Abstract
Based on a “filter press backwash–chemical precipitation–gaseous membrane absorption” process, treatment of harmless cyanide tailings was conducted using cyanide tailings from a gold smelting enterprises (Yunnan Province, China) as the research object. The effects of air-drying time, backwash water parameters, initial pH of acidification, NaHS dosage, cyanide-containing water flow rate, and gaseous membrane stages on the process were investigated. Chemical composition, X-ray diffraction, and X-ray photoelectron spectroscopy analyses of the copper products were carried out. Results showed that the copper content in the copper product was 54.56%, and the chemical composition was mainly CuSCN, CuS, Cu2S, and CaSO4. Five cycles of experiments were carried out under optimal conditions; the results showed that the process can make the treated cyanide tailings meet the requirements of the technical specification for pollution control of cyanide leaching residue in the gold industry (TSPC) standard for storage in a tailings pond and a have certain stability. The average recovery rate of copper and total cyanide in elution water was 97.8% and 99.89%, respectively, and the average removal rate of thiocyanate was 94.09%.
1. Introduction
The cyanide gold extraction method was proposed by the British scientist MacArthur in 1890 [1]. It is a gold extraction process that first dissolves the gold in the ore with a dilute cyanide solution, replaces it with zinc powder, and then smelts it into gold ingots [2]. The cyanide extraction process inevitably produces a large amount of cyanide-containing waste, and the annual discharge of cyanide tailings of China’s gold industry exceeds 24.5 million tons. The technical specification for pollution controls of cyanide leaching residue in the gold industry (TSPC) was not promulgated in China until 1 August 2018 [3]. The cyanide tailings produced in the gold industry are mainly disposed of in open-air stockpiling and tailings pond storage, which not only occupies a large amount of land, but also affects the environment and sustainable development of the mining area [4,5]. Cyanide tailings are also a secondary resource, containing a large number of valuable metals such as gold, silver, copper, iron, sulfur, and so on [6,7]. However, if valuable resources are directly recovered, the residual cyanide in the cyanide tailings will not only harm the health of the workers, but also affect the recovery rate of the valuable metal resources. Therefore, harmless treatment of cyanide tailings is not only beneficial to environmental protection, but also beneficial to the recovery of valuable resources.
Under the current call of energy conservation, emission reduction, and environmental protection, it is of great practical significance to study the cyanide treatment method in the cyanide tailings of gold enterprises [8]. At present, cyanide treatment technologies mainly include electrochemical oxidation, chemical oxidation, adsorption, biological methods, etc. [7,9,10,11,12]. These methods mainly convert cyanide into non-toxic, harmless, and recyclable substances through chemical or physical reactions, and then the treated harmless tailings and water are recycled to reduce the harm of cyanide to the environment. Chen et al. [10] used electrochemical oxidation to oxidize cyanide in the cyanide tailings pulp to CO2 and N2, and the metal cations were reduced and deposited at the cathode. The removal rates of total cyanide (TCN), free cyanide (CN−), Cu, and Fe were 80.17%, 84.91%, 84.10%, and 90.91%, respectively. Hou et al. [11] found that the cyanide tailings could meet the backfilling requirements of TSPC after being co-treated with sodium metabisulfite and hydrogen peroxide. The best conditions for the decomposition of cyanide from cyanide tailings are treatment in 0.5 g/L Na2S2O5 at pH 10 for 3 h and then 2 mL/L H2O2 is added to the tailings at pH 9 for 4 h. Bahrami et al. [12] utilized gilsonite to adsorb cyanide in cyanide wastewater; a maximum adsorption of 61.64% was obtained in the size range of −1 + 0.5 and −2 + 1 mm of gilsonite. However, these methods have some disadvantages, such as a high consumption of ingredients, harsh operating conditions, difficulty achieving industrial application, no recovery of cyanide in cyanide tailings, and so on.
In this study, green treatment of cyanide tailings with the “filter press backwash–chemical precipitation–gaseous membrane absorption” method results in the treated cyanide tailings meeting the requirements of TSPC to enter the tailing pond for storage. In this process, a filter press with a reverse washing function is used to realize the harmless treatment of cyanide tailing pulp, the copper element in elution water is recovered by acidification precipitation technology combined with vulcanization precipitation technology, the cyanide in eluent water is recovered by a hollow fiber hydrophobic membrane, and the water after membrane treatment is returned to the pressure filter backwash process as backwash water to realize the recycling of water in the system. The process not only provides an efficient and environmentally friendly method for harmless cyanide tailings but also recovers a certain amount of copper and cyanide during the treatment process, realizing the water recycling in the system without the generation of secondary pollutants. Furthermore, there is also an opportunity for the subsequent recovery of valuable metals from the treated cyanide tailings.
2. Materials and Methods
2.1. Materials and Equipment
The object processed in this study is cyanide tailings slurry with a slurry concentration of 38%, obtained from a mining company in Yunnan. The experimental sulfuric acid was industrial grade 98% concentrated sulfuric acid from the plant. The industrial grade (70%) sodium hydrosulfide was provided by Nantong Ruijia Chemical Co., LTD., (Nantong, China). The 99% flake sodium hydroxide was provided by Wujiang Xianglong Chemical Co., LTD., (Suzhou, China).
The experimental equipment included a CJWA-5/4/30 countercurrent washing machine; 1 m3 PPH reaction tank with a stirring device; Φ500 × 2500 lye spray system; 2 m3 diaphragm plate and frame filter press; and 5-um-filter-hole precision filter. The model number of the gaseous membrane assembly is ETN-6X28 (including two hollow fiber gaseous membranes, cyanide-containing water storage tank, lye storage tank, lye pump and cyanide-containing water pump, and two security filters).
