Featured Application
Ru(terpy)-based conducting polymers can be used as modifying agents in enzyme-based am-perometric biosensors to significantly enhance selectivity and sensitivity thanks to the effi-cient electron-transfer process promoted by the simultaneous presence of the organic con-ducting framework and the metal center.
Abstract
A heteroleptic [Ru(terpy)2]2+ (terpy = 2,2′:6′,2″-terpyridine) complex was electrochemically polymerized to give the corresponding metal-containing conducting polymer on gold and glassy carbon electrodes. The polymerization of the Ru(II) complex was allowed by a terthiophene functionalization on one of the two terpy coordinating fragments, whereas the presence of -COOH substituents on the second terpy ligand enabled the film to immobilize a tyrosinase enzyme by cross-linking with glutaraldehyde. Then, the Ru(terpy) conducting polymer worked as a transducer as well as an immobilizing agent in the design of amperometric biosensors for the determination of epinephrine. The electrochemical behavior of enzymatic sensors containing Ru(terpy)-based conducting polymers was investigated by differential pulse voltammetry and chronoamperometry. Analytical performances and kinetic parameters were calculated, suggesting a potential application of the reported biosensors in the determination of epinephrine in pharmaceutical products.
1. Introduction
Chemical sensors are a broad class of analytical devices in which a chemical recognition system, known as receptor, is combined with a physicochemical transducer to transform chemical information into an analytical signal. When the receptor is a biological recognition element, the recognition system is based on a biochemical mechanism, and these sensors are more properly called biosensors [1]. Usually, bioreceptors can be yeasts, enzymes, whole cells, bacteria, proteins, or antibodies that allow the sensor to be able to detect an analyte or a specific class of analytes with high selectivity. Various types of biosensors can be recognized according to the biological mechanism that determines their specificity or to the mode of signal transduction. In particular, the biocatalytic mechanisms promoted by the presence of enzymes as biological receptors are of considerable interest in scientific research. On the other hand, electrochemical methods often allow highly selective and sensitive determinations and low levels of interference. The most frequent drawbacks in the development of electrochemical biosensors are in the effectiveness of immobilization of the biological component on the electrode surface and its stability on replicated measurements. An efficient immobilization can be achieved by different approaches: physical adsorption, cross-linking, covalent bonding, or entrapment in gels or membranes. All these methods aim to obtain a stable anchoring of the enzyme, then the possibility to use the biosensor many times while retaining a constant quality in the analytical response in terms of sensitivity, linear dynamic range, limit of detection, and selectivity. In recent years, the use of conducting polymers (CPs) as immobilizing agents as well as transducer elements in biosensors has attracted much interest. Entrapping an enzyme into a CP film can be achieved during the electrochemical polymerization in a solution containing both the monomer and the enzyme, or through a suitable functionalization of the polymer film [2,3,4,5]. Such an approach allows an effective immobilization of the enzyme, but it is sometimes not suitable because the conditions of polymerization can often cause a total or partial loss of the catalytic activity of the enzyme [6,7]. In this context, the use of CPs-based electrochemical biosensors in the detection of neurotransmitters is becoming more relevant.
Neurotransmitters are commonly investigated because of their central role in transferring signals across chemical synapses from one nerve cell to another or to non-neuronal cells. Changes in neurotransmitter concentrations induce a number of diseases, including Parkinson’s, Alzheimer’s, and some types of cancer, so a fast-responsive and sensitive analytical device is of particular interest to determine these chemical species. In this field, CPs have been used alone or combined with materials such as carbon nanotubes, pyrolytic graphite, and metal-containing nanoparticles as modifying agents for electrode surfaces. The most common CPs, such as polypyrrole, polyaniline, and polythiophene derivatives, have been considered [8,9,10]. Among neurotransmitters, epinephrine (EP, also known as adrenaline) is widely used in the treatment of heart diseases, allergic reactions, anaphylactic shock, and asthma. From the chemical point of view, epinephrine belongs to the catecholamines group along with norepinephrine and dopamine. Its detection is often based on the activity of the enzyme tyrosinase, which is a Cu-based metal-protein able to catalyze the oxidation of mono- and of o-diphenols to the corresponding quinones and o-diquinones, respectively, in the presence of O2 [11,12]. The adequate anchoring of the enzyme on an electrode surface allows to determine the analyte using electrochemical techniques. In particular, differential pulse voltammetry (DPV) and chronoamperometry (CA) are often used to minimize the effect of interfering substances and, at the same time, to reach a high sensitivity [13].
