Molecular Characterization of the Organic Fraction of Municipal Solid Waste and Compositional Evolution during Oxidative Processes Assessed by HR-MAS 13C NMR Spectroscopy
Abstract
1. Introduction
2. Materials and Methods
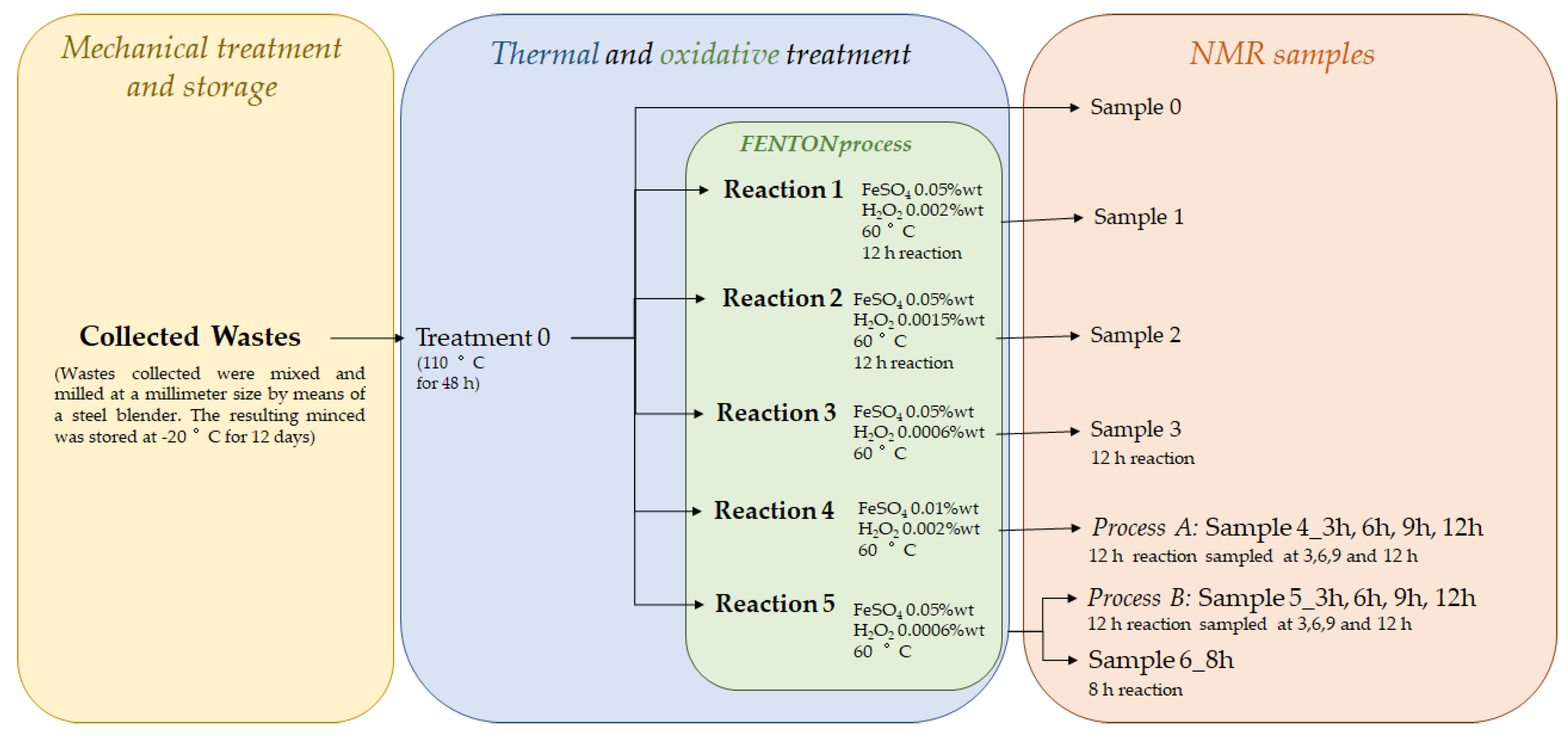
2.1. Sample Collection and Preparation for NMR
2.2. NMR Spectroscopy and Data Processing
3. Results and Discussion
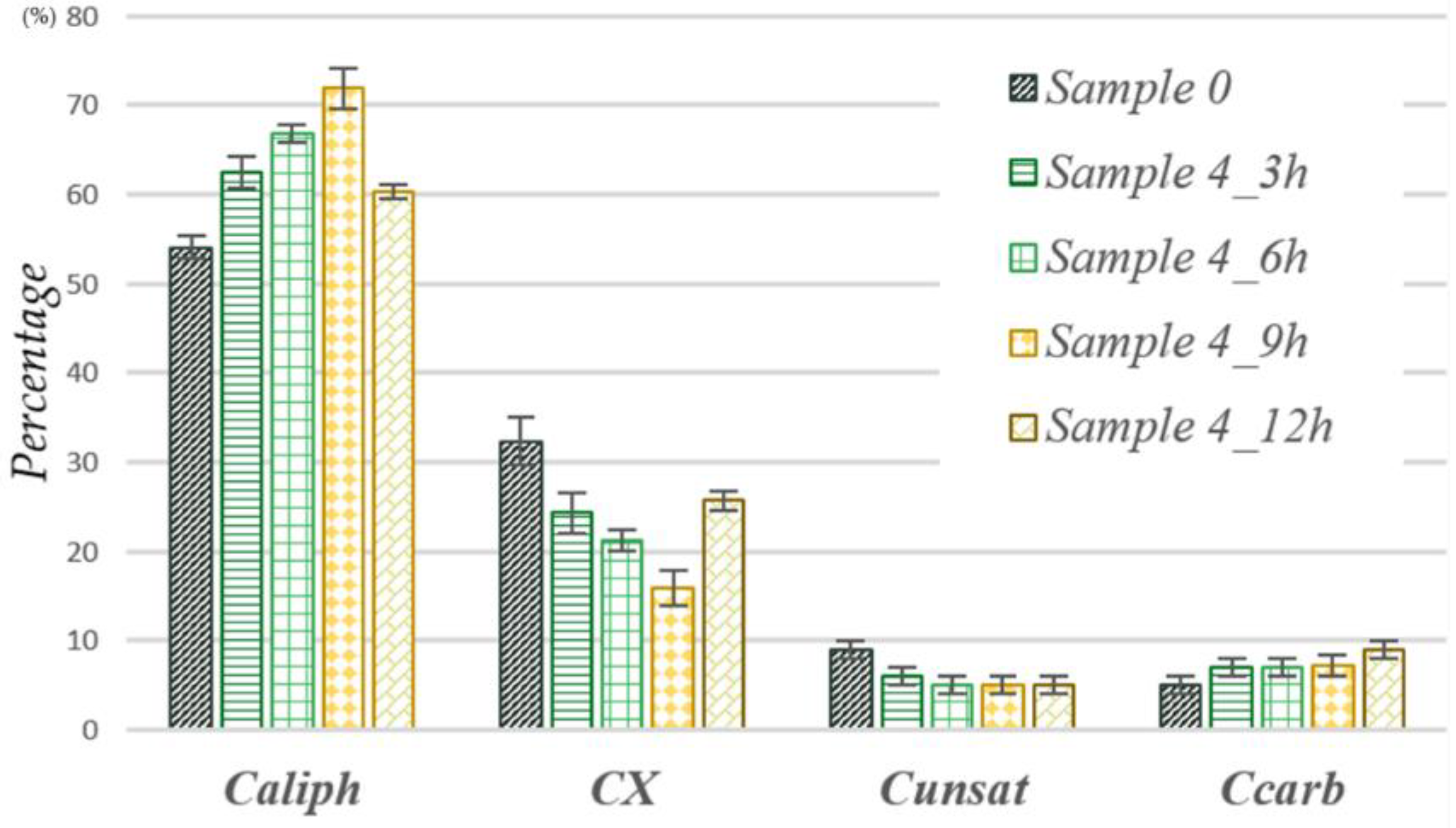
- Process A. From the data reported in Table 3 and Figure 4, it can be deduced that the reaction proceeds according to two main steps. The first stage, stage I, goes from 0 up to 9 h: during this time, as the reaction proceeds, a decrease in the content of carbon atoms linked to heteroatoms is observed. Since the data present in the literature on organic waste show that the amount of carbons linked to oxygen (C–O) is greater than the amount of any other C–X [23], it is probable that this also occurs for our samples and, therefore, this decrease can be attributable, for example, to the oxidation of alcoholic groups, mostly present in sugars, to carbonyl groups. On the other hand, however, there is no substantial variation in the areas related to the carbon atoms of the carbonyl groups and this suggests that probably, in this period of time, further oxidation of carbonyl groups with loss of CO2 occurs. It should be emphasized that it cannot be deduced in any way from the recorded spectra as to whether the CO2 produced comes from the newly formed or pre-existing carbonyl groups. Stage II occurs around 12 h: at this time, there is an evident increase in the area of carbon atoms linked to heteroatoms due, probably, to an oxidation of aliphatic carbons and, to a lesser extent, to the unsaturated ones. As evidence of this, a decrease in the areas due to these carbon groups can be appreciated.
