Quantitative Microbiological Analysis of Artisanal Stretched Cheese Manufacture
Abstract
1. Introduction
2. Materials and Methods
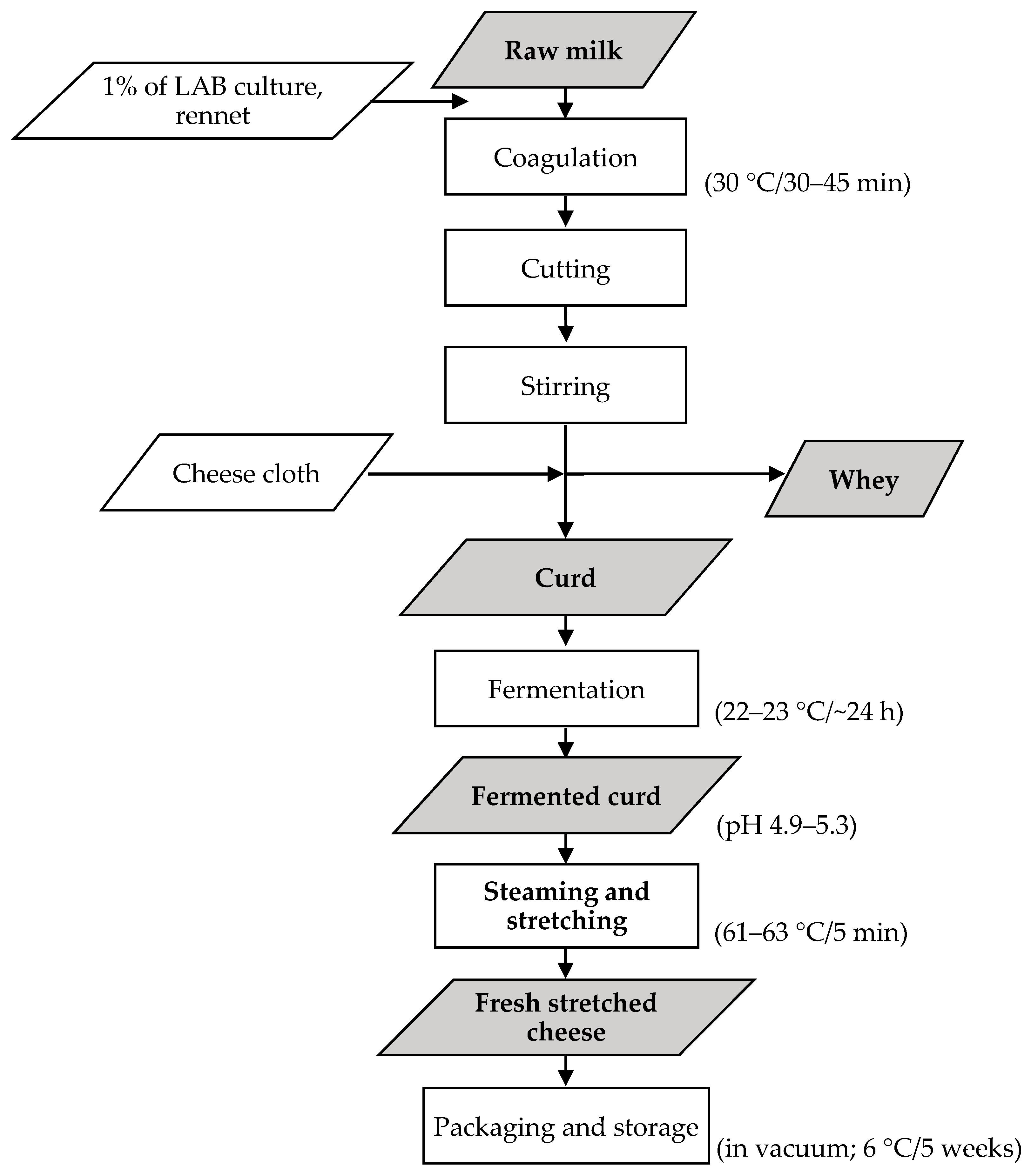
2.1. Cheese Preparation
2.2. Microbiological Analysis
2.3. Additional Analysis
2.4. Statistical Analysis
3. Results
