Investigation of the LCST-Thermoresponsive Behavior of Novel Oligo(Ethylene Glycol)-Modified Pentafluorostyrene Homopolymers
Abstract
Featured Application
Abstract
1. Introduction
2. Materials and Methods
2.1. Synthesis of the Monomer EFS-8
2.2. Synthesis of the Homopolymer pEFS8-x
3. Results and Discussion
3.1. Polymer Synthesis and Characterization
3.2. Size Characterization in Aqueous Solutions
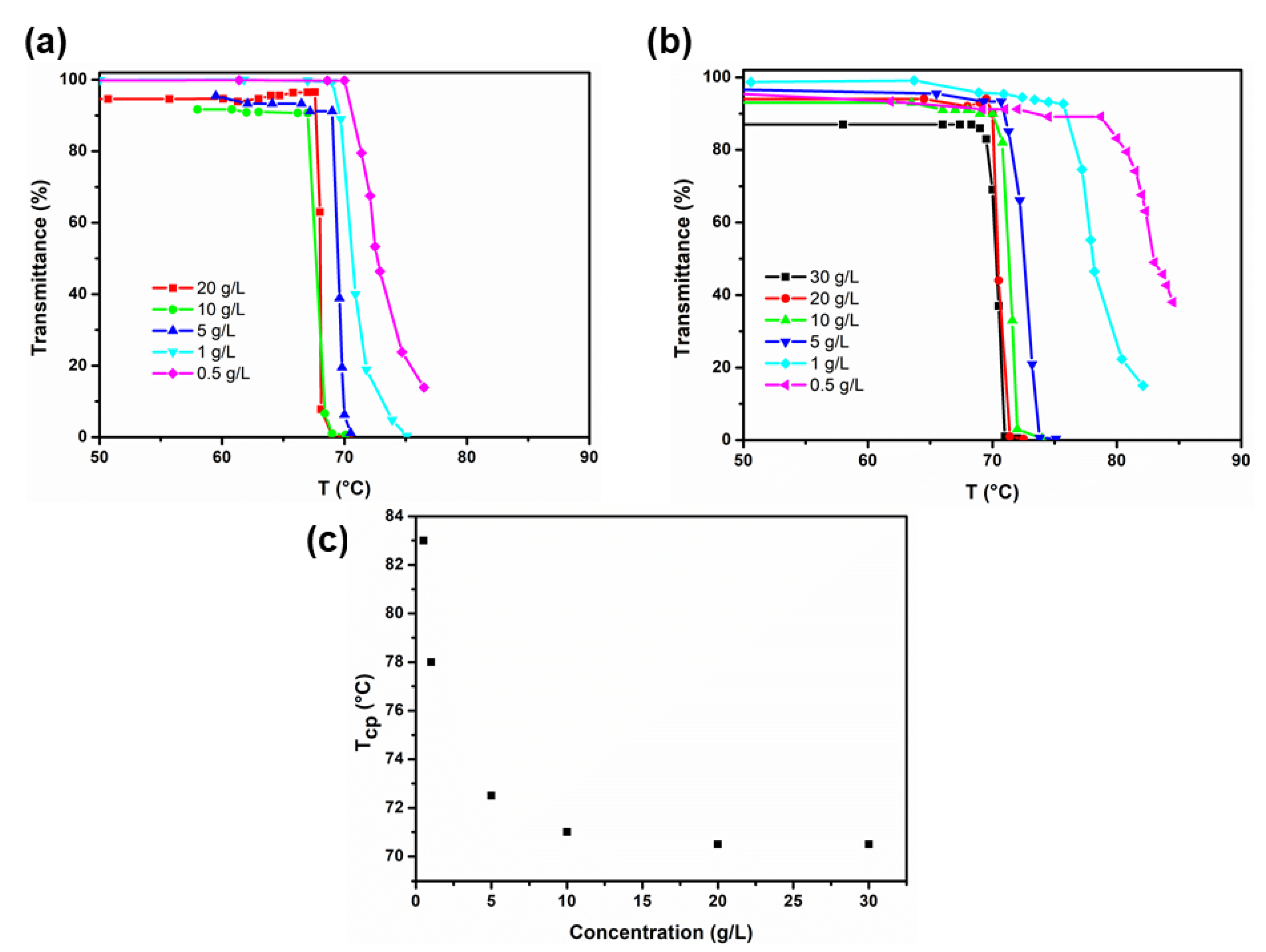
3.3. Characterization of the Phase Transition
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Wei, M.; Gao, Y.; Li, X.; Serpe, M.J. Stimuli-Responsive Polymers and Their Applications. Polym. Chem. 2016, 8, 127–143. [Google Scholar] [CrossRef]
- Priya James, H.; John, R.; Alex, A.; Anoop, K.R. Smart Polymers for the Controlled Delivery of Drugs—A Concise Overview. Acta Pharm. Sin. B 2014, 4, 120–127. [Google Scholar] [CrossRef] [PubMed]
- Municoy, S.; Álvarez Echazú, M.I.; Antezana, P.E.; Galdopórpora, J.M.; Olivetti, C.; Mebert, A.M.; Foglia, M.L.; Tuttolomondo, M.V.; Alvarez, G.S.; Hardy, J.G.; et al. Stimuli-Responsive Materials for Tissue Engineering and Drug Delivery. Int. J. Mol. Sci. 2020, 21, 4724. [Google Scholar] [CrossRef] [PubMed]
- Hu, L.; Zhang, Q.; Li, X.; Serpe, M.J. Stimuli-Responsive Polymers for Sensing and Actuation. Mater. Horiz. 2019, 6, 1774–1793. [Google Scholar] [CrossRef]
- Nagappan, S.; Moorthy, M.S.; Rao, K.M.; Ha, C.-S. Stimuli-Responsive Smart Polymeric Coatings: An Overview. In Industrial Applications for Intelligent Polymers and Coatings; Hosseini, M., Makhlouf, A.S.H., Eds.; Springer International Publishing: Cham, Switzerland, 2016; pp. 27–49. ISBN 978-3-319-26893-4. [Google Scholar]
- Kim, Y.-J.; Matsunaga, Y.T. Thermo-Responsive Polymers and Their Application as Smart Biomaterials. J. Mater. Chem. B 2017, 5, 4307–4321. [Google Scholar] [CrossRef]
- Bordat, A.; Boissenot, T.; Nicolas, J.; Tsapis, N. Thermoresponsive Polymer Nanocarriers for Biomedical Applications. Adv. Drug Deliv. Rev. 2019, 138, 167–192. [Google Scholar] [CrossRef] [PubMed]
- Schattling, P.; Jochum, F.D.; Theato, P. Multi-Stimuli Responsive Polymers—The All-in-One Talents. Polym. Chem. 2013, 5, 25–36. [Google Scholar] [CrossRef]
- de la Rosa, V.R.; Woisel, P.; Hoogenboom, R. Supramolecular Control over Thermoresponsive Polymers. Mater. Today 2016, 19, 44–55. [Google Scholar] [CrossRef]
- Umapathi, R.; Reddy, P.M.; Rani, A.; Venkatesu, P. Influence of Additives on Thermoresponsive Polymers in Aqueous Media: A Case Study of Poly(N-Isopropylacrylamide). Phys. Chem. Chem. Phys. 2018, 20, 9717–9744. [Google Scholar] [CrossRef]