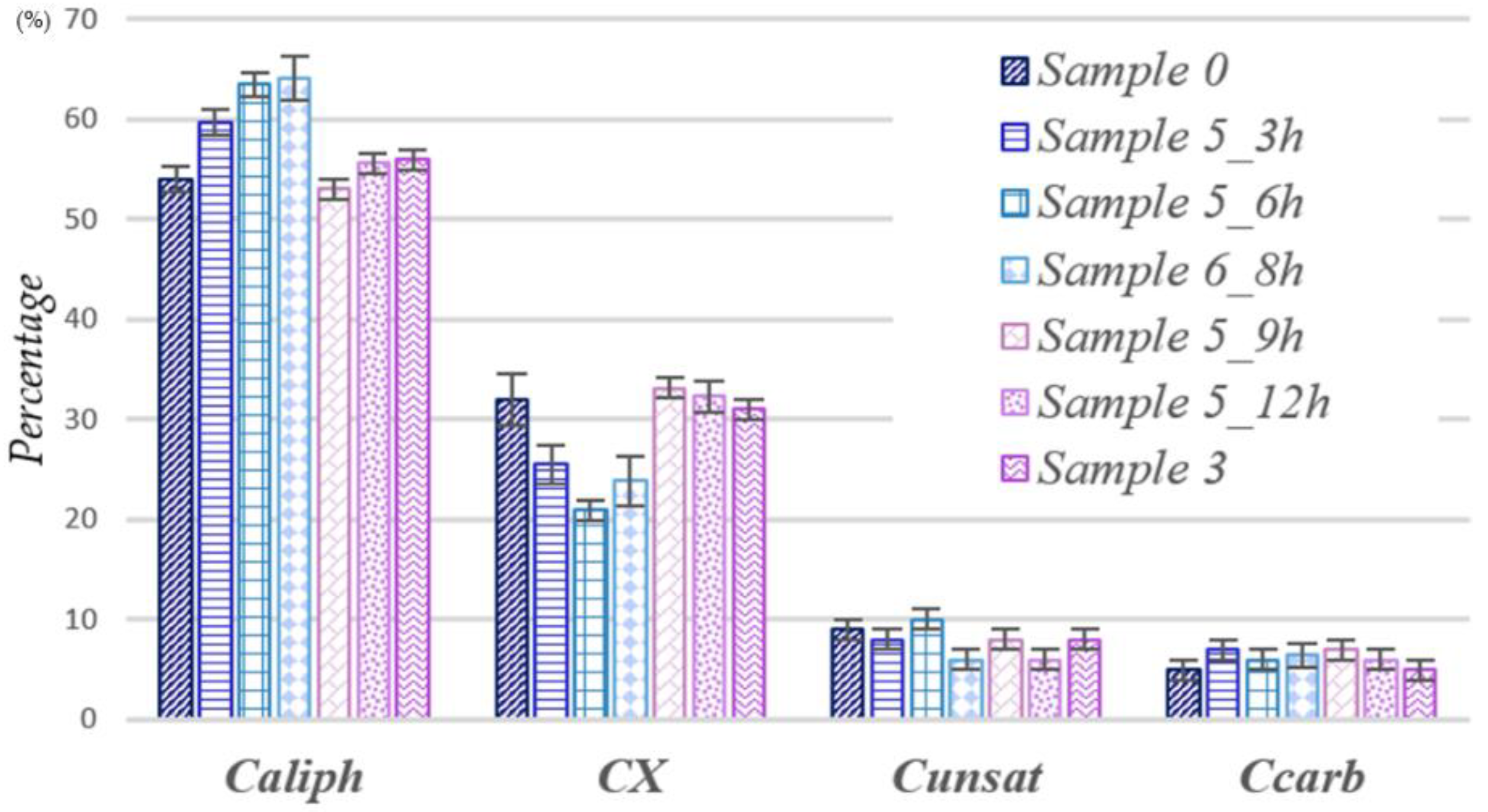
- Process B. From the data reported in Table 4 and from the relative distribution of Figure 5, it is possible to infer that the process B proceeds in the same way as for process A, but the two steps described above start and finish at different times. Indeed, Stage I of Sample 5 goes from 0 to 6 h, unlike Sample 4, in which it continues up to 9 h, while Stage II occurs at the time of about 8 h compared to 12 h for process A. Moreover, for Sample 5, it is possible to hypothesize a third stage between 9 and 12 h: at this moment, the trend previously observed in Stage I, i.e., an oxidation of the alcoholic groups to carbonyls with simultaneous loss of CO2, occurs. In Table 4 and Figure 5 are also reported the NMR data obtained for the Sample 6_8 h, in which the same concentrations of Sample 5 are used but the reaction proceeds continuously for 8 h. As can be seen from Table 4 and Figure 4, in Sample 6_8 h, there is a slight increase in the carbon atoms bound to the heteroatoms compared to the situation observed at 6 h. A decrease in the aliphatic content at 8 h is not observed, but a decrease in the unsaturation content is appreciable and suggests that this process (Stage II) begins with the oxidation of the double bonds and that it only subsequently proceeds with the oxidation of the saturated chains, as highlighted in Sample 5_9 h.
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Minelgaite, A.; Liovikiene, G. Waste Problem in European Union and its Influence on Waste Management Behaviours. Sci. Total Environ. 2019, 667, 86–93. [Google Scholar] [CrossRef]
- Sankoh, F.P. Understandig Solid Waste Managemente Practices in Developing Countries: From Waste Disposal to Recovery of Resources. Am. J. Environ. Prot. 2020, 9, 44–48. [Google Scholar] [CrossRef]
- Zorpas, A.A. Strategy Development in the Framework of Waste Management. Sci. Total Environ. 2020, 716, 137088. [Google Scholar] [CrossRef]
- Hamoda, M.F. Air Pollutants Emissions from Waste Treatment and Disposal Facilities. J. Environ. Sci. Health Part A 2006, 41, 77–85. [Google Scholar] [CrossRef]
- Das, S.; Lee, S.-H.; Kumar, P.; Kim, K.-H.; Lee, S.S. Solid Waste Management: Scope and the Challenge of Sustainability. J. Clean. Prod. 2019, 228, 658–678. [Google Scholar] [CrossRef]
