Abstract
This work aims to study the qualitative composition of biologically active substance (BAS) extracts in vitro callus, cell suspension, and root cultures of the medicinal plant Rhaponticum carthamoides. The research methodology is based on high-performance liquid chromatography, and 1H nuclear magnetic resonance (NMR) spectra, to study the qualitative and quantitative analysis of BAS. The results of the qualitative composition analysis of the dried biomass extracts of in vitro callus, cell suspension and root cultures showed that the main biologically active substances in the medicinal plant Rhaponticum carthamoides are 2-deoxy-5,20,26-trihydroxyecdyson (7 mg, yield 0.12%), 5,20,26-trihydroxyecdyson 20,22-acetonide (15 mg, yield 0.25%), 2-deoxy-5,20,26-trihydroxyecdyson 20,22-acetonide (6 mg, yield 0.10%), 20,26-dihydroxyecdyson 20,22-acetonidecdyson 20,22-acetonide (5 mg, yield 0.09%), and ecdyson 20,22-acetonide (6 mg, yield 0.10%). In the future, it is planned to study the antimicrobial, antioxidant, and antitumor activity of BAS of extracts of in vitro callus, cell suspension, and root cultures of the medicinal plant Rhaponticum carthamoides, for the production of pharmaceuticals and dietary supplements with antitumor, antimicrobial and antioxidant effects.
1. Introduction
Medicinal plants are sources of large amounts of biologically active substances (BAS), such as antioxidants, alkaloids, phenylpropanoids, terpenoids, and many others. Each of these compounds, to a greater or lesser extent, have medicinal properties and potential pharmaceutical applications [1,2,3,4]. Some compounds have several properties at once; for example, antioxidant, adaptogenic, anticancer, and even anti-aging properties are attributed to ginsenosides [5]. Ginseng is good at reducing fatigue and increasing resistance to cancer [5]. Tanshinones (diterpenoids) and salvianolic acid (phenylpropanoids) have antioxidant, anti-inflammatory, antibacterial, antitumor, and cytotoxic properties, and are actively used in the comprehensive treatment of cardiovascular diseases [6]. Sesquiterpene-artemisinin is effectively used in antimalarial therapy [7].
It is believed that plants have many natural compounds, such as terpenoids (isoprenoids, more than 50,000 different structures are distinguished), the production of which has two pathways (the cytosolic mevalonate or plastid methylerythritol phosphate) [8,9]. The cytosolic mevalonate pathway is considered to be the main pathway for producing sesquiterpenoids (in particular artemisinin) and triterpenoids (ginsenosides) [7,10,11,12,13,14], while the plastid methylerythritol phosphate pathway is considered to be the main pathway for diterpenoids (such as tanshinones and taxol) and monoterpenoids (such as limonene) [9,13,15]. Phenylpropanoids (anthocyanin, catechin, salvianolic acid, etc.) are derivatives of the shikimate pathway, synthesized from phenylalanine and tyrosine [6,16].
It is known that plants often synthesize biologically active compounds in small amounts [1], and only in some of the tissues [10,17]. It was established that compounds such as tanshinones, ginsenosides and flavones are formed and accumulate in the roots of Salvia miltiorrhiza, ginseng (Panax), and Scutellaria baicalensis [10,18,19]. It is known that catharantin accumulates in all tissues and organs, while vinblastine and vincristine only accumulate in the aerial parts of the Catharanthus roseus plant [20,21]. In Artemisia annua, artemisinin is formed in the glandular secretory trichomes of leaves [7]. Natural medicinal plants often require many years of growth before biologically active compounds are synthesized in their parts, for example, in the roots, usually in very small doses [22]. Moreover, the environmental and ecological problems lead to a deterioration in the quality characteristics of both plants and their derivatives [23,24]. The study and research of alternative methods of obtaining compounds with useful and unique characteristics from plant materials is relevant. The biosynthesis of in vitro transformed callus, cell suspension, and root cultures (hairy roots), from the point of view of the controlled production of biologically active substances with desired properties, has potential, and is of interest to both researchers and producers [25].
It is known that hairy roots grow faster than any roots of intact plants [26,27] and accumulate a higher content of valuable compounds in their cells [19,28,29]. The total content of tanshinone in the hairy roots of transgenic S. miltiorrhiza was several times higher than the content in the roots of field plants (15.4 and 1.7–9.7 mg/g dry weight, respectively) [19,28]. The total content of vilforin in the hairy roots of Tripterygium wilfordii Hook.f. was significantly higher than in the adventitious roots [29]. There is an opinion that various BAS with new characteristics can be produced in vitro using hairy root cultures. New compounds of cadaverine and natural triterpene saponins, which were absent in both the leaves and roots of intact plants, were found in hairy roots of Brugmansia candida and Medicago truncatula when varying the parameters of the biosynthesis process.
It was also found that in vitro hairy root cultures can be used as model systems, both for identifying new genes and for fast characterization of their functions. It is possible to control BAS biosynthesis using genetic engineering methods (genome editing), and obtain genetically modified hairy root cultures. Blocking via the RNA biotransformation of the initial precursor, with the simultaneous optimization of substrates with unique compositions, makes it possible to construct hairy root cultures and produce specific BAS artificially.