2.2. Experimental Set-Up and Operation
2.2.1. Filter Press Backwash Process
The whole process of the “filter press backwash–chemical precipitation–gaseous membrane absorption” method is illustrated in Figure 1. The cyanide tailings were transported to the mixing barrel in front of the filter press in the form of slurry and were driven into the countercurrent washing machine through the slurry pump. The feeding pressure was about 0.6 MPa. When there was no obvious filtrate outflow in the filtrate pipeline, the filter chamber was judged to be basically full. The feeding time was 5–10 min. After the feeding was complete, an air compressor blew air for 0.5–3 min (primary air-drying), and the filter press filtrate and the primary air-dried blow-off liquid were returned to the cyanide process together as the filter barren solution. Then, the backwash pump was turned on, the backwash water parameters were adjusted, and a backwash pressure of 0.8 MPa was used for backwashing the cyanide tailings. After washing, secondary air-drying was conducted for 3–25 min. After air-drying, the filter cake was removed and a leaching toxicity test carried; the washing solution and the secondary air-dried blowout solution were used as eluent to enter the chemical treatment process. The influences of primary air-drying time, secondary air-drying time, and backwash water parameters (pH value, total cyanide content, multiple (ratio of the amount of backwash water to the mass of primary air-dried cyanide tailings)) on the harmless effect of cyanide tailings were studied in the filter press backwash process.
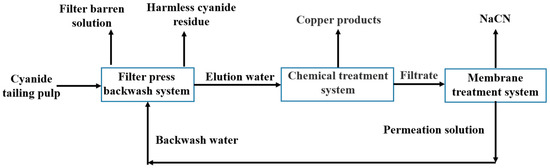
Figure 1.
The process of the “filter press backwash–chemical precipitation–gaseous membrane absorption” method.
2.2.2. Chemical Treatment Process
The elution water obtained in the filter press backwash process was pumped into the chemical reaction tank in which sulfuric acid was added to preliminary sink the copper, and after acidifying the sink, sodium hydrosulfide was added to deeply sink the copper. After the copper was deposited, the solution was pressed by the diaphragm plate and frame filter press, and the filter cake was a copper product. The filtrate was filtered by a precision filter and pumped into the cyanide-containing water storage tank of the gaseous membrane module. The chemical reaction tank was a closed device connected to the alkali spray system through negative pressure, and the spilled HCN in the chemical treatment process was absorbed by the alkali liquor. In the chemical treatment process, the influence of the initial pH value of the acidification reaction and the amount of sodium hydrosulfide on the copper precipitation effect was studied.
2.2.3. Membrane Treatment Process
The cyanide-containing water was pumped into the outside of the hollow fiber gaseous membrane, and the NaOH solution was pumped into the inside of the hollow fiber gaseous membrane. The two flowed in opposite directions. There were two security filters in front of the membrane to prevent debris from blocking the membrane hole. Because hollow fiber has “breathable and impermeable” characteristic, the liquids on both sides cannot be mutually soluble [13]. HCN in cyanide-containing water passed through the micropores of the hollow fiber membrane in gaseous form and was absorbed by NaOH to generate NaCN, thereby reducing the cyanide content in cyanide-containing water [14].
2.3. Analysis and Characterization
The total metal ion contents were measured by inductively coupled plasma-atomic emission spectrometry (ICP-OES, Optima 8000). XRD (MPDDY2094, PANalytical, Netherlands) with Cu Ka radiation (l = 0.15418 nm) was conducted with a scanning rate of 5° min−1 from 5° to 90°. Copper products were characterized by X-ray photoelectron spectroscopy (XPS) for analysis of chemical and electronic properties. The pH value was detected by a pH meter (pHs-3E, Leici, China). A leaching toxic solution of tailings was prepared according to HJ/T 299-2007 “Solid Waste Leaching Toxic Leaching Method—Sulfuric acid nitric acid Method” [15]. The total cyanide in the water sample and leaching toxic solution was analyzed by a colorimetric method.
3. Results
3.1. Raw Material Analysis
The current cyanide tailing slurry treatment method is to use a common plate and frame filter press to filter the cyanide tailing slurry. The cyanide tailings after the filter press are directly stored in the tailing pond, and the filtrate is returned to the cyanide gold extraction system. The actual research goal aims to reduce the toxicity of the cyanide tailings before washing to meet the standards for entering the tailings pond in the TSPC. Therefore, the chemical composition and leaching toxicity identification of the cyanide tailings after air-drying was carried out, and the element analysis of the filter barren solution was carried out. The cyanide tailings after primary air-drying were dried at 105 °C, and their chemical composition was determined; the results are presented in Table 1. According to the mass ratio of samples before and after drying, the moisture content of cyanide tailings was 20.32%. The standard for cyanide tailings in TSPC to enter the tailings pond for storage [3,16], the elemental analysis of the leached toxic solution of cyanide tailings (after primary air-drying), and the filter barren solution are shown in Table 2.

Table 1.
Chemical composition analysis of cyaniding tailings after primary air-drying.

Table 2.
Storage standard of cyanide tailings and element content table of cyanide tailing slurry.
It can be seen from Table 2 that the total cyanide content in the toxic liquid extracted from the cyanide tailings after primary air-drying was 53.3 mg/L, the free cyanide content was 3.9 mg/L, the Cu content was 34.7 mg/L, and the Fe content was 0.3 mg/L. The results show that the main reason for the substandard leaching toxicity of the cyanide tailings was the copper–cyanide complex in the cyanide tailings. It can be seen from Table 1 that the moisture content of the cyanide tailings after primary air-drying was 20.32%, the Fe content and Cu content in the dry base of cyanidation tailings were 30.16% and 0.372%, respectively, while the Fe content in the toxic leaching solution was only 0.3 mg/L. Combining the content of total cyanide, free cyanide, and Cu and Fe ions in the filter barren solution in Table 2, it can be seen that the main reason for the excessive toxicity of the cyanide tailings was the copper–cyanide complex in the cyanide tailings. And the copper–cyanide complex was mainly distributed in the moisture of cyanide tailings. Therefore, the idea of this research is to replace the remaining high-concentration cyanide-containing water with low-concentration cyanide-containing water in the cyanide tailings after primary air-drying to reduce the toxicity of the cyanidation tailings and make them reach the standard for entering the tailings pond. The elution water undergoes chemical treatment and membrane treatment to recover copper and cyanide and then returns to the filter press backwash process to realize the recycling of the backwash water.
3.2. Filter Press Backwash Process
3.2.1. The Effect of the Primary Air-Drying Time
In order to explore the influence of the primary air-drying time on the filter press backwash effect, the primary air-drying time was tested under the conditions of 0.8 times the back washing water, pH 9 of the back washing water, 10 min of the secondary air-drying time, and 1.17 mg/L of the total cyanide concentration of the back washing water. Figure 2 shows the leaching toxicity results of backwashed cyanide tailings under different primary air-drying times. It can be seen from Figure 2 that when the backwash water multiple was 0.8, the leaching toxicity of the backwashed cyanide tailings was greater than 5 mg/L when the primary air-drying time was less than 0.73 min or more than 2.55 min. If the primary air-drying time was too short under the same conditions, then the moisture content of the filter cake before backwashing was higher, and more high-concentration cyanide-containing water needed to be replaced, resulting in a high cyanide content of backwashed cyanide tailings. The filter cake easily formed cracks when the primary air-drying time exceeded 1.5 min, which will cause the backwash water to pass through the gap preferentially during backwashing, thus losing the backwash effect. Therefore, the best time for primary air-drying was 1.5 min.
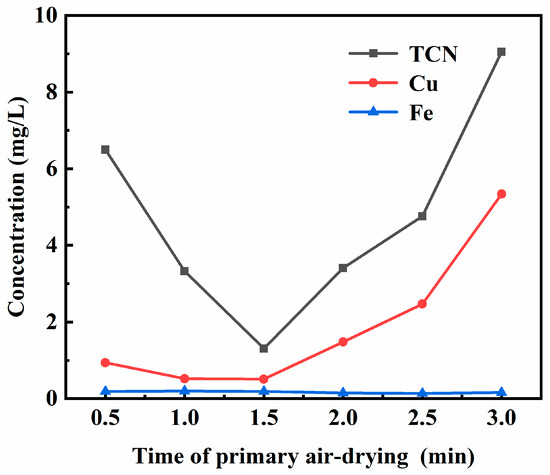
Figure 2.
Effect of primary air-drying time on the leaching toxicity of backwashed cyanide tailings.
3.2.2. The Effect of Multiple Backwashing Conditions
Figure 3 illustrates the leaching toxicity results of the backwashed cyanide tailings under different backwash conditions. The experiments were carried out under the following conditions: pH 9 of the backwash water, 1.17 mg/L of the total cyanide concentration of the backwash water, 1.5 min of the primary air-drying time, and 10 min of the secondary air-drying time. As observed in Figure 3, the leaching toxicity of backwashed cyanide tailings decreased with the increase in backwashing multiples. When the backwash water multiple increased from 0.5 to 1, the concentration of TCN in the leached toxic solution from backwashed cyanide tailings decreased from 6.8 mg/L to 0.374 mg/L, and the concentration of Cu also decreased from 6.09 to 0.15 mg/L. When the backwash water multiples were 0.5 and 0.6, the total cyanide concentration in the toxic liquid leached from the backwashed cyanide tailings was 6.8 mg/L and 5.26 mg/L, respectively, both of which are higher than 5 mg/L. In order to ensure that the leaching toxicity of the backwashed cyanide tailings meets the technical requirements for the disposal of cyanide tailings in the TSPC and to reduce the water consumption, the optimal multiple of backwash water should be 0.7.
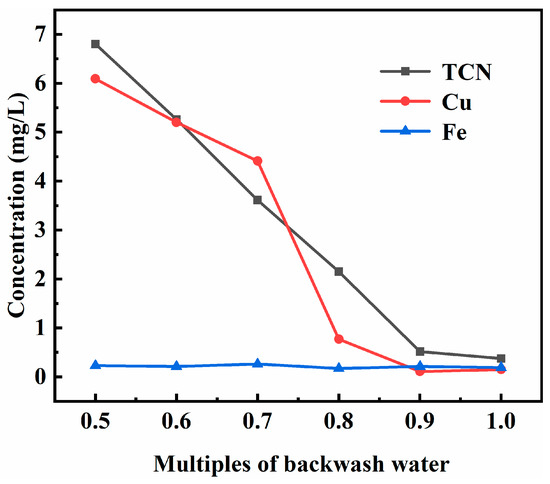
Figure 3.
Effect of backwash water multiples on the leaching toxicity of backwashed cyanide tailings.
3.2.3. The Effect of the pH Value of Backwash Water
The leaching toxicity results of backwashed cyanide tailings under different pH conditions of the backwash water are shown in Figure 4a, and the corresponding elution water components are given in Figure 4b. A set of runs was conducted under the following conditions: 0.7 times the backwash water, 1.17 mg/L of total cyanide in the backwash water, 1.5 min of primary air-drying, and 10 min of secondary air-drying. Referring to Figure 4, when the pH value of backwash water was 1 and 1.5, the leaching toxicity of backwashed cyanide tailings exceeded the standard, while when the pH value of backwash water was greater than 2, the leaching toxicity of backwashed tailings met the expected requirements. It is seen that when the pH was 1 and 1.5 (see Figure 4b), the total cyanide and copper concentrations of the corresponding elution water were lower, and as the pH of the backwash water continued to increase, total cyanide and copper concentrations in the eluent remained stable after increasing to a certain extent. When the pH of the backwash water was less than 2, the acidity of the backwash water was too high, and it reacted directly with the copper–cyanide complex in the cyanide tailings during backwashing, resulting in a low copper content in the elution water and obvious turbidity of the effluent [17]. There was an off-white precipitate, which was a mixture of CaSO4, CuCN, etc. The lower content of copper ions in the elution water indicates that part of copper sediment was left in the backwashed cyanide tailings; therefore, the leaching toxicity of backwashed cyanide tailings could not meet the technical requirements for the disposal of cyanide tailings in the TSPC. The copper content in the elution water increased with the increase in the pH value of the backwash water, and the leaching toxicity of the backwashed cyanide tailings decreased. Therefore, the pH value of the backwash water should not be lower than 2.
