this study, the HPO-loaded PLA electrospun nonwoven fabric as a potential antibacterial medium was successfully fabricated by a green strategy. The incorporation of HPO onto the PLA fibers showed good antibacterial behavior against Staphylococcus aureus and Escherichia coli bacteria. The diameters of the electrospun nonwoven fibers were determined to be in the micrometer range. ATR-FTIR results confirmed the presence of HPO incorporated within ultrafine PLA fibers. In the interaction between HPO and PLA, the incorporation of HPO slightly influenced the nonwoven fabric’s thermal stability. However, little difference in air permeability and thickness characteristics was observed between PLA and HPO/PLA samples. Overall, our findings indicated that the successful preparation of such HPO loaded-PLA nonwoven fabrics with good antibacterial properties could serve as a model for designing and developing innovative antibacterial materials for various biomedical fields such as drug delivery and wound dressing.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/app11178219/s1, Figure S1: Images of the petri dishes showing the antibacterial activity of samples.
Author Contributions
Conceptualization, A.P.; methodology, A.P.; formal analysis, A.P., J.S., D.S. (Dawid Stawski), N.T., A.B., D.S. (Dominik Sikorski), C.H., S.S.; investigation, A.P. and J.S.; resources, Z.D., D.S. (Dawid Stawski), I.K. and B.G; data curation, A.P. and Z.D; writing—original draft preparation, A.P.; writing—review and editing, A.P. and Z.D.; visualization, A.P.; supervision, Z.D.; funding acquisition, I.K. and B.G. All authors have read and agreed to the published version of the manuscript.
Funding
The article processing charge (APC) was funded by Lodz University of Technology. This work was financed by the statutory activity of the Institute of Materials Science of Textiles and Polymer Composites I-42/501/4-42-1-3.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
The authors would like to thank the Lodz University of Technology’s Institute of Materials Science of Textiles and Polymer Composites, as well as the Faculty of Biotechnology and Food Sciences, for their assistance with the research.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Hagen, R. Polylactic Acid. In Polymer Science: A Comprehensive Reference, 1st ed.; Matyjaszewski, K., Möller, M., Eds.; Elsevier: Amsterdam, The Netherlands, 2012; pp. 231–236. [Google Scholar]
- Puchalski, M.; Kwolek, S.; Szparaga, G.; Chrzanowski, M.; Krucińska, I. Investigation of the influence of PLA molecular structure on the crystalline Forms (α’ and α) and mechanical properties of wet spinning fibres. Polymers 2017, 9, 18. [Google Scholar] [CrossRef] [PubMed]
- Giełdowska, M.; Puchalski, M.; Szparaga, G.; Krucińska, I. Investigation of the Influence of PLA molecular and supramolecular structure on the kinetics of thermal-supported hydrolytic degradation of wet spinning fibres. Materials 2020, 13, 2111. [Google Scholar] [CrossRef] [PubMed]