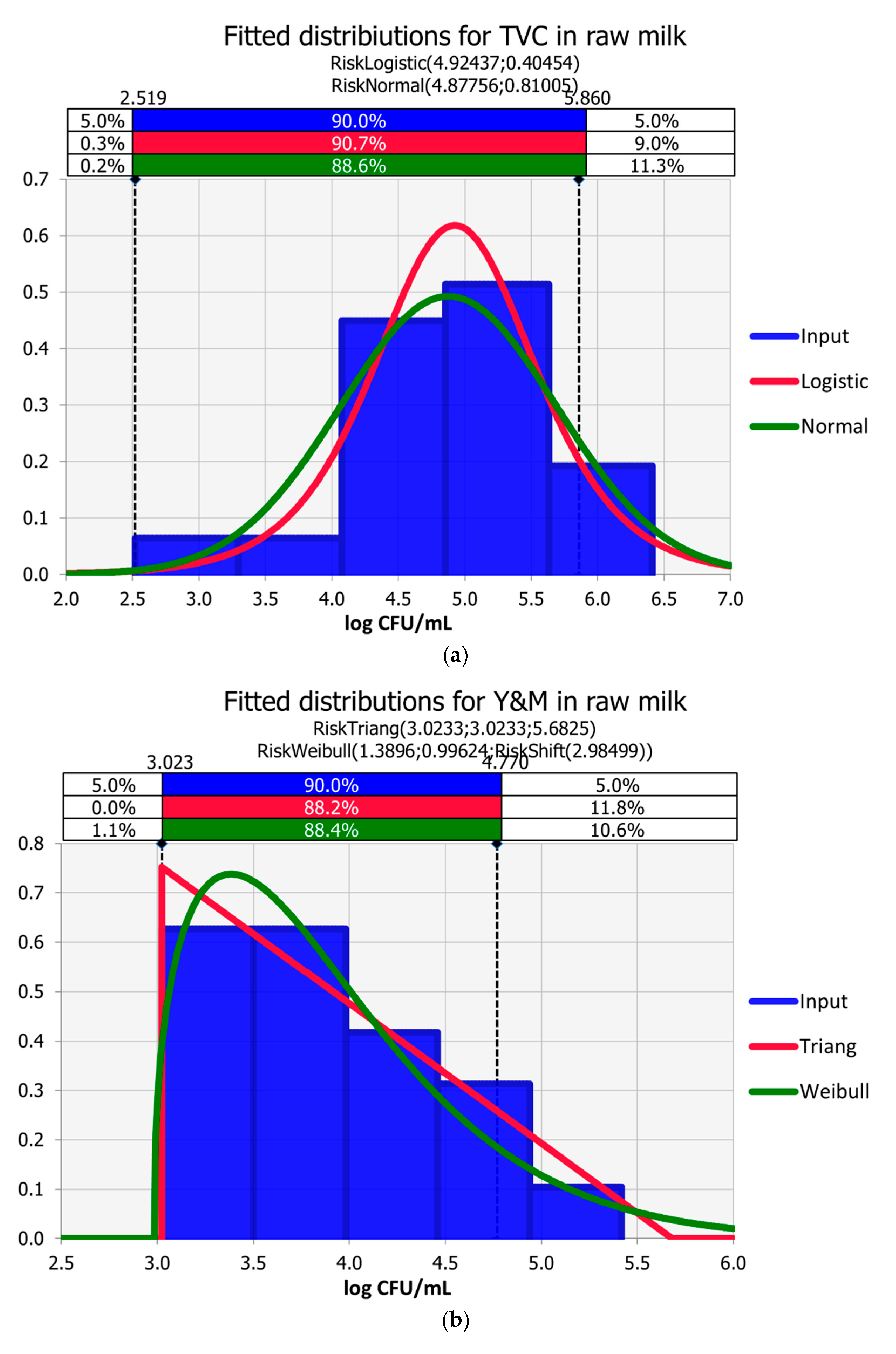
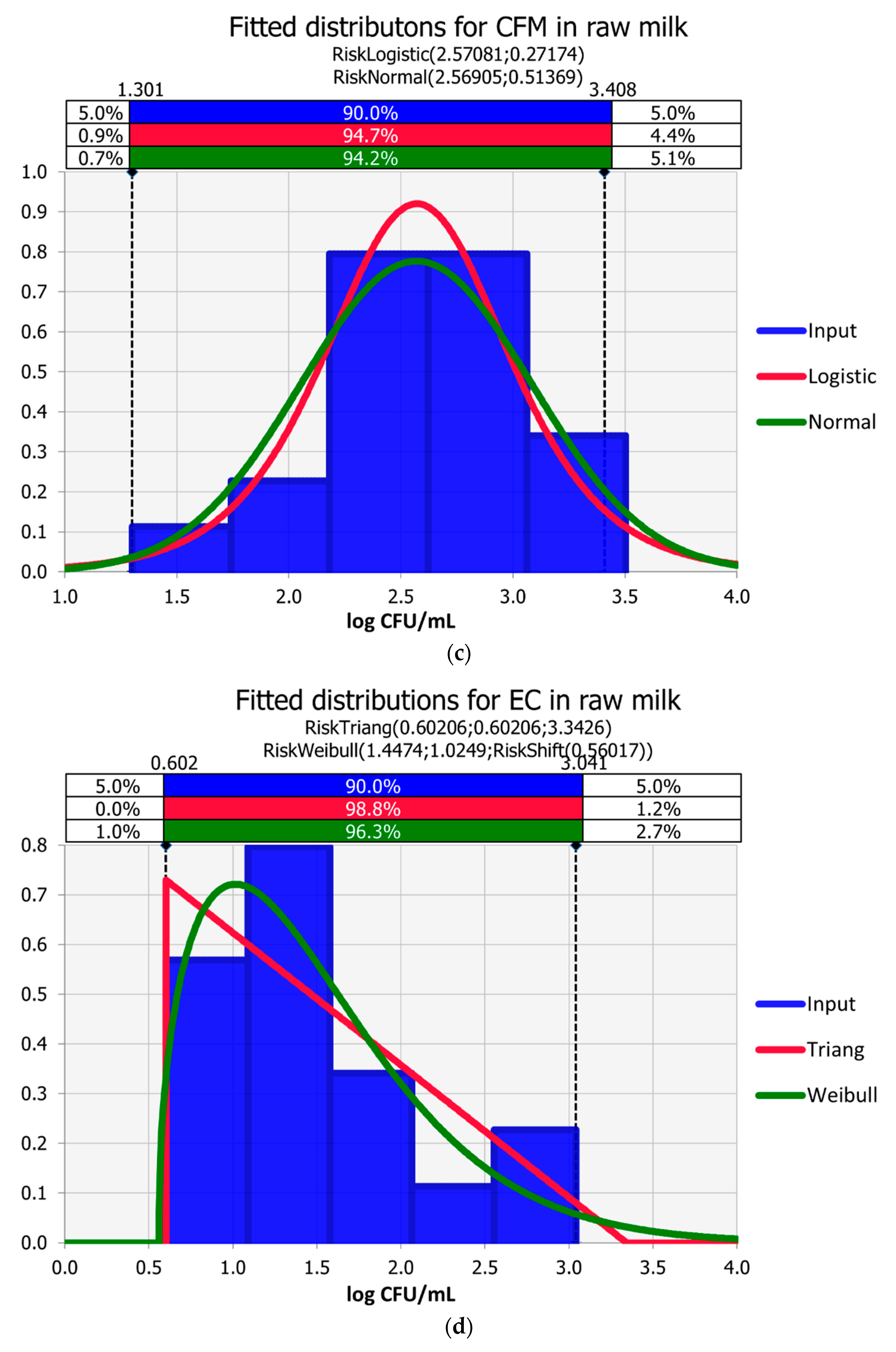
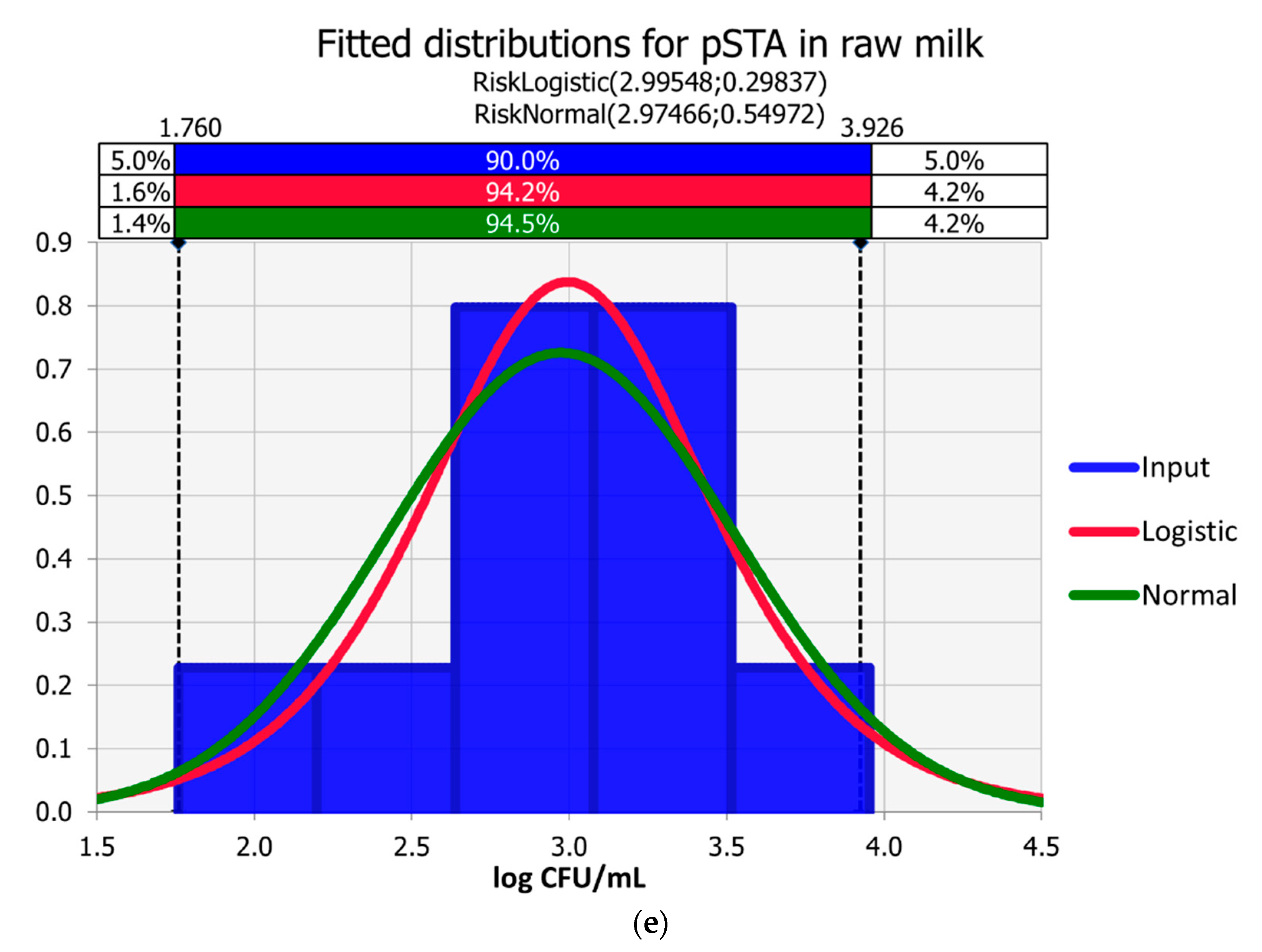
3.1. Raw Milk
3.2. Coagulation
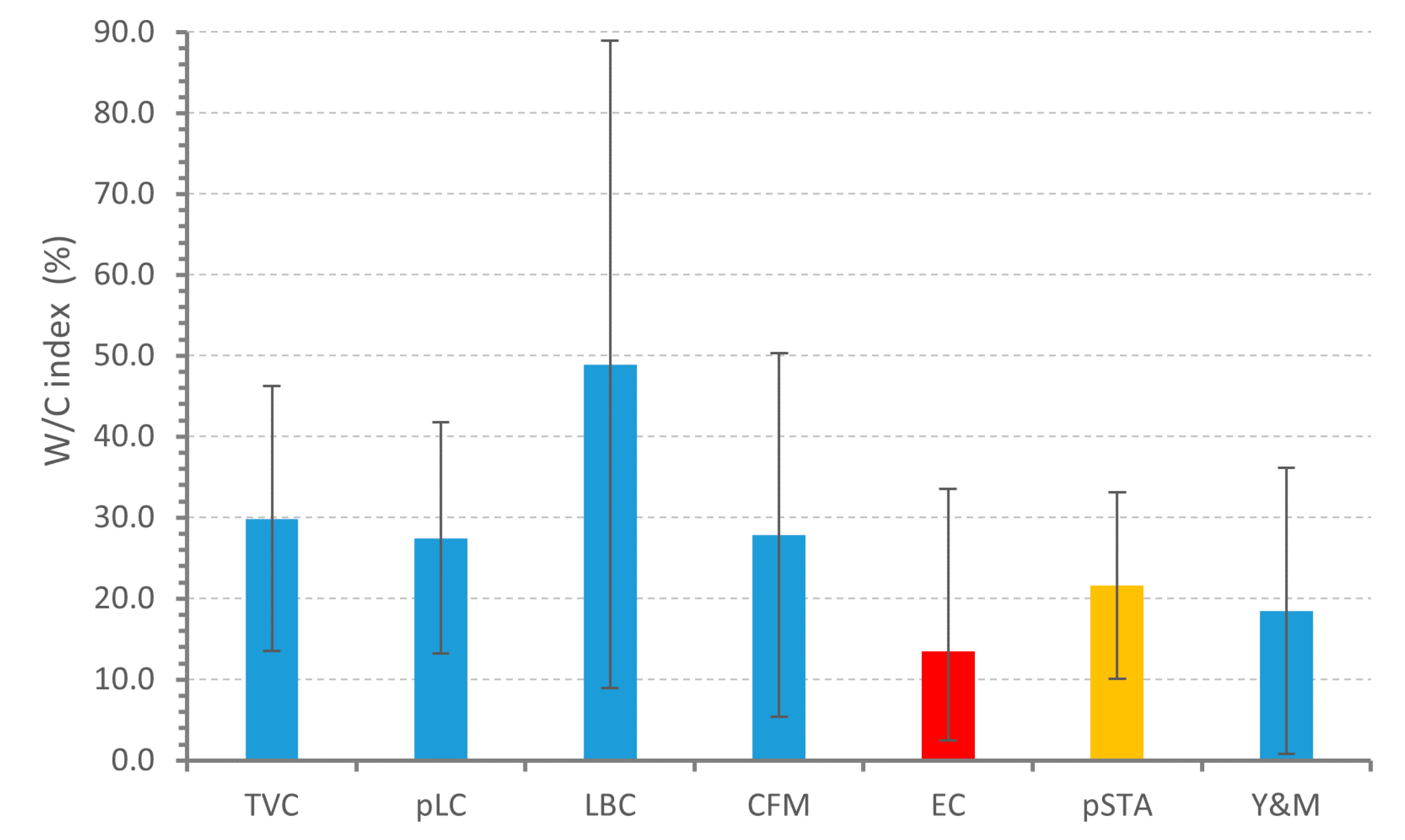
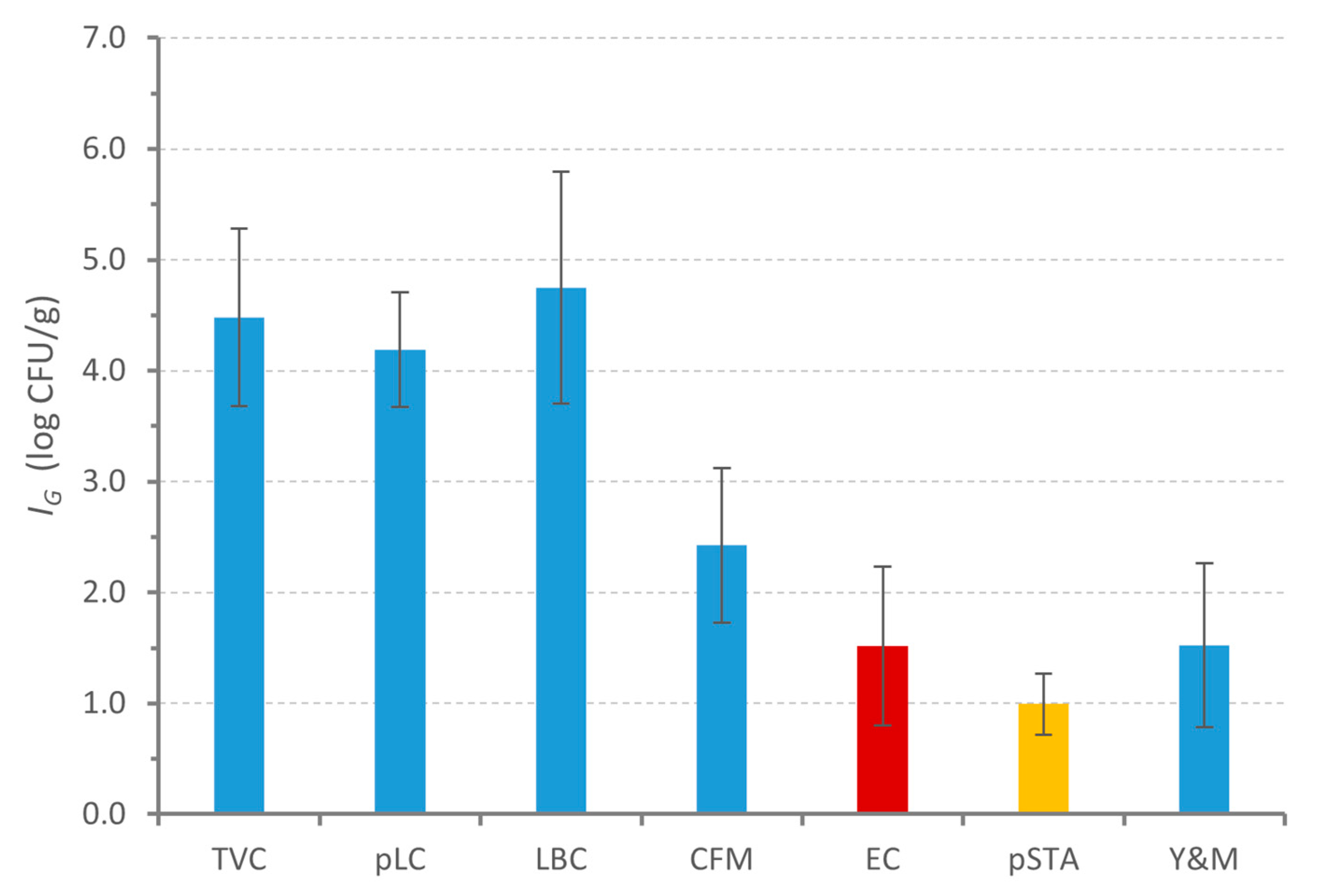
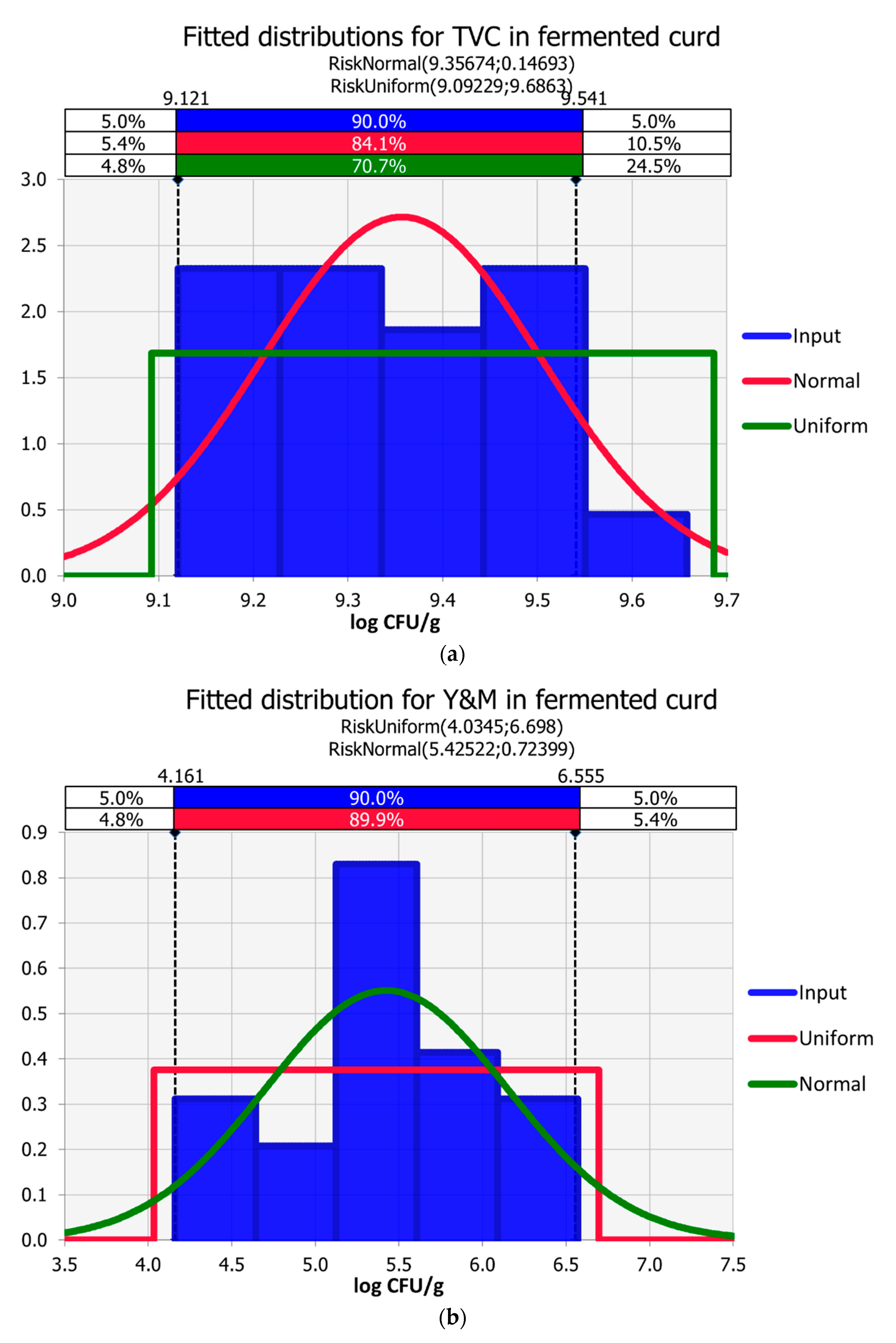
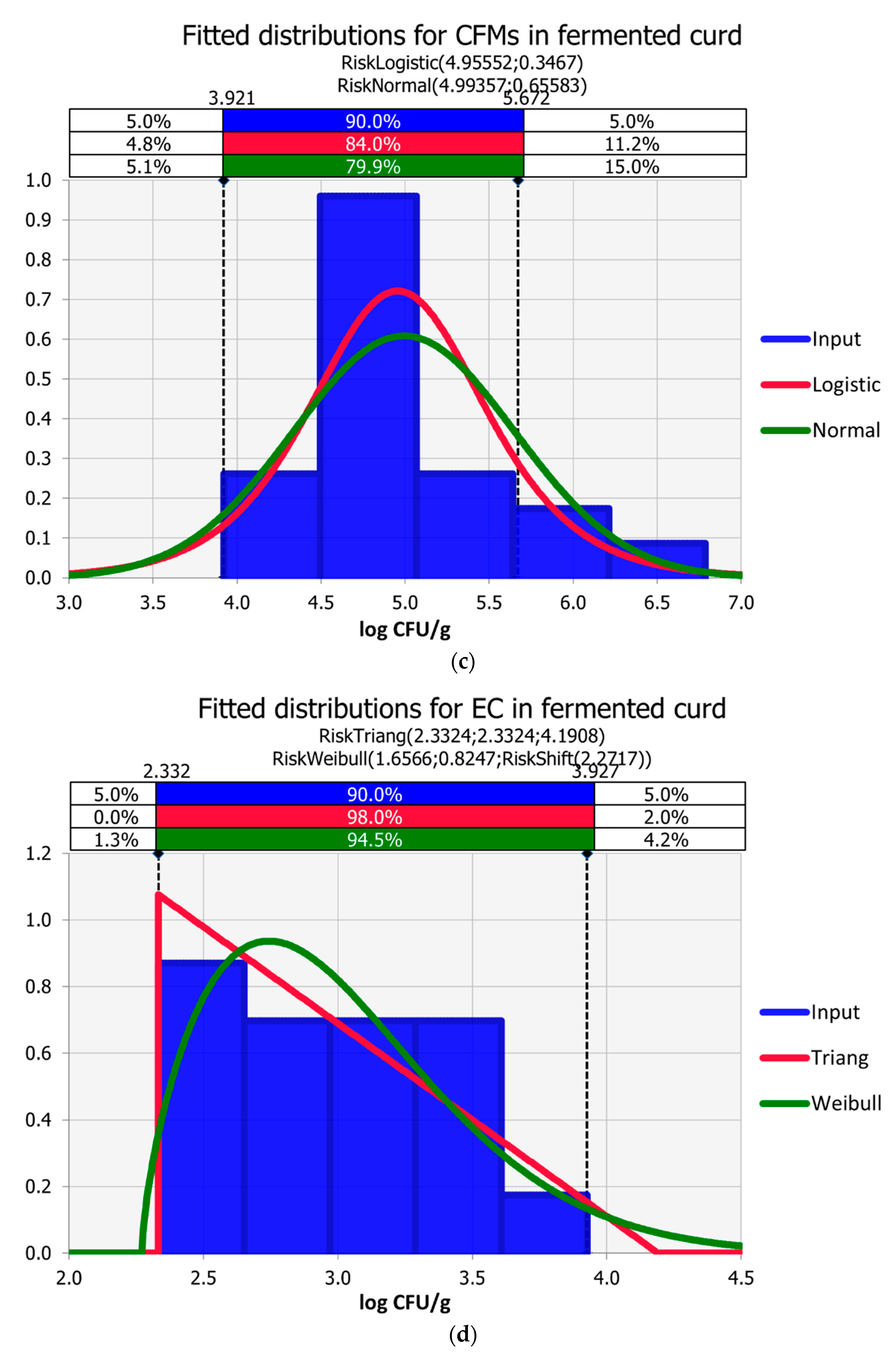