- All, U.; Sajid, N.; Khalld, A.; Riaz, L.; Rabbani, M.M.; Hussain Syed, J.; Malik, R.N. A Review on Vermicomposting of Organi Wastes. Environ. Prog. Sustain. Energy 2015, 34, 1050–1062. [Google Scholar] [CrossRef]
- Soudejani, H.; Kazemian, H.; Inglezakis, V.; Zorpas, A. Application of Zeolites in Organic Waste Composting: A Review. Biocatal. Agric. Biotechnol. 2019, 22, 101396. [Google Scholar] [CrossRef]
- Diaz, L.F.; Savage, G.M.; Eggerth, L.; Golueke, C. Composting and Recycling Municipal Solid Waste; CRC Press: Boca Raton, FL, USA, 1993. [Google Scholar]
- Hailu, Y.; Hanchiso, T.; Bereta, A. Municipal Solid Waste Source Identification, Characterization and Physical Composition Analysis, Case Study Wolkite Town, Ethiopia. Am. J. Environ. Prot. 2019, 8, 48–53. [Google Scholar] [CrossRef]
- Mata-Alvarez, J.; Macè, S.; Llabrès, P. Anaerobic Digestion of Organic Solid Wasts. An Overview of Research Achievements and Perspectives. Bioresour. Technol. 2000, 74, 3–16. [Google Scholar] [CrossRef]
- Khalid, A.; Arshad, M.; Anjum, M.; Mahmood, T.; Dawson, L. The Anaerobic Digestion of Solid Organic Waste. Waste Manag. 2011, 31, 1737–1744. [Google Scholar] [CrossRef]
- Li, Y.; Park, S.; Zhu, J. Soli-State Anaerobic Digestion for Methane Production from Organic Waste. Renew. Sustain. Energy Rev. 2011, 15, 821–826. [Google Scholar] [CrossRef]
- Pramanik, S.K.; Suja, F.B.; Zain, S.; Pramanik, B.K. The Anaerobic Digestion Process of Biogas Production from Food Waste: Prospects and Constraints. Bioresour. Technol. Rep. 2019, 8, 100310. [Google Scholar] [CrossRef]
- Soumaré, M.; Tack, F.; Verloo, M. Effect of a Municipal Solid Waste Compost and Mineral Fertilization on Plan Growth in two Tropical Agricultural Soils of Mali. Bioresour. Technol. 2003, 86, 15–20. [Google Scholar] [CrossRef]
- Hargreaves, J.; Adl, M.; Warman, P. A Review of the Use of Composted Municipal Solid Waste in Agriculture. Agric. Ecosyst. Environ. 2008, 123, 1–14. [Google Scholar] [CrossRef]
- Kumar, S. Composting of Municipal Solid Waste. Crit. Rev. Biotechnol. 2011, 31, 112–136. [Google Scholar] [CrossRef]
- Cerda, A.; Artola, A.; Font, X.; Barrena, R.; Gea, T.; Sanchez, A. Composting of Food Wastes: Status and Challenges. Bioresour. Technol. 2018, 248, 57–67. [Google Scholar] [CrossRef] [PubMed]
- Koppenol, W. The Centennial of the Fenton Reaction. Free Radic. Biol. Med. 1993, 15, 645–651. [Google Scholar] [CrossRef]
