Erythrocytes and Nanoparticles: New Therapeutic Systems
Abstract
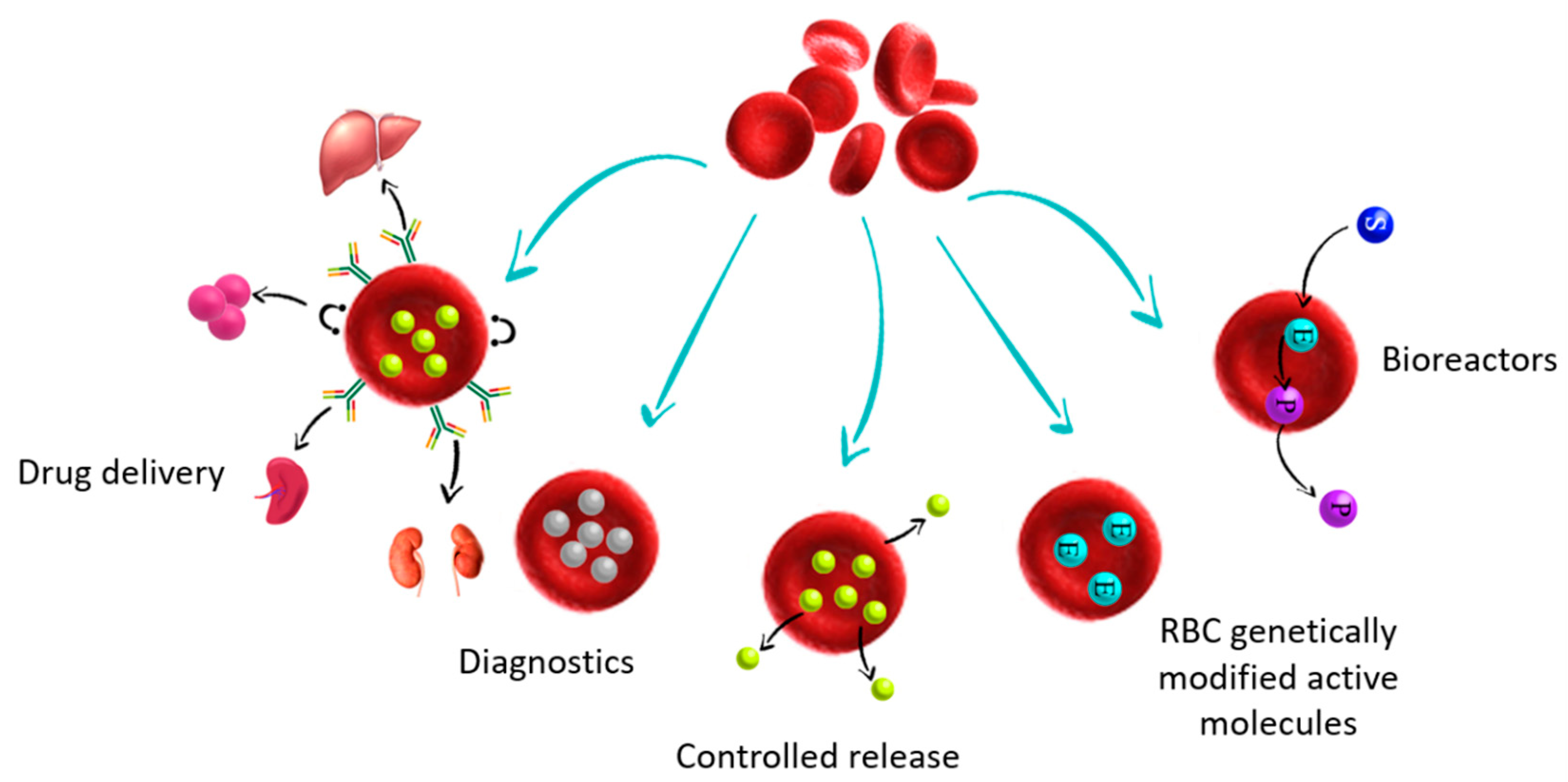
:1. Introduction
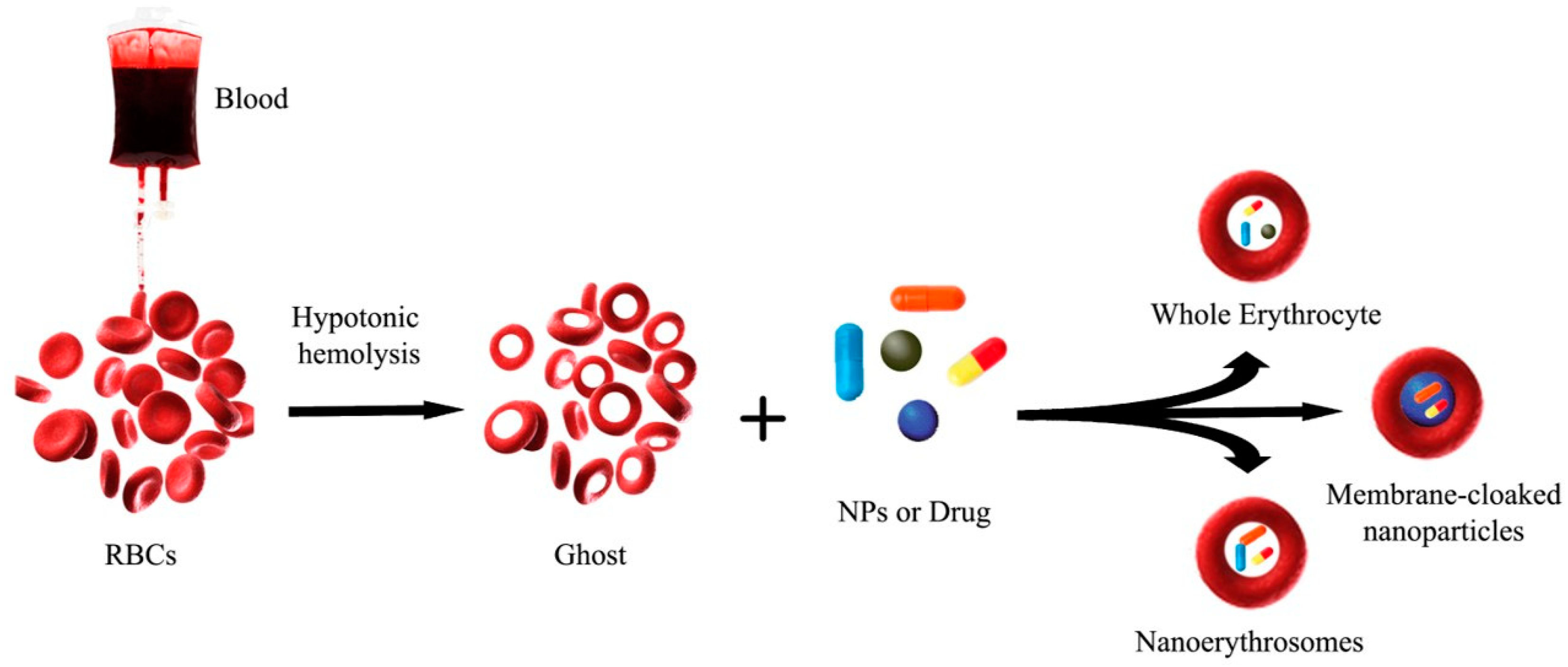
2. Bio Hybrid Carrier Using RBC
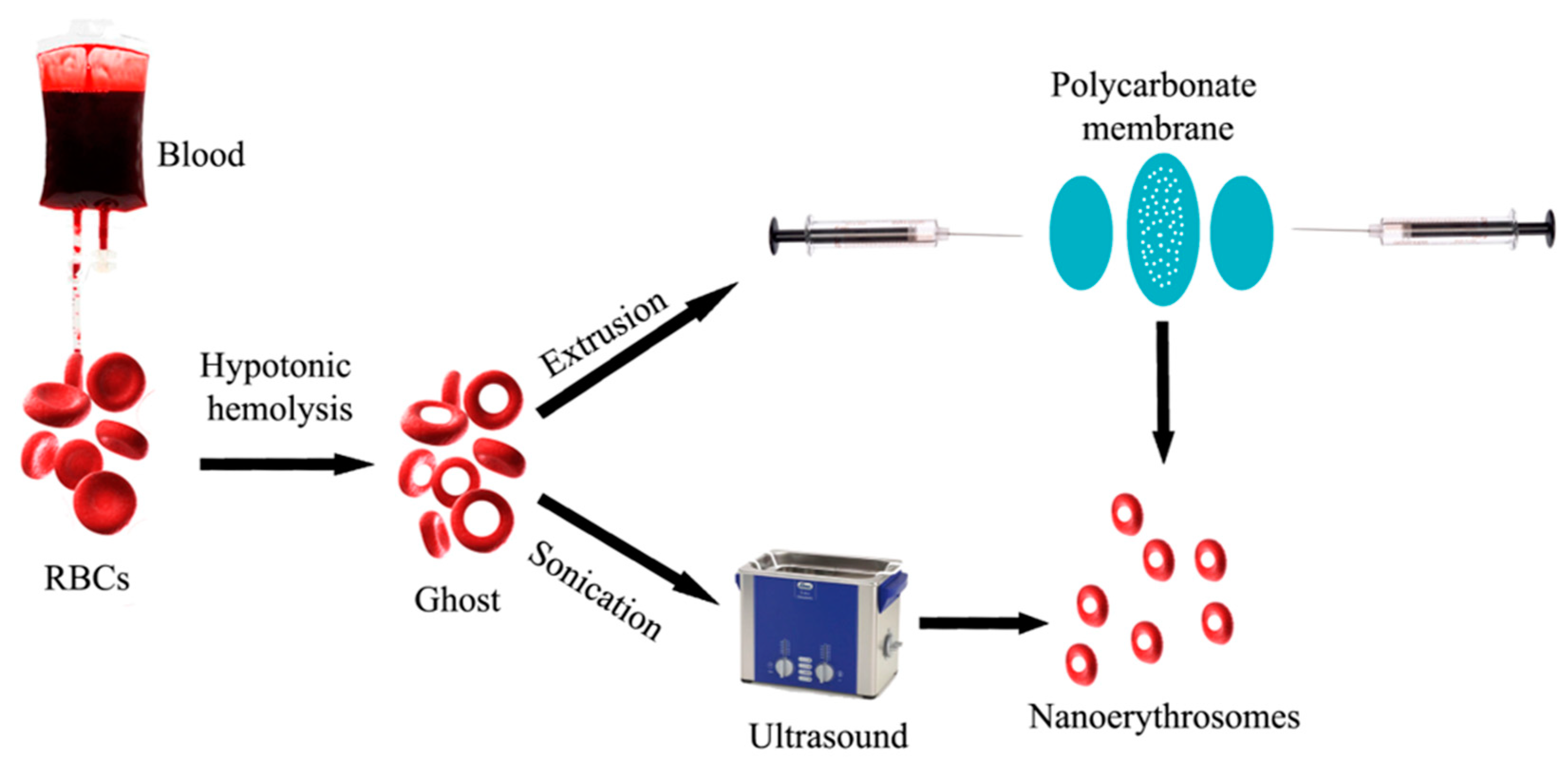
Nanoerythrosomes (NERs)
3. NPs and RBC
3.1. RBC and Solid Lipid NPs (SLN)/Nanostructured Lipid Carriers (NLC)
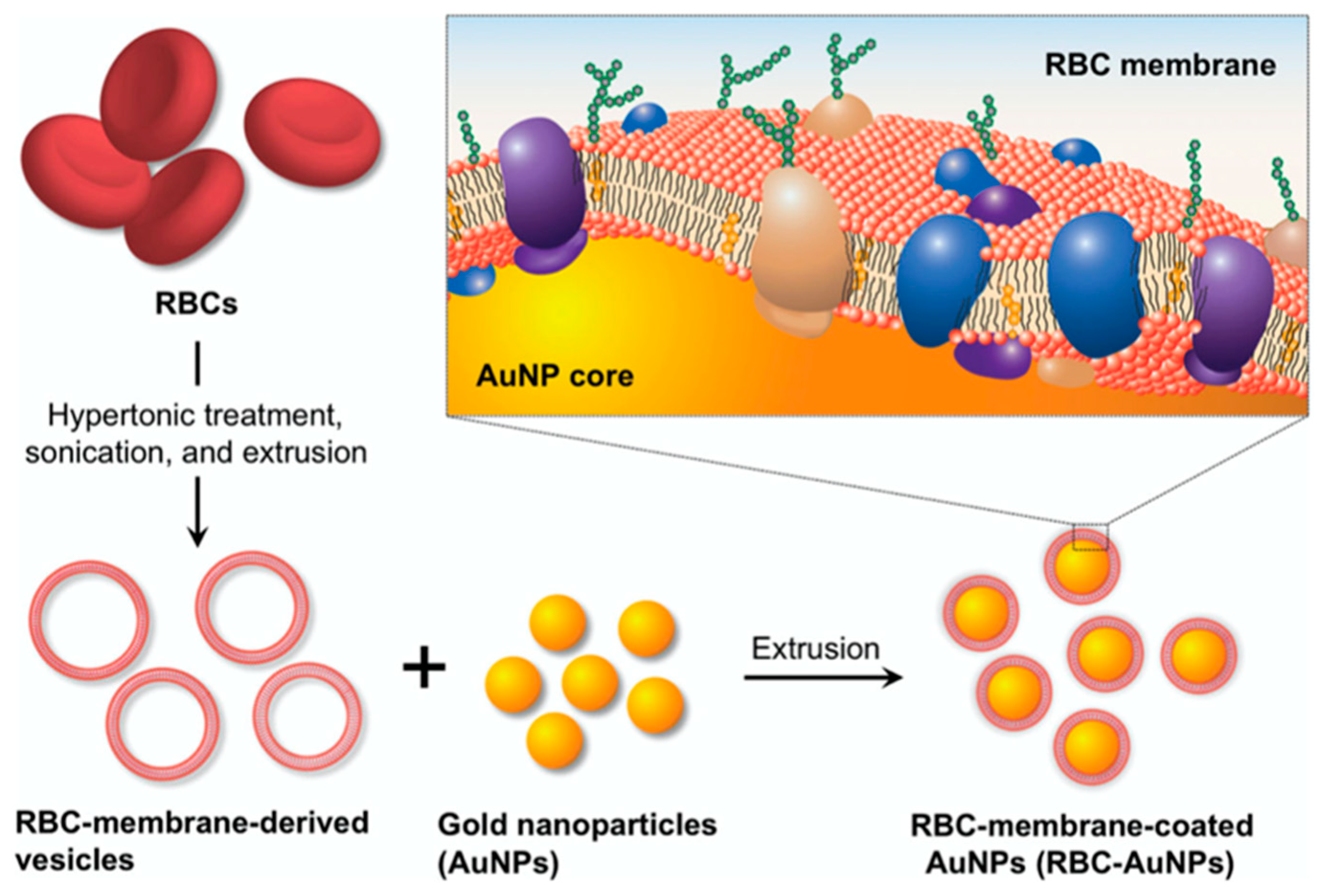
3.2. RBC and Inorganic NPs
3.3. RBC and Polymeric NPs
3.4. RBC-Mimicking Particles
4. Innovative Application of RBC-Coated NPs versus Nano-RBCs
5. RBCs Loaded with Biologically Active Compounds and NPs: New Perspective
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Farokhzad, O.C.; Langer, R. Impact of Nanotechnology on Drug Delivery. ACS Nano 2008, 3, 16–20. [Google Scholar] [CrossRef] [PubMed]
- Cortese, B.; D’Amone, S.; Testini, M.; Ratano, P.; Palamà, I.E. Hybrid Clustered Nanoparticles for Chemo-Antibacterial Combinatorial Cancer Therapy. Cancers 2019, 11, 1338. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Davis, M.E.; Chen, Z.G.; Shin, D.M. Nanoparticle therapeutics: An emerging treatment modality for cancer. Nat. Rev. Drug Discov. 2008, 7, 771–782. [Google Scholar] [CrossRef]
- Fernandes, D.A.; Kolios, M.C. Near-infrared absorbing nanoemulsions as nonlinear ultrasound contrast agents for cancer theranostics. J. Mol. Liq. 2019, 287, 110848. [Google Scholar] [CrossRef]
- Fernandes, D.A.; Kolios, M.C. Perfluorocarbon bubbles as photoacoustic signal amplifiers for cancer theranostics. Opt. Mater. Express 2019, 9, 4532–4544. [Google Scholar] [CrossRef]
- Shohet, S.B. Red Blood Cells. In Lipid Metabolism in Mammals; Snyder, F., Ed.; Springer: Boston, MA, USA, 1977; pp. 191–208. [Google Scholar] [CrossRef]
- Kuhn, V.; Diederich, L.; Keller, T.S.; Kramer, C.M.; Lückstädt, W.; Panknin, C.; Suvorava, T.; Isakson, B.E.; Kelm, M.; Cortese-Krott, M.M. Red Blood Cell Function and Dysfunction: Redox Regulation, Nitric Oxide Metabolism, Anemia. Antioxid. Redox Signal. 2017, 26, 718–742. [Google Scholar] [CrossRef]
- Magnani, M.; Rossi, L. Approaches to erythrocyte-mediated drug delivery. Expert Opin. Drug Deliv. 2014, 11, 677–687. [Google Scholar] [CrossRef] [PubMed]
- Hu, C.-M.J.; Fang, R.H.; Zhang, L. Erythrocyte-Inspired Delivery Systems. Adv. Healthc. Mater. 2012, 1, 537–547. [Google Scholar] [CrossRef]
- Goodhead, L.K.; Macmillan, F.M. Measuring osmosis and hemolysis of red blood cells. Adv. Physiol. Educ. 2017, 41, 298–305. [Google Scholar] [CrossRef]
- Yarmush, M.L.; Golberg, A.; Serša, G.; Kotnik, T.; Miklavčič, D. Electroporation-Based Technologies for Medicine: Principles, Applications, and Challenges. Annu. Rev. Biomed. Eng. 2014, 16, 295–320. [Google Scholar] [CrossRef] [Green Version]
- Rao, L.; Cai, B.; Bu, L.-L.; Liao, Q.-Q.; Guo, S.-S.; Zhao, X.-Z.; Dong, W.-F.; Liu, W. Microfluidic Electroporation-Facilitated Synthesis of Erythrocyte Membrane-Coated Magnetic Nanoparticles for Enhanced Imaging-Guided Cancer Therapy. ACS Nano 2017, 11, 3496–3505. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.-P.; Guan, Y.-S.; Jin, X.-R.; Jiang, S.-S.; Lu, Z.-J.; Wu, Y.; Li, Y.; Li, M.; Luo, F. Development of novel 5-fluorouracil carrier erythrocyte with pharmacokinetics and potent antitumor activity in mice bearing malignant ascites. J. Gastroenterol. Hepatol. 2010, 25, 985–990. [Google Scholar] [CrossRef]
- Wadhwa, R.; Aggarwal, T.; Thapliyal, N.; Kumar, A.; Priya; Yadav, P.; Kumari, V.; Reddy, B.S.C.; Chandra, P.; Maurya, P.K. Red blood cells as an efficient in vitro model for evaluating the efficacy of metallic nanoparticles. 3 Biotech 2019, 9, 279. [Google Scholar] [CrossRef] [PubMed]
- Mukthavaram, R.; Shi, G.; Kesari, S.; Simberg, D. Targeting and depletion of circulating leukocytes and cancer cells by lipophilic antibody-modified erythrocytes. J. Control. Release 2014, 183, 146–153. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xia, Q.; Zhang, Y.; Li, Z.; Hou, X.; Feng, N. Red blood cell membrane-camouflaged nanoparticles: A novel drug delivery system for antitumor application. Acta Pharm. Sin. B 2019, 9, 675–689. [Google Scholar] [CrossRef] [PubMed]
- Zaitsev, S.; Danielyan, K.; Murciano, J.-C.; Ganguly, K.; Krasik, T.; Taylor, R.P.; Pincus, S.; Jones, S.; Cines, D.B.; Muzykantov, V.R. Human complement receptor type 1–directed loading of tissue plasminogen activator on circulating erythrocytes for prophylactic fibrinolysis. Blood 2006, 108, 1895–1902. [Google Scholar] [CrossRef] [Green Version]
- Kina, T.; Ikuta, K.; Takayama, E.; Wada, K.; Majumdar, A.S.; Weissman, I.L.; Katsura, Y. The monoclonal antibody TER-119 recognizes a molecule associated with glycophorin A and specifically marks the late stages of murine erythroid lineage. Br. J. Haematol. 2000, 109, 280–287. [Google Scholar] [CrossRef] [PubMed]
- Oudin, S.; Libyh, M.T.; Goossens, D.; Dervillez, X.; Philbert, F.; Réveil, B.; Bougy, F.; Tabary, T.; Rouger, P.; Klatzmann, D.; et al. A Soluble Recombinant Multimeric Anti-Rh(D) Single-Chain Fv/CR1 Molecule Restores the Immune Complex Binding Ability of CR1-Deficient Erythrocytes. J. Immunol. 2000, 164, 1505–1513. [Google Scholar] [CrossRef] [Green Version]
- Zaitsev, S.; Kowalska, M.A.; Neyman, M.; Carnemolla, R.; Tliba, S.; Ding, B.-S.; Stonestrom, A.; Spitzer, D.; Atkinson, J.P.; Poncz, M.; et al. Targeting recombinant thrombomodulin fusion protein to red blood cells provides multifaceted thromboprophylaxis. Blood 2012, 119, 4779–4785. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oldenborg, P.-A.; Zheleznyak, A.; Fang, Y.-F.; Lagenaur, C.F.; Gresham, H.D.; Lindberg, F.P. Role of CD47 as a Marker of Self on Red Blood Cells. Science 2000, 288, 2051–2054. [Google Scholar] [CrossRef] [PubMed]
- Khoory, J.; Estanislau, J.; Elkhal, A.; Lazaar, A.; Melhorn, M.I.; Brodsky, A.; Illigens, B.; Hamachi, I.; Kurishita, Y.; Ivanov, A.R.; et al. Ligation of Glycophorin a Generates Reactive Oxygen Species Leading to Decreased Red Blood Cell Function. PLoS ONE 2016, 11, e0141206. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, H. Erythrocytes in nanomedicine: An optimal blend of natural and synthetic materials. Biomater. Sci. 2016, 4, 1024–1031. [Google Scholar] [CrossRef]
- Colino, C.I.; Lanao, J.M.; Gutierrez-Millan, C. Recent advances in functionalized nanomaterials for the diagnosis and treatment of bacterial infections. Mater. Sci. Eng. C 2021, 121, 111843. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, X.; He, D.; Zhou, Y.; Qin, L. Surface-Modified Nanoerythrocyte Loading DOX for Targeted Liver Cancer Chemotherapy. Mol. Pharm. 2018, 15, 5728–5740. [Google Scholar] [CrossRef]
- Hamidi, M.; Rafiei, P.; Azadi, A.; Mohammadi-Samani, S. Encapsulation of Valproate-Loaded Hydrogel Nanoparticles in Intact Human Erythrocytes: A Novel Nano-cell Composite for Drug Delivery. J. Pharm. Sci. 2011, 100, 1702–1711. [Google Scholar] [CrossRef]
- Jiang, A.; Song, B.; Ji, X.; Peng, F.; Wang, H.; Su, Y.; He, Y. Doxorubicin-loaded silicon nanoparticles impregnated into red blood cells featuring bright fluorescence, strong photostability, and lengthened blood residency. Nano Res. 2017, 11, 2285–2294. [Google Scholar] [CrossRef]
- Pan, D.C.; Myerson, J.W.; Brenner, J.S.; Patel, P.N.; Anselmo, A.C.; Mitragotri, S.; Muzykantov, V. Nanoparticle Properties Modulate Their Attachment and Effect on Carrier Red Blood Cells. Sci. Rep. 2018, 8, 1–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pan, D.; Vargas-Morales, O.; Zern, B.; Anselmo, A.C.; Gupta, V.; Zakrewsky, M.; Mitragotri, S.; Muzykantov, V. The Effect of Polymeric Nanoparticles on Biocompatibility of Carrier Red Blood Cells. PLoS ONE 2016, 11, e0152074. [Google Scholar] [CrossRef] [Green Version]
- Delcea, M.; Sternberg, N.; Yashchenok, A.M.; Georgieva, R.; Bäumler, H.; Möhwald, H.; Skirtach, A.G.; Moehwald, H. Nanoplasmonics for Dual-Molecule Release through Nanopores in the Membrane of Red Blood Cells. ACS Nano 2012, 6, 4169–4180. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Sun, X.; Cheng, L.; Yin, S.; Yang, G.; Li, Y.; Liu, Z. Multifunctional Theranostic Red Blood Cells For Magnetic-Field-Enhanced in vivo Combination Therapy of Cancer. Adv. Mater. 2014, 26, 4794–4802. [Google Scholar] [CrossRef] [PubMed]
- Brähler, M.; Georgieva, R.; Buske, N.; Müller, A.; Müller, S.; Pinkernelle, J.; Teichgräber, U.; Voigt, A.; Bäumler, H. Magnetite-Loaded Carrier Erythrocytes as Contrast Agents for Magnetic Resonance Imaging. Nano Lett. 2006, 6, 2505–2509. [Google Scholar] [CrossRef]
- Gupta, N.; Patel, B.; Ahsan, F. Nano-Engineered Erythrocyte Ghosts as Inhalational Carriers for Delivery of Fasudil: Preparation and Characterization. Pharm. Res. 2014, 31, 1553–1565. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Balasubramanian, V.; Poillucci, A.; Correia, A.; Zhang, H.; Celia, C.; Santos, H.A. Cell Membrane-Based Nanoreactor To Mimic the Bio-Compartmentalization Strategy of a Cell. ACS Biomater. Sci. Eng. 2018, 4, 1471–1478. [Google Scholar] [CrossRef]
- Ben-Akiva, E.; Meyer, R.A.; Yu, H.; Smith, J.T.; Pardoll, D.M.; Green, J.J. Biomimetic anisotropic polymeric nanoparticles coated with red blood cell membranes for enhanced circulation and toxin removal. Sci. Adv. 2020, 6, eaay9035. [Google Scholar] [CrossRef]
- Deák, R.; Mihály, J.; Szigyártó, I.C.; Beke-Somfai, T.; Turiák, L.; Drahos, L.; Wacha, A.; Bóta, A.; Varga, Z. Nanoerythrosomes tailoring: Lipid induced protein scaffolding in ghost membrane derived vesicles. Mater. Sci. Eng. C 2020, 109, 110428. [Google Scholar] [CrossRef] [PubMed]
- Pouliot, R.; Saint-Laurent, A.; Chypre, C.; Audet, R.; Vitté-Mony, I.; C-Gaudreault, R.; Auger, M. Spectroscopic characterization of nanoErythrosomes in the absence and presence of conjugated polyethyleneglycols: An FTIR and (31)P-NMR study. Biochim. Biophys. Acta BBA Bioenerg. 2002, 1564, 317–324. [Google Scholar] [CrossRef] [Green Version]
- Harisa, G.I.; Badran, M.M.; Alanazi, F.K. Erythrocyte nanovesicles: Biogenesis, biological roles and therapeutic approach: Erythrocyte nanovesicles. Saudi Pharm. J. 2017, 25, 8–17. [Google Scholar] [CrossRef] [Green Version]
- Fornasier, M.; Porcheddu, A.; Casu, A.; Raghavan, S.R.; Jönsson, P.; Schillén, K.; Murgia, S. Surface-modified nanoerythrosomes for potential optical imaging diagnostics. J. Colloid Interface Sci. 2021, 582, 246–253. [Google Scholar] [CrossRef]
- Millán, C.G.; Bravo, D.G.; Lanao, J.M. New erythrocyte-related delivery systems for biomedical applications. J. Drug Deliv. Sci. Technol. 2017, 42, 38–48. [Google Scholar] [CrossRef]
- Han, X.; Shen, S.; Fan, Q.; Chen, G.; Archibong, E.; Dotti, G.; Liu, Z.; Gu, Z.; Wang, C. Red blood cell–derived nanoerythrosome for antigen delivery with enhanced cancer immunotherapy. Sci. Adv. 2019, 5, eaaw6870. [Google Scholar] [CrossRef] [Green Version]
- Buss, N.; Yasa, O.; Alapan, Y.; Akolpoglu, M.B.; Sitti, M. Nanoerythrosome-functionalized biohybrid microswimmers. APL Bioeng. 2020, 4, 026103. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kiparissides, C.; Kammona, O. Nanoscale carriers for targeted delivery of drugs and therapeutic biomolecules. Can. J. Chem. Eng. 2012, 91, 638–651. [Google Scholar] [CrossRef]
- Trapani, A.; Tripodo, G.; Mandracchia, D.; Cioffi, N.; Ditaranto, N.; De Leo, V.; Cordero, H.; Esteban, M.A. Glutathione-loaded solid lipid nanoparticles based on Gelucire® 50/13: Spectroscopic characterization and interactions with fish cells. J. Drug Deliv. Sci. Technol. 2018, 47, 359–366. [Google Scholar] [CrossRef]
- Matougui, N.; Boge, L.; Groo, A.-C.; Umerska, A.; Ringstad, L.; Bysell, H.; Saulnier, P. Lipid-based nanoformulations for peptide delivery. Int. J. Pharm. 2016, 502, 80–97. [Google Scholar] [CrossRef] [PubMed]
- Peres, L.B.; de Araújo, P.H.H.; Sayer, C. Solid lipid nanoparticles for encapsulation of hydrophilic drugs by an organic solvent free double emulsion technique. Colloids Surf. B Biointerfaces 2016, 140, 317–323. [Google Scholar] [CrossRef]
- Ali, J.; Ali, M.; Baboota, S.; Sahani, J.K.; Ramassamy, C.; Dao, L. Bhavna Potential of Nanoparticulate Drug Delivery Systems by Intranasal Administration. Curr. Pharm. Des. 2010, 16, 1644–1653. [Google Scholar] [CrossRef]
- Muga, J.O.; Gathirwa, J.W.; Tukulula, M.; Jura, W.G.Z.O. In vitro evaluation of chloroquine-loaded and heparin surface-functionalized solid lipid nanoparticles. Malar. J. 2018, 17, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.-R.; Hua, S.-C.; Yang, Y.-L.; Fang, J.-Y. Development and evaluation of lipid nanoparticles for camptothecin delivery: A comparison of solid lipid nanoparticles, nanostructured lipid carriers, and lipid emulsion. Acta Pharmacol. Sin. 2008, 29, 1094–1102. [Google Scholar] [CrossRef] [Green Version]
- Gartziandia, O.; Herran, E.; Pedraz, J.L.; Carro, E.; Igartua, M.; Hernandez, R.M. Chitosan coated nanostructured lipid carriers for brain delivery of proteins by intranasal administration. Colloids Surf. B Biointerfaces 2015, 134, 304–313. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharyya, S.; Kudgus, R.A.; Bhattacharya, R.; Mukherjee, P. Inorganic Nanoparticles in Cancer Therapy. Pharm. Res. 2010, 28, 237–259. [Google Scholar] [CrossRef] [Green Version]
- Sharifi, S.; Seyednejad, H.; Laurent, S.; Atyabi, F.; Saei, A.A.; Mahmoudi, M. Superparamagnetic iron oxide nanoparticles forin vivomolecular and cellular imaging. Contrast Media Mol. Imaging 2015, 10, 329–355. [Google Scholar] [CrossRef] [PubMed]
- Rao, L.; Bu, L.-L.; Xu, J.-H.; Cai, B.; Yu, G.-T.; Yu, X.; He, Z.; Huang, Q.; Li, A.; Guo, S.-S.; et al. Red Blood Cell Membrane as a Biomimetic Nanocoating for Prolonged Circulation Time and Reduced Accelerated Blood Clearance. Small 2015, 11, 6225–6236. [Google Scholar] [CrossRef]
- Zhou, Y.; Quan, G.; Wu, Q.; Zhang, X.; Niu, B.; Wu, B.; Huang, Y.; Pan, X.; Wu, C. Mesoporous silica nanoparticles for drug and gene delivery. Acta Pharm. Sin. B 2018, 8, 165–177. [Google Scholar] [CrossRef]
- Su, J.; Sun, H.; Meng, Q.; Zhang, P.; Yin, Q.; Li, Y. Enhanced Blood Suspensibility and Laser-Activated Tumor-specific Drug Release of Theranostic Mesoporous Silica Nanoparticles by Functionalizing with Erythrocyte Membranes. Theranostics 2017, 7, 523–537. [Google Scholar] [CrossRef] [PubMed]
- Ancona, A.; Sportelli, M.; Trapani, A.; Picca, R.; Palazzo, C.; Bonerba, E.; Mezzapesa, F.; Tantillo, G.; Cioffi, N. Synthesis and characterization of hybrid copper–chitosan nano-antimicrobials by femtosecond laser-ablation in liquids. Mater. Lett. 2014, 136, 397–400. [Google Scholar] [CrossRef]
- Toth, M.; Fridman, R. Assessment of Gelatinases (MMP-2 and MMP-9) by Gelatin Zymography. Methods Mol. Med. 2001, 57, 163–174. [Google Scholar] [CrossRef] [Green Version]
- Li, L.-L.; Xu, J.-H.; Qi, G.-B.; Zhao, X.; Yu, F.; Wang, H. Core–Shell Supramolecular Gelatin Nanoparticles for Adaptive and “On-Demand” Antibiotic Delivery. ACS Nano 2014, 8, 4975–4983. [Google Scholar] [CrossRef] [PubMed]
- Giljohann, D.A.; Seferos, D.S.; Daniel, W.L.; Massich, M.D.; Patel, P.C.; Mirkin, C.A. Gold Nanoparticles for Biology and Medicine. Angew. Chem. Int. Ed. 2010, 49, 3280–3294. [Google Scholar] [CrossRef] [Green Version]
- Dreaden, E.C.; Alkilany, A.M.; Huang, X.; Murphy, C.J.; El-Sayed, M.A. The golden age: Gold nanoparticles for biomedicine. Chem. Soc. Rev. 2011, 41, 2740–2779. [Google Scholar] [CrossRef] [Green Version]
- Piao, J.-G.; Wang, L.; Gao, F.; You, Y.-Z.; Xiong, Y.; Yang, L. Erythrocyte Membrane Is an Alternative Coating to Polyethylene Glycol for Prolonging the Circulation Lifetime of Gold Nanocages for Photothermal Therapy. ACS Nano 2014, 8, 10414–10425. [Google Scholar] [CrossRef]
- Gao, W.; Hu, C.-M.J.; Fang, R.H.; Luk, B.T.; Su, J.; Zhang, L. Surface Functionalization of Gold Nanoparticles with Red Blood Cell Membranes. Adv. Mater. 2013, 25, 3549–3553. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sechi, M.; Sanna, V.; Pala, N. Targeted therapy using nanotechnology: Focus on cancer. Int. J. Nanomed. 2014, 9, 467–483. [Google Scholar] [CrossRef] [Green Version]
- Guido, C.; Testini, M.; D’Amone, S.; Cortese, B.; Grano, M.; Gigli, G.; Palamà, I.E. Capsid-like biodegradable poly-glycolic acid nanoparticles for a long-time release of nucleic acid molecules. Mater. Adv. 2021, 2, 310–321. [Google Scholar] [CrossRef]
- Palamà, I.E.; Cortese, B.; D’Amone, S.; Arcadio, V.; Gigli, G. Coupled delivery of imatinib mesylate and doxorubicin with nanoscaled polymeric vectors for a sustained downregulation of BCR-ABL in chronic myeloid leukemia. Biomater. Sci. 2014, 3, 361–372. [Google Scholar] [CrossRef]
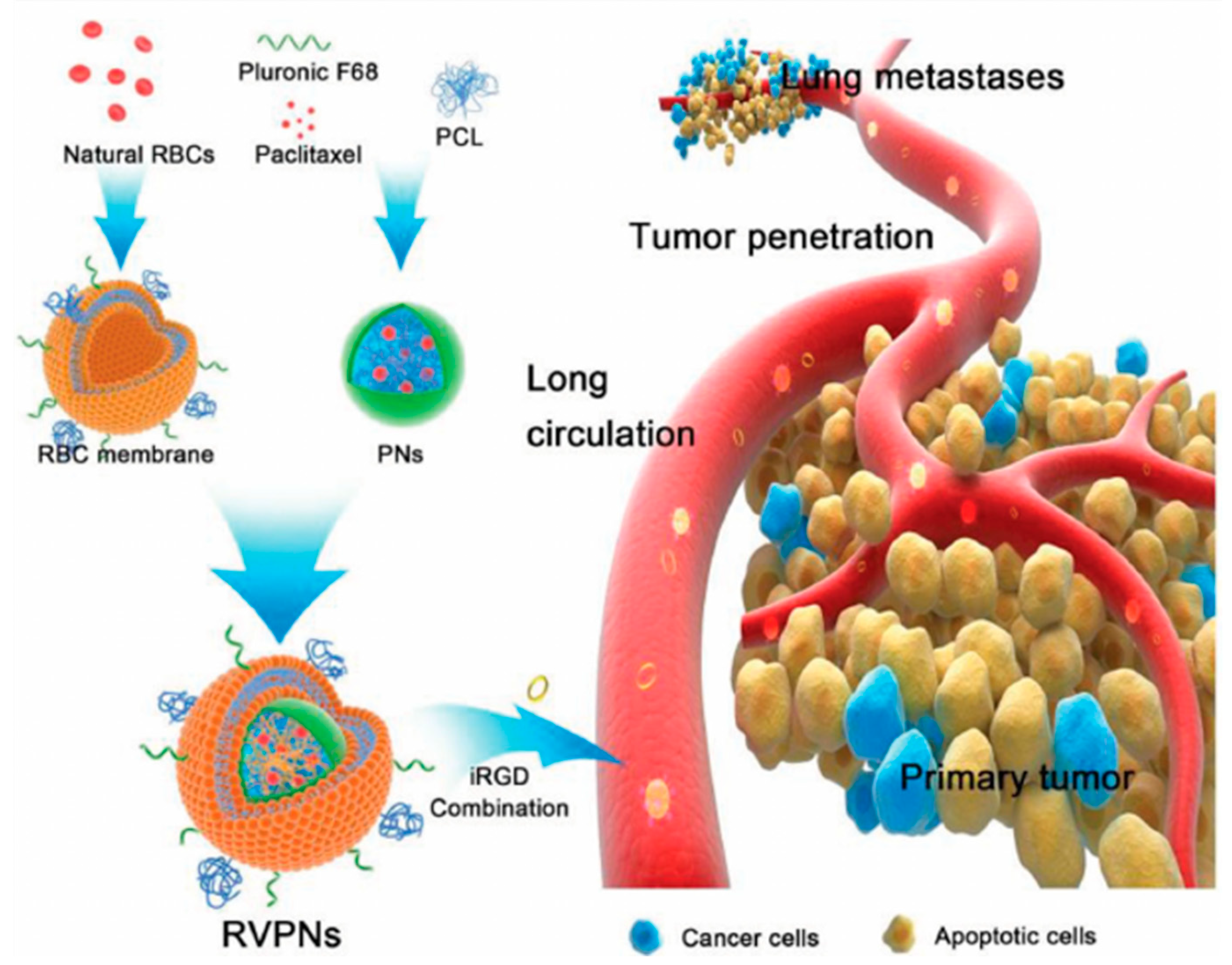
- Su, J.; Sun, H.; Meng, Q.; Yin, Q.; Tang, S.; Zhang, P.; Chen, Y.; Zhang, Z.; Yu, H.; Li, Y. Long Circulation Red-Blood-Cell-Mimetic Nanoparticles with Peptide-Enhanced Tumor Penetration for Simultaneously Inhibiting Growth and Lung Metastasis of Breast Cancer. Adv. Funct. Mater. 2016, 26, 1243–1252. [Google Scholar] [CrossRef]
- Tawakkal, I.S.M.A.; Cran, M.J.; Miltz, J.; Bigger, S.W. A Review of Poly(Lactic Acid)-Based Materials for Antimicrobial Packaging. J. Food Sci. 2014, 79, R1477–R1490. [Google Scholar] [CrossRef] [PubMed]
- Gao, M.; Liang, C.; Song, X.; Chen, Q.; Jin, Q.; Wang, C.; Liu, Z. Erythrocyte-Membrane-Enveloped Perfluorocarbon as Nanoscale Artificial Red Blood Cells to Relieve Tumor Hypoxia and Enhance Cancer Radiotherapy. Adv. Mater. 2017, 29. [Google Scholar] [CrossRef] [PubMed]
- Hu, C.-M.J.; Zhang, L.; Aryal, S.; Cheung, C.; Fang, R.H. Erythrocyte membrane-camouflaged polymeric nanoparticles as a biomimetic delivery platform. Proc. Natl. Acad. Sci. USA 2011, 108, 10980–10985. [Google Scholar] [CrossRef] [Green Version]
- Doshi, N.; Zahr, A.S.; Bhaskar, S.; Lahann, J.; Mitragotri, S. Red blood cell-mimicking synthetic biomaterial particles. Proc. Natl. Acad. Sci. USA 2009, 106, 21495–21499. [Google Scholar] [CrossRef] [Green Version]
- Guido, C.; Maiorano, G.; Cortese, B.; D’Amone, S.; Palamà, I.E. Biomimetic Nanocarriers for Cancer Target Therapy. Bioengineering 2020, 7, 111. [Google Scholar] [CrossRef]
- Kroll, A.V.; Fang, R.H.; Zhang, L. Biointerfacing and Applications of Cell Membrane-Coated Nanoparticles. Bioconjugate Chem. 2017, 28, 23–32. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roggers, R.A.; Joglekar, M.; Valenstein, J.S.; Trewyn, B.G. Mimicking Red Blood Cell Lipid Membrane To Enhance the Hemocompatibility of Large-Pore Mesoporous Silica. ACS Appl. Mater. Interfaces 2014, 6, 1675–1681. [Google Scholar] [CrossRef]
- Hayashi, K.; Ono, K.; Suzuki, H.; Sawada, M.; Moriya, M.; Sakamoto, W.; Yogo, T. Electrosprayed Synthesis of Red-Blood-Cell-Like Particles with Dual Modality for Magnetic Resonance and Fluorescence Imaging. Small 2010, 6, 2384–2391. [Google Scholar] [CrossRef]
- Merkel, T.J.; Jones, S.W.; Herlihy, K.P.; Kersey, F.R.; Shields, A.R.; Napier, M.; Luft, J.C.; Wu, H.; Zamboni, W.C.; Wang, A.Z.; et al. Using mechanobiological mimicry of red blood cells to extend circulation times of hydrogel microparticles. Proc. Natl. Acad. Sci. USA 2011, 108, 586–591. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Haghgooie, R.; Toner, M.; Doyle, P.S. Squishy Non-Spherical Hydrogel Microparticles. Macromol. Rapid Commun. 2009, 31, 128–134. [Google Scholar] [CrossRef] [Green Version]
- Kozlovskaya, V.; Alexander, J.F.; Wang, Y.; Kuncewicz, T.; Liu, X.; Godin, B.; Kharlampieva, E. Internalization of Red Blood Cell-Mimicking Hydrogel Capsules with pH-Triggered Shape Responses. ACS Nano 2014, 8, 5725–5737. [Google Scholar] [CrossRef] [PubMed]
- Colasuonno, M.; Palange, A.L.; Aid, R.; Ferreira, M.; Mollica, H.; Palomba, R.; Emdin, M.; Del Sette, M.; Chauvierre, C.; Letourneur, D.; et al. Erythrocyte-Inspired Discoidal Polymeric Nanoconstructs Carrying Tissue Plasminogen Activator for the Enhanced Lysis of Blood Clots. ACS Nano 2018, 12, 12224–12237. [Google Scholar] [CrossRef] [Green Version]
- Dong, X.; Niu, Y.; Ding, Y.; Wang, Y.; Zhao, J.; Leng, W.; Qin, L. Formulation and Drug Loading Features of Nano-Erythrocytes. Nanoscale Res. Lett. 2017, 12, 202. [Google Scholar] [CrossRef] [Green Version]
- Platt, F.M.; D’Azzo, A.; Davidson, B.L.; Neufeld, E.F.; Tifft, C.J. Lysosomal storage diseases. Nat. Rev. Dis. Prim. 2018, 4, 27. [Google Scholar] [CrossRef]
- Liu, J.; Lkhagva, E.; Chung, H.-J.; Kim, H.-J.; Hong, S.-T. The Pharmabiotic Approach to Treat Hyperammonemia. Nutrients 2018, 10, 140. [Google Scholar] [CrossRef] [Green Version]
- Protasov, E.S.; Borsakova, D.V.; Alexandrovich, Y.G.; Korotkov, A.V.; Kosenko, E.A.; Butylin, A.A.; Ataullakhanov, F.I.; Sinauridze, E.I. Erythrocytes as bioreactors to decrease excess ammonium concentration in blood. Sci. Rep. 2019, 9, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Riaz, M.I.; Sarwar, H.S.; Rehman, M.; Gohar, U.F.; Raza, S.A.; Siddique, M.I.; Shahnaz, G.; Sohail, M.F. Study of erythrocytes as a novel drug carrier for the delivery of artemether. Braz. J. Pharm. Sci. 2019, 55, 55. [Google Scholar] [CrossRef]
- Koleva, L.; Bovt, E.; Ataullakhanov, F.; Sinauridze, E. Erythrocytes as Carriers: From Drug Delivery to Biosensors. Pharmaceutics 2020, 12, 276. [Google Scholar] [CrossRef] [Green Version]
- Chang, T.M.S. ARTIFICIAL CELL evolves into nanomedicine, biotherapeutics, blood substitutes, drug delivery, enzyme/gene therapy, cancer therapy, cell/stem cell therapy, nanoparticles, liposomes, bioencapsulation, replicating synthetic cells, cell encapsulation/scaffold, biosorbent/immunosorbent haemoperfusion/plasmapheresis, regenerative medicine, encapsulated microbe, nanobiotechnology, nanotechnology. Artif. Cells Nanomed. Biotechnol. 2019, 47, 997–1013. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Batool, T.; Makky, E.A.; Jalal, M.; Yusoff, M.M. A Comprehensive Review on l-Asparaginase and Its Applications. Appl. Biochem. Biotechnol. 2015, 178, 900–923. [Google Scholar] [CrossRef] [Green Version]
- Biagiotti, S.; Paoletti, M.F.; Fraternale, A.; Rossi, L.; Magnani, M. Drug delivery by red blood cells. IUBMB Life 2011, 63, 621–631. [Google Scholar] [CrossRef] [PubMed]
- Hunault-Berger, M.; Leguay, T.; Huguet, F.; Leprêtre, S.; Deconinck, E.; Ojeda-Uribe, M.; Bonmati, C.; Escoffre-Barbe, M.; Bories, P.; Himberlin, C.; et al. A Phase 2 study of L-asparaginase encapsulated in erythrocytes in elderly patients with Philadelphia chromosome negative acute lymphoblastic leukemia: The GRASPALL/GRAALL-SA2-2008 study. Am. J. Hematol. 2015, 90, 811–818. [Google Scholar] [CrossRef] [Green Version]
- Bachet, J.-B.; Gay, F.; Maréchal, R.; Galais, M.-P.; Adenis, A.; Salako, M.D.; Cros, J.; Demetter, P.; Svrcek, M.; Bardier-Dupas, A.; et al. Asparagine Synthetase Expression and Phase I Study With L-Asparaginase Encapsulated in Red Blood Cells in Patients with Pancreatic Adenocarcinoma. Pancreas 2015, 44, 1141–1147. [Google Scholar] [CrossRef]
- Booser, D.J.; Hortobagyi, G.N. Anthracycline antibiotics in cancer therapy. Focus on drug resistance. Drugs 1994, 47, 223–258. [Google Scholar] [CrossRef]