A possible improvement in the performances of a CP-based biosensor in the determination of EP can be achieved by metal-containing CPs, where the metal center usually enhances the efficiency of the electron-transfer process. In this context, here we report the application of a Ru-containing conducting polymer in the construction of tyrosinase-based biosensors for the selective determination of epinephrine. The conducting polymer considered was obtained from the electropolymerization of a heteroleptic Ru(II)-complex (Figure 1) as previously reported [14]. It is characterized by a [Ru(terpy)2]2+ (terpy = 2,2′:6′,2″-terpyridine) core, and its features are due to the different structure of the two coordinating terpy units. In particular, a 2,2′:5′,2″-terthiophene fragment was attached to the central ring of one terpy through an ethynyl tether. Terthiophene is able to polymerize on the α positions of the terminal rings, giving a metal-containing polymer that joins the redox properties of the metal center and the conducting properties of the organic backbone. On the other hand, the second terpy ligand brings −COOH functionalization to the fourth position of each pyridine ring, which act as active sites in cross-linking processes.
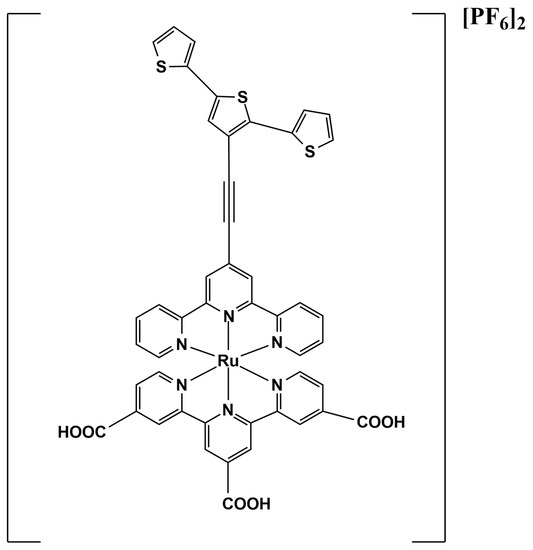
Figure 1.
Heteroleptic Ru-(II) complex [(TAT)Ru(Tpy-COOH)][PF6]2.
The biosensors were tested for the determination of epinephrine, evaluating their ability to minimize typical interferences such as those caused by ascorbic and uric acids, tryptophan and L-cysteine, whereas the analytical performances are reported in terms of sensibility, LoD, LoQ, and linear dynamic range.
2. Materials and Methods
2.1. Chemicals
The tyrosinase (Tyr) from mushroom (lyophilized powder ≥ 1000 U/mg solid protein), (−)-epinephrine (EP), tetraethylammonium hexafluorophosphate (TEAPF6), dichloromethane (CH2Cl2) and acetonitrile (MeCN) (anhydrous ≥ 99.8%), glutaraldehyde (GA, technical 50% in H2O, 5.6 mol dm−3), hydrochloric acid 37%, Tris (Trizma® base), and uric acid (UA) were purchased from Sigma-Aldrich; the potassium dihydrogen phosphate (KH2PO4), sodium hydrogen phosphate (Na2HPO4), acetic acid, and sodium acetate from Carlo Erba; the citric acid from Riedel-De Haën; the ascorbic acid (AA) from Lancaster; the L-cysteine (L-cys) from Fluka; and the tryptophan (Trp) from Alfa Aesar. The ruthenium complex [(TAT)Ru(TpyCOOH)][PF6]2 (RuTt; TAT = 4′-[(2,2′:5′,2″-terthien-3′-ethynyl]-2,2′:6′,2″-terpyridine, TpyCOOH = 4,4′,4″-tricarboxylate-2,2′:6′,2″-terpyridine) was synthesized according to a literature method [14]. All chemicals were used without any further purification. The tyrosinase was stored at −20 °C, and the epinephrine was stored at 2–8 °C. All aqueous solutions were prepared with double-distilled water. All the experiments were performed at room temperature and in an open-air atmosphere.
2.2. Instruments
All electrochemical tests were carried out using a CHI-650 potentiostat interfaced with a computer using the specific software CHI-650 in a conventional three electrodes voltammetric cell. A gold disk (diameter 2 mm) or a glassy carbon (GC, diameter 3 mm) electrode was the working electrode used for the preparation of the biosensor. An Ag/AgCl (on a 3 mol dm−3 KCl aqueous solution) was the reference electrode, and a graphite bar was the counter electrode. Working electrodes were polished with alumina powder (0.3 μm), placed in an ultrasonic bath for 15 min, then rinsed with water and anhydrous acetone. All the electrochemical measurements for the epinephrine determination were carried out in a phosphate buffer solution (0.1 mol dm−3, pH = 7.0) in an open-air atmosphere. All potential values refer to the aqueous Ag/AgCl electrode.