- Dalgakiran, E.; Tatlipinar, H. The Role of Hydrophobic Hydration in the LCST Behaviour of POEGMA 300 by All-Atom Molecular Dynamics Simulations. Phys. Chem. Chem. Phys. 2018, 20, 15389–15399. [Google Scholar] [CrossRef]
- Sambe, L.; Stoffelbach, F.; Lyskawa, J.; Delattre, F.; Fournier, D.; Bouteiller, L.; Charleux, B.; Cooke, G.; Woisel, P. Host–Guest Modulation of the Micellization of a Tetrathiafulvalene-Functionalized Poly(N-Isopropylacrylamide). Macromolecules 2011, 44, 6532–6538. [Google Scholar] [CrossRef]
- Li, Z.; Kyeremateng, S.O.; Fuchise, K.; Kakuchi, R.; Sakai, R.; Kakuchi, T.; Kressler, J. Aggregation Behavior of Poly(N-Isopropylacrylamide) Semitelechelics with a Perfluoroalkyl Segment in Watera. Macromol. Chem. Phys. 2009, 210, 2138–2147. [Google Scholar] [CrossRef]
- Matsumoto, K.; Terashima, T.; Sugita, T.; Takenaka, M.; Sawamoto, M. Amphiphilic Random Copolymers with Hydrophobic/Hydrogen-Bonding Urea Pendants: Self-Folding Polymers in Aqueous and Organic Media. Macromolecules 2016, 49, 7917–7927. [Google Scholar] [CrossRef]
- Koda, Y.; Terashima, T.; Sawamoto, M. Multimode Self-Folding Polymers via Reversible and Thermoresponsive Self-Assembly of Amphiphilic/Fluorous Random Copolymers. Macromolecules 2016, 49, 4534–4543. [Google Scholar] [CrossRef]
- Hattori, G.; Takenaka, M.; Sawamoto, M.; Terashima, T. Nanostructured Materials via the Pendant Self-Assembly of Amphiphilic Crystalline Random Copolymers. J. Am. Chem. Soc. 2018, 140, 8376–8379. [Google Scholar] [CrossRef]
- Zhang, Q.; Weber, C.; Schubert, U.S.; Hoogenboom, R. Thermoresponsive Polymers with Lower Critical Solution Temperature: From Fundamental Aspects and Measuring Techniques to Recommended Turbidimetry Conditions. Mater. Horiz. 2017, 4, 109–116. [Google Scholar] [CrossRef]
- Van Durme, K.; Van Assche, G.; Van Mele, B. Kinetics of Demixing and Remixing in Poly(N-Isopropylacrylamide)/Water Studied by Modulated Temperature DSC. Macromolecules 2004, 37, 9596–9605. [Google Scholar] [CrossRef]
- Pham, Q.-T.; Yao, Z.-H.; Chang, Y.-T.; Wang, F.-M.; Chern, C.-S. LCST Phase Transition Kinetics of Aqueous Poly(N-Isopropylacrylamide) Solution. J. Taiwan Inst. Chem. Eng. 2018, 93, 63–69. [Google Scholar] [CrossRef]
- Liu, P.; Song, L.; Li, N.; Lin, J.; Huang, D. Time Dependence of Phase Separation Enthalpy Recovery Behavior in Aqueous Poly(N-Isopropylacrylamide) Solution. J. Therm. Anal. Calorim. 2017, 130, 843–850. [Google Scholar] [CrossRef]
- Yan, Y.; Huang, L.; Zhang, Q.; Zhou, H. Concentration Effect on Aggregation and Dissolution Behavior of Poly(N-Isopropylacrylamide) in Water. J. Appl. Polym. Sci. 2015, 132. [Google Scholar] [CrossRef]