- Połowiński, S.; Jantas, R.; Szumilewicz, J.; Stawski, D.; Herczyńska, L.; Draczyński, Z. Modification of PLA Fibers with Bioacceptable Hydrophilic Polymers. Fibres Text. East. Eur. 2012, 20, 78–85. [Google Scholar]
- Szuman, K.; Krucińska, I.; Boguń, M.; Draczyński, Z. PLA/PHA- biodegradable blends for pneumothermic fabrication of nonwovens. Autex Res. J. 2016, 16, 119–127. [Google Scholar] [CrossRef] [Green Version]
- Herrero-Herrero, M.; Gómez-Tejedor, J.; Vallés Lluch, A. PLA/PCL electrospun membranes of tailored fibres diameter as drug delivery systems. Eur. Polym. J. 2018, 99, 445–455. [Google Scholar] [CrossRef] [Green Version]
- Lascano, D.; Moraga, G.; Ivorra-Martinez, J.; Rojas-Lema, S.; Torres-Giner, S.; Balart, R.; Boronat, T.; Quiles-Carrillo, L. Development of Injection-Molded Polylactide Pieces with High Toughness by the Addition of Lactic Acid Oligomer and Characterization of Their Shape Memory Behavior. Polymers 2019, 11, 2099. [Google Scholar] [CrossRef] [Green Version]
- Ramakrishna, S.; Fujihara, K.; Teo, W.; Lim, T.; Ma, Z. An Introduction to Electrospinning and Nanofibers; World Scientific Publishing, Co.: Singapore, 2005. [Google Scholar]
- Huang, C.; Thomas, N.L. Fabrication of porous fibers via electrospinning: Strategies and applications. Polym. Rev. 2020, 60, 595–647. [Google Scholar] [CrossRef]
- Sundhari, D.; Dhineshbabu, N.R.; Sutha, S.; Saravanan, M.E.R. Encapsulation of bioactive agent (Curcumin, Moringa) in electrospun nanofibers—Some insights into recent research trends. Mater. Today-Proc. 2021, 46, 7–2682. [Google Scholar]
- Pakolpakçıl, A.; Draczynski, Z. Preparation and characterization of the advanced alginate-based nanofibrous nonwoven using EDC/NHS coupling agent by electrospinning. J. Text. Inst. 2021, 1–9. [Google Scholar] [CrossRef]
- Zafar, M.; Najeeb, S.; Khurshid, Z.; Vazirzadeh, M.; Zohaib, S.; Najeeb, B.; Sefat, F. Potential of Electrospun Nanofibers for Biomedical and Dental Applications. Materials 2016, 9, 73. [Google Scholar] [CrossRef] [PubMed]
- Qasim, S.B.; Zafar, M.S.; Najeeb, S.; Khurshid, Z.; Shah, A.H.; Husain, S.; Rehman, I.U. Electrospinning of Chitosan-Based Solutions for Tissue Engineering and Regenerative Medicine. Int. J. Mol. Sci. 2018, 19, 407. [Google Scholar] [CrossRef] [Green Version]
- Kakoria, A.; Sinha-Ray, S. A Review on Biopolymer-Based Fibers via Electrospinning and Solution Blowing and Their Applications. Fibers 2018, 6, 45. [Google Scholar] [CrossRef] [Green Version]
- Nagam Hanumantharao, S.; Rao, S. Multi-Functional Electrospun Nanofibers from Polymer Blends for Scaffold Tissue Engineering. Fibers 2019, 7, 66. [Google Scholar] [CrossRef] [Green Version]
- Chiu, C.-M.; Nootem, J.; Santiwat, T.; Srisuwannaket, C.; Pratumyot, K.; Lin, W.-C.; Mingvanish, W.; Niamnont, N. Enhanced Stability and Bioactivity of Curcuma comosa Roxb. Extract in Electrospun Gelatin Nanofibers. Fibers 2019, 7, 76. [Google Scholar] [CrossRef] [Green Version]