- Oliveros, E.; Legrini, O.; Hohl, M.; Muller, T.; Braun, A.M. Industrial Waste Water Treatment: Large Scale Development of a Light-Enhanced Fenton Reaction. Chem. Eng. Process. 1997, 36, 397–405. [Google Scholar] [CrossRef]
- Chamarro, E.; Marco, A.; Esplugas, S. Use of FENTON Reagent to Improve Organic Chemical Biodegradability. Water Res. 2001, 35, 1047–1051. [Google Scholar] [CrossRef]
- Neyens, E.; Baeyens, J. A Review of Classic FENTON’s Peroxidation as an Advanced Oxidation Technique. J. Hazard. Mater. 2003, 98, 33–50. [Google Scholar] [CrossRef]
- Roccotelli, A.; Araniti, F.; Tursi, A.; Di Rauso Simeone, G.; Rao, M.A.; Lania, I.; Chidichimo, G.; Abenavoli, M.R.; Gelsomino, A. Organic Matter Characterization and Phytotoxic Potential Assessment of a Solid Anaerobic Digestate Following Chemical Stabilization by an Iron-Based Fenton Reaction. J. Agric. Food Chem. 2020, 68, 9461–9474. [Google Scholar] [CrossRef]
- Chefetz, B.; Hatcher, P.G.; Hadar, Y.; Chen, Y. Chemical and Biological Characterization of Organic Matter during Composting of Municipal Solid Waste. J. Environ. Qual. 1996, 25, 776–785. [Google Scholar] [CrossRef]
- Muller, M.; Pereira Milori, D.M.B.; Déléris, S.; Steyer, J.-P.; Dudal, Y. Solid-Phase Fluorescence Spectroscopy to Characterize Organic Wates. Waste Manag. 2011, 31, 1916–1923. [Google Scholar] [CrossRef]
- Baldock, J.A.; Oades, J.M.; Nelson, O.N.; Skene, T.M.; Golchin, A.; Clarke, P. Assessing the Extent of Decomposition of Natural Organic Materials Using Solid-State 13C NMR Spectroscopy. Aust. J. Soil Res. 1997, 35, 1061–1083. [Google Scholar] [CrossRef]
- Pichler, M.; Knicker, H.; Kogel-Knabner, I. Changes in the Chemical Structure of Municipal Solid Waste during Composting as Studied by Solid-State Dipolar Dephasing and PSRE 13C NMR ad Solid-State 15N NMR Spectroscopy. Environ. Sci. Technol. 2000, 34, 4034–4038. [Google Scholar] [CrossRef]
- Pichler, M.; Knicker, H.; Kögel-Knabner, I. Solid-State 13C NMR Spectroscopic, Chemolytic and Biological Assessment of Pretreated Municipal Solid Waste. J. Ind. Microbiol. Biotechnol. 2001, 26, 83–89. [Google Scholar] [CrossRef] [PubMed]
- Castaldi, P.; Alberti, G.M.R.M.P. Study of the Organic Matter Evolution During Municipal Solid Waste Composting Aimed at Identifying Suitable Parameters for the Evaluation of Compost Maturity. Waste Manag. 2001, 25, 209–213. [Google Scholar] [CrossRef] [PubMed]