At present, it is of great interest to use in vitro callus, cell suspension, and root cultures of medicinal plants as biologically active additives to increase the economic efficiency of the pharmaceutical industry. One of such plants is Rhaponticum carthamoides. It contains the following amino acids: aspartic (3.5–3.9 g/100 g), glutamic (2.5–3.0 g/100 g), leucine (1.9–2.0 g/100 g), tyrosine (1.2–1.4 g/100 g), lysine (1.6 g/100 g), vitamin C (62.0–77.0 mg/%), vitamin E (6.2 mg/%), carotenoids (65.0–113.0 mg/%), PP (vitamin PP is nicotinic acid, 11.5 mg/%), ecdysteroids (418.0–2170.0 mg/kg), and flavonoids (3.0–7.2% of dry matter). Due to the presence of various biologically active substances, the study of the qualitative and quantitative composition of in vitro callus, cell suspension, and root cultures of Rhaponticum carthamoides is a relevant task [30].
This work aims to study the possibility of producing and using extracts of BAS from Rhaponticum carthamoides callus, cell suspension, and root cultures as pharmaceutical substances.
2. Materials and Methods
2.1. Research Objects
BAS complexes isolated from extracts of freeze-dried biomass of in vitro callus, cell suspension, and root cultures of the medicinal plant Rhaponticum carthamoides (family Compositae), collected in the Kemerovo region (Siberia, Russia) in 2020, were the objects of this research. To obtain the biomass of in vitro callus, cell suspension, and root cultures, the seeds of Rhaponticum carthamoides were pre-washed with a detergent (acetic acid 3%), then immersed for 1 min in a 75% ethanol solution, transferred to a laminar box, and sterilized for 15 min in a 20% sodium hypochlorite solution (5% of active chlorine). All chemical reagents were purchased from Akvilon (Moscow, Russia). After sterilization, the sterilizing substance was washed off; for this, the seeds were washed for 20 min in distilled sterile water three times. The explants were then placed in a sterile 100 mL flask with 30 mL of Murashige-Skoog culture medium containing 3% sucrose, 0.7% agar-agar (Laverna XXI vek, Moscow, Russia), without growth stimulants, and were illuminated by compact fluorescent lamps (Economy 11W/865 11W E27 3U 6500K 6y CDL Philips 871150031502110 (Philips, Eindhoven, The Netherlands)) while maintaining a temperature of 25 °C. Seedlings that were 1.5-month-old were used for transformation. Lyophilization of germinated biomass of in vitro callus, cell suspension, and root cultures was carried out using a Triad freeze-dryer by Labconco (Kansas City, Missouri, USA). Lyophilization conditions: vacuum 0.05 mbar and cooler temperature –80 °C. The extracts were obtained as follows: a weighed portion of the studied biomass sample was weighed on an analytical balance (Oxaus PX85, New York, NY, USA), and transferred into a polyethylene Falcon tube. An organic solvent (ethanol) was added in an amount of 1:5 according to the experimental procedure, and the extraction process was carried out. The duration and temperature of the experiment varied up to 360 min and from 25 °C to boiling, respectively. Furthermore, the filtration process was carried out, followed by centrifugation of the filtrate at a rotor speed of 3900 ± 100 rpm. The filtrate was centrifuged in a PE-6900 centrifuge (Ekros, Moscow, Russia) to remove suspended particles. The solvent was evaporated from the extract on an IKA RV 8 V rotary evaporator (IKA, Staufen, Germany), under reduced pressure from a 100 mL flask pre-weighed on a CAS CUW420H balance (CAS Corporation Ltd., Seoul, Korea). The flask was weighed, and the yield of the extract was determined [31].
2.2. Drying of the BAS Complex
The drying of the BAS complex, isolated from lyophilized biomass extracts of in vitro callus, cell suspension, and root cultures, was also carried out by lyophilization. Lyophilization was performed using a Triad freeze-dryer by Labconco (USA). Lyophilization conditions made it possible to optimize the temperature and drying time of the samples. The stable conditions for lyophilization were selected: vacuum 0.05 mbar and temperature of the cooler −80 °C. The temperature regime and duration of the lyophilization process were individually selected for each sample. The residual solvent content was the controlled parameter [3].
2.3. Separation and Identification of Individual BAS
The isolated BAS complexes, from the lyophilized biomass extracts of in vitro callus, cell suspension, and root cultures of Rhaponticum carthamoides, were additionally separated by preparative HPLC, using a Shimadzu chromatograph (Shimadzu, Kyoto, Japan), flow rate 10 mL/min, phase A–B gradient 1–90% in 15 min, phase A—0.1% trifluoroacetic acid, B—acetonitrile (Laverna XXI vek, Moscow, Russia) [32], and a column ZORBAX Eclipse XDB-C18 Semi-Preparative 250 mm × 9.4 mm × 5 μm. Each fraction was evaporated to dryness, weighed, the yield was determined, and the structure of the compounds was identified by 1H NMR spectrometry.
To identify the BAS of in vitro callus, cell suspension, and root culture extracts of Rhodiola rosea, a mixed stock solution was prepared immediately before the experiment, containing 1 mg/mL of each biologically active substance in ethanol. Standard solutions, prepared by serial dilution to the final concentration (from 0.1 to 100.0 μg/mL) of the stock solution with ethanol, were used to construct the calibration curve with R2 0.987.