- Wróblewska-Krepsztul, J.; Rydzkowski, T.; Michalska-Pożoga, I.; Thakur, V.K. Biopolymers for Biomedical and Pharmaceutical Applications: Recent Advances and Overview of Alginate Electrospinning. Nanomaterials 2019, 9, 404. [Google Scholar] [CrossRef] [Green Version]
- Fattahi, F.S.; Khoddami, A.; Avinc, O.O. Poly(Lactic Acid) Nano Structure Mats as Potential Wound Dressings. Pamukkale Univ Muh Bilim Derg. 2020, 26, 1193–1203. [Google Scholar]
- Mozaffari, A.; Parvinzadeh Gashti, M.; Mirjalili, M.; Parsania, M. Argon and Argon–Oxygen Plasma Surface Modification of Gelatin Nanofibers for Tissue Engineering Applications. Membranes 2021, 11, 31. [Google Scholar] [CrossRef] [PubMed]
- Quoc Pham, L.; Uspenskaya, M.V.; Olekhnovich, R.O.; Olvera Bernal, R.A. A Review on Electrospun PVC Nanofibers: Fabrication, Properties, and Application. Fibers 2021, 9, 12. [Google Scholar] [CrossRef]
- Pakolpakçıl, A.; Karaca, E.; Becerir, B. Investigation of a natural pH-indicator dye for nanofibrous wound dressings. IOP Conf. Ser. Mater. Sci. Eng. 2018, 460, 012020. [Google Scholar] [CrossRef]
- Pakolpakçıl, A.; Osman, B.; Özer, E.T.; Şahan, Y.; Becerir, B.; Göktalay, G.; Karaca, E. Halochromic composite nanofibrous mat for wound healing monitoring. Mater. Res. Express 2019, 6, 1250c3. [Google Scholar] [CrossRef]
- Pakolpakçıl, A.; Osman, B.; Göktalay, G.; Özer, E.T.; Şahan, Y.; Becerir, B.; Karaca, E. Design and in vivo evaluation of alginate-based pH-sensing electrospun wound dressing containing anthocyanins. J. Polym. Res. 2021, 28, 50. [Google Scholar] [CrossRef]
- Parin, F.N.; Terzioğlu, P.; Sicak, Y.; Yildirim, K.; Öztürk, M. Pine honey–loaded electrospun poly (vinyl alcohol)/gelatin nanofibers with antioxidant properties. J. Text. Inst. 2021, 112, 628–635. [Google Scholar] [CrossRef]
- Ulag, S.; Ilhan, E.; Demirhan, R.; Sahin, A.; Yilmaz, B.K.; Aksu, B.; Sengor, M.; Ficai, D.; Titu, A.M.; Ficai, A.; et al. Propolis-Based Nanofiber Patches to Repair Corneal Microbial Keratitis. Molecules 2021, 26, 2577. [Google Scholar] [CrossRef] [PubMed]
- Pakolpakçıl, A.; Draczynski, Z. Green approach to develop bee pollen-loaded alginate based nanofibrous mat. Materials 2021, 14, 2775. [Google Scholar] [CrossRef]
- Zare, M.R.; Khorram, M.; Barzegar, S.; Asadian, F.; Zareshahrabadi, Z.; Saharkhiz, M.J.; Ahadian, S.; Zomorodian, K. Antimicrobial core–shell electrospun nanofibers containing Ajwain essential oil for accelerating infected wound healing. Int. J. Pharm. 2021, 603, 120698. [Google Scholar] [CrossRef] [PubMed]
- Mele, E. Electrospinning of Essential Oils. Polymers 2020, 12, 908. [Google Scholar] [CrossRef] [Green Version]
- De Luca, I.; Pedram, P.; Moeini, A.; Cerruti, P.; Peluso, G.; Di Salle, A.; Germann, N. Nanotechnology development for formulating essential oils in wound dressing materials to promote the wound-healing process: A Review. Appl. Sci. 2021, 11, 1713. [Google Scholar] [CrossRef]
- Ozturk, N.; Korkmaz, S.; Ozturk, Y. Wound-healing activity of St. John’s Wort (Hypericum perforatum L.) on chicken embryonic fibroblasts. J. Ethnopharmacol. 2007, 111, 33–39. [Google Scholar] [CrossRef]