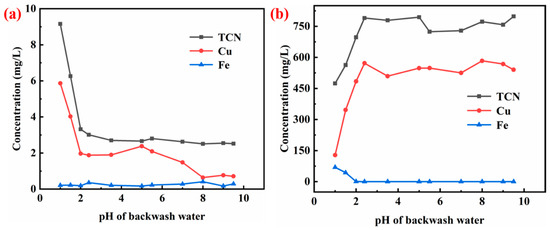
Figure 4.
Effect of the pH value of the backwash water on the filter press backwash process: (a) leaching toxicity of backwashed cyanide tailings; (b) element content of elution water.
3.2.4. Effect of the Total Cyanide Content in Backwash Water
Under today’s environmental protection requirements, the production water in a factory is prohibited from being discharged. If clean water is utilized in the press filter washing process of cyanide tailings, it will inevitably cause the expansion and waste of water resources in the plant area. Therefore, the cyanide tailings backwashing process needs to use treated cyanide-containing water as backwash water. It is necessary to study the effect of the TCN content in the backwash water on the filter press backwash effect.
The conditions of the TCN content in the backwash water were tested under the following conditions: 0.7 times the amount of backwash water, the pH of the backwash water at 9, primary air-drying time for 1.5 min, and secondary air-drying time for 10 min. The leaching toxicity of backwashed cyanide tailings under different conditions of the TCN content of backwash water is shown in Figure 5. Figure 5 shows that the leaching toxicity of backwashed cyanide tailings increased with the increase in the TCN content in the backwash water. Under the same experimental conditions, the higher the TCN content in the backwash water, the higher the cyanide content in the water content of the backwashed cyanide tailings. In order to make the TCN content in the leached toxic liquid of the backwashed cyanide tailings less than 5 mg/L, the TCN content in the backwash water should be less than 21.75 mg/L.
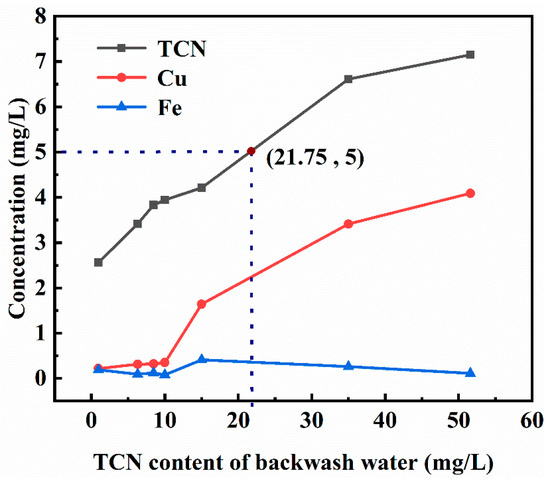
Figure 5.
Effect of the TCN content in the backwash water on the leaching toxicity of backwashed cyanide tailings.
3.2.5. The Effect of the Secondary Air-Drying Time
To better understand the influence of the secondary air-drying time on the filter press backwash effect, the secondary air-drying time of cyanide tailings was tested under the following conditions: the backwash water dosage was 0.7 times, the pH value of the backwash water was 9, the total cyanide concentration of the backwash water was 1.17 mg/L, and the primary air-drying time was 1.5 min. The leaching toxicity results of backwashed cyanide tailings under different secondary air-drying times are shown in Figure 6. As discussed for Figure 6, the leaching toxicity of backwashed cyanide tailings decreased first and then stabilized with the increase in the secondary air-drying time. When the secondary air-drying time was 5 min and 10 min, the total cyanide content in the leached toxic solution of the backwashed cyanide tailings was 5.42 mg/L and 2.29 mg/L, respectively. After the secondary air-drying time exceeded 10 min, leaching toxicity was not significantly reduced, indicating that the moisture content of cyanide tailings decreased slightly after more than 10 min. Therefore, the secondary air-drying time is preferably 10 min.
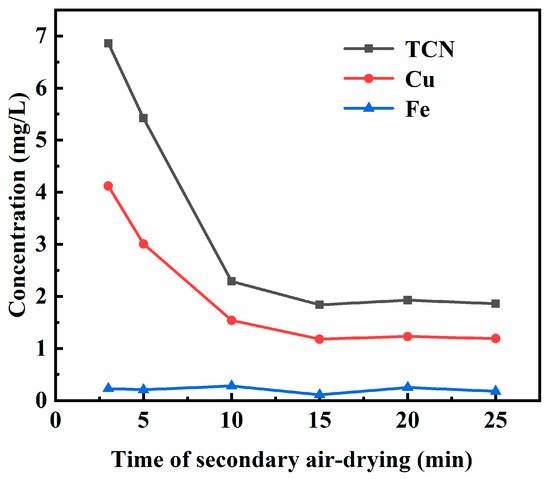
Figure 6.
Effect of secondary air-drying time on the leaching toxicity of backwashed cyanide tailings.
3.3. Chemical Treatment Process
3.3.1. The Effect of the Initial pH of Acidification Conditions
In order to study the influence of the initial pH value of acidification conditions on the effect of copper precipitation, the initial pH value of elution was adjusted by sulfuric acid, and the addition of sodium hydrosulfide was controlled to be 0 mg/L. The elemental content analysis of the solution after full reaction with different initial pH values is shown in Figure 7. Figure 7 shows that the acidification treatment caused the volatilization of HCN to a certain extent, resulting in a decrease in the total cyanide concentration of the solution [18]. The acidification process in elution water at room temperature could be described as [19]:
2NaCN + H2SO4 → Na2SO4 + 2HCN
4NaCu(CN)2 + 2H2SO4 → 2Cu2(CN)2 + 2Na2SO4 + 4HCN↑
Cu2(CN)2 + 2NaSCN → 2CuSCN↓ + 2NaCN
Ca2+ + H2SO4 → CaSO4↓ + 2H+
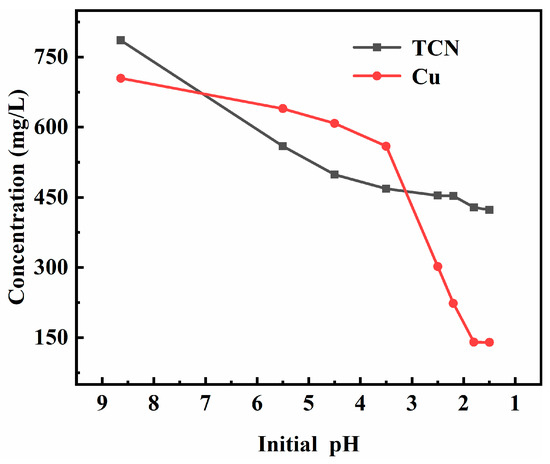
Figure 7.
Effect of initial pH value of the acidification reaction on the copper precipitation effect.
The effect of acidizing copper precipitation increased with the decrease in the initial pH value. When the initial pH of acidification reaction decreased from 8.64 to 1.5, the concentration of copper ions in the filtrate after the reaction decreased from 704.58 mg/L to 139.86 mg/L. When the pH value was reduced to 1.8, the copper ion concentration did not decrease as the pH value decreased, so the initial pH value of the optimal acidification reaction was 1.8.
3.3.2. The Effect of Sodium Hydrosulfide Dosage
After the acidification reaction, 140 mg/L copper ions were in the solution, which may adversely affect the effect of subsequent membrane treatment and the service life of the membrane. Therefore, in this study, sodium hydrosulfide was added for deep copper deposition to improve the copper recovery rate [20]. In order to study the influence of the amount of sodium hydrosulfide on the effect of copper deposition, the conditional experiment of the amount of sodium hydrosulfide was carried out under the condition that the initial pH value of the acidification reaction was 1.8. The vulcanization process in elution water at room temperature could be described as [21,22]:
S2− + Cu2+ → CuS↓
2Cu(CN)32− + 3H2SO4 + S2− → Cu2S↓ + 6HCN +3SO42−
The elemental content analysis of the solution after treatment with different sodium hydrosulfide dosages is shown in Figure 8. It can be seen from Figure 8 that when the dosage of sodium hydrosulfide was 50 mg/L, the concentration of copper ions in the solution was reduced from 140.3 mg/L to 70 mg/L, and the copper sinking effect was obvious. With the increase in the amount of sodium hydrosulfide, the concentration of copper ions after treatment was maintained at about 10 mg/L, so the appropriate amount of sodium hydrosulfide was 100 mg/L.
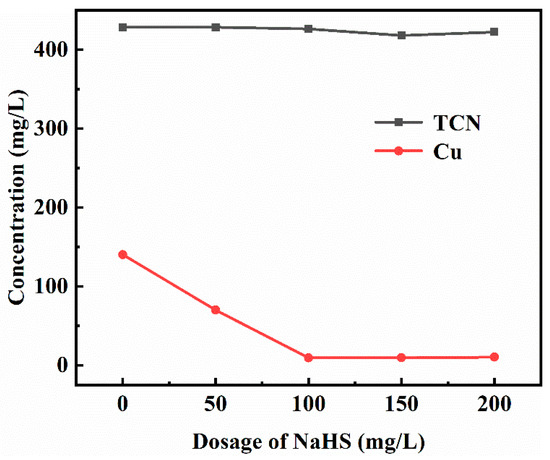
Figure 8.
Effect of sodium hydrosulfide dosage on the copper precipitation effect.
3.3.3. Characterization of Copper Products
The chemical composition of the copper products is shown in Table 3. The chemical analysis showed that copper products were mainly composed of copper (54.56%) and sulfur (16.58%) and a small number of elements such as calcium and iron. Analysis of copper products by X-ray diffraction (see Figure 9) revealed that the main components of the copper products were cuprous thiocyanate (CuSCN), copper sulfide (CuS), cuprous sulfide (Cu2S), and calcium sulfate (CaSO4) [23]. This is consistent with the element analysis results in Table 3. Next, surface chemical states of copper products were characterized by X-ray photoelectron spectroscopy (XPS). The XPS survey spectra shown in Figure 10 indicated the presence of the expected elements: copper, sulfur, carbon, nitrogen, and oxygen. The high-resolution Cu 2p spectra in Figure 10b indicated that the asymmetric peaks of Cu 2p3/2 were overlapped by two peaks, which were assigned to Cu+ (932.5 eV) and Cu2+ (933.2 eV) [24,25,26]. Considering Figure 10c, the spectrum in the sulfur 2p region was dominated by the peaks of SCN−, including S 2p1/2 (164.4 eV) and S 2p3/2 (163.2 eV) [25,26], and the second peak at 163.2 eV suggested some contribution from S2− [27]. The minor peak at 169 eV is likely the trace of the oxidized sulfur (SO42−) [24]. The N 1s peak (see Figure 10d) had a binding energy at 398.2 eV, which probably corresponds to C≡N bonds [23,28].

Table 3.
Chemical composition of copper products.
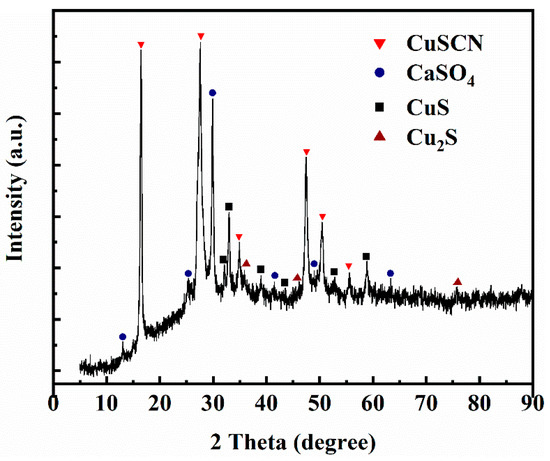
Figure 9.
XRD patterns of copper products.
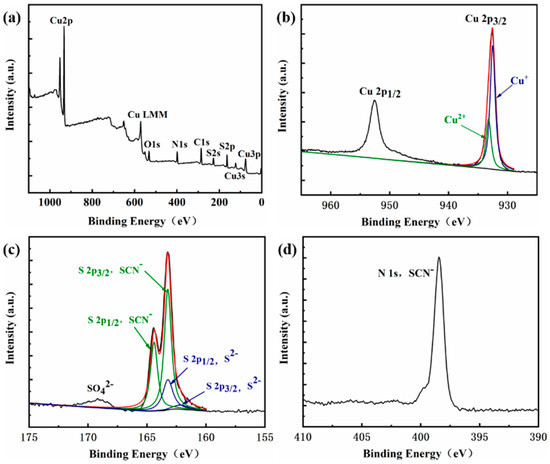
Figure 10.