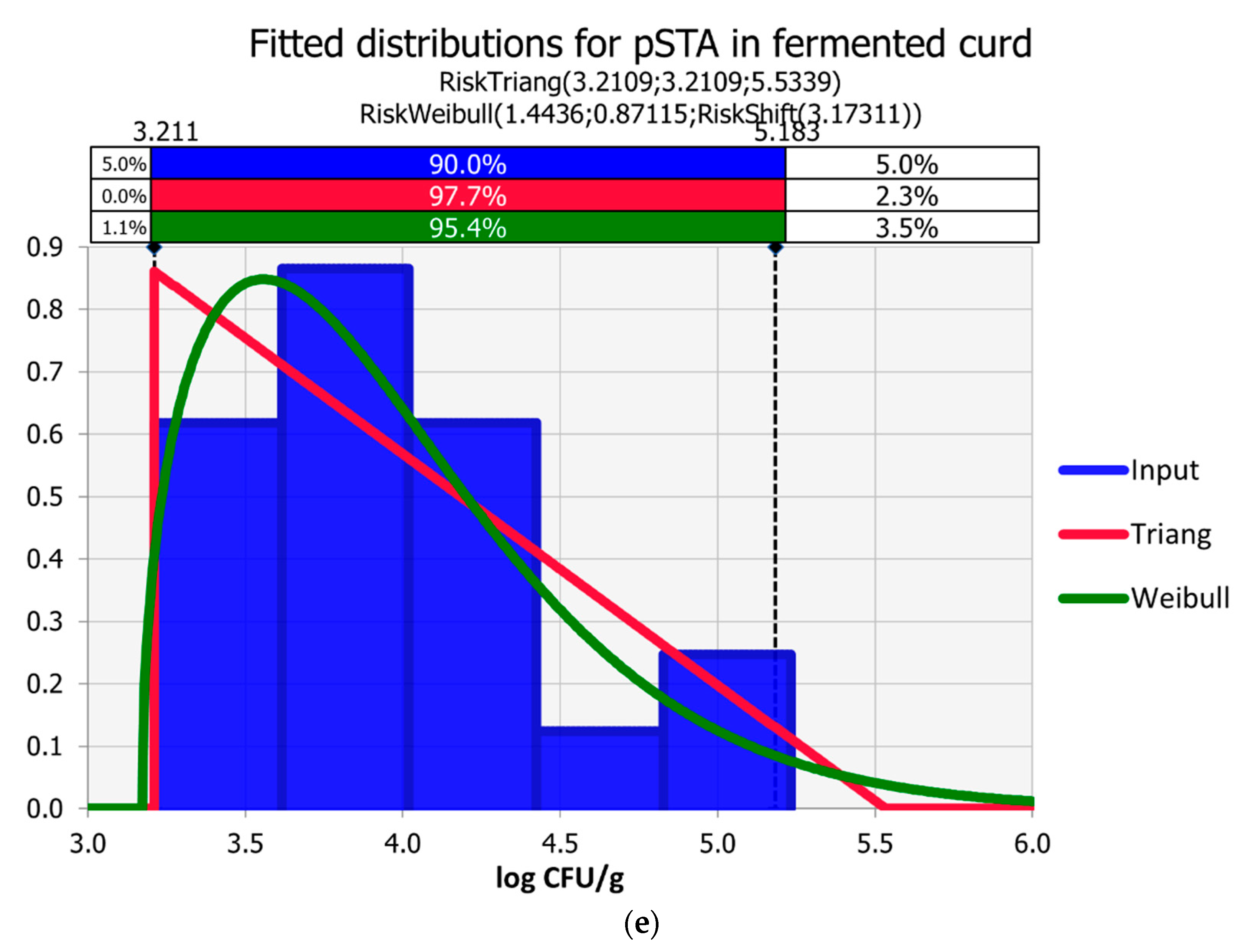
3.3. Curd Fermentation
3.4. Steaming and Stretching Process
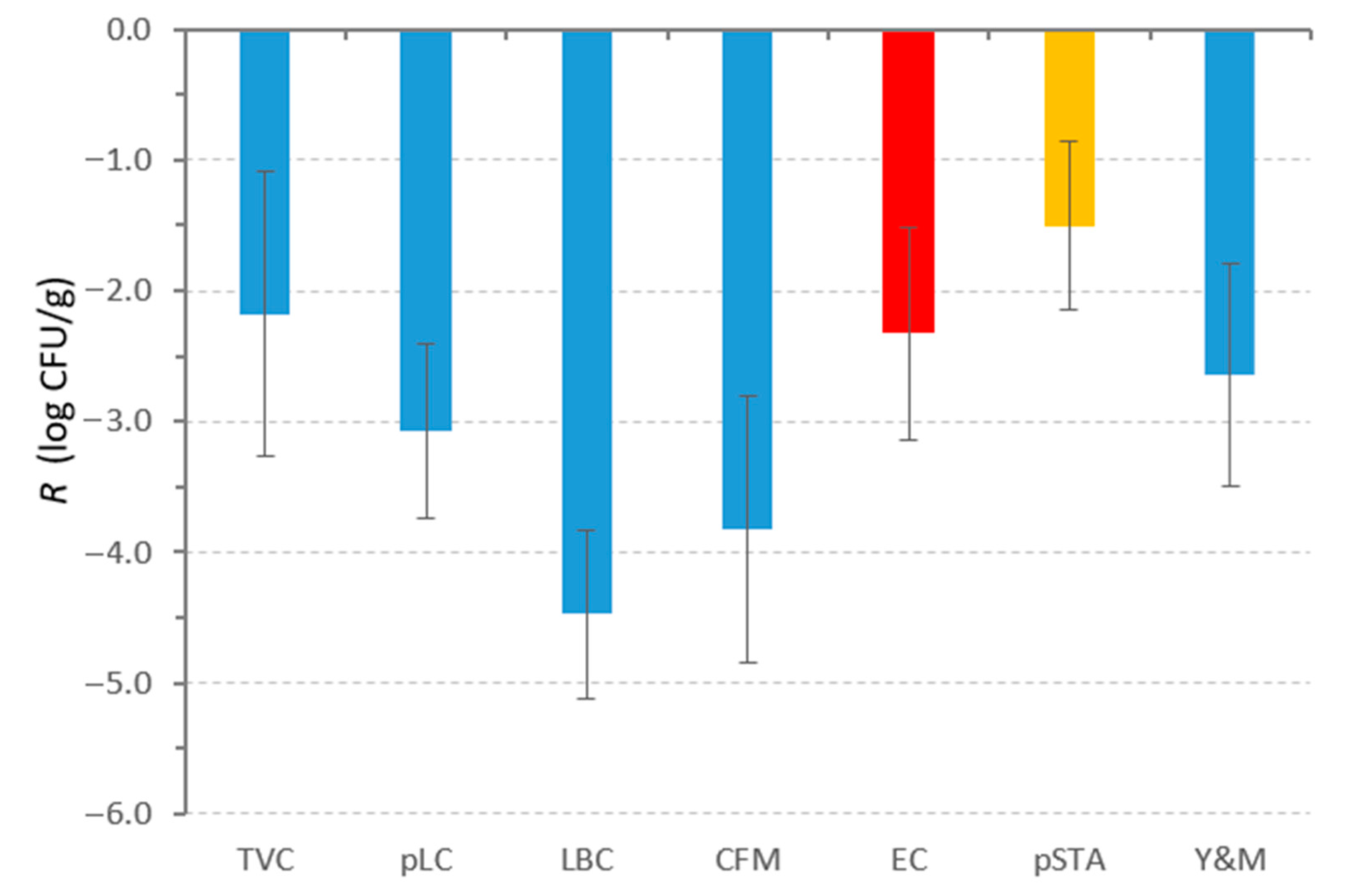
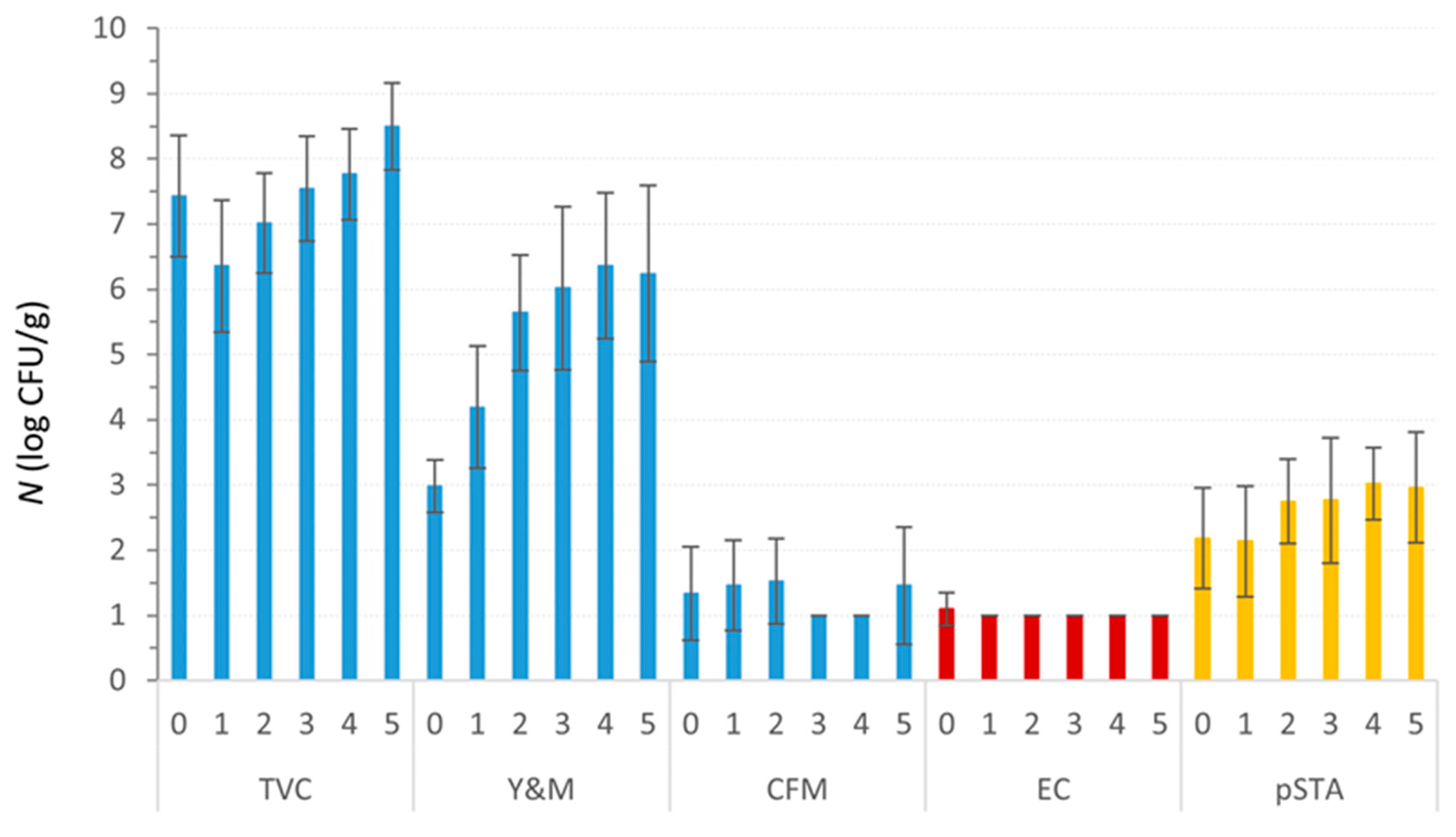
3.5. Storage Test
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- De Angelis, M.; Gobbetti, M. Pasta-filata cheeses: Traditional Pasta-filata cheese. In Encyclopedia of Dairy Sciences, 2nd ed.; Fuquay, J.W., Ed.; Elsevier: New York, NY, USA, 2011; Volume 1, pp. 745–752. [Google Scholar]
- McMahon, D.J.; Oberg, C.J. Pasta-filata cheeses. In Cheese: Chemistry, Physics and Microbiology, 4th ed.; McSweeney, P.L.H., Fox, P.F., Cotter, P.D., Everett, D.W., Eds.; Academic Press: Cambridge, MA, USA, 2017; pp. 1041–1068. [Google Scholar]
- Commission Regulation (EC) No 943/2008 entering certain names in the Register of protected designations of origin and protected geographical indications (Presunto de Campo Maior e Elvas or Paleta de Campo Maior e Elvas (PGI), Presunto de Santana da Serra or Paleta de Santana da Serra (PGI), Slovenský oštiepok (PGI)). Off. J. Eur. Communities 2008, 258, 52–53. Available online: https://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=OJ:L:2008:258:0052:0053:EN:PDF (accessed on 2 February 2021).