- Gao, Y.; Yang, J.; Ding, Y.; Ye, X. Effect of Urea on Phase Transition of Poly(N-Isopropylacrylamide) Investigated by Differential Scanning Calorimetry. J. Phys. Chem. B 2014, 118, 9460–9466. [Google Scholar] [CrossRef]
- Bischofberger, I.; Calzolari, D.C.E.; De Los Rios, P.; Jelezarov, I.; Trappe, V. Hydrophobic Hydration of Poly-N-Isopropyl Acrylamide: A Matter of the Mean Energetic State of Water. Sci. Rep. 2014, 4, 4377. [Google Scholar] [CrossRef]
- Ding, Y.; Ye, X.; Zhang, G. Microcalorimetric Investigation on Aggregation and Dissolution of Poly(N-Isopropylacrylamide) Chains in Water. Macromolecules 2005, 38, 904–908. [Google Scholar] [CrossRef]
- Lutz, J.-F.; Akdemir, Ö.; Hoth, A. Point by Point Comparison of Two Thermosensitive Polymers Exhibiting a Similar LCST: Is the Age of Poly(NIPAM) Over? J. Am. Chem. Soc. 2006, 128, 13046–13047. [Google Scholar] [CrossRef] [PubMed]
- Grishkewich, N.; Akhlaghi, S.P.; Zhaoling, Y.; Berry, R.; Tam, K.C. Cellulose Nanocrystal-Poly(Oligo(Ethylene Glycol) Methacrylate) Brushes with Tunable LCSTs. Carbohydr. Polym. 2016, 144, 215–222. [Google Scholar] [CrossRef] [PubMed]
- Koda, Y.; Terashima, T.; Sawamoto, M.; Maynard, H.D. Amphiphilic/Fluorous Random Copolymers as a New Class of Non-Cytotoxic Polymeric Materials for Protein Conjugation. Polym. Chem. 2014, 6, 240–247. [Google Scholar] [CrossRef]
- Martinelli, E.; Guazzelli, E.; Galli, G.; Telling, M.T.F.; Poggetto, G.D.; Immirzi, B.; Domenici, F.; Paradossi, G. Prolate and Temperature-Responsive Self-Assemblies of Amphiphilic Random Copolymers with Perfluoroalkyl and Polyoxyethylene Side Chains in Solution. Macromol. Chem. Phys. 2018, 219, 1800210. [Google Scholar] [CrossRef]
- Guazzelli, E.; Masotti, E.; Biver, T.; Pucci, A.; Martinelli, E.; Galli, G. The Self-Assembly over Nano- to Submicro-Length Scales in Water of a Fluorescent Julolidine-Labeled Amphiphilic Random Terpolymer. J. Polym. Sci. Part Polym. Chem. 2018, 56, 797–804. [Google Scholar] [CrossRef]
- Becer, C.R.; Kokado, K.; Weber, C.; Can, A.; Chujo, Y.; Schubert, U.S. Metal-Free Synthesis of Responsive Polymers: Cloud Point Tuning by Controlled “Click” Reaction. J. Polym. Sci. Part Polym. Chem. 2010, 48, 1278–1286. [Google Scholar] [CrossRef]
- Zuppardi, F.; Chiacchio, F.R.; Sammarco, R.; Malinconico, M.; Gomez d’Ayala, G.; Cerruti, P. Fluorinated Oligo(Ethylene Glycol) Methacrylate-Based Copolymers: Tuning of Self Assembly Properties and Relationship with Rheological Behavior. Polymer 2017, 112, 169–179. [Google Scholar] [CrossRef]