- Süntar, I.P.; Akkol, E.K.; Yilmazer, D.; Baykal, T.; Kirmizibekmez, H.; Alper, M.; Yeşilada, E. Investigations on the in vivo wound healing potential of Hypericum perforatum L. J. Ethnopharmacol. 2010, 27, 468–477. [Google Scholar] [CrossRef]
- Kiyan, G.S.; Uyanikgil, Y.; Altunci, Y.A.; Çavuşoğlu, T.; Uyanikgil, E.Ö.Ç.; Karabey, F. Investigation of acute effects of Hypericum perforatum (St. John’s Wort-Kantaron) treatment in experimental thermal burns and comparison with silver sulfadiazine treatment. Ulus. Travma ve Acil Cerrahi Derg. 2015, 21, 323–336. [Google Scholar]
- Altan, A.; Aras, M.H.; Damlar, İ.; Gökçe, H.; Özcan, O.; Alpaslan, C. The effect of Hypericum Perforatum on wound healing of oral mucosa in diabetic rats. Eur Oral Res. 2018, 52, 143–149. [Google Scholar] [CrossRef]
- Pourhojat, F.; Sohrabi, M.; Shariati, S.; Asadpour, L. Evaluation of poly ε-caprolactone electrospun nanofibers loaded with Hypericum perforatum extract as a wound dressing. Res. Chem. Intermed. 2017, 43, 297–320. [Google Scholar] [CrossRef]
- Pourhojat, F.; Shariat, S.; Sohrabi, M.; Mahdavi, H.; Asadpour, L. Preparation of antibacterial electrospun Poly lactic-co–glycolic acid nanofibers containing Hypericum Perforatum with bedsore healing property and evaluation of its drug release performance. Int. J. Nano Dimens. 2018, 9, 286–297. [Google Scholar]
- Eğri, O.; Erdemir, N. Production of Hypericumperforatum oil loaded membranes for wound dressing material and in vitro tests. Artif. Cells Nanomed. Biotechnol. 2019, 47, 1404–1415. [Google Scholar] [CrossRef] [Green Version]
- Avci, H.; Gergeroglu, H. Synergistic effects of plant extracts and polymers on structural and antibacterial properties for wound healing. Polym. Bull. 2019, 76, 3709–3731. [Google Scholar] [CrossRef]
- Akşit, N.N.; Gürdap, S.; İşoğlu, S.D.; İşoğlu, İ.A. Preparation of antibacterial electrospun poly(D, L-lactide-co-glycolide)/gelatin blend membranes containing Hypericumcapitatum var. capitatum. Int. J. Polym. Mater. 2020, 70, 797–809. [Google Scholar] [CrossRef]
- Gunes, S.; Tamburaci, S.; Tihminlioglu, F. A novel bilayer zein/MMT nanocomposite incorporated with H. perforatum oil for wound healing. J. Mater. Sci. Mater. Med. 2020, 31, 7. [Google Scholar] [CrossRef] [PubMed]
- Maver, T.; Kurečič, M.; Pivec, T.; Maver, U.; Gradisnik, L.; Gasparic, P.; Kaker, B.; Bratusa, A.; Hribernik, S.; Kleinschek, K.S. Needleless electrospun carboxymethyl cellulose/polyethylene oxide mats with medicinal plant extracts for advanced wound care applications. Cellulose 2020, 27, 4487–4508. [Google Scholar] [CrossRef]
- Mouro, C.; Gomes, A.P.; Gouveia, I.C. Double-layer PLLA/PEO_Chitosan nanofibrous mats containing Hypericum perforatum L. as an effective approach for wound treatment. Polym. Adv. Technol. 2021, 32, 1493–1506. [Google Scholar] [CrossRef]
- Guleken, Z.; Depciuch, J.; Ege, H.; İlbay, G.; Kalkandelen, C.; Ozbeyli, D.; Bulut, H.; Sener, G.; Tarhan, N.; Kuruca, S.E. Spectrochemical and biochemical assay comparison study of the healing effect of the Aloe vera and Hypericum perforatum loaded nanofiber dressings on diabetic wound. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2021, 254, 119639. [Google Scholar] [CrossRef] [PubMed]
- Au, H.T.; Pham, L.N.; Vu, T.H.T.; Park, J.S. Fabrication of an antibacterial non-woven mat of a poly(lactic acid)/chitosan blend by electrospinning. Macromol. Res. 2012, 20, 51–58. [Google Scholar] [CrossRef]
- Karami, Z.; Rezaeian, I.; Zahedi, P.; Abdollahi, M. Preparation and performance evaluations of electrospun poly(ε-caprolactone), poly(lactic acid), and their hybrid (50/50) nanofibrous mats containing thymol as an herbal drug for effective wound healing. J. Appl. Polym. Sci. 2013, 129, 756–766. [Google Scholar] [CrossRef]
- Sutjarittangtham, K.; Sanpa, S.; Tunkasiri, T.; Chantawannakul, P.; Intatha, U.; Eitssayeam, S. Bactericidial effects of propolis/PLA nanofibres obtained via electrospinning. J. Apic. Res. 2014, 53, 109–115. [Google Scholar] [CrossRef]
- Munteanu, B.S.; Aytac, Z.; Pricope, G.M.; Uyar, T.; Vasile, C. Polylactic acid (PLA)/Silver-NP/VitaminE bionanocomposite electrospun nanofibers with antibacterial and antioxidant activity. J. Nanopart. Res. 2014, 16, 2643. [Google Scholar] [CrossRef] [Green Version]
- Bonan, R.F.; Bonan, P.R.F.; Batista, A.U.D.; Sampaio, F.C.; Albuquerque, A.J.R.; Moraes, M.C.B.; Mattoso, L.H.C.; Glenn, G.M.; Medeiros, E.S.; Oliveira, J.E. In vitro antimicrobial activity of solution blow spun poly(lactic acid)/polyvinylpyrrolidone nanofibers loaded with Copaiba (Copaifera sp.) oil. Mater. Sci. Eng. C 2015, 48, 372–377. [Google Scholar] [CrossRef] [PubMed]
- Jiang, S.; Lv, J.; Ding, M.; Li, Y.; Wang, H.; Jiang, S. Release behavior of tetracycline hydrochloride loaded chitosan/poly(lactic acid) antimicrobial nanofibrous membranes. Mater. Sci. Eng. C 2016, 59, 86–91. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, S.; Zhang, R.; Lan, W.; Qin, W. Development of poly(lactic acid)/chitosan fibers loaded with essential oil for antimicrobial applications. Nanomaterials 2017, 7, 194. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gomaa, S.F.; Madkour, T.M.; Moghannem, S.; El-Sherbiny, I.M. New polylactic acid/ cellulose acetate-based antimicrobial interactive single dose nanofibrous wound dressing mats. Int. J. Biol. Macromol. 2017, 105, 1148–1160. [Google Scholar] [CrossRef]
- Zhang, W.; Huang, C.; Kusmartseva, O.; Thomas, N.L.; Mele, E. Electrospinning of polylactic acid fibres containing tea tree and manuka oil. React. Funct. Polym. 2017, 117, 106–111. [Google Scholar] [CrossRef]
- Scaffaro, R.; Lopresti, F.; D’Arrigo, M.; Marino, A.; Nostro, A. Efficacy of poly(lactic acid)/carvacrol electrospun membranes against Staphylococcus aureus and Candida albicans in single and mixed cultures. Appl. Microbiol. Biotechnol. 2018, 102, 4171–4181. [Google Scholar] [CrossRef]
- Wang, P.; Mele, E. Effect of Antibacterial Plant Extracts on the Morphology of Electrospun Poly(Lactic Acid) Fibres. Materials 2018, 11, 923. [Google Scholar] [CrossRef] [Green Version]