- Commission Regulation (EC) No 656/2008 registering certain names in the Register of protected designations of origin and protected geographical indications (Chamomilla Bohemica (PDO), Vlaams-Brabantse tafeldruif (PDO), Slovenská parenica (PGI), Cipollotto Nocerino (PDO)). Off. J. Eur. Communities 2008, 183, 15–16. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=uriserv%3AOJ.L_.2008.183.01.0015.01.ENG&toc=OJ%3AL%3A2008%3A183%3AFULL (accessed on 2 February 2021).
- Commission Regulation (EC) No 243/2011 entering a name in the register of protected designations of origin and protected geographical indications (Oravský korbáčik (PGI)). Off. J. Eur. Communities 2011, 66, 19–20. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX%3A32011R0243 (accessed on 2 February 2021).
- Commission Regulation (EC) No 238/2011 entering a name in the register of protected designations of origin and protected geographical indications (Zázrivský korbáčik (PGI)). Off. J. Eur. Communities 2011, 66, 9–10. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX%3A32011R0238 (accessed on 2 February 2021).
- Commission Regulation (EC) No 963/2014 entering a name in the register of protected designations of origin and protected geographical indications [Zázrivské vojky (PGI)]. Off. J. Eur. Communities 2014, 271, 15. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=uriserv%3AOJ.L_.2014.271.01.0015.01.ENG&toc=OJ%3AL%3A2014%3A271%3AFULL (accessed on 2 February 2021).
- Licitra, G.; Radulovic, Z.; Miocinovic, J.; Uzunsoy, I.; Özer, B.; Bintsis, T.; Alichanidis, E.; Herian, K.; Jelen, P. Pasta-filata cheeses. In Global Cheesemaking Technology: Cheese Quality and Characteristics; Papademas, P., Bintsis, T., Eds.; John Wiley & Sons Ltd.: Oxford, UK, 2017; pp. 379–382. [Google Scholar]
- Verraes, C.; Vlaemynck, G.; Van Weyenberg, S.; De Zutter, L.; Daube, G.; Sindic, M.; Uyttendaele, M.; Herman, L. A review of the microbiological hazards of dairy products made from raw milk. Int. Dairy J. 2015, 50, 32–44. [Google Scholar] [CrossRef]
- Hui, Y.H.; Evranuz, E.Ö.; Chandan, R.C. Handbook of Animal-Based Fermented Food and Beverage Technology, 1st ed.; CRC Press: Boca Raton, FL, USA, 2012; 814p. [Google Scholar]
- Gernigon, G.; Schuck, P.; Jeantet, R. Processing of Mozzarella cheese wheys and stretchwaters: A preliminary review. Dairy Sci. Technol. 2010, 90, 27–46. [Google Scholar] [CrossRef]
- Trevisani, M.; Valero, A.; Mancusi, R. Effectiveness of the Thermal Treatments Used for Curd Stretching in the Inactivation of Shiga Toxin-Producing O157 and O26 Escherichia coli. BioMed Res. Int. 2017, 2017, 1609836. [Google Scholar] [CrossRef]
- Spano, G.; Goffredo, E.; Beneduce, L.; Tarantino, D.; Dupuy, A.; Massa, S. Fate of Escherichia coli O157:H7 during the manufacture of Mozzarella cheese. Lett. Appl. Microbiol. 2003, 36, 73–76. [Google Scholar] [CrossRef]
- Mucchetti, G.; Bonvini, B.; Remagni, M.C.; Ghiglietti, R.; Locci, F.; Barzaghi, S.; Francolino, S.; Perrone, A.; Rubiloni, A.; Campo, P.; et al. Influence of cheese-making technology on composition and microbiological characteristics of Vastedda cheese. Food Control 2008, 19, 119–125. [Google Scholar] [CrossRef]
- Onipchenko, N.; Doležalová, M.; Procházková, E.; Martinková, I.; Hrabě, J. Změny mikroflóry během výroby pařených sýrů. Mlékařské Listy 2012, 132, 1–4. [Google Scholar]
- Samelis, J.; Kakouri, A.; Kondyli, E.; Pappa, E.C. Effects of curd heating with or without previous milk pasteurisation on the microbiological quality and safety of craft-made ‘Pasta Filata’ Kashkaval cheese curds. Int. J. Dairy Technol. 2019, 72, 447–455. [Google Scholar] [CrossRef]
- Den Besten, H.M.W.; Wells-Bennik, M.H.J.; Zwietering, M.H. Natural diversity in heat resistance of bacteria and bacterial spores: Impact on food safety and quality. Annu. Rev. Food Sci. Technol. 2018, 9, 383–410. [Google Scholar] [CrossRef]
- Publication of an application pursuant to Article 6(2) of Council Regulation (EC) No 510/2006 on the protection of geographical indications and designations of origin for agricultural products and foodstuffs. Off. J. Eur. Communities 2010, 188. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX%3A52010XC0713%2803%29&qid=1606380518398 (accessed on 2 February 2021).
- Publication of an application pursuant to Article 50(2)(a) of Regulation (EU) No 1151/2012 of the European Parliament and of the Council on quality schemes for agricultural products and foodstuffs. Off. J. Eur. Communities 2014, 109. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX%3A52014XC0411%2801%29&qid=1606380970157 (accessed on 2 February 2021).
- ISO 6887-5. Microbiology of food and animal feeding stuffs. Preparation of test samples, initial suspension and decimal dilutions for microbiological examination. In Part 5: Specific Rules for the Preparation of Milk and Milk Products; International Organization of Standardization (ISO): Geneva, Switzerland, 2010; pp. 1–12. [Google Scholar]
- ISO 15214. Microbiology of Food and Animal Feeding Stuffs. In Horizontal Method for the Enumeration of Mesophilic Lactic Acid Bacteria. Colony-Count Technique at 30 °C; International Organization of Standardization (ISO): Geneva, Switzerland, 2002; pp. 1–12. [Google Scholar]
- ISO 4832:2006. Microbiology of Food and Animal Feeding Stuffs—Horizontal Method for the Enumeration of Coliforms—Colony-Count Technique; International Organization of Standardization (ISO): Geneva, Switzerland, 2006; pp. 1–6. [Google Scholar]
- ISO 6888-1. Microbiology of food and animal feeding stuffs. Horizontal method for the enumeration of coagulase-positive staphylococci (Staphylococcus aureus and other species). In Part 1: Technique Using Baird-Parker Agar Medium; International Organization of Standardization (ISO): Geneva, Switzerland, 2001; pp. 1–22. [Google Scholar]
- ISO 4833-1. Microbiology of food chain. Horizontal method for the enumeration of microorganisms. In Part 1: Colony Count at 30 Degrees C by the Pour Plate Technique; International Organization of Standardization (ISO): Geneva, Switzerland, 2014; pp. 1–16. [Google Scholar]
- ISO 6611. Milk and Milk Products—Enumeration of Colony-Forming Units of Yeasts and/or Moulds—Colony-Count Technique at 25 Degrees C; Organization of Standardization (ISO): Geneva, Switzerland, 2004; pp. 1–7. [Google Scholar]
- Calamari, L.; Gobbi, L.; Bani, P. Improving the prediction ability of FT-MIR spectroscopy to assess titratable acidity in cow’s milk. Food Chem. 2016, 192, 477–484. [Google Scholar] [CrossRef]
- International Commission for the Microbiological Specifications of Foods. Microorganisms in Foods 7; Kluwer Academic/Plenum Publishers: New York, NY, USA, 2002; 362p. [Google Scholar]
- Carrascosa, C.; Millán, R.; Saavedra, P.; Jaber, J.R.; Raposo, A.; Sanjuán, E. Identification of the risk factors associated with cheese production to implement the hazard analysis and critical control points (HACCP) system on cheese farms. J. Dairy Sci. 2016, 99, 2606–2616. [Google Scholar] [CrossRef] [PubMed]
- National Agricultural and Food Centre: Research Institute of Agriculture and Food Economics. Commodity situation and Outlook Report—Milk. 2012. Available online: http://www.vuepp.sk/dokumenty/komodity/2020/Mlieko05_2020_v2.pdf (accessed on 2 February 2021).