The solutions were chromatographed and eluted. We used a H2O:MeCN eluent system, with an acetonitrile gradient of 0–20% with a step of 2%. Trifluoroacetic acid (Lavrena XXI vek, Moscow, Russia), in an amount of 0.1%, was used as a modifier. The content of each BAS was calculated based on the calibration curves of the relationship between the peak regions and the concentrations of the standard solutions.
1H NMR spectra were obtained using a Bruker Avance NMR spectrometer (Bruker, Leipzig, Germany), with an operating frequency of 500 MHz [33]; CDCl3 (Chloroform-d) was used as a solvent for all compounds (Laverna XXI vek, Moscow, Russia).
2.4. Statistical Analysis
All experimentations were achieved in triplicates and results were given as a mean. The differences in the extracts were investigated by using student t-test (p < 0.05), and this test was performed in Statistica 10.0 (StatSoft Inc., 2007, Tulsa, OK, USA).
3. Results
Figure 1 shows a chromatogram after fractionation of the extract of in vitro callus, cell suspension, and root cultures of Rhaponticum carthamoides by HPLC.
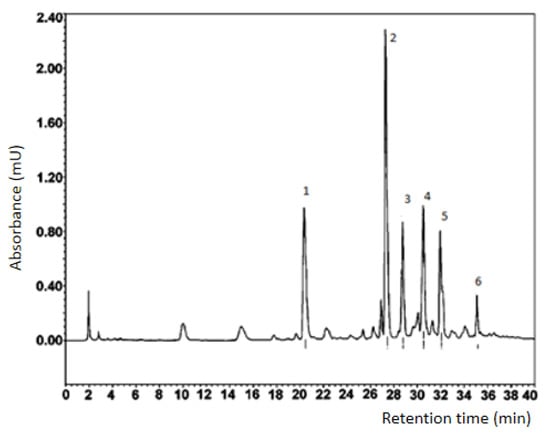
Figure 1.
Results of preparative separation of the ecdysteroid fraction of in vitro callus, cell suspension, and root cultures of Rhaponticum carthamoides.
For each BAS compound of in vitro callus, cell suspension and root cultures of Rhaponticum carthamoides, which corresponds to one of the numbered peaks (for small peaks, the yield of compounds was less than 0.5 mg, which did not allow identifying their structure by NMR), the structure was established by 1H NMR (see ESI with correlation of proton signals in NMR spectra and number, Figures S1–S5). The formulas of the identified substances are presented in Figure 2.
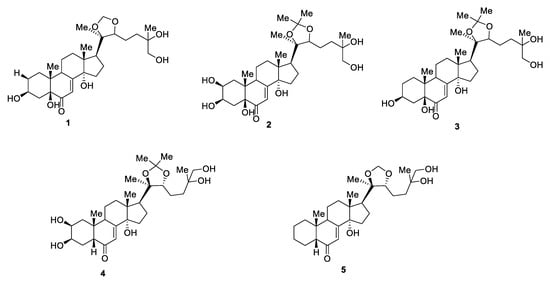
Figure 2.
Formulas of compounds present in the extract of callus, cell suspension, and root cultures of Rhaponticum carthamoides, identified by NMR spectra (the formula number corresponds to the peak number).
Identification of compounds of in vitro callus, cell suspension, and root cultures of BAS complex extracts from Rhaponticum carthamoides dried biomass by HPLC (Figure 1) resulted in a determination of the ecdysteroid fractions, in particular, 2,3,5,14,20,22,25-Heptahydroxycholest-7-en-6-one, with a mobility factor of 0.5 units.
For compound No. 1 (2-deoxy-5,20,26-trihydroxyecdyson), 7 mg of the substance was obtained, and the yield was 0.12% (Appendix A). For compound No. 2 (5,20,26-trihydroxyecdyson 20,22-acetonide), 15 mg of the substance was obtained, and the yield was 0.25% (Appendix B). For compound No. 3 (2-deoxy-5,20,26-trihydroxyecdyson 20,22-acetonide), 6 mg was isolated, and the yield was 0.10% (Appendix C). For compound No. 4 (20,26-dihydroxyecdyson 20,22-acetonide), 5 mg was isolated, and the yield was 0.09% (Appendix D). For compound No. 5 (ecdyson 20,22-acetonide), 6 mg was isolated, and the yield was 0.10% (Appendix E). The identified compounds are presented in Appendix A, Appendix B, Appendix C, Appendix D and Appendix E
It was not possible to identify compound No. 6 of the chromatogram (Figure 1); the NMR and mass spectrum have complex sets of signals, indicating a mix of two substances.