2.3. Biosensors Preparation
The biosensors were obtained by the modification of a gold or glassy carbon disk electrode with a two-step procedure. In the first step, the electrode surface was modified by a film of poly[(TAT)Ru(TpyCOOH)] (pRuTt). Next, the tyrosinase was immobilized on the modified electrode by cross-linking it with glutaraldehyde.
The electrochemical polymerization of [(TAT)Ru(TpyCOOH)][PF6]2 was carried out on the working electrode in an anhydrous CH2Cl2 solution containing 0.1 mol dm−3 of TEAPF6 as supporting electrolyte and 0.005 mol dm−3 of monomer, purging argon in the cell for 20 min before the experiment. A film of pRuTt was obtained by chronoamperometry by applying a constant potential value to the working electrode until a density charge (Qdep) of 1 mC or 2 mC (for the gold or the glassy carbon electrode, respectively) was reached. The film was neutralized by keeping it at 0 V in the same solution for 60 s. The characterization was performed by cyclic voltammetry in CH3CN/TEAPF6. The film thickness (d) was estimated according to Semenikhin et al. [15], assuming a linear relationship between d (nm) and the electrodeposition charge Qdep (mC cm−2) with α = 2.6 nm cm2 mC−1.
In the second step, the tyrosinase was immobilized on the modified electrode according to Baluta et al. [16] through a physical absorption process followed by cross-linking between enzyme molecules using glutaraldehyde as a coupling agent. With this aim, 0.040 cm3 of tyrosinase solution (2 mg of enzyme in 1 cm3 of phosphate-citrate buffer solution, pH 5.2) were dropped on the pRuTt layer, ensuring that the surface of the electrode did not dry out. After 2 h, 0.040 cm−3 of 40% glutaraldehyde solution in a phosphate buffer were added to the surface to cross-link the enzyme. After a further 10 min, the unbound protein was washed away by dipping the electrode first in a phosphate buffer at pH 7.0 (2 × 15 min), then in an acetate buffer at pH 5.2 (2 × 15 min), and, finally, in a Tris-HCl buffer at pH 7.2 (45 min). The biosensor was stored at 4 °C in a phosphate buffer until use.
2.4. Epinephrine Sensing
The epinephrine sensing tests were carried out at room temperature in an open-air atmosphere in a cell containing 30 cm3 of phosphate buffer (0.1 mol dm−3, pH 7.0). The electrochemical tests were performed by differential pulse voltammetry (DPV) and by chronoamperometry (CA). Differential pulse voltammograms were recorded between −0.6 V and +0.6 V (step potential: 0.004 V, amplitude: 0.05 V). According to the voltammetric responses, the chronoamperometric measurements were recorded while a constant potential of 0.18 V was applied and the solution was gently and constantly stirred. When the background current reached a constant value, incremental amounts of epinephrine aqueous solution were injected into the cell with a time interval of about 200 s, and the current/time response was recorded in a concentration range from 1 µmol dm−3 to 1 mmol dm−3.
2.5. Influence of Interfering Substances
The influence of common interfering substances (ascorbic acid (AA), uric acid (UA), L-cysteine (L-cys), and tryptophan (Trp)) on the determination of 1 × 10−5 mol dm−3 EP was investigated. The concentration of the interfering agents was varied in a ratio (EP:interferent) between 1:1 and 1:10.
3. Results and Discussion
3.1. Electrodeposition and Voltammetric Behavior of pRuTt on the Au Electrode
On a gold disk electrode, a film of pRuTt was obtained from a solution of RuTt in a CH2Cl2/0.1 mol·dm−3 TEAPF6 solvent system, repeatedly cycling the potential between −0.5 and 1.3 V vs. Ag/AgCl. The increase in the current peak and the presence of a deep red colored layer on the gold surface evidenced the growth of a polymer film, confirming that polymerization occurred (Figure S1).
The film used as an immobilizing agent for the enzyme was then obtained by potentiostatic deposition; a constant potential of 1.05 V to an unmodified Au electrode was applied as described in Section 2.3. Films with different thickness were tested in the determination of EP, suggesting an optimal charge value for the film of 1.0 mC (32 mC cm−2), corresponding to a film thickness of about 80 nm. The neutral pRuTt film was characterized in a CH3CN solution containing 0.1 mol dm−3 of TEAPF6. A doping/dedoping process was evident at about 1.0/0.87 V as confirmation of the presence of a conductive layer on the electrode surface (Figure 2).