- Nepomuceno, N.C.; Barbosa, M.A.; Bonan, R.F.; Oliveira, J.E.; Sampaio, F.C.; Medeiros, E.S. Antimicrobial activity of PLA/PEG nanofibers containing terpinen-4-ol against Aggregatibacter actinomycetemcomitans. J. Appl. Polym. Sci. 2018, 135, 1–9. [Google Scholar] [CrossRef]
- Xu, F.; Wang, H.; Zhang, J.; Jiang, L.; Zhang, W.; Hu, Y. A facile design of EGF conjugated PLA/gelatin electrospun nanofibers for nursing care of in vivo wound healing applications. J. Ind. Text. 2020. [Google Scholar] [CrossRef]
- Kost, B.; Svyntkivska, M.; Brzeziński, M.; Makowski, T.; Piorkowska, E.; Rajkowska, K.; Kunicka-Styczyńska, A.; Biela, T. PLA/β-CD-based fibres loaded with quercetin as potential antibacterial dressing materials. Colloids Surf. B 2020, 190, 110949. [Google Scholar] [CrossRef] [PubMed]
- Sharifi, M.; Bahrami, S.H.; Nejad, N.H.; Milan, P.B. Electrospun PCL and PLA hybrid nanofibrous scaffolds containing Nigella sativa herbal extract for effective wound healing. J. Appl Polym Sci. 2020, 137, e49528. [Google Scholar] [CrossRef]
- Argui, H.; Suner, S.C.; Periz, Ç.D.; Ulusoy, S.; Ben-Attia, M.; Oral, A.; Coşkun, Y.; Oral, A.; Said, H. Fabrication, Characterization, In Vitro Release, and Some Biological Activities of Eucalyptus Essential Oil Loaded Poly (Lactic Acid) Nanofibers. ASMI 2021, 4, 54–63. [Google Scholar]
- Alisir, S.H.; Ozdemir, N.; Burgaz, E.; Dege, N.; Canavar, Y.E. Fabrication and Antimicrobial Activity of Poly(lactic acid) Nanofibers Containing Firstly Synthesized Silver Diclofenac Complex with (2-methylimidazole) for Wound Dressing Applications. Fibers Polym. 2021, 1–12. [Google Scholar] [CrossRef]
- Milanesi, G.; Vigani, B.; Rossi, S.; Sandri, G.; Mele, E. Chitosan-Coated Poly(lactic acid) Nanofibres Loaded with Essential Oils for Wound Healing. Polymers 2021, 13, 2582. [Google Scholar] [CrossRef]
- Ciftci, F.; Ayan, S.; Duygulu, N.; Yilmazer, Y.; Karavelioglu, Z.; Vehapi, M.; Koc, R.C.; Sengor, M.; Yılmazer, H.; Ozcimen, D.; et al. Selenium and clarithromycin loaded PLA-GO composite wound dressings by electrospinning method. Int. J. Polym. Mater. 2021, 1–12. [Google Scholar] [CrossRef]
- Hajikhani, M.; Emam-Djomeh, Z.; Askari, G. Fabrication and characterization of mucoadhesive bioplastic patch via coaxial polylactic acid (PLA) based electrospun nanofibers with antimicrobial and wound healing application. Int. J. Biol. Macromol. 2021, 172, 143–153. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Cheng, X.; Xiao, L.; Wang, Q.; Yan, K.; Su, Z.; Wang, L.; Ma, C.; Wang, Y. Inside-outside Ag nanoparticles-loaded polylactic acid electrospun fiber for long-term antibacterial and bone regeneration. Int. J. Biol. Macromol. 2021, 167, 1338–1348. [Google Scholar] [CrossRef]
- Zhang, R.; Lan, W.; Ji, T.; Sameen, D.E.; Ahmed, S.; Qin, W.; Liu, Y. Development of polylactic acid/ZnO composite membranes prepared by ultrasonication and electrospinning for food packaging. LWT 2021, 135, 110072. [Google Scholar] [CrossRef]