XPS spectra of copper products: (a) survey; (b) copper; (c) sulfur; (d) nitrogen.
3.4. Membrane Treatment Process
3.4.1. The Influence of Gaseous Membrane Stages
The influence of the number of membrane stages on the effect of membrane treatment was studied under the conditions of 0.3 m3/h of cyanide-containing water and 0.6 m3/h of lye side flow. Figure 11 indicates that when the flow rate of cyanine-containing water was 0.3 m3/h, the total cyanide concentration in the solution could be reduced from 421.21 mg/L to 9.26 mg/L through the primary membrane treatment. After a two-stage membrane treatment, the total cyanide concentration was 1.24 mg/L, and with a continued increase in the stage of membrane, the total cyanide concentration changed little. In order to ensure the effect of the filter press backwash, the total cyanide content in the cyanide-containing water should be reduced as much as possible, so the number of membrane stages should be 2.
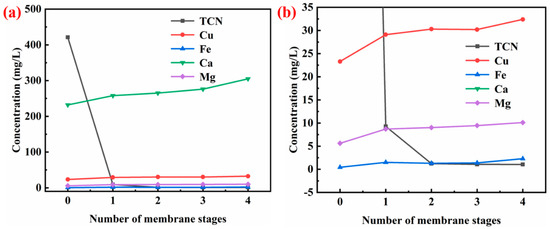
Figure 11.
Effect of number of membrane stages on the membrane treatment process: (a) general view; (b) the range of −5 to 35 mg/L in (a).
3.4.2. The Influence of the Flow Rate of Cyanide-Containing Water
The experimental results of the flow rate conditions of cyanide-containing water under the condition of membrane stage 2 are shown in Figure 12. As can be seen from Figure 12, when the flow rate of cyanine-containing water increased from 0.3 m3/h to 0.9 m3/h, the total cyanide content in cyanine-containing water increased from 10 mg/L to 118.66 mg/L after the treatment with the primary membrane and from 2.66 mg/L to 31.15 mg/L after the treatment with the secondary membrane. When the flow rate of the cyanide-containing water was low, the HCN in the cyanide-containing water had sufficient time to pass through the membrane pores in the form of gas to the other side of the membrane and was neutralized and absorbed by the counter-current NaOH solution inside the hollow fiber membrane. In order to ensure the effect of backwashing, the flow rate of cyanide-containing water during gaseous membrane treatment should be 0.3 m3/h.
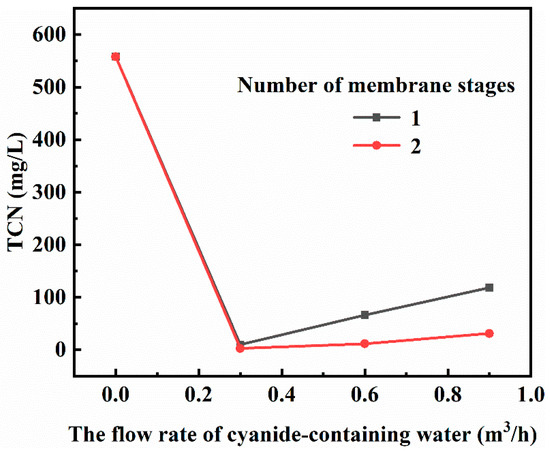
Figure 12.
Effect of the flow rate of cyanide-containing water on the membrane treatment process.
3.5. Results of the Circulation Experiment
In order to verify the feasibility of the whole process, the three processes were connected to carry out the circulation experiment. The process flow is shown in Figure 1. The cyanide-containing water was pumped into the chemical reaction tank, sulfuric acid was added to adjust the initial pH of the acidification reaction to 1.8, and after the full reaction, 0.1 g/L of sodium hydrosulfide was added for deep copper precipitation. After copper sinking, the solution was pumped into the plate and frame filter press through a pneumatic diaphragm pump, and the filter residue became copper products. The filtrate was further filtered through a precision filter, and the precision filter filtrate was pumped into the gaseous membrane module through an acid-resistant pump. During membrane treatment, the flow rate of cyanide-containing water was 0.3 m3/h, the flow rate of lye was 0.6 m3/h, and the number of membrane stages was two. After membrane treatment, cyanide-containing water was pumped as backwash water into the backwash tank of the countercurrent washing machine for backwashing the cyanide tailings. In the filter press backwash, the primary air-drying time was 1.5 min, the backwash water multiple was 0.7 times, and the secondary air-drying time was 10 min. The elution water after the filter press and backwashing was returned to the chemical treatment process for the circulation experiment, and the total cycle number was five. During the experiment, the leaching toxicity of the backwashed cyanide tailings was tested, and the elemental content analysis of the leached toxic solution (LTS), elution water, solution after acidification (SA), solution after vulcanization (SV), solution after primary membrane treatment (M1), solution after secondary membrane treatment (M2), and the lye after gaseous membrane treatment (ML) was carried out. The results of the circulation experiment are shown in Table 4.

Table 4.
Results of the circulation experiment.