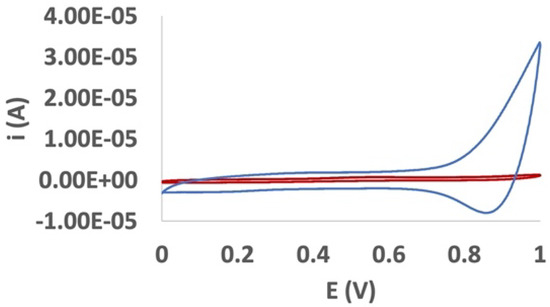
Figure 2.
Cyclic voltammetry characterization of a pRuTt film on a gold disk electrode in CH3CN/0.1 mol dm−3 TEAPF6 solvent system, Qdep = 32 mC cm−2 (blue line). The cyclic voltammetry (CV) curve of the gold disk electrode in the same conditions is reported for comparison (red line).
3.2. Determination of EP with a Au/pRuTt/Tyr Biosensor
An efficient immobilization of the enzyme on an electrode surface is the most critical step in biosensors development. In this context, the design of the polymer film here proposed includes the presence of −COOH groups with the aim to induce a chemical bond between the polymer-modified electrode surface and the enzyme. The use of glutaraldehyde as a coupling reagent between enzyme molecules in a classical cross-linking reaction further contributes to the immobilization of the enzyme [17]. In this perspective, Tyr was firstly deposited on the gold or glassy carbon electrode surface modified by the pRuTt film and then immobilized with the help of glutaraldehyde (as described in Section 2.3).
The electrochemical behavior of the Au/pRuTt/Tyr biosensor was preliminarily investigated by cyclic voltammetry. The CV response of a 550 µmol dm−3 of EP solution in the phosphate buffer evidences a broad and ill-resolved oxidation process at about 0.2 V (Figure S2).
In order to better ascertain the analytical behavior of the reported biosensor, DPV tests were performed between −0.6 and 0.6 V in an EP concentration range between 1 and 100 µmol dm−3. The DPV responses show two peaks (Ep = −0.18 and 0.16 V, respectively), with the more anodic one being significantly more intense (Figure 3 Left). These peaks are reasonably attributable to two different oxidation processes: from EP to adrenalinequinone (an open-chain quinone structure) and from the cyclized form of this quinone to adrenochrome [18,19,20]. Calibration tests were performed on the more anodic (and more intense and sharper) peak, evidencing a linear increase in the current at an increasing EP concentration in the whole range considered (Figure 3 Right) with a good linear correlation (R2 = 0.998). The DPV responses at Au/pRuTt electrode in the same EP concentration range (Figure S3) show a decrease of an order of magnitude in the current intensity, confirming the electrocatalytic effect due to the presence of the enzyme.
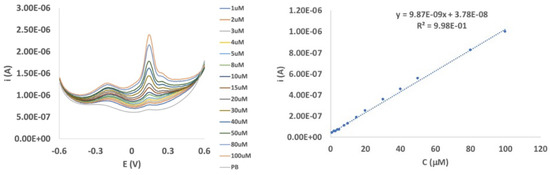
Figure 3.
Differential pulse voltammetry (DPV) responses (Left) and calibration curve (Right) of epinephrine (EP) between 1 and 100 µmol dm−3 in 0.1 M phosphate buffer (pH 7.0) at the Au/pRuTt/Tyr biosensor.
Limit of detection (LoD) was calculated according to Equation (1) [21]:
where σB is the standard deviation of the blank response (calculated on 11 replicates of the blank), and b is the slope of the regression line. LoD was calculated to be 0.67 µmol dm−3.
LoD = 3.29σB/b
Analogously, limit of quantification (LoQ) was calculated according to Equation (2)
and was found to be 1.02 µmol dm−3.
LoQ = 5σB/b
Finally, sensitivity, calculated as the ratio of the slope of the calibration curve to the area of the electrode surface, was 3.1·10−7 A µmol−1 dm3 cm−2.
The analytical behavior of the Au/pRuTt/Tyr biosensor was also investigated by chronoamperometry (Figure 4), and the results were compared to those obtained by DPV. The working potential was chosen at 0.18 V, according to the DPV response, and a concentration range between 1 µmol dm−3 and 1 mmol dm−3 was investigated. An increase in current at the increasing EP concentration was observed with a good linear correlation in the range 1–100 µmol dm−3 (R2 = 0.998).