- Vernozy-Rozand, C.; Mazuy-Cruchaudet, C.; Bavai, C.; Montet, M.P.; Bonin, V.; Dernburg, A.; Richard, Y. Growth and survival of Escherichia coli O157:H7 during the manufacture and ripening of raw goat milk lactic cheeses. Int. J. Food Microbiol. 2005, 105, 83–88. [Google Scholar] [CrossRef] [PubMed]
- Brooks, J.C.; Martinez, B.; Stratton, J.; Bianchini, A.; Krokstrom, R.; Hutkins, R. Survey of raw milk cheeses for microbiological quality and prevalence of foodborne pathogens. Food Microbiol. 2012, 31, 154–158. [Google Scholar] [CrossRef]
- Le Marc, Y.; Valík, Ľ.; Medveďová, A. Modelling the effect of the starter culture on the growth of Staphylococcus aureus in milk. Int. J. Food Microbiol. 2009, 129, 306–311. [Google Scholar] [CrossRef]
- Yoon, Y.; Lee, S.; Choi, K. Microbial benefits and risks of raw milk cheese. Food Control 2016, 63, 201–215. [Google Scholar] [CrossRef]
- Charlier, C.; Cretenet, M.; Even, S.; Le Loir, Y. Interactions between Staphylococcus aureus and lactic acid bacteria: An old story with new perspectives. Int. J. Food Microbiol. 2009, 131, 30–39. [Google Scholar] [CrossRef]
- Kongo, J.M. Lactic Acid Bacteria as Starter-Cultures for Cheese Processing: Past, Present and Future Developments. In Lactic Acid Bacteria—For Food, Health and Livestock Purposes; Kongo, J.M., Ed.; IntechOpen: London, UK, 2013; pp. 3–22. [Google Scholar]
- Ačai, P.; Medveďová, A.; Mančušková, T.; Valík, Ľ. Growth prediction of two bacterial populations in co-culture with lactic acid bacteria. Food Sci. Technol. Int. 2019, 8, 692–700. [Google Scholar] [CrossRef] [PubMed]
- Mangia, N.P.; Fancello, F.; Deiana, P. Microbiological characterization using combined culture dependent and independent approaches of Casizolu pasta filata cheese. J. Appl. Microbiol. 2015, 120, 329–345. [Google Scholar] [CrossRef] [PubMed]
- Fuentes, L.; Mateo, J.; Quinto, E.J.; Caro, I. Changes in quality of nonaged pasta filata Mexican cheese during refrigated vacuum storage. J. Dairy. Sci. 2015, 98, 2833–2842. [Google Scholar] [CrossRef]
- Todaro, M.; Palmeri, M.; Settanni, L.; Scatassa, M.L.; Mazza, F.; Bonanno, A.; Di Grigoli, A. Effect of refrigerated storage on microbiological, chemical and sensory characteristics of a ewes’ raw milk stretched cheese. Food Packag. Shelf Life 2017, 11, 67–73. [Google Scholar]
- Kenedy, J.; Blair, I.S.; McDowell, D.A.; Bolton, D.J. An investigation of the thermal inactivation of Staphylococcus aureus and the potential for increased thermotolerance as a result of chilled storage. J. Appl. Microbiol. 2005, 99, 1229–1235. [Google Scholar] [CrossRef]
- Li, H.; Gänzle, M. Some like it hot: Heat resistance of Escherichia coli in food. Front. Microbiol. 2016, 7, 1736. [Google Scholar] [CrossRef]
| Microbial Group | Raw Milk | Fresh Curd | Whey | Fermented Cheese Curd (Before Stretching) | Stretched Cheese |
|---|---|---|---|---|---|
| [log CFU/mL] | [log CFU/g] | [log CFU/mL] | [log CFU/g] | ||
| Total viable count | 4.88 (±0.50) | 7.18 (±0.69) | 6.58 (±0.38) | 9.36 (±0.64) | 7.44 (±0.98) |
| Presumptive lactococci 2 | 4.55 (±0.25) | 6.89 (±0.66) | 6.29 (±0.92) | 8.74 (±0.44) | 5.50 (±0.56) |
| Lactobacillus sp. 2 | 4.06 (±0.75) | 6.68 (±0.41) | 6.16 (±0.38) | 8.81 (±0.24) | 4.34 (±0.41) |
| Coliforms | 2.57 (±0.20) | 2.91 (±0.45) | 2.27 (±0.37) | 4.99 (±0.24) | 1.02 (±0.50) |
| E. coli | 1.49 (±0.54) | 1.82 (±0.50) | 0.73 (±0.49) | 3.01 (±0.53) | 0.64 (±0.81) |
| Presumptive S. aureus | 2.97 (±0.79) | 3.19 (±0.39) | 2.45 (±0.37) | 3.97 (±0.14) | 2.46 (±1.27) |
| Yeasts and molds | 3.90 (±0.64) | 4.43 (±0.69) | 3.70 (±0.69) | 5.43 (±0.71) | 2.81 (±0.64) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lehotová, V.; Antálková, V.; Medveďová, A.; Valík, Ľ. Quantitative Microbiological Analysis of Artisanal Stretched Cheese Manufacture. Appl. Sci. 2021, 11, 2680. https://doi.org/10.3390/app11062680
Lehotová V, Antálková V, Medveďová A, Valík Ľ. Quantitative Microbiological Analysis of Artisanal Stretched Cheese Manufacture. Applied Sciences. 2021; 11(6):2680. https://doi.org/10.3390/app11062680
Chicago/Turabian StyleLehotová, Veronika, Veronika Antálková, Alžbeta Medveďová, and Ľubomír Valík. 2021. "Quantitative Microbiological Analysis of Artisanal Stretched Cheese Manufacture" Applied Sciences 11, no. 6: 2680. https://doi.org/10.3390/app11062680
APA StyleLehotová, V., Antálková, V., Medveďová, A., & Valík, Ľ. (2021). Quantitative Microbiological Analysis of Artisanal Stretched Cheese Manufacture. Applied Sciences, 11(6), 2680. https://doi.org/10.3390/app11062680