4. Discussion
The results of the qualitative composition analysis of the dried biomass extracts of in vitro callus, cell suspension, and root cultures, showed that the main BAS of the medicinal plant Rhaponticum carthamoides are 2-deoxy-5,20,26-trihydroxyecdyson, 5,20,26-trihydroxyecdyson 20,22-acetonide, 2-deoxy-5,20,26-trihydroxyecdyson 20,22-acetonide, 20,26-dihydroxyecdyson 20,22-acetonidecdyson and 20,22-acetonide. Stereochemistry for corresponding isomers of ecdysone was determined by the value of the spin–spin constant; for compound 4 and 5, value J increased, and this fact and conclusion led to the conclusion about the diastereomeric nature of the obtained compounds. We also took into account the values of the spin–spin interaction constant of the methylene group and protons of the cyclopentanoperhydrophenanthrene system. In this case, a decrease in the value of the spin–spin interaction constant was observed, which indicates the trans-configuration of methylene groups. In the side chain, a close value of the spin–spin interaction and chemical shift constants, with already known compounds, was found. From this, an assumption was made about a similar character of stereoisomerism for the obtained compounds.
In the root culture of Leuzea carthamoides, Yu et al. [33] found three new ecdysteroids (polypodine B 20,22-acetonide, 20-hydroxyecdysone 2,3; 20,22-diacetonide and isovitexiron together with 20-hydroxyecdysone, 20-hydroxyecdysone 2,3-acetonide, 20-hydroxyecdysone 20,22-acetonide, ajugasterone C, macisterone A and polypodyne B).
The described data confirm the presence of ecdysteroids in the Rhaponticum carthamoides root cultures. The molecular structure of each compound was determined by NMR spectroscopy and HRMS analysis. A method for the isolation of an individual compound with high brightness and selectivity, and subsequent identification by NMR spectra, which characterize the magnitude of the chemical shift signal and the multiplet structure, is described.
The BAS profile of callus, cell suspension, and root cultures of Thevetia peruviana (an ornamental shrub growing in many tropical regions of the world) is described [34]. The biologically active compounds of this plant have unique characteristics and can be used as pharmaceutical substances for drugs under development. The research objects were the 50% aqueous ethanol and ethyl acetate extracts. The work used thin-layer chromatography and standard chemical tests for phytochemical analysis. High-performance liquid chromatography was used to analyze the phenolic chemical profile. The total amount of phenolic and flavonoid, the total amount of cardiac glycosides, and the total antioxidant activity in callus, cell suspension, and root cultures, were determined. All the samples under study had common biological activity; antioxidants, amino acids, alkaloids, flavonoids, phenols, cardiac glycosides, leukoanthocyanidins, triterpenes, and sugars were found in their composition. Dihydroquercetin, which has anticancer activity, has been identified using high-performance liquid chromatography. The presented results demonstrate the usefulness of T. peruviana callus, cell suspension, and root cultures for the production of valuable pharmaceutical compounds. The data described in [34] confirm the high accumulation of flavonoids and ecdysteroids in in vitro callus, cell suspension, and root cultures of medicinal plants.
In [35], ginseng callus, cell suspension and root cultures, and their extracts (alcohol content 30–70%), were studied. Organic compounds were determined by thin-layer chromatography. For each plant, quercetin, magneferin, luteolin, rutin, quercetin-2-D-glucoside, malvidin, caffeic, cinnamic, ferulic, and sinapic acids were identified. The described results confirm the high accumulation of flavonoids and ecdysteroids in the extracts of in vitro callus, cell suspension, and root cultures of medicinal plants as well.
5. Conclusions
The qualitative composition analysis of extracts of callus, cell suspension, and root cultures showed that ecdysteroids and flavonoids are the most promising BAS, from the point of view of industrial and technological production. These compounds make the greatest contribution to the BAS complex of extracts of callus, cell suspension, and root cultures; their biological activity has been established, and their technological production is cost-effective, since it allows these compounds to be sold on the existing market, thereby reducing economic risk. Many people nowadays prefer natural dietary supplements to synthetic medicines. Therefore, the in vitro biotechnological production of callus, cell suspension, and root cultures, under controlled conditions, represents a cost-effective way for the commercial mass production of phytochemicals. The studied extracts of callus, cell suspension, and root cultures of the medicinal plant Rhaponticum carthamoides are readily available and are considered effective, with fewer side effects compared to modern drugs, in the treatment of various diseases.
Substances such as flavonoids, ecdysteroids and anthocyanins found in the in vitro callus, cell suspension, and root cultures of Rhaponticum carthamoides have antioxidant activity, the ability to trap free radicals, and cardioprotective, antidiabetic, anti-inflammatory and anti-allergic effects, while some other phytonoid and ecdysteroid compounds show potential antiviral activity. Recently, the flavonoids and ecdysteroids of Rhaponticum carthamoides were proved to be the most effective anticancer agents, due to apoptosis, which causes cell cycle arrest and the inhibition of key enzymes involved in tumor promotion. The use of flavonoids and ecdysteroids as potential drugs for the prevention of many chronic diseases is a topical trend in pharmacology [36].
In the future, it is planned to study the antimicrobial, antioxidant, and antitumor activity of the BAS of extracts of in vitro callus, cell suspension, and root cultures of the medicinal plant Rhaponticum carthamoides for the production of pharmaceuticals and dietary supplements with antitumor, antimicrobial and antioxidant effects.