- Zuppardi, F.; Malinconico, M.; D’Agosto, F.; D’Ayala, G.G.; Cerruti, P. Well-Defined Thermo-Responsive Copolymers Based on Oligo(Ethylene Glycol) Methacrylate and Pentafluorostyrene for the Removal of Organic Dyes from Water. Nanomaterials 2020, 10, 1779. [Google Scholar] [CrossRef]
- Hvilsted, S. The Pentafluorostyrene Endeavours with Atom Transfer Radical Polymerization—Quo Vadis? Polym. Int. 2014, 63, 814–823. [Google Scholar] [CrossRef]
- Bartels, J.W.; Cheng, C.; Powell, K.T.; Xu, J.; Wooley, K.L. Hyperbranched Fluoropolymers and Their Hybridization into Complex Amphiphilic Crosslinked Copolymer Networks. Macromol. Chem. Phys. 2007, 208, 1676–1687. [Google Scholar] [CrossRef]
- Powell, K.T.; Cheng, C.; Wooley, K.L. Complex Amphiphilic Hyperbranched Fluoropolymers by Atom Transfer Radical Self-Condensing Vinyl (Co)Polymerization. Macromolecules 2007, 40, 4509–4515. [Google Scholar] [CrossRef] [PubMed]
- Martinelli, E.; Pelusio, G.; Yasani, B.R.; Glisenti, A.; Galli, G. Surface Chemistry of Amphiphilic Polysiloxane/Triethyleneglycol-Modified Poly(Pentafluorostyrene) Block Copolymer Films Before and After Water Immersion. Macromol. Chem. Phys. 2015, 216, 2086–2094. [Google Scholar] [CrossRef]
- Imbesi, P.M.; Gohad, N.V.; Eller, M.J.; Orihuela, B.; Rittschof, D.; Schweikert, E.A.; Mount, A.S.; Wooley, K.L. Noradrenaline-Functionalized Hyperbranched Fluoropolymer-Poly(Ethylene Glycol) Cross-Linked Networks as Dual-Mode, Anti-Biofouling Coatings. ACS Nano 2012, 6, 1503–1512. [Google Scholar] [CrossRef]
- Oliva, M.; Martinelli, E.; Galli, G.; Pretti, C. PDMS-Based Films Containing Surface-Active Amphiphilic Block Copolymers to Combat Fouling from Barnacles B. Amphitrite and B. Improvisus. Polymer 2017, 108, 476–482. [Google Scholar] [CrossRef]
- Martinelli, E.; Hill, S.D.; Finlay, J.A.; Callow, M.E.; Callow, J.A.; Glisenti, A.; Galli, G. Amphiphilic Modified-Styrene Copolymer Films: Antifouling/Fouling Release Properties against the Green Alga Ulva Linza. Prog. Org. Coat. 2016, 90, 235–242. [Google Scholar] [CrossRef]
- Michnik, A.; Drzazga, Z.; Kluczewska, A.; Michalik, K. Differential Scanning Microcalorimetry Study of the Thermal Denaturation of Haemoglobin. Biophys. Chem. 2005, 118, 93–101. [Google Scholar] [CrossRef]
- Terashima, T.; Sugita, T.; Fukae, K.; Sawamoto, M. Synthesis and Single-Chain Folding of Amphiphilic Random Copolymers in Water. Macromolecules 2014, 47, 589–600. [Google Scholar] [CrossRef]
- Martinelli, E.; Annunziata, L.; Guazzelli, E.; Pucci, A.; Biver, T.; Galli, G. The Temperature-Responsive Nanoassemblies of Amphiphilic Random Copolymers Carrying Poly(Siloxane) and Poly(Oxyethylene) Pendant Chains. Macromol. Chem. Phys. 2018, 219, 1800082. [Google Scholar] [CrossRef]