- Tambone, F.; Genevini, P.; D’Imporzano, G.; Adani, F. Assessing Amendment Properties of Digestate by Sudying the Organic Matter Composition and the Degree of Biological Stability During the Anaerobic Digestion of the Organic Fraction of MSW. Bioresour. Technol. 2009, 100, 3140–3142. [Google Scholar] [CrossRef]
- Tambone, F.; Adani, F.; Gigliotti, G.; Volpe, D.; Fabbri, C.; Provenzano, M.R. Organic Matter Characterization During the Anaerobic Digestion of Different Biomasses by Means of CPMAS 13C NMR Spectroscopy. Biomass Bioenergy 2013, 48, 111–120. [Google Scholar] [CrossRef]
- Tang, H.; Hills, B.P. Use of 13C MAS NMR to Study Domain Structure and Dynamics of Polysaccarides in the Native Starch Granules. Biomacromolecules 2003, 4, 1269–1276. [Google Scholar] [CrossRef] [PubMed]
- Huhn, S.; Szabo, C.; Gass, J.; Manzi, A. Metabolic Profiling of Normal and Hypertensive Rat Kidney Tissues by HRMAS-NMR Spectroscopy. Anal. Bioanal. Chem. 2004, 378, 1511–1519. [Google Scholar] [CrossRef]
- Beckonert, O.; Coen, M.; Keun, H.C.; Wang, Y.; Ebbels, T.M.D.; Holmes, E.; Lindon, J.C.; Nicholson, J.K. High-Resolution Magic-Angle-Spinning NMR Spectroscopy for Metabolic Profiling of Intact Tissues. Nat. Protoc. 2010, 5, 1019–1032. [Google Scholar] [CrossRef]
- Farooq, H.; Courtier-Murias, D.; Soong, R.; Bermel, W.; Kingery, W.; Simpson, A.J. HR-MAS NMR Spectroscopy: A Pratical Guide for Natural Samples. Curr. Org. Chem. 2013, 17, 3013–3031. [Google Scholar] [CrossRef]
- Corsaro, C.; Cicero, N.; Mallamace, D.; Vasi, S.; Naccari, C.; Salvi, A.; Giofrè, V.; Dugo, G. HR-MAS and NMR towards Foodomics. Food Res. Int. 2016, 89, 1085–1094. [Google Scholar] [CrossRef]
- Mazzei, P.; Piccolo, A. HRMAS NMR Spectroscopy Applications in Agriculture. Chem. Biol. Technol. Agric. 2017, 4, 1–13. [Google Scholar] [CrossRef]
- Ferro, M.; Castiglione, F.; Panzeri, W.; Dispenza, R.; Santini, L.; Karlsson, H.J.; de Wit, P.P.; Mele, A. Non-Destructive and Direct Determination of the Degree of Substitution of Carboxymethyl Cellulose by HR-MAS 13C NMR Spectroscopy. Carbohydr. Polym. 2017, 169, 16–22. [Google Scholar] [CrossRef]
- TopSpin. Available online: https://www.bruker.com/en/products-and-solutions/mr/nmr-software/topspin.html (accessed on 1 February 2021).