- Maroufi, L.Y.; Ghorbani, M.; Mohammadi, M.; Pezeshki, A. Improvement of the physico-mechanical properties of antibacterial electrospun poly lactic acid nanofibers by incorporation of guar gum and thyme essential oil. Colloids Surf. A Physicochem Eng. Asp. 2021, 622, 126659. [Google Scholar] [CrossRef]
- Aman, M.M.; Ramezani, S.; Hosseini, H.; Mortazavian, A.M.; Hosseini, S.M.; Ghorbani, M. Electrospun Antibacterial and Antioxidant Zein/Polylactic Acid/Hydroxypropyl Methylcellulose Nanofibers as an Active Food Packaging System. Food Bioprocess. Technol. 2021, 14, 1529–1541. [Google Scholar] [CrossRef]
- Fan, T.; Daniels, R. Preparation and Characterization of Electrospun Polylactic Acid (PLA) Fiber Loaded with Birch Bark Triterpene Extract for Wound Dressing. AAPS PharmSciTech 2021, 22, 205. [Google Scholar] [CrossRef]
- Zhu, P.; Zhang, X.; Wang, Y.; Li, C.; Wang, X.; Tie, J.; Wang, Y. Electrospun polylactic acid nanofiber membranes containing Capparis spinosa L. extracts for potential wound dressing applications. J. Appl Polym Sci. 2021, 138, e50800. [Google Scholar] [CrossRef]
- Ibili, H.; Dasdemir, M.; Çankaya, İ.İ.T.; Orhan, M.; Güneşoğlu, C.; Arabacı, A.S. Investigation of poly(lactic acid) nanocapsules containing the plant extract via coaxial electrospraying method for functional nonwoven applications. J. Ind. Text. 2021. [Google Scholar] [CrossRef]
- Jahanmardi, Y.; Tavanaie, M.A.; Tehrani-Bagha, A.R. Curcumin release from blended polycaprolactone/polylactic acid electrospun nanofibrous meshes. J. Ind. Text. 2021, 50, 1065–1078. [Google Scholar] [CrossRef]
- Fernandes, D.M.; Barbosa, W.S.; Rangel, W.S.P.; Valle, I.M.M.; Matos, A.P.S.; Melgaço, F.G.; Dias, M.L.; Júnior, E.R.; Silva, L.C.P.; Abreu, L.C.L.; et al. Polymeric membrane based on polylactic acid and babassu oil for wound healing. Mater. Today Commun. 2021, 26, 102173. [Google Scholar] [CrossRef]
- Argui, H.; Suner, S.; Periz, Ç.; Ulusoy, S.; Türker, G.; Ben-Attia, M.; Büyükkaya, F.; Oral, A.; Coşkun, Y.; Said, H. Preparation of cypress (Cupressus sempervirens L.) essential oil loaded poly(lactic acid) nanofibers. Open Chem. 2021, 19, 796–805. [Google Scholar] [CrossRef]
- Štular, D.; Kruse, M.; Župunski, V.; Kreinest, L.; Medved, J.; Gries, T.; Blaeser, A.; Jerman, I.; Simončič, B.; Tomšič, B. Smart Stimuli-Responsive Polylactic Acid-Hydrogel Fibers Produced via Electrospinning. Fibers Polym. 2019, 20, 1857–1868. [Google Scholar] [CrossRef]
- Mao, Y.; Guidoin, R.; Li, Y.; Brochu, G.; Zhang, Z.; Wang, L. Soybean-derived phospholipids complexed poly (lactic-co-glycolic acid) nanofibrous scaffolds for tissue engineering applications. Mater. Des. 2021, 205, 109737. [Google Scholar] [CrossRef]
- İçoğlu, H.; Oğulata, R. Effect of ambient parameters on morphology of electrospun poly (trimethylene terephthalate) (PTT) fibers. Tekstil ve Konfeksiyon 2017, 27, 215–223. [Google Scholar]
- Kim, M.S.; Kim, D.; Kang, J.K.; Lee, J.H.; Kim, H.L.; Koo, M.A.; Lee, M.H.; Park, J.C. Migration of human dermal fibroblast is affected by the diameter of the electrospun PLGA fiber. Biomater Res. 2012, 16, 135–139. [Google Scholar]