- Laukkanen, A.; Valtola, L.; Winnik, F.M.; Tenhu, H. Formation of Colloidally Stable Phase Separated Poly(N-Vinylcaprolactam) in Water: A Study by Dynamic Light Scattering, Microcalorimetry, and Pressure Perturbation Calorimetry. Macromolecules 2004, 37, 2268–2274. [Google Scholar] [CrossRef]
- Sanchez-Ruiz, J.M. Theoretical Analysis of Lumry-Eyring Models in Differential Scanning Calorimetry. Biophys. J. 1992, 61, 921–935. [Google Scholar] [CrossRef]
- Benjwal, S.; Verma, S.; Röhm, K.-H.; Gursky, O. Monitoring Protein Aggregation during Thermal Unfolding in Circular Dichroism Experiments. Protein Sci. Publ. Protein Soc. 2006, 15, 635–639. [Google Scholar] [CrossRef]
- Borzova, V.A.; Markossian, K.A.; Chebotareva, N.A.; Kleymenov, S.Y.; Poliansky, N.B.; Muranov, K.O.; Stein-Margolina, V.A.; Shubin, V.V.; Markov, D.I.; Kurganov, B.I. Kinetics of Thermal Denaturation and Aggregation of Bovine Serum Albumin. PLoS ONE 2016, 11, e0153495. [Google Scholar] [CrossRef]
- Pelosi, C.; Saitta, F.; Wurm, F.R.; Fessas, D.; Tinè, M.R.; Duce, C. Thermodynamic Stability of Myoglobin-Poly(Ethylene Glycol) Bioconjugates: A Calorimetric Study. Thermochim. Acta 2019, 671, 26–31. [Google Scholar] [CrossRef]
- Domenici, F.; Guazzelli, E.; Masotti, E.; Mahmoudi, N.; Gabrielli, S.; Telling, M.T.F.; Martinelli, E.; Galli, G.; Paradossi, G. Understanding the Temperature-Responsive Self-Assemblies of Amphiphilic Random Copolymers by SANS in D2O Solution. Macromol. Chem. Phys. 2021. [Google Scholar] [CrossRef]
- Sezonenko, T.; Qiu, X.-P.; Winnik, F.M.; Sato, T. Dehydration, Micellization, and Phase Separation of Thermosensitive Polyoxazoline Star Block Copolymers in Aqueous Solution. Macromolecules 2019, 52, 935–944. [Google Scholar] [CrossRef]
- Qiu, X.; Koga, T.; Tanaka, F.; Winnik, F.M. New Insights into the Effects of Molecular Weight and End Group on the Temperature-Induced Phase Transition of Poly(N-Isopropylacrylamide) in Water. Sci. China Chem. 2013, 56, 56–64. [Google Scholar] [CrossRef]
- Ieong, N.S.; Hasan, M.; Phillips, D.J.; Saaka, Y.; O’Reilly, R.K.; Gibson, M.I. Polymers with Molecular Weight Dependent LCSTs Are Essential for Cooperative Behaviour. Polym. Chem. 2012, 3, 794–799. [Google Scholar] [CrossRef]
- Christova, D.; Velichkova, R.; Loos, W.; Goethals, E.J.; Prez, F.D. New Thermo-Responsive Polymer Materials Based on Poly(2-Ethyl-2-Oxazoline) Segments. Polymer 2003, 44, 2255–2261. [Google Scholar] [CrossRef]
- Yamamoto, S.-I.; Pietrasik, J.; Matyjaszewski, K. The Effect of Structure on the Thermoresponsive Nature of Well-Defined Poly(Oligo(Ethylene Oxide) Methacrylates) Synthesized by ATRP. J. Polym. Sci. Part Polym. Chem. 2008, 46, 194–202. [Google Scholar] [CrossRef]