Supplementary Materials
The following are available online at https://www.mdpi.com/2076-3417/11/6/2555/s1, Figure S1: 1H NMR spectrum of identified compound 2-deoxy-5,20,26-trihydroxyecdyson; Figure S2: 1H NMR spectrum of identified compound 5,20,26-trihydroxyecdyson 20,22-acetonide/; Figure S3: 1H NMR spectrum of identified compound 2-deoxy-5,20,26-trihydroxyecdyson 20,22-acetonide; Figure S4: 1H NMR spectrum of identified compound 20,26-dihydroxyecdyson 20,22-acetonide; Figure S5: 1H NMR spectrum of identified compound ecdyson 20,22-acetonide.
Author Contributions
S.I., A.P. and O.B. conceived and designed the research; L.A., L.D. and S.S. analyzed and interpreted the data; L.D., E.U. and S.S. contributed reagents, materials, analysis tools or data; S.I., A.P., E.C., E.U. and O.B. wrote the paper. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by RUSSIAN SCIENCE FOUNDATION, grant number 18-75-10066.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Appendix A. The Identified Compound No. 1 (1 2-deoxy-5,20,26-trihydroxyecdyson)
+41 (c 0.05 MeOH); UV (MeOH) λmax (log ε) 243 nm (4.02) nm; 1H NMR 1H NMR (500 MHz, Chloroform-d) δ 6.02 (d, J = 1.1 Hz, 1H), 4.81 (s, 1H), 4.74 (d, J = 12.4 Hz, 1H), 4.59–4.51 (m, 2H), 4.43 (d, J = 7.9 Hz, 1H), 4.27 (s, 1H), 4.10–3.99 (m, 2H), 3.73–3.66 (m, 1H), 3.58–3.47 (m, 2H), 2.20–2.08 (m, 2H), 2.08–2.01 (m, 2H), 1.92–1.50 (m, 15H), 1.50–1.40 (m, 1H), 1.33 (t, J = 1.5 Hz, 3H), 1.26 (s, 3H), 1.10 (d, J = 1.4 Hz, 3H), 0.86 (s, 3H).
HRESIMS m = z 497.3108 (calculated for C27H45O8, 497.3102).
Appendix B. The Identified Compound No. 2 (5,20,26-trihydroxyecdyson 20,22-acetonide)
+89 (c 0.05 MeOH); UV (MeOH) λmax (log ε) 242 (3.76) nm; 1H NMR (500 MHz, Chloroform-d) δ 5.86 (1H, d, J 2.8 Hz, CH), 3.99 (1H, q, J 3.0 Hz, CH), 3.95 (1H, ddd, J 10.0, 7.4, 3.6 Hz, CH), 3.695 (1H, t, J 6.0 Hz, CH), 3.375 (1H, d, J 11.0 Hz, CH), 3.355 (1H, d, J 11.0 Hz, CH), 3.19 (1H, ddd, J 11.3, 7.0, 2.7 Hz, OH), 2.31 (1H, dd, J 9.4, 8.1 Hz, OH), 2.12 (1H, td, J 13.1, 5.0 Hz, CH), 2.075 (1H, dd, J 14.7, 3.0 Hz, CH), 2.03 (1H, m, CH), 1.96 (1H, dd, J 12.4, 6.5 Hz, CH), 1.87 (1H, m, CH), 1.86 (1H, m, CH), 1.81 (1H, m, CH), 1.77 (1H, dd, J 14.9, 3.0 Hz, CH), 1.74 (1H, m, CH), 1.73 (2H, m, CH), 1.71 (1H, m, CH), 1.61 (1H, m, CH), 1.55 (1H, m, CH), 1.53 (1H, m, CH), 1.52 (1H, m, CH), 1.39 (3H, s, CH3), 1.32 (3H, s, CH3), 1.18 (3H, s, CH3), 1.15 (3H, s, CH3), 0.915 (3H, s, CH3), 0.83 (3H, s, CH3); 13C NMR (CD3OD, 125 MHz) δ 202.5, 167.4, 120.7, 108.2, 86.0, 85.3, 83.6, 80.4, 73.6, 70.7, 70.4, 68.6, 50.5, 48.7, 45.5, 39.2, 37.2, 36.3, 34.3, 32.6, 31.8, 29.5, 27.3, 24.0, 23.9, 22.7, 22.65, 22.5, 17.8, 17.1; ESIMS m/z 575 [M + Na]+ (46), 553 [M + H]+ (100), 537 [M - CH3]+ (5), 535 [M + H - H2O]+ (2), 520 [M + H - H2O - CH3]+ (2), 495 [M + H - acetone]+ (59), 481 (1), 477 (3), 437 (3), 359 (3), 328 (14).
HRESIMS m=z 553.3366 (calculated for C30H49O9, 553.3363).