- Caricasole, P.; Provenzano, M.; Hatcher, P.; Senesi, N. Evolution of Organic Matter During Composting of Different Organic Wastes Asessed by CPMAS 13C NMR Spectroscopy. Waste Manag. 2011, 31, 411–415. [Google Scholar] [CrossRef]
- Wong, A.; Lucas-Torres, C. High-Resolution Magic-Angle-Spinning (HR-MAS) NMR Spectroscopy. In NMR-Based Metabolomics; Keun, H.C., Ed.; Royal Society of Chemistry: Croydon, UK, 2018; Chapter 5; pp. 113–147. [Google Scholar]

| Chemical Shift Range (ppm) | Assignment |
|---|---|
| 220 to 190 | C=O of ketones and aldehydes |
| 190 to 160 | C=O in carboxylic acids, esters and amides |
| 160 to 140 | C–O–R and C–N–R aromatics; C–1 in polystyrene |
| 140 to 110 | Aromatic C–H; C–2, C–3, C–4, C–5 and C–6 in polystyrene |
| 110 to 90 | C–1 in carbohydrates; C–2 and C–6 in the lignin syringes unit |
| 90 to 60 | C–2 and C–6 in carbohydrates; side chain in lignin |
| 60 to 50 | Methoxy groups, Ca in amino acids |
| 50 to 40 | Methylene groups (lipids, proteins, polyethylene, polyisoprene, polyamide). Long chain aliphatic C, also biopolymers |
| 40 to 25 | Cα and Cβ in polymers such as polyvinylchloride, polypropylene, polystyrene |
| 25 to 0 | Methyl groups (lipids, peptides, polypropylene). Short chain aliphatic C |
| Caliph (%) | CX (%) | Cunsat (%) | Ccarb (%) | |
|---|---|---|---|---|
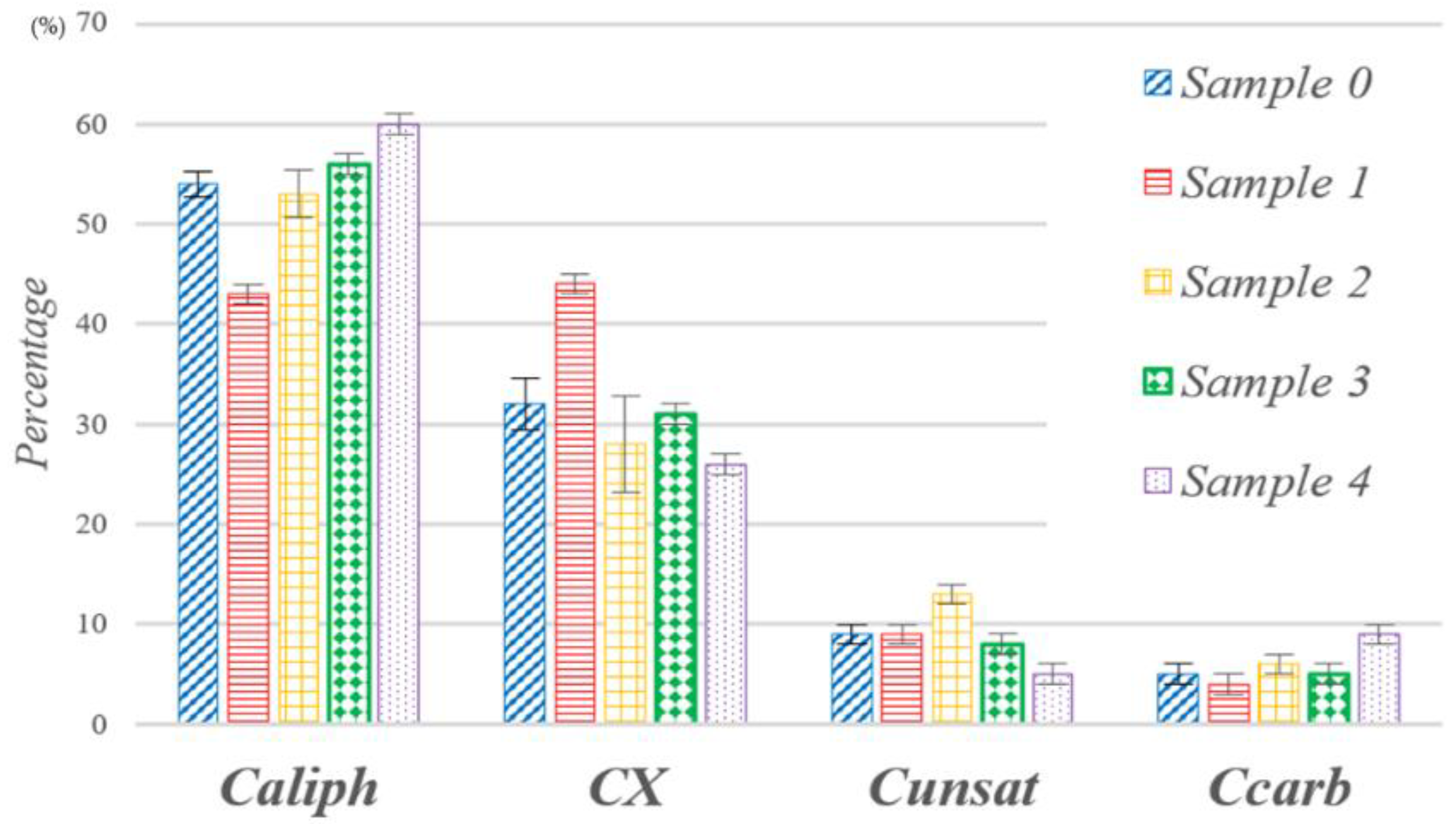
| Sample 0 | 54 ± 1 | 32 ± 3 | 9 ± 1 | 5 ± 1 |
| Sample 1 | 43 ± 1 | 44 ± 1 | 9 ± 1 | 4 ± 1 |
| Sample 2 | 53 ± 2 | 28 ± 5 | 13 ± 1 | 6 ± 1 |
| Sample 3 | 56 ± 1 | 31 ± 1 | 8 ± 1 | 5 ± 1 |
| Sample 4 | 60 ± 1 | 26 ± 1 | 5 ± 1 | 9 ± 1 |
| Process A | Caliph (%) | CX (%) | Cunsat (%) | Ccarb (%) |
|---|---|---|---|---|
| Sample 0 | 54 ± 1 | 32 ± 3 | 9 ± 1 | 5 ± 1 |
| Sample 4_3 h | 63 ± 2 | 24 ± 2 | 6 ± 1 | 7 ± 1 |