- Prabhu, N.V.; Sharp, K.A. Heat Capacity in Proteins. Annu. Rev. Phys. Chem. 2004, 56, 521–548. [Google Scholar] [CrossRef] [PubMed]
- Gómez, J.; Hilser, V.J.; Xie, D.; Freire, E. The Heat Capacity of Proteins. Proteins Struct. Funct. Bioinforma. 1995, 22, 404–412. [Google Scholar] [CrossRef] [PubMed]







| Polymer | Atmosphere | Monomer/ Initiator Mole Ratio | Conversion (%) | x 1 | Mn NMR 2 (g/mol) | Mn GPC 3 (g/mol) | Ð3 |
|---|---|---|---|---|---|---|---|
| pEFS8-26 | N2 | 24 | 99 | 26 | 14,410 | 10,800 | 1.26 |
| pEFS8-46 | Vacuum | 60 | 93 | 46 | 25,490 | 19,800 | 1.25 |
| Dh at 25 °C (nm) | Dh at 80 °C (nm) | |
|---|---|---|
| pEFS8-26 (5 g/L) | 6 ± 1 | 3000 ± 1000 |
| pEFS8-46 (5 g/L) | 7 ± 2 | 1500 ± 300 |
| pEFS8-46 (0.5 g/L) | 7 ± 2 | 340 ± 60 |
| pEFS8-46 (10 g/L) | 7 ± 2 | 2000 ± 600 |
| First Heating Scan | Second Heating Scan | ||||||
|---|---|---|---|---|---|---|---|
| Concentration (g/L) | Tonset1 (°C) | Tmax2 (°C) | Peak Area (KJ/mol) 3 | Tcp4 (°C) | Tonset1 (°C) | Tmax2 (°C) | |
| pEFS8-26 | 0.5 | 71 | 75.2 | 7.6 | 72.5 | 69.5 | 74.4 |
| 1 | 70.4 | 73.7 | 7.2 | 71 | 68.4 | 72.2 | |
| 5 | 68.7 | 71.7 | 4.5 | 70 | 65.3 | 70.2 | |
| pEFS8-46 | 0.5 | 75.6 | 80.2 | 6.8 | 83 | 75 | 79.8 |
| 1 | 74.8 | 79.1 | 5.5 | 78 | 73.2 | 78.3 | |
| 5 | 71.8 | 76.8 | / | 73 | 70.7 | 75.2 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pelosi, C.; Guazzelli, E.; Calosi, M.; Bernazzani, L.; Tiné, M.R.; Duce, C.; Martinelli, E. Investigation of the LCST-Thermoresponsive Behavior of Novel Oligo(Ethylene Glycol)-Modified Pentafluorostyrene Homopolymers. Appl. Sci. 2021, 11, 2711. https://doi.org/10.3390/app11062711
Pelosi C, Guazzelli E, Calosi M, Bernazzani L, Tiné MR, Duce C, Martinelli E. Investigation of the LCST-Thermoresponsive Behavior of Novel Oligo(Ethylene Glycol)-Modified Pentafluorostyrene Homopolymers. Applied Sciences. 2021; 11(6):2711. https://doi.org/10.3390/app11062711
Chicago/Turabian StylePelosi, Chiara, Elisa Guazzelli, Matteo Calosi, Luca Bernazzani, Maria Rosaria Tiné, Celia Duce, and Elisa Martinelli. 2021. "Investigation of the LCST-Thermoresponsive Behavior of Novel Oligo(Ethylene Glycol)-Modified Pentafluorostyrene Homopolymers" Applied Sciences 11, no. 6: 2711. https://doi.org/10.3390/app11062711
APA StylePelosi, C., Guazzelli, E., Calosi, M., Bernazzani, L., Tiné, M. R., Duce, C., & Martinelli, E. (2021). Investigation of the LCST-Thermoresponsive Behavior of Novel Oligo(Ethylene Glycol)-Modified Pentafluorostyrene Homopolymers. Applied Sciences, 11(6), 2711. https://doi.org/10.3390/app11062711