- Silverajah, V.S.G.; Ibrahim, N.A.; Zainuddin, N.; Yunus, W.M.Z.W.; Hassan, H.A. Mechanical, thermal and morphological properties of poly(lactic acid)/epoxidized palm olein blend. Molecules 2012, 17, 11729–11747. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kister, G.; Cassanas, G.; Vert, M.; Pauvert, B.; Térol, A. Vibrational analysis of poly(Llactic acid). J. Raman Spectrosc. 1995, 26, 307–311. [Google Scholar] [CrossRef]
- Alharbi, H.F.; Luqman, M.; Khalil, K.A.; Elnakady, Y.A.; Abd-Elkader, O.H.; Rady, A.M.; Alharthi, N.H.; Karim, M.R. Fabrication of core-shell structured nanofibers of poly (lactic acid) and poly (vinyl alcohol) by coaxial electrospinning for tissue engineering. Eur. Polym. J. 2018, 98, 483–491. [Google Scholar] [CrossRef]
- Restrepo, I.; Medina, C.; Meruane, V.; Fakhrabadi, A.A.; Flores, P.; Llamazare, S.R. The effect of molecular weight and hydrolysis degree of poly(vinyl alcohol)(PVA) on the thermal and mechanical properties of poly(lactic acid)/PVA blends. Polímeros 2018, 28, 2. [Google Scholar] [CrossRef] [Green Version]
- Burkov, A.; Kraev, A.; Grishin, M.; Vesnin, R.; Fomin, S.; Iordanskii, A. Structural Features and Properties’ Characterization of Polylactic Acid/Natural Rubber Blends with Epoxidized Soybean Oil. Polymers 2021, 13, 1101. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Hu, H. Spacer fabric-based exuding wound dressing—Part II: Comparison with commercial wound dressings. Text. Res. J. 2017, 87, 1481–1493. [Google Scholar] [CrossRef]
- Rodriguez, P.G.; Felix, F.N.; Woodley, D.T.; Shim, E.K. The role of oxygen in wound healing: A review of the literature. Dermatol Surg. 2008, 34, 1159–1169. [Google Scholar] [CrossRef]
- Liu, Y.Q.; Feng, J.W.; Zhang, C.C.; Teng, Y.; Liu, Z.; He, J.H. Air permeability of nanofiber membrane with hierarchical structure. Therm. Sci. 2018, 22, 1637–1643. [Google Scholar] [CrossRef]
- Afshari, M. Electrospun Nanofibers, 1st ed.; Woodhead Publishing: Cambridge, UK, 2016; p. 162. [Google Scholar]
- Saddiqe, Z.; Naeem, I.; Maimoona, A. A review of the antibacterial activity of Hypericum perforatum L. J. Ethnopharmacol. 2010, 131, 511–521. [Google Scholar] [CrossRef] [PubMed]
- Crockett, S.L. Essential oil and volatile components of the genus Hypericum (Hypericaceae). Nat. Prod. Commun. 2010, 5, 1493–1506. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alebachew, T.; Yismaw, G.; Derabe, A.; Sisay, Z. Staphylococcus aureus burn wound infection among patients attending yekatit 12 hospital burn unit, addis ababa, ethiopia. Ethiop J. Health Sci. 2012, 22, 209–213. [Google Scholar] [PubMed]
- Forson, O.A.; Ayanka, E.; Olu-Taiwo, M.; Pappoe-Ashong, P.J.; Ayeh-Kumi, P.J. Bacterial infections in burn wound patients at a tertiary teaching hospital in Accra, Ghana. Ann. Burns Fire Disasters 2017, 30, 116–120. [Google Scholar]
- Cetik, Y.S.; Demir, C.; Cengiz, M.; Ayhanci, A. Protective properties of kefir on burn wounds of mice that were infected with S. aureus, P. auroginasa and E. coli. Cell Mol. Biol. 2019, 65, 60–65. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).