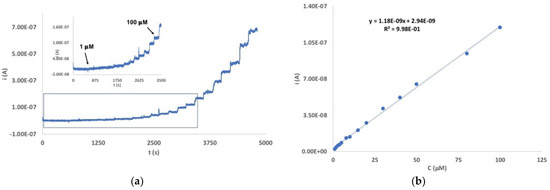
Figure 4.
(a) Current/time responses of the Au/pRuTt/Tyr biosensor at 0.18 V in 0.1 mol dm−3 phosphate buffer in the EP concentration range 1 µmol dm−3 ÷ 1 mmol dm−3 (main graph) and 1 µmol dm−3 ÷ 100 µmol dm−3 (inset). (b) Calibration curve in the EP concentration range 1 µmol dm−3 ÷ 100 µmol dm−3.
The calculated LoD, LoQ, and sensitivity were 0.47 µmol dm−3, 0.71 µmol dm−3, and 3.7 × 10−8 A µmol−1 dm3 cm−2 respectively.
The comparison between the analytical results obtained by DPV and CA show lower values for LoD and LoQ by CA than DPV. This is possibly due to the main feature of CA working at a steady-state potential, whereas DPV requires the potential to be continuously scanned.
3.3. Selectivity
In order to evaluate the selectivity of the Au/pRuTt/Tyr biosensor, the effect of the simultaneous presence of some typical interfering substances was studied. Ascorbic acid (AA), uric acid (UA), tryptophan (Trp), and L-cysteine (L-cys) were considered since these are typical components of biological fluids. The DPV curves reported in Figure 5 and in Figure S4 suggest that the oxidation potentials of the EP and the considered interferents, although quite close, are different enough to allow a selective determination of the main analyte. Unlike the other interfering substances investigated, L-cys was not detected in a DPV scan in these experimental conditions, likely because the presence of the -SH groups in its structure deactivates the enzyme.
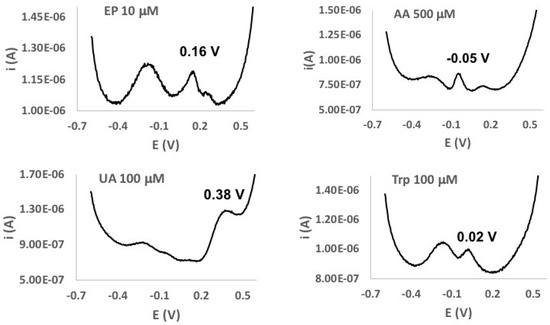
Figure 5.
DPV curves of solutions of EP (10 µmol dm−3, Top Left), ascorbic acid (AA) (500 µmol dm−3, Top Right), uric acid (UA) (100 µmol dm−3, Bottom Left), and tryptophan (Trp) (100 µmol dm−3, Bottom Right) in 0.1 mol dm−3 phosphate buffer at the Au/pRuTt/Tyr biosensor.
As described in Section 2.5, changes in the concentration of interferents compared to a 10 µmol dm−3 EP solution were considered, and the results are summarized in Figure 6.
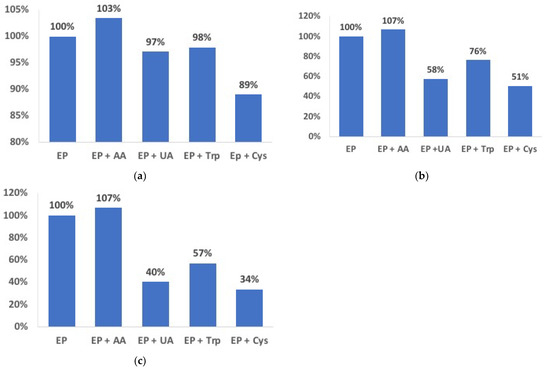
Figure 6.
Effect of AA, UA, Trp, and L-cys in a 10 µmol dm−3 EP solution at EP:interferent ratios = 1:1 (a), 1:5 (b), 1:10 (c) in 0.1 mol dm−3 phosphate buffer at the Au/pRuTt/Tyr biosensor.
As Figure 6 shows, the effect of the interfering substances is acceptably low (from +3% to −11%) when EP and interferents have the same concentration. On the other hand, when increasing the concentration of interfering molecules, their effect is also more evident, causing a decrease of up to 66% in the case of EP:L-cys 1:10. Furthermore, as is predictable according to its chemical structure, L-cys seems to have the highest interfering effect on the determination of EP.
3.4. Analytical Applