Nanomedicine Interventions in Clinical Trials for the Treatment of Metastatic Breast Cancer
Abstract
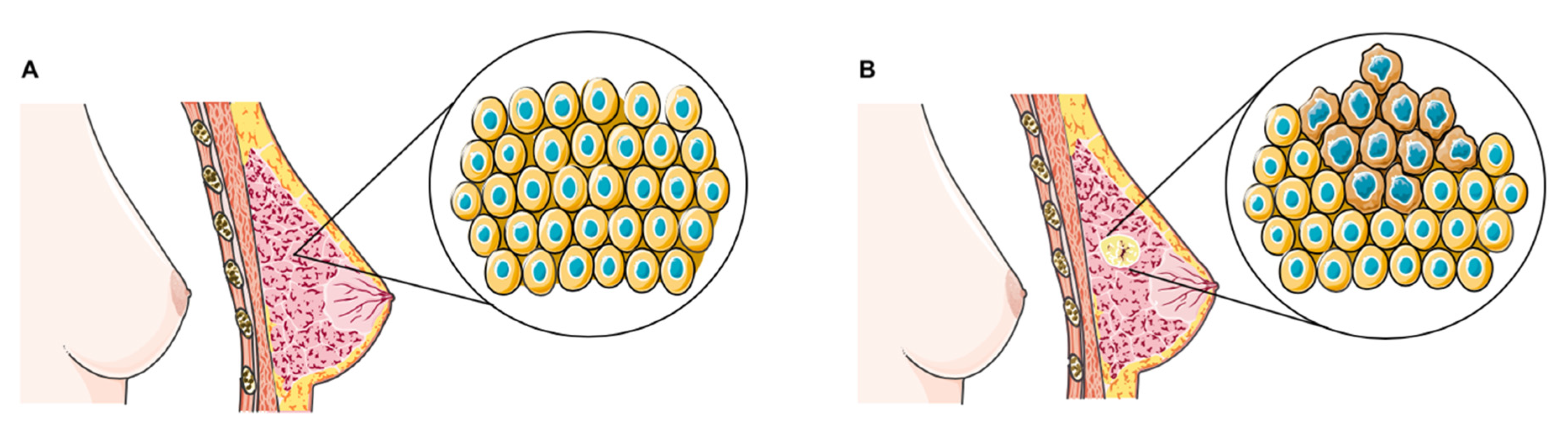
1. Introduction
2. Materials and Methods
2.1. Literature Search
2.2. Inclusion/Exclusion Criteria
2.3. Quality of Methods Assessment
3. Results
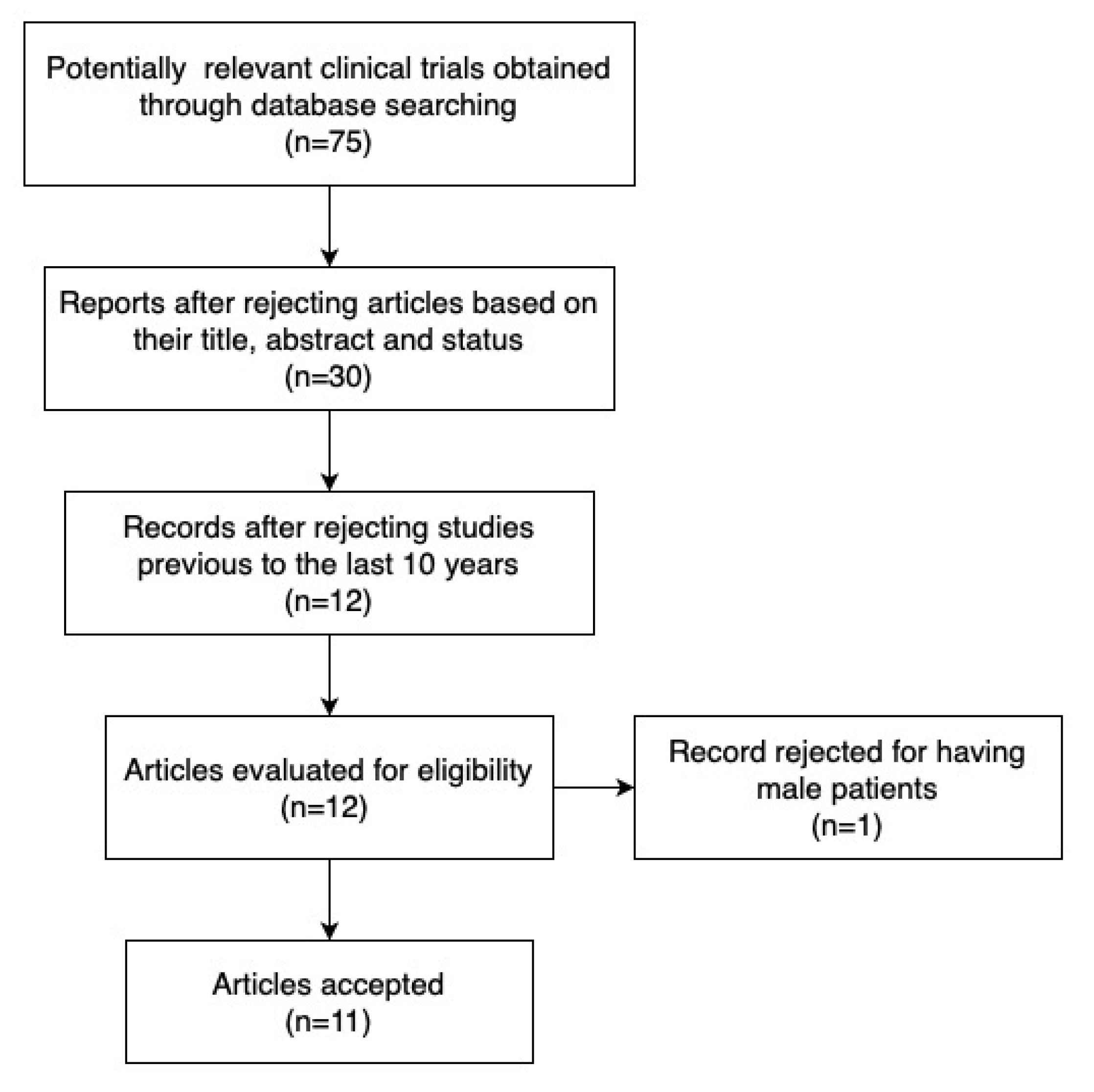
3.1. Study Selection
3.2. Study Characteristics
3.3. Synthesis of Study Results
3.4. Nanoparticle Albumin-Stabilized Paclitaxel
3.5. Micelle Nanoparticles
3.6. Quality of Used Clinical Trials
4. Discussion
4.1. Limitations
4.2. Future Research
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Woolston, C. Breast cancer. Nature 2015, 527, S101. [Google Scholar] [CrossRef] [PubMed]
- Harbeck, N.; Penault-Llorca, F.; Cortes, J.; Gnant, M.; Houssami, N.; Poortmans, P. Breast cancer. Nat. Rev. Dis. Primers. 2019, 5, 66. [Google Scholar] [CrossRef]
- World Health Organization. World Cancer Report 2014; World Health Organization: Geneva, Switzerland, 2014. [Google Scholar]
- Autier, P.; Boniol, M.; La Vecchia, C.; Vatten, L.; Gavin, A.; Héry, C.; Heanue, M. Disparities in breast cancer mortality trends between 30 European countries: Retrospective trend analysis of WHO mortality database. BMJ 2010, 341, c3620. [Google Scholar] [CrossRef] [PubMed]
- Sun, T.; Zhang, Y.S.; Pang, B.; Hyun, D.C.; Yang, M.; Xia, Y. Engineered nanoparticles for drug delivery in cancer therapy. Angew. Chem. Int. Ed. Engl. 2014, 53, 12320–12364. [Google Scholar]
- Giuliano, A.E.; Edge, S.B.; Hortobagyi, G.N. Eighth Edition of the AJCC Cancer Staging Manual: Breast Cancer. Ann. Surg. Oncol. 2018, 25, 1783–1785. [Google Scholar] [CrossRef]
- Steeg, P.S. Tumor metastasis: Mechanistic insights and clinical challenges. Nat. Med. 2006, 12, 895–904. [Google Scholar] [CrossRef] [PubMed]
- Cardoso, F.; Senkus, E.; Costa, A.; Papadopoulos, E.; Aapro, M.; Andre, F. 4th ESO-ESMO International Consensus Guidelines for Advanced Breast Cancer (ABC 4) dagger. Ann. Oncol. 2018, 29, 1634–1657. [Google Scholar] [CrossRef]
- Waks, A.G.; Winer, E.P. Breast Cancer Treatment: A Review. JAMA 2019, 321, 288–300. [Google Scholar] [CrossRef] [PubMed]
- Hassan, M.S.; Ansari, J.; Spooner, D.; Hussain, S.A. Chemotherapy for breast cancer (Review). Oncol. Rep. 2010, 24, 1121–1131. [Google Scholar] [CrossRef]
- Sonnenblick, A.A.; Pondé, N.N.; Piccart, M. Metastatic breast cancer: The Odyssey of personalization. Mol. Oncol. 2016, 10, 1147–1159. [Google Scholar] [CrossRef] [PubMed]
- Foulkes, W.D.; Smith, I.E.; Reis-Filho, J.S. Triple-Negative Breast Cancer. N. Engl. J. Med. 2010, 363, 1938–1948. [Google Scholar] [CrossRef]
- Unal, O.; Akkoc, Y.; Kocak, M.; Nalbat, E.; Dogan-Ekici, A.I.; Acar, H.Y.; Gozuacik, D. Treatment of breast cancer with autophagy inhibitory microRNAs carried by AGO2-conjugated nanoparticles. J. Nanobiotechnol. 2020, 18, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Talluri, S.; Malla, R.R. Superparamagnetic Iron Oxide Nanoparticles (SPIONs) for Diagnosis and Treatment of Breast, Ovarian and Cervical Cancers. Curr. Drug Metab. 2020, 20, 942–945. [Google Scholar] [CrossRef] [PubMed]
- Taruno, K.; Kurita, T.; Kuwahata, A.; Yanagihara, K.; Enokido, K.; Katayose, Y.; Nakamura, S.; Takei, H.; Sekino, M.; Kusakabe, M. Multicenter clinical trial on sentinel lymph node biopsy using superparamagnetic iron oxide nanoparticles and a novel handheld magnetic probe. J. Surg. Oncol. 2019, 120, 1391–1396. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Kantoff, P.W.; Wooster, R.; Farokhzad, J.S.O.C. Cancer nanomedicine: Progress, challenges and opportunities. Nat. Rev. Cancer 2017, 17, 20–37. [Google Scholar] [CrossRef] [PubMed]
- Harpern, S.H.; Douglas, M.J. Jadad scale for reporting randomized controlled trials. Evid. Based Obstet. Anesth. 2005, 237. [Google Scholar]
- Kaklamani, V.; Siziopikou, K.; Scholtens, D.; Lacouture, M.E.; Gordon, J.; Uthe, R.; Meservey, C.; Hansen, N.; Khan, S.A.; Jeruss, J.S.; et al. Pilot neoadjuvant trial in HER2 positive breast cancer with combination of nab-paclitaxel and lapatinib. Breast Cancer Res. Treat. 2011, 132, 833–842. [Google Scholar] [CrossRef]
- ClinicalTrials.gov. National Library of Medicine (US). Identifier NCT00856492, S0800, Nab-Paclitaxel, Doxorubicin, Cyclophosphamide, and Pegfilgrastim With or Without Bevacizumab in Treating Women With Inflammatory or Locally Advanced Breast Cancer. 2009. Available online: https://ClinicalTrials.gov/show/NCT00856492 (accessed on 29 December 2020).
- ClinicalTrials.gov. National Library of Medicine (US). Identifier NCT00733408, Nab-Paclitaxel and Bevacizumab Followed By Bevacizumab and Erlotinib in Metastatic Breast Cancer. 2008. Available online: https://ClinicalTrials.gov/show/NCT00733408 (accessed on 29 December 2020).
- Yardley, D.A.; Hart, L.; Bosserman, L.; Salleh, M.N.; Waterhouse, D.M.; Hagan, M.K.; Richards, P.; DeSilvio, M.L.; Mahoney, J.M.; Nagarwala, Y. Phase II study evaluating lapatinib in combination with nab-paclitaxel in HER2-overexpressing metastatic breast cancer patients who have received no more than one prior chemotherapeutic regimen. Breast Cancer Res. Treat. 2013, 137, 457–464. [Google Scholar] [CrossRef]
- Mrozek, E.; Layman, R.; Ramaswamy, B.; Lustberg, M.; Vecchione, A.; Knopp, M.V.; Shapiro, C.L. Phase II trial of neoadjuvant weekly nanoparticle albumin-bound paclitaxel, carboplatin, and biweekly bevacizumab therapy in women with clinical stage II or III HER2-negative breast cancer. Clin. Breast Cancer 2014, 14, 228–234. [Google Scholar] [CrossRef]
- Northfelt, D.W.; Dueck, A.C.; Flynn, T.P.; Zander, P.J.; Stella, P.J.; Melnik, M.; Pavey, E.S.; Perez, E.A. Phase II trial combining nab-paclitaxel (NP), gemcitabine (G), and bevacizumab (B) in patients (pts) with metastatic breast cancer (MBC): NCCTG N0735. J. Clin. Oncol. 2011, 29, 1126. [Google Scholar] [CrossRef]
- Hamilton, E.; Kimmick, G.; Hopkins, J.; Marcom, P.K.; Rocha, G.; Welch, R.; Broadwater, G.; Blackwell, K. Nab-Paclitaxel/Bevacizumab/Carboplatin Chemotherapy in First-Line Triple Negative Metastatic Breast Cancer. Clin. Breast Cancer 2013, 13, 416–420. [Google Scholar] [CrossRef]
- ClinicalTrials.gov. National Library of Medicine (US). Identifier NCT00407888, Doxorubicin Hydrochloride, Cyclophosphamide, and Filgrastim Followed By Paclitaxel Albumin-Stabilized Nanoparticle Formulation With or Without Trastuzumab in Treating Patients With Breast Cancer Previously Treated With Surgery. 2006. Available online: https://ClinicalTrials.gov/show/NCT00407888 (accessed on 29 December 2020).
- ClinicalTrials.gov. National Library of Medicine (US). Identifier NCT00254592, Neoadjuvant Treatment of Breast Cancer. 2005. Available online: https://ClinicalTrials.gov/show/NCT00254592 (accessed on 29 December 2020).
- Conlin, A.K.; Seidman, A.; Bach, A.; Lake, D.; Dickler, M.; D’Andrea, G.; Traina, T.; Danso, M.; Brufsky, A.M.; Saleh, M.; et al. Phase II Trial of Weekly Nanoparticle Albumin-Bound Paclitaxel With Carboplatin and Trastuzumab as First-line Therapy for Women With HER2-Overexpressing Metastatic Breast Cancer. Clin. Breast Cancer 2010, 10, 281–287. [Google Scholar] [CrossRef] [PubMed]
- Fujiwara, Y.; Mukai, H.; Saeki, T.; Ro, J.; Lin, Y.-C.; Nagai, S.E.; Lee, K.S.; Watanabe, J.; Ohtani, S.; Kim, S.B.; et al. A multi-national, randomised, open-label, parallel, phase III non-inferiority study comparing NK105 and paclitaxel in metastatic or recurrent breast cancer patients. Br. J. Cancer 2019, 120, 475–480. [Google Scholar] [CrossRef]
- Guan, Z.; Xu, B.; DeSilvio, M.L.; Shen, Z.; Arpornwirat, W.; Tong, Z.; Lorvidhaya, V.; Jiang, Z.; Yang, J.; Makhson, A.; et al. Randomized Trial of Lapatinib Versus Placebo Added to Paclitaxel in the Treatment of Human Epidermal Growth Factor Receptor 2–Overexpressing Metastatic Breast Cancer. J. Clin. Oncol. 2013, 31, 1947–1953. [Google Scholar] [CrossRef]
- Jagiello-Gruszfeld, A.; Tjulandin, S.; Dobrovolskaya, N.; Manikhas, A.; Pienkowski, T.; DeSilvio, M.; Ridderheim, M.; Abbey, R. A Single-Arm Phase II Trial of First-Line Paclitaxel in Combination with Lapatinib in HER2-Overexpressing Metastatic Breast Cancer. Oncology 2010, 79, 129–135. [Google Scholar] [CrossRef]
- Hamaguchi, T.; Matsumura, Y.; Suzuki, M.; Shimizu, K.; Goda, R.; Nakamura, I.; Nakatomi, I.; Yokoyama, M.; Kataoka, K.; Kakizoe, T. NK105, a paclitaxel-incorporating micellar nanoparticle formulation, can extend in vivo antitumour activity and reduce the neurotoxicity of paclitaxel. Br. J. Cancer 2005, 92, 1240–1246. [Google Scholar] [CrossRef]
- Tharkar, P.; Madani, A.U.; Lasham, A.; Shelling, A.N.; Al-Kassas, R. Nanoparticulate carriers: An emerging tool for breast cancer therapy. J. Drug Target. 2014, 23, 97–108. [Google Scholar] [CrossRef]
- Singh, S.K.; Singh, S.; Lillard, J.W., Jr.; Singh, R. Drug delivery approaches for breast cancer. Int. J. Nanomed. 2017, 12, 6205–6218. [Google Scholar] [CrossRef] [PubMed]
- Liyanage, P.Y.; Hettiarachchi, S.D.; Zhou, Y.; Ouhtit, A.; Seven, E.S.; Oztan, C.Y.; Celik, E.; Leblanc, R.M. Nanoparticle-mediated targeted drug delivery for breast cancer treatment. Biochim. Biophys. Acta 2019, 1871, 419–433. [Google Scholar] [CrossRef]
- Boussen, H.; Cristofanilli, M.; Zaks, T.; DeSilvio, M.; Salazar, V.; Spector, N. Phase II Study to Evaluate the Efficacy and Safety of Neoadjuvant Lapatinib Plus Paclitaxel in Patients With Inflammatory Breast Cancer. J. Clin. Oncol. 2010, 28, 3248–3255. [Google Scholar] [CrossRef] [PubMed]
- Yan, L.; Shen, J.; Wang, J.; Yang, X.; Dong, S.; Lu, S. Nanoparticle-Based Drug Delivery System: A Patient-Friendly Chemotherapy for Oncology. Dose-Response 2020, 18. [Google Scholar] [CrossRef] [PubMed]
- Brufsky, A.; Hoelzer, K.; Beck, T.; Whorf, R.; Keaton, M.; Nadella, P.; Krill-Jackson, E.; Kroener, J.; Middleman, E.; Frontiera, M.; et al. A Randomized Phase II Study of Paclitaxel and Bevacizumab With and Without Gemcitabine as First-Line Treatment for Metastatic Breast Cancer. Clin. Breast Cancer 2011, 11, 211–220. [Google Scholar] [CrossRef] [PubMed]
- Farjadian, F.; Ghasemi, A.; Gohari, O.; Roointan, A.; Karimi, M.; Hamblin, M.R. Nanopharmaceuticals and nanomedicines currently on the market: Challenges and opportunities. Nanomedicine 2019, 14, 93–126. [Google Scholar] [CrossRef] [PubMed]
- Anselmo, A.C.; Mitragotri, S. Nanoparticles in the clinic: An update. Bioeng. Transl. Med. 2019, 4, e10143. [Google Scholar] [CrossRef] [PubMed]
- Perez, E.A.; Suman, V.J.; Rowland, K.M.; Ingle, J.N.; Salim, M.; Loprinzi, C.L.; Flynn, P.J.; Mailliard, J.A.; Kardinal, C.G.; Krook, J.E.; et al. Two Concurrent Phase II Trials of Paclitaxel/Carboplatin/Trastuzumab (Weekly or Every-3-Week Schedule) as First-Line Therapy in Women with HER2-Overexpressing Metastatic Breast Cancer: NCCTG Study 983252. Clin. Breast Cancer 2005, 6, 425–432. [Google Scholar] [CrossRef] [PubMed]
| Drug/Agent | Mechanisms | Half-Life | Route of Elimination | Common Side-Effects | Reference |
|---|---|---|---|---|---|
| Endocrine therapy | |||||
| Anastrazole | Non–steroidal aromatase inhibitor | 50 h | 85% in feces 10% in urine | Hot flashes, arthralgias or myalgias | [1,2] |
| Fulvestrant | Estrogen receptor antagonist | 40 days | ≈90% in feces <1% in urine | Alopecia, constipation, diarrhea, nausea and vomiting | [1,3] |
| Octreotide | Somatostatin analogue | 2.3–2.7 h | 32% in urine 30–40% in feces | Gastrointestinal, dizziness, dry skin and depressed mood | [1,3] |
| Tamoxifen | Selective estrogen receptor modulator | 5–7 days (14 days for its metabolite) | Mainly in feces | Hot flashes | [2] |
| Cytotoxic Chemotherapy | |||||
| Abraxane | Alkylating agente | 13–27 h | Mainly in bile | Hipersensitivity reaction, neutropenia, neuropathy and sepsis | [4] |
| Carboplatin | Alkylating agente | 1.1–5.9 h | Mainly in urine | Hypersensitivity reaction, nausea, vomiting, anemia and genitourinary symptoms | [1,3] |
| Cyclophosphamide | Alkylating agente | 3–12 h | Primarily in the form of metabolites 10–20% in urine unchanged | Neutropenia, febrile neutropenia, fever, alopecia, náusea, vomiting and diarrhea | [1] |
| Doxorubicin | Cytotoxic anthracycline antibiotic | 20–48 h | 40% in bile 5–12% in urine | Cardiomyopathy, myelosuppression, infection and septic shock | [1] |
| Gemcitabine | Anti–metabolite (nucleoside analog) | Short infusions: 42–94 min Long infusions: 245–638 min | 92–98% in urine | Alopecia, myelosuppression, nausea, vomiting and diarrhea | [1,3] |
| Paclitaxel | Antimicrotubule agent | 52.7 h (with a 24 h infusion) | ≈71% in feces ≈14% in urine | Bone marrow suppression, peripheral neurotoxicity and mucositis | [1] |
| Targeted Therapy | |||||
| Bevacizumab | Recombinant humanized IgG1 monoclonal antibody that against VEGF | ≈20 days | Information not found | Proteinuria, arterial thromboembolic events, GI bleeding and sepsis | [1] |
| Erlotinib | Inhibitor of EGFR tyrosine kinase | 36.2 h | 83% in feces 8% in urine | Diarrhea, rash and liver transaminase elevation | [1,3] |
| Irinotecan | Topoisomerase I inhibitor | 6–12 h | Bile and urine | Nausea, vomiting, abdominal cramping, diarrhea and infection | [1] |
| Lapatinib | 4–anilinoquinazoline kinase inhibitor of intracellular tyrosine kinase domains of HER1/EGFR/ERBB1 and HER2/ERBB2 | 14.2 h | 14% in feces | Diarrhea and vomiting | [1] |
| Trastuzumab | Recombinant humanized IgG1 monoclonal antibody against the HER2 receptor | ≈28 days | Information not found | Ventricular dysfunction and congestive heart failure | [1] |
| Immunotherapy | |||||
| Atezolizumab | Humanized IgG monoclonal antibody that prevents interaction of PD-L1 and PD-1 | 27 days | Information not found | Fatigue, decreased appetite, nausea, urinary tract infection, pyrexia and constipation | [1] |
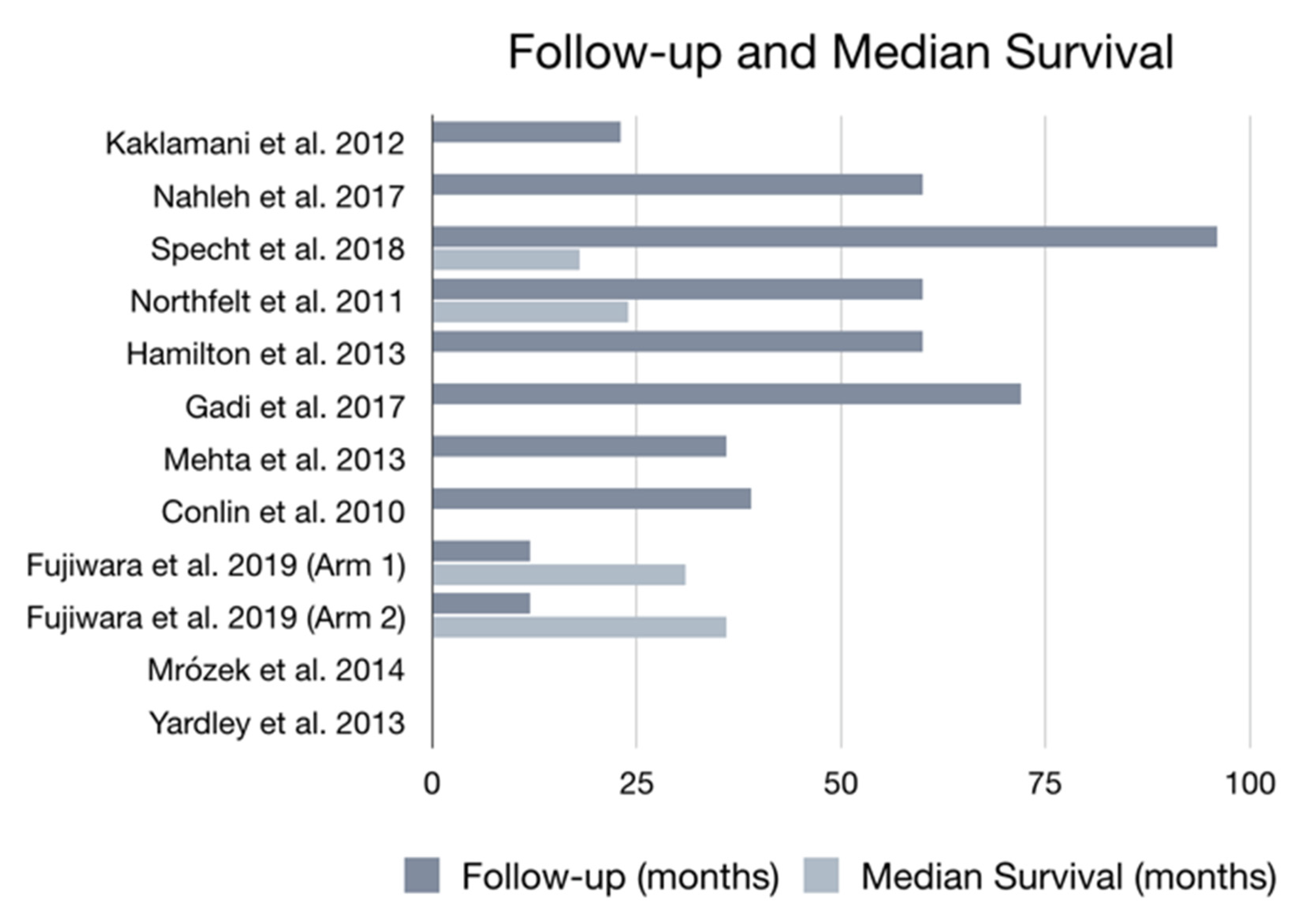
| Country/Region | Sample Size | Age Range (Years) | Previous Treatments | Follow-Up | Mortality | Assessment | Reference |
|---|---|---|---|---|---|---|---|
| United States | n = 30 | 27–70 | None | 22.6 months | 5 total deaths (16.67%) | Abraxane + lapatinib | [18] |
| United States and Puerto Rico | n = 211 | 22–75 | Not specified | 5 years | 31 total deaths 14 deaths in Arm 1 17 deaths in Arm 2/3 | Arm 1: nab-paclitaxel + bevacizumab followed by doxorubicin + cyclophosphamide + pegfilgrastim Arm 2: nab-paclitaxel followed by doxorubicin + cyclophosphamide + pegfilgrastim Arm 3: doxorubicin + cyclophosphamide + pegfilgrastim followed by nab-paclitaxel | [19] |
| United States | n = 55 | Not stated | Chemotherapy | 8 years | 3 total deaths (5.45%) | Nab-paclitaxel + bevacizumab, followed by bevacizumab + erlotinib hydrochloride | [20] |
| United States | n = 60 | 28–80 | Only one prior chemotherapeutic regimen | Not specified | 3 total deaths (5%) | Lapatinib + nab-paclitaxel | [21] |
| United States | n = 33 | 28–74 | Chemotherapy | Not specified | Not analyzed | Nab-paclitaxel + carboplatin and bevacizumab as neoadjuvant chemotherapy, followed by surgery and bevacizumab as adjuvant chemotherapy | [22] |
| United States | n = 48 | 27–77 | Chemotherapy, hormonal therapy, radiotherapy and immunologic therapy | 5 years | Not analyzed | Nab-paclitaxel + gemcitabine + bevacizumab | [23] |
| United States | n = 41 | 30–76 | Surgery, radiation and adjuvant chemotherapy | 5 years | Not analyzed | Abraxane + carboplatin + bevacizumab | [24] |
| United States | n = 60 | 29–69 | Surgery | 6 years | Not analyzed | Doxorubicin hydrochloride + cyclophosphamide + filgrastim followed by paclitaxel albumin-stabilized nanoparticle, patients with HER-2/NEU positive also receive trastuzumab | [25] |
| United States | n = 43 | Not stated | Not specified | 36 months | Not analyzed | Doxorubicin + cyclophosphamide + GM-CSF, followed by carboplatin + paclitaxel | [26] |
| United States | n = 32 | 29–76 | Surgery, radiation and chemotherapy | 39 months | 10 total deaths (31%) | Abraxane + carboplatin + trastuzumab | [27] |
| Japan, Korea and Taiwan | n= 427 | 20–74 | Chemotherapy | 12 months | 4 total deaths 3 in the paclitaxel group 1 in the treatment group | Arm 1: NK 105 Arm 2: Paclitaxel | [28] |
| Authors | Study Design | Nanoparticle Formulation | Selection Criteria | Main Objective | Participants | Overall Median Survival/Outcome | Adverse Reactions | Conclusion | Reference |
|---|---|---|---|---|---|---|---|---|---|
| Kaklamani et al. | Phase I Single Group Assignment | Abraxane (nanoparticle albumin-stabilized paclitaxel) | Stage I, II and III invasive breast cancer | Determining the efficacy of abraxane + lapatinib as neoadjuvant therapy in patients with stage I, II and III breast cancer. | n = 30. Age 27–70. | Not specified. pCR was obtained in 5 (17.9%) patients. | Diarrhea, neuropathy, fatigue, rash, bone pain, anemia, pruritus, fever, mucositis and vomiting | In general, the combination was well tolerated with minimal grade 3 toxicity and showed good efficacy. | [18] |
| Nahleh et al. | Phase II Randomized Clinical Trial | Nab-paclitaxel (nanoparticle albumin-stabilized paclitaxel) | Inflammatory and locally advanced HER2-/NEU negative breast cancer | Compare nab-paclitaxel, doxorubicin, cyclophosphamide and pegfilgrastim given with or without bevacizumab in the treatment of HER2-/NEU negative breast cancer. | n = 211. Age 22–75 | Not analyzed. Arm 1: pCR was obtained in 35 patients (35.7%) Arm 2/3: pCR was obtained in 24 patients (21.2%) | Anemia, febrile neutropenia, fatigue, watering eyes, constipation, diarrhea, nausea and mucositis oral | - | [19] |
| Specht et al. | Phase II Single Group Assignment | Nab-paclitaxel (nanoparticle albumin-stabilized paclitaxel) | Metastatic breast cancer | Evaluating maintenance treatment with erlotinib and bevacizumab after nab-paclitaxel and bevacizumab in women with metastatic breast cancer. | n = 55 | 18.1 months (95% CI, 15.6–21.7) | Infection, neutropenia, fatigue and neuropathy | - | [20] |
| Yardley et al. | Phase II Single Group Assignment | Nab-paclitaxel (nanoparticle albumin-stabilized paclitaxel) | HER2-positive metastatic breast cancer | Evaluate the efficacy and safety of nab-paclitaxel + lapatinib in women with HER2-postitive metastatic breast cancer who had received no more than one prior chemotherapeutic regimen. | n = 60. Age 28–80. | Not analyzed. PFS of 39.7 weeks (95% CI, 34.1–63.9) | Dehydration, diarrhea, anemia, cellulitis, febrile neutropenia, hypokalemia and acute renal failure | There is a clinical benefit for treatment with lapatinib and nab-paclitaxel. Toxicity was manageable and predictable. | [21] |
| Mrozek et al. | Phase II Single Group Assignment | Nab-paclitaxel (nanoparticle albumin-stabilized paclitaxel) | Stage II or III HER2-negative breast cancer | Determining the efficacy and safety of adding bevacizumab to the treatment with nab-paclitaxel and carboplatin in women with stage II or III HER2-negative breast cancer. | n = 33. Age 28–74. | Not analyzed. pCR was obtained in 6 patients. | Leukopenia, anemia, thrombocytopenia and neutropenia | The endpoint for efficacy was not reached. Regimen might be effective in TNBC. | [22] |
| Northfelt et al. | Phase II Single Group Assignment | Nab-paclitaxel (nanoparticle albumin-stabilized paclitaxel) | Metastatic breast cancer | Studying efficacy of treatment with nab-paclitaxel + gemcitabine + bevacizumab in metastatic breast cancer. | n = 48. Age 27–77. | 24.4 months (95% CI, 18.2–29.3) | Neutropenia, leukopenia, thrombocytopenia, anemia, dyspnea, diarrhea, nausea and nasal hemorrhage | The combination of nab-paclitaxel + gemcitabine + bevacizumab met the endpoint of 6 months PFS > 60%. Toxicity was manageable. | [23] |
| Hamilton et al. | Phase II Single Group Assignment | Abraxane (nanoparticle albumin-stabilized paclitaxel) | Triple-negative metastatic breast cancer (TNMBC) | Evaluate de efficacy of treatment with abraxane+ carboplatin + bevacizumab in TNMBC. | n = 41. Age 30–76. | Not specified. PFS of 9.2 months (95% CI, 7.8–25.1) | Neutropenia, fatigue, constipation, neuropathy, anemia, thrombocytopenia, alopecia and anorexia | The combination abraxane + carboplatin + bevacizumab is an active and tolerable regimen for first line TNMBC treatment. | [24] |
| Gadi et al. | Phase II Single Group Assignment | Nanoparticle albumin-stabilized paclitaxel | Breast cancer | Studying the efficacy of doxorubicin hydrochloride + cyclophosphamide + filgrastrim, followed by nanoparticle albumin-stabilized paclitaxel with or without trastuzumab in patients with breast cancer previously treated with surgery. | n = 60. Age 29–69. | 59 surviving patients in 2 years 53 surviving patients in 6 years | Febrile neutropenia, fever, gastrointestinal disorders, dehydration and respiratory disorders | - | [25] |
| Mehta et al. | Phase II Single Group Assignment | Nab-paclitaxel (nanoparticle albumin-stabilized paclitaxel) | Breast cancer with 2cm and/or lymph node positive | Measuring the efficacy of treatment with doxorubicin + cyclophosphamide with GM-CSF, followed by carboplatin + nab-paclitaxel in breast cancer with 2 cm and/or lymph node positive. | n = 43 | Not specified. Overall clinical response was obtained in 43 patients. | Cardiovascular disease and neutropenic fever | - | [26] |
| Conlin et al. | Phase II Single Group Assignment | Abraxane (nanoparticle albumin-stabilized paclitaxel) | HER2-positive metastatic breast cancer | Evaluating the efficacy and safety of abraxane + carboplatin + trastuzumab in the treatment of HER2-positive metastatic breast cancer. | n = 32. Age 29–76. | NA; 81.3% of patients achieved t al response (95% CI, 67.7–94.8) | Neutropenia, anemia, nausea, thrombocytopenia, vomiting, diarrhea, constipation, fatigue, neuropathy and alopecia | The therapeutic regimen of abraxane + carboplatin + transtuzumab has high efficacy in HER2-overexpressing metastatic breast cancer, highlighting the advantage of a weekly taxane. | [27] |
| Fujiwara et al. | Phase III Randomized Clinical Trial | NK 105 (paclitaxel-incorporating micellar nanoparticle) | Metastatic or recurrent adenocarcinoma of the breast | Verify the non-inferiority of NK105 to paclitaxel in the treatment of metastatic or recurrent adenocarcinoma of the breast. | n = 427. Age 20–74. | Arm 1: 31.2 months (95% CI, 27.1–39.3) Arm 2: 36.2 months (95% CI, 30.3–NA) | Neutropenia, leukopenia, alopecia, neuropathy, rash, nausea, nasopharyngitis, diarrhea, fatigue, stomatitis, nail discoloration, myalgia and dysgeusia | Non inferiority of NK105 to paclitaxel was not demonstrated. Neuropathy profile was favorable. | [28] |
| Author/Principal Investigator and Year | Randomization | Blinding | An Account of All Patients | Total Score |
|---|---|---|---|---|
| Kaklamani et al. 2012 | 0 | 0 | 1 | 1 |
| Nahleh et al. 2017 | 1 | 0 | NA | 1 |
| Specht et al. 2018 | 0 | 0 | NA | 0 |
| Yardley et al. 2013 | 0 | 0 | 1 | 1 |
| Mrózek et al. 2014 | 0 | 0 | 1 | 1 |
| Northfelt et al. 2011 | 0 | 0 | NA | 0 |
| Hamilton et al. 2013 | 0 | 0 | 1 | 1 |
| Gadi et al. 2017 | 0 | 0 | 1 | 1 |
| Mehta et al. 2013 | 0 | 0 | 1 | 1 |
| Conlin et al. 2010 | 0 | 0 | 1 | 1 |
| Fujiwara et al. 2019 | 2 | 0 | 1 | 3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moreira, R.; Granja, A.; Pinheiro, M.; Reis, S. Nanomedicine Interventions in Clinical Trials for the Treatment of Metastatic Breast Cancer. Appl. Sci. 2021, 11, 1624. https://doi.org/10.3390/app11041624
Moreira R, Granja A, Pinheiro M, Reis S. Nanomedicine Interventions in Clinical Trials for the Treatment of Metastatic Breast Cancer. Applied Sciences. 2021; 11(4):1624. https://doi.org/10.3390/app11041624
Chicago/Turabian StyleMoreira, Rita, Andreia Granja, Marina Pinheiro, and Salette Reis. 2021. "Nanomedicine Interventions in Clinical Trials for the Treatment of Metastatic Breast Cancer" Applied Sciences 11, no. 4: 1624. https://doi.org/10.3390/app11041624
APA StyleMoreira, R., Granja, A., Pinheiro, M., & Reis, S. (2021). Nanomedicine Interventions in Clinical Trials for the Treatment of Metastatic Breast Cancer. Applied Sciences, 11(4), 1624. https://doi.org/10.3390/app11041624