| Sample 4_6 h | 67 ± 1 | 21 ± 1 | 5 ± 1 | 7 ± 1 |
| Sample 4_9 h | 72 ± 2 | 16 ± 2 | 5 ± 1 | 7 ± 1 |
| Sample 4_12 h | 60 ± 1 | 26 ± 1 | 5 ± 1 | 9 ± 1 |
| Process B | Caliph (%) | CX (%) | Cunsat (%) | Ccarb (%) |
|---|---|---|---|---|
| Sample 0 | 54 ± 1 | 32 ± 3 | 9 ± 1 | 5 ± 1 |
| Sample 3 | 56 ± 1 | 31 ± 1 | 8 ± 1 | 5 ± 1 |
| Sample 5_3 h | 60 ± 1 | 25 ± 2 | 8 ± 1 | 7 ± 1 |
| Sample 5_6 h | 63 ± 1 | 21 ± 1 | 10 ± 1 | 6 ± 1 |
| Sample 5_9 h | 53 ± 1 | 33 ± 1 | 8 ± 1 | 7 ± 1 |
| Sample 5_12 h | 56 ± 1 | 32 ± 2 | 6 ± 1 | 6 ± 1 |
| Sample 6_8 h | 64 ± 2 | 24 ± 2 | 6 ± 1 | 6 ± 1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Salvino, R.A.; Celebre, G.; De Luca, G. Molecular Characterization of the Organic Fraction of Municipal Solid Waste and Compositional Evolution during Oxidative Processes Assessed by HR-MAS 13C NMR Spectroscopy. Appl. Sci. 2021, 11, 2267. https://doi.org/10.3390/app11052267
Salvino RA, Celebre G, De Luca G. Molecular Characterization of the Organic Fraction of Municipal Solid Waste and Compositional Evolution during Oxidative Processes Assessed by HR-MAS 13C NMR Spectroscopy. Applied Sciences. 2021; 11(5):2267. https://doi.org/10.3390/app11052267
Chicago/Turabian StyleSalvino, Rosachiara A., Giorgio Celebre, and Giuseppina De Luca. 2021. "Molecular Characterization of the Organic Fraction of Municipal Solid Waste and Compositional Evolution during Oxidative Processes Assessed by HR-MAS 13C NMR Spectroscopy" Applied Sciences 11, no. 5: 2267. https://doi.org/10.3390/app11052267
APA StyleSalvino, R. A., Celebre, G., & De Luca, G. (2021). Molecular Characterization of the Organic Fraction of Municipal Solid Waste and Compositional Evolution during Oxidative Processes Assessed by HR-MAS 13C NMR Spectroscopy. Applied Sciences, 11(5), 2267. https://doi.org/10.3390/app11052267