- Skorokhod, O.A.; Kulikova, E.V.; Galkina, N.M.; Medvedev, P.V.; Zybunova, E.E.; Vitvitsky, V.M.; Pivnik, A.V.; Ataullakhanov, F.I. Doxorubicin pharmacokinetics in lymphoma patients treated with doxorubicin-loaded eythrocytes. Haematologica 2007, 92, 570–571. [Google Scholar] [CrossRef] [Green Version]
- Delaby, C.; Pilard, N.; Hetet, G.; Driss, F.; Grandchamp, B.; Beaumont, C.; Canonne-Hergaux, F. A physiological model to study iron recycling in macrophages. Exp. Cell Res. 2005, 310, 43–53. [Google Scholar] [CrossRef]
- Yuan, S.-H.; Ge, W.-H.; Huo, J.; Wang, X.-H. Slow release properties and liver-targeting characteristics of methotrexate erythrocyte carriers. Fundam. Clin. Pharmacol. 2009, 23, 189–196. [Google Scholar] [CrossRef]
- Magnani, M.; Rossi, L.; Fraternale, A.; Casabianca, A.; Brandi, G.; Benatti, U.; De Flora, A. Targeting antiviral nucleotide analogues to macrophages. J. Leukoc. Biol. 1997, 62, 133–137. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mandracchia, D.; Rosato, A.; Trapani, A.; Chlapanidas, T.; Montagner, I.M.; Perteghella, S.; Di Franco, C.; Torre, M.L.; Trapani, G.; Tripodo, G. Design, synthesis and evaluation of biotin decorated inulin-based polymeric micelles as long-circulating nanocarriers for targeted drug delivery. Nanomed. Nanotechnol. Biol. Med. 2017, 13, 1245–1254. [Google Scholar] [CrossRef] [PubMed]
- Chiarantini, L.; Argnani, R.; Zucchini, S.; Stevanato, L.; Zabardi, P.; Grossi, M.; Magnani, M.; Manservigi, R. Red blood cells as delivery system for recombinant HSV-1 glycoprotein B: Immunogenicity and protection in mice. Vaccine 1997, 15, 276–280. [Google Scholar] [CrossRef]
- Hendrickson, J.E.; Roback, J.D.; Hillyer, C.D.; Easley, K.A.; Zimring, J.C. Discrete Toll-like receptor agonists have differential effects on alloimmunization to transfused red blood cells. Transfusion 2008, 48, 1869–1877. [Google Scholar] [CrossRef]
- Cremel, M.; Guérin, N.; Campello, G.; Barthe, Q.; Berlier, W.; Horand, F.; Godfrin, Y. Innovative approach in Pompe disease therapy: Induction of immune tolerance by antigen-encapsulated red blood cells. Int. J. Pharm. 2015, 491, 69–77. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guido, C.; Maiorano, G.; Gutiérrez-Millán, C.; Cortese, B.; Trapani, A.; D’Amone, S.; Gigli, G.; Palamà, I.E. Erythrocytes and Nanoparticles: New Therapeutic Systems. Appl. Sci. 2021, 11, 2173. https://doi.org/10.3390/app11052173
Guido C, Maiorano G, Gutiérrez-Millán C, Cortese B, Trapani A, D’Amone S, Gigli G, Palamà IE. Erythrocytes and Nanoparticles: New Therapeutic Systems. Applied Sciences. 2021; 11(5):2173. https://doi.org/10.3390/app11052173
Chicago/Turabian StyleGuido, Clara, Gabriele Maiorano, Carmen Gutiérrez-Millán, Barbara Cortese, Adriana Trapani, Stefania D’Amone, Giuseppe Gigli, and Ilaria Elena Palamà. 2021. "Erythrocytes and Nanoparticles: New Therapeutic Systems" Applied Sciences 11, no. 5: 2173. https://doi.org/10.3390/app11052173
APA StyleGuido, C., Maiorano, G., Gutiérrez-Millán, C., Cortese, B., Trapani, A., D’Amone, S., Gigli, G., & Palamà, I. E. (2021). Erythrocytes and Nanoparticles: New Therapeutic Systems. Applied Sciences, 11(5), 2173. https://doi.org/10.3390/app11052173