As we can see in Table 4, after chemical treatment and membrane treatment of cyanide water in the five cycle tests, the total content of cyanide in the permeating liquid was about 2 mg/L, which can meet the requirements of reverse washing of oxidized mineral cyanide residue. The concentration of thiocyanate and copper in the solution could be reduced to less than 5 mg/L and 15 mg/L, respectively, through the chemical reaction process. During the whole cycle, calcium ions increased from 300 mg/L to about 800 mg/L and then stabilized at about 800 mg/L. This may have occurred because in the backwashing process, acidic backwash water reacts with lime or calcium hydroxide in oxidized mineral cyanidation tailings to form calcium sulfate [29,30]. In addition, part of the calcium sulfate precipitation is discharged with the filter cake, and at the same time, the concentration of calcium and sulfate ions in the solution is close to the saturated solubility of calcium sulfate. In addition, the backwashed cyanide tailings in the five cycle experiments met the requirements for storage in a tailing pond in TSPC. Compared with the existing AVR and SART processes, this process adopts the first acidification and then vulcanization for copper precipitation, which had the advantages of good copper precipitation effect, low consumption of sodium hydride sulfide and no secondary wastewater generation [20,31,32,33]. And the treated acidic water in this process was used as backwash water to wash the alkaline cyanide tailings, so there was no need to add alkali for neutralization, and there is no acid wastewater discharge. What’s more, the process adopts gaseous membrane to absorb HCN, which had high absorption efficiency and low energy consumption compared with the traditional inflatable volatilization reabsorption [17]. In summary, the “filter press backwash–chemical precipitation–gaseous membrane absorption” method can realize the green disposal of cyanide tailings.
4. Conclusions
It is feasible to use the “filter press backwash–chemical precipitation–gaseous membrane absorption” method for green treatment of cyanide tailings. The main conclusions are summarized as follows:
- The main reason for the excessive toxicity of the cyanide tailings was the high concentration of copper-cyanide complexes in the cyanide tailings.
- The effect of the filter press backwash was mainly affected by air-drying time and backwash water parameters. To decrease the total cyanide concentration in the leached toxic solution of backwashed cyanide tailings to less than 5 mg/L, the primary air-drying time should be 1.5 min; in addition, the secondary air- drying time should be 10 min, the pH value of the backwash water must be greater than 2, the backwash water multiple must be greater than or equal to 0.7, and the total cyanide concentration in the backwash water must be greater than 21.75 mg/L.
- In the process of chemical treatment, the copper element in the elution water was recovered by acidification and vulcanization. The optimal reaction conditions were as follows: initial pH value of the acidification reaction was 1.8 and NaHS dosage of 100 mg/L. The copper products were composed of CuSCN, CuS, Cu2S, and CaSO4.
- The effect of gaseous membrane treatment was mainly affected by the flow rate of cyanide-containing water and the number of membrane stages. The best conditions were that the flow rate of cyanide-containing water was 0.3 m3/h and the number of membrane stages was two.
- The process circulation experiment was carried out under optimal conditions to verify the feasibility and stability of the process. In the circulation experiment, the leaching toxicity of the backwashed cyanide tailings reached the TSPC standard for storage in a tailings pond. The average recovery rate of copper and total cyanide in elution water was 97.8% and 99.89%, respectively, and the average removal rate of thiocyanate was 94.09%.
Author Contributions
Conceptualization, J.Y. and S.Y.; methodology, J.Y. and Y.W.; validation, Y.W., P.H. and J.Y.; investigation, J.Y. and X.L.; resources, S.Y. and Y.T.; data curation, J.Y.; writing—original draft preparation, J.Y.; writing—review and editing, J.Y. and S.Y.; supervision, P.H.; project administration, Y.W. and X.L.; funding acquisition, S.Y. All authors have read and agreed to the published version of the manuscript.
Funding
We are grateful for the Chinese Academy of Science Project (No. KFJ-STS-QYZD-044) and the National Key R&D Program during the 13th Five-year Plan Period (2019YFC1908405) for support of this work.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding author. The data are not publicly available due to project requirements.
Acknowledgments
Thanks are extended to the Yunnan Gold Group for the support of experimental raw materials.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Habashi, F. 100 years of cyanidation. Can. Min. Metall. Bull. 1987, 80, 108–114. [Google Scholar]
- Li, H.; Li, S.; Srinivasakannan, C.; Zhang, L.; Yin, S.; Yang, K.; Xie, H. Efficient cleaning extraction of silver from spent symbiosis lead-zinc mine assisted by ultrasound in sodium thiosulfate system. Ultrason. Sonochem. 2018, 49, 118–127. [Google Scholar] [CrossRef] [PubMed]
- Chinese Standard HJ 943-2018. Technical Specification for Pollution Control of Cyanide Leaching Residue in Gold Industry; Ministry of Environmental Protection: Beijing, China, 2018. [Google Scholar]
- Korte, F.; Coulston, F. Some considerations on the impact on ecological chemical principles in practice with emphasis on gold mining and cyanide. Ecotoxicol. Environ. Saf. 1998, 41, 119–129. [Google Scholar] [CrossRef] [PubMed]
- Donato, D.B.; Madden-Hallett, D.M.; Smith, G.B.; Gursansky, W. Heap leach cyanide irrigation and risk to wildlife: Ramifications for the international cyanide management code. Ecotoxicol. Environ. Saf. 2017, 140, 271–278. [Google Scholar] [CrossRef] [PubMed]