Appendix C. The Identified Compound No. 3 (2-deoxy-5,20,26-trihydroxyecdyson 20,22-acetonide)
+25 (c 0.05 MeOH); UV (MeOH) λmax (log ε) 238 (4.08) nm; 1H NMR (CD3OD, 500 MHz, Chloroform-d) δ 5.86 (1H, s, br, OH), 4.08 (1H, s, br, CH), 3.70 (1H, m, OH), 3.37 (1H, d, J 11.0 Hz, CH), 3.36 (1H, d, J 11.0 Hz, CH), 3.28 (1H, m, CH), 2.32 (1H, t, J 8.7 Hz, CH), 2.12 (1H, td, J 12.4, 5.7 Hz, CH), 2.04 (1H, m, CH), 2.035 (1H, m, CH), 1.97 (1H, m, CH), 1.96 (1H, m, CH), 1.88 (1H, m, CH), 1.86 (1H, m, CH), 1.84 (1H, m, CH), 1.77 (1H, m, CH), 1.73 (1H, m, CH), 1.72 (1H, m, CH) 1.61 (2H, m, CH2), 1.55 (2H, m, CH2), 1.53 (1H, m, CH), 1.50 (1H, m, CH), 1.39 (3H, s, CH3), 1.32 (3H, s, CH3), 1.18 (3H, s, CH3), 1.15 (3H, s, CH3), 0.89 (3H, s, CH3), 0.83 (3H, s, CH3); 13C NMR (CD3OD, 125 MHz) δ 167.9, 120.7, 108.2, 86.0, 85.4, 83.6, 81.2, 73.6, 70.7, 67.2, 50.6, 48.7, 43.25, 38.1, 37.2, 36.9, 32.6, 31.8, 29.5, 29.3, 27.3, 25.6, 24.05, 23.8, 22.7, 22.5, 22.5, 17.8, 17.3; ESIMS m/z 559 [M + Na]+ (100), 537 [M + H]+ (36), 518 [M - H2O]+ (3), 541 [M + Na - H2O]+ (12), 501 [M + H - 2H2O]+ (2), 445 (10), 385 [M + H - H2O - C6O3H14]+ (3), 315 (12), 304 (24).
HRESIMS m=z 537.3420 (calculated for C30H49O8, 537.3414).
Appendix D. The Identified Compound No. 4 (20,26-dihydroxyecdyson 20,22-acetonide)
+145 (c 0.005 MeOH); UV (MeOH) λmax (log ε) 242 (4.01) nm; 1H NMR (CD3OD, 500 MHz, Chloroform-d) δ 5.86 (1H, d, J 2.6 Hz, CH), 3.70 (1H, m, OH), 3.38 (1H, d, J 10.9 Hz, CH), 3.36 (1H, d, J 11.0 Hz, CH), 2.42 (1H, dd, J 12.6, 4.0 Hz, CH), 2.33 (1H, dd, J 9.2, 8.6 Hz, CH), 1.39 (3H, s, CH3), 1.32 (3H, s, CH3=), 1.18 (3H, s, CH3), 1.15 (3H, s, CH3), 0.96 (3H, s, CH3), 0.83 (3H, s, CH3); 13C NMR (CD3OD, 125 MHz) δ 121.8, 85.9, 85.5, 83.6, 73.2, 70.7, 50.5, 49.3, 37.1, 32.55, 29.4, 27.3, 24.4, 23.7, 22.7, 17.8; ESIMS m/z 575 [M + K]+ (14), 560 [M + H + Na]+ (6), 559 [M + Na]+ (5), 542 (100), 521 [M - CH3]+ (23), 519 [M + H - H2O]+ (2), 503 [M - CH3 - H2O]+ (7), 501 [M + H - 2H2O]+ (23), 478 [M - acetone]+ (4), 445 (14), 413 (6), 314 (10), 304 (55).
HRESIMS m=z 537.3418 (calculated for C30H48O8, 537.3414).
Appendix E. The Identified Compound No. 5 (ecdyson 20,22-acetonide)
UV (MeOH) λmax (log ε) 245 (6.01) nm; 1H NMR (CD3OD, 500 MHz, Chloroform-d) δ 3.70 (1H, m, OH), 3.38 (1H, d, J 10.9 Hz, CH), 3.36 (1H, d, J 11.0 Hz, CH), 2.42 (1H, dd, J 12.6, 4.0 Hz, CH), 2.33 (1H, dd, J 9.2, 8.6 Hz, CH), 1.39 (3H, s, CH), 1.32 (3H, s, CH), 1.18 (3H, s, CH), 1.15 (3H, s, CH), 0.96 (3H, s, CH), 0.83 (3H, s, CH); 13C NMR (CD3OD, 125 MHz) δ 121.8, 85.9, 85.5, 83.6, 73.2, 70.7, 50.5, 49.3, 37.1, 32.55, 29.4, 27.3, 24.4, 23.7, 22.7, 17.8.
HRESIMS m=z 476.3278 (calculated for C28H44O6, 476.3138).