- Garcia, V.; Hayrynen, P.; Landaburu-Aguirre, J.; Pirila, M.; Keiski, R.L.; Urtiaga, A. Purification techniques for the recovery of valuable compounds from acid mine drainage and cyanide tailings: Application of green engineering principles. J. Chem. Technol. Biotechnol. 2014, 89, 803–813. [Google Scholar] [CrossRef]
- Guo, X.; Qin, H.; Tian, Q.; Zhang, L. The efficacy of a new iodination roasting technology to recover gold and silver from refractory gold tailing. J. Clean. Prod. 2020, 261, 121147. [Google Scholar] [CrossRef]
- Dong, K.; Xie, F.; Wang, W.; Chang, Y.; Chen, C.; Gu, X. Calcination of calcium sulphoaluminate cement using pyrite-rich cyanide tailings. Crystals 2020, 10, 971. [Google Scholar] [CrossRef]
- Aliprandini, P.; Veiga, M.M.; Marshall, B.G.; Scarazzato, T.; Espinosa, D.C.R. Investigation of mercury cyanide adsorption from synthetic wastewater aqueous solution on granular activated carbon. J. Water Process Eng. 2020, 34, 101154. [Google Scholar] [CrossRef]
- Chen, Y.; Song, Y.; Chen, Y.; Zhang, X.; Lan, X. Comparative experimental study on the harmless treatment of cyanide tailings through slurry electrolysis. Sep. Purif. Technol. 2020, 251, 117314. [Google Scholar] [CrossRef]
- Hou, D.; Liu, L.; Yang, Q.; Zhang, B.; Qiu, H.; Ruan, S.; Chen, Y.; Li, H. Decomposition of cyanide from gold leaching tailingsby using sodium metabisulphite and hydrogen peroxide. Adv. Mater. Sci. Eng. 2020, 2020, 1–7. [Google Scholar] [CrossRef]
- Bahrami, A.; Kazemi, F.; Alighardashi, A.; Ghorbani, Y.; Abdollahi, M.; Parvizian, A. Isolation and removal of cyanide from tailing dams in gold processing plant using natural bitumen. J. Environ. Manag. 2020, 262, 110286. [Google Scholar] [CrossRef] [PubMed]
- Estay, H.; Ortiz, M.; Romero, J. A novel process based on gas filled membrane absorption to recover cyanide in gold mining. Hydrometallurgy 2013, 134, 166–176. [Google Scholar] [CrossRef]
- Estay, H.; Troncoso, E.; Romero, J. Design and cost estimation of a gas-filled membrane absorption (GFMA) process as alternative for cyanide recovery in gold mining. J. Membr. Sci. 2014, 466, 253–264. [Google Scholar] [CrossRef]
- Chinese Standard HJ/T 299-2007. Solid Waste-Extraction Procedure for Leaching Toxicity-Sulphuric Acid & Acid Method; State Environmental Protection Administration: Beijing, China, 2007. [Google Scholar]
- Chinese Standard GB/T 18598-2001. Standard for Pollution Control on the Security Landfill Site for Hazardous Wastes; State Environmental Protection Administration: Beijing, China, 2001. [Google Scholar]
- Estay, H.; Gim-Krumm, M.; Quilaqueo, M. Two-stage SART process: A feasible alternative for gold cyanidation plants with high zinc and copper contents. Minerals 2018, 8, 392. [Google Scholar] [CrossRef]
- Alonso-Gonzalez, O.; Nava-Alonso, F.; Uribe-Salas, A. Copper removal from cyanide solutions by acidification. Miner. Eng. 2009, 22, 324–329. [Google Scholar] [CrossRef]
- Dai, X.; Simons, A.; Breuer, P. A review of copper cyanide recovery technologies for the cyanidation of copper containing gold ores. Miner. Eng. 2012, 25, 1–13. [Google Scholar] [CrossRef]
- Riveros, P.A.; Molnar, R.E.; McNamara, V.M. Alternative-technology to decrease the environmental-impact of gold milling—A progress report on canmet research activitiesin this filed. CIM Bull. 1993, 86, 167–171. [Google Scholar]
- Xie, F.; Dreisinger, D. Studies on solvent extraction of copper and cyanide from waste cyanide solution. J. Hazard. Mater. 2009, 169, 333–338. [Google Scholar] [CrossRef]
- Xie, F.; Dreisinger, D.; Doyle, F. A review on recovery of copper and cyanide from waste cyanide solutions. Miner. Process. Extr. Metall. Rev. 2013, 34, 387–411. [Google Scholar] [CrossRef]
- Er, U.; Icli, K.C.; Ozenbas, M. Spin-coated copper(I) thiocyanate as a hole transport layer for perovskite solar cells. J. Solid State Electrochem. 2020, 24, 293–304. [Google Scholar] [CrossRef]
- Wu, H.; Or, V.W.; Gonzalez-Calzada, S.; Grassian, V.H. CuS nanoparticles in humid environments: Adsorbed water enhances the transformation of CuS to CuSO4. Nanoscale 2020, 12, 19350–19358. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Yang, H.; Zhu, Y.; Luo, S.; Ma, J. Interfacial N-Cu-S coordination mode of CuSCN/C3N4 with enhanced electrocatalytic activity for hydrogen evolution. Nanoscale 2019, 11, 12938–12945. [Google Scholar] [CrossRef]
- Aldakov, D.; Chappaz-Gillot, C.; Salazar, R.; Delaye, V.; Welsby, K.A.; Ivanova, V.; Dunstan, P.R. Properties of electrodeposited CuSCN 2D layers and nanowires influenced by their mixed domain structure. J. Phys. Chem. C 2014, 118, 16095–16103. [Google Scholar] [CrossRef]
- Panzeri, G.; Cristina, M.; Jagadeesh, M.S.; Bussetti, G.; Magagnin, L. Modification of large area Cu2O/CuO photocathode with CuS non-noble catalyst for improved photocurrent and stability. Sci. Rep. 2020, 10, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Chai, B.; Wang, M.; Wang, Z.; Wang, Y.; Zhu, Y. Synthesis and characterization of ball-in-ball CuSCN hollow architecture. Mater. Lett. 2013, 93, 56–59. [Google Scholar] [CrossRef]
- Gayevskii, V.R.; Kochmarskii, V.Z.; Gayevska, S.G. Nucleation and crystal growth of calcium sulfate dihydrate from aqueous solutions: Speciation of solution components, kinetics of growth, and interfacial tension. J. Cryst. Growth 2020, 548, 125844. [Google Scholar] [CrossRef]
- Jia, C.; Wu, L.; Chen, Q.; Ke, P.; De Yoreo, J.J.; Guan, B. Structural evolution of amorphous calcium sulfate nanoparticles into crystalline gypsum phase. Crystengcomm 2020, 22, 6805–6810. [Google Scholar] [CrossRef]
- Prosyanikov, E.D.; Tsybikova, B.A.; Batoeva, A.A.; Ryazantsev, A.A. Extraction of hydrogen cyanide from waste solutions of cyaniding circuit for sulfide flotation concentrates. J. Min. Sci. 2009, 45, 80–86. [Google Scholar] [CrossRef]
- Adams, M.D. Impact of recycling cyanide and its reaction products on upstream unit operations. Miner. Eng. 2013, 53, 241–255. [Google Scholar] [CrossRef]
- Estay, H.; Becker, J.; Carvajal, P.; Arriagada, F. Predicting HCN gas generation in the SART process. Hydrometallurgy 2012, 113, 131–142. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).