References
- Ashour, M.; Wink, M.; Gershenzon, J. Biochemistry of terpenoids: Monoterpenes, sesquiterpenes and diterpenes. Annu. Plant Rev. 2010, 40, 258–303. [Google Scholar]
- Atanasov, A.G.; Waltenberger, B.; Pferschy-Wenzig, E.-M.; Linder, T.; Wawrosch, C.; Uhrin, P. Discovery and resupply of pharmacologically active plant-derived natural products: A review. Biotechnol. Adv. 2015, 33, 1582–1614. [Google Scholar] [CrossRef] [PubMed]
- Babich, O.; Prosekov, A.; Zaushintsena, A.; Sukhikh, A.; Dyshlyuk, L.; Ivanova, S. Identification and quantification of phenolic compounds of Western Siberia Astragalus danicus in different regions. Heliyon 2019, 5, e02245. [Google Scholar] [CrossRef] [PubMed]
- Babich, O.; Sukhikh, S.; Prosekov, A.; Asyakina, L.; Ivanova, S. Medicinal Plants to Strengthen Immunity during a Pandemic. Pharmaceuticals 2020, 13, 313. [Google Scholar] [CrossRef]
- Babich, O.; Sukhikh, S.; Pungin, A.; Ivanova, S.; Asyakina, L.; Prosekov, A. Modern Trends in the In Vitro Production and Use of Callus, Suspension Cells and Root Cultures of Medicinal Plants. Molecules 2020, 25, 5805. [Google Scholar] [CrossRef]
- Banerjee, S.; Singh, S.; Ur Rahman, L. Biotransformation studies using hairy root cultures—A review. Biotechnol. Adv. 2012, 30, 461–468. [Google Scholar] [CrossRef] [PubMed]
- Butler, N.M.; Shelley, H.J.; Jiang, J. First generation genome editing in potato using hairy root transformation. Plant Biotechnol. J. 2020, 18, 2201–2209. [Google Scholar] [CrossRef] [PubMed]
- Caputi, L.; Franke, J.; Farrow, S.C.; Chung, K.; Payne, R.M.E.; Nguyen, T.-D.; Dang, T.-T.T.; Carqueijeiro, I.S.T.; Koudounas, K.; de Bernonville, T.D.; et al. Missing enzymes in the biosynthesis of the anticancer drug vinblastine in Madagascar periwinkle. Science 2018, 360, 1235–1239. [Google Scholar] [CrossRef] [PubMed]
- Cardon, F.; Pallisse, R.; Bardor, M.; Caron, A.; Vanier, J.; Ele Ekouna, J.P.; Lerouge, P.; Boitel-Conti, M.; Guillet, M. Brassica rapa hairy root based expression system leads to the production of highly homogenous and reproducible profiles of recombinant human alpha-L-iduronidase. Plant Biotechnol. J. 2019, 17, 505–516. [Google Scholar] [CrossRef] [PubMed]
- Carqueijeiro, I.; Langley, C.; Grzech, D.; Koudounas, K.; Papon, N.; O’Connor, S.E.; Courdavault, V. Beyond the semi-synthetic artemisinin: Metabolic engineering of plant-derived anticancer drugs. Curr. Opin. Biotechnol. 2020, 65, 17–24. [Google Scholar] [CrossRef] [PubMed]
- Carrizo, C.N.; Pitta-Alvarez, S.I.; Kogan, M.J.; Giulietti, A.M.; Tomaro, M.L. Occurrence of cadaverine in hairy roots of Brugmansia candida. Phytochemistry 2001, 57, 759–763. [Google Scholar] [CrossRef]
- Chahardoli, M.; Fazeli, A.; Ghabooli, M. Recombinant production of bovine Lactoferrin-derived antimicrobial peptide in tobacco hairy roots expression system. Plant Physiol. Biochem. 2018, 123, 414–421. [Google Scholar] [CrossRef]
- Dang, T.T.T.; Franke, J.; Tatsis, E.; O’Connor, S.E. Dual catalytic activity of a cytochrome P450 controls bifurcation at a metabolic branch point of alkaloid biosynthesis in Rauwolfia serpentine. Angew Chem. Int. Ed. Engl. 2017, 56, 9440–9444. [Google Scholar] [CrossRef] [PubMed]
- Dudareva, N.; Klempien, A.; Muhlemann, J.K.; Kaplan, I. Biosynthesis, function and metabolic engineering of plant volatile organic compounds. New Phytol. 2013, 198, 16–32. [Google Scholar] [CrossRef] [PubMed]
- Dyshlyuk, L.; Dmitrieva, A.; Ivanova, S.; Golubcova, Y.; Ostroumov, L. Panax ginseng callus, suspension, and root cultures: Extraction and qualitative analysis. Foods Raw Mater. 2020, 8, 369–376. [Google Scholar] [CrossRef]
- Georgiev, M.I.; Agostini, E.; Ludwig-Müller, J.; Xu, J. Genetically transformed roots: From plant disease to biotechnological resource. Trends Biotechnol. 2012, 30, 528–537. [Google Scholar] [CrossRef] [PubMed]
- Hidalgo, D.; Georgiev, M.; Marchev, A.; Bru-Martínez, R.; Cusido, R.M. Purificación Corchete & Javier Palazon Tailoring tobacco hairy root metabolism for the production of stilbenes. Sci. Rep. 2017, 7, 17976. [Google Scholar] [CrossRef]
- Jeziorek, M.; Sykłowska-Baranek, K.; Pietrosiuk, A. Hairy root cultures for the production of anticancer naphthoquinone compounds. Curr. Med. Chem. 2018, 25, 4718–4739. [Google Scholar] [CrossRef] [PubMed]
- Kai, G.Y.; Xu, H.; Zhou, C.; Liao, P.; Xiao, J.; Luo, X.; You, L.; Zhang, L. Metabolic engineering tanshinone biosynthetic pathway in Salvia miltiorrhiza hairy root cultures. Metab. Eng. 2011, 13, 319–327. [Google Scholar] [CrossRef]
- Kim, Y.J.; Zhang, D.; Yang, D.-C. Biosynthesis and biotechnological production of ginsenosides. Biotechnol. Adv. 2015, 33, 717–735. [Google Scholar] [CrossRef]
- Li, F.S.; Weng, J.K. Demystifying traditional herbal medicine with modern approaches. Nat. Plants 2017, 3, 17109. [Google Scholar] [CrossRef] [PubMed]
- Liao, P.; Hemmerlin, A.; Bach, T.J.; Chye, M.-L. The potential of the mevalonate pathway for enhanced isoprenoid production. Biotechnol. Adv. 2016, 34, 697–713. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.N.; Xu, D.-B.; Li, L.; Zhang, F.; Fu, X.-Q.; Shen, Q.; Lyu, X.-Y.; Wu, Z.-K.; Pan, Q.-F.; Shi, P.; et al. Jasmonate promotes artemisinin biosynthesis by activating the TCP14-ORA complex in Artemisia annua. Sci. Adv. 2018, 4, eaas9357. [Google Scholar] [CrossRef]
- Massa, S.; Paolini, F.; Marino, C.; Franconi, R.; Venuti, A. Bioproduction of a therapeutic vaccine against human Papillomavirus in tomato hairy root cultures. Front. Plant Sci. 2019, 10, 452. [Google Scholar] [CrossRef]
- Mendoza, D.; Pablo, J.; Cuaspud, O.; Arias, M. Phytochemical Screening of Callus and Cell Suspensions Cultures of Thevetia peruviana. Braz. Arch. Biol. Technol. 2020, 63. [Google Scholar] [CrossRef]
- Miao, G.; Han, J.; Feng, J.T.; Zhu, C.-S.; Zhang, X. A MDR transporter contributes to the different extracellular production of sesquiterpene pyridine alkaloids between adventitious root and hairy root liquid cultures of Tripterygium wilfordii Hook.f. Plant Mol. Biol. 2017, 95, 51–62. [Google Scholar] [CrossRef] [PubMed]
- Nag, S.A.; Qin, J.-J.; Wang, W.; Wang, M.-H.; Wang, H.; Zhang, R. Ginsenosides as anticancer agents: In vitro and in vivo activities, structure–activity relationships, and molecular mechanisms of action. Front. Pharmacol. 2012, 3, 25. [Google Scholar] [CrossRef] [PubMed]
- Normile, D. Asian medicine: The new face of traditional Chinese medicine. Science 2003, 299, 188–190. [Google Scholar] [CrossRef] [PubMed]
- Paek, K.Y.; Hosakatte, N.M.; Hahn, E.-J.; Zhong, J.-J. Large scale culture of ginseng adventitious roots for production of ginsenosides. Adv. Biochem. Eng. Biotechnol. 2009, 113, 151–176. [Google Scholar] [PubMed]
- Latushkina, N.A.; Ivanovsky, A.A.; Timkina, E.Y. Issledovanie himicheskogo sostava i toksicheskih svoystv fitokompleksa, soderzhaschego biologicheski aktivnye veschestva. Agrarnaya Nauka Evro-Severo-Vostoka [Agric. Sci. Euro North East] 2017, 4, 58–62. (In Russian) [Google Scholar]
- Asyakina, L.; Sukhikh, S.; Ivanova, S.; Prosekov, A.; Ulrikh, E.; Chupahin, E.; Babich, O. Determination of the qualitative composition of biologically-active substances of extracts of in vitro callus, cell suspension, and root cultures of the medicinal plant Rhodiola rosea. Biomolecules 2021, 11, 365. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Asyakina, L.K.; Babich, O.O.; Dyshlyuk, L.S.; Sukhykh, S.A.; Popov, A.D.; Kostiushina, N.V. Izuchenie fiziko-himicheskih svoystv i biologicheskoy aktivnosti ekstraktov iz vysushennoy biomassy kallusnyh, suspenzionnyh kletok I kornevyh kultur in vitro. Tehnika I Tehnologiay Pishchevyh Proizvodstv [Tech. Technol. Food Prod.] 2020, 50, 480–492. (In Russian) [Google Scholar] [CrossRef]
- Yu, K.W.; Hahn, E.J.; Paek, K.Y. Ginsenoside production by hairy root cultures of Panax ginseng: Influence of temperature and light quality. Biochem. Eng. J. 2005, 23, 53–56. [Google Scholar] [CrossRef]
- Zaushintsena, A.V.; Milentyeva, I.; Babich, O.; Noskova, S.Y.; Kiseleva, T.F.; Popova, D.G.; Bakin, I.A.; Lukin, A. Quantitative and qualitative profile of BASextracted from purple echinacea (Echinacea purpurea L.) growing in the Kemerovo region: Functional foods application. Foods Raw Mater. 2019, 7, 84–92. [Google Scholar] [CrossRef]
- Zhang, J.H.; Wider, B.; Shang, H.; Li, X.; Ernst, E. Quality of herbal medicines: Challenges and solutions. Complement. Ther. Med. 2012, 20, 100–106. [Google Scholar] [CrossRef] [PubMed]
- Prithviraj, K. Biological activities of flavonoids: An overview. Int. J. Pharm. Sci. Res. 2019, 10, 1567–1574. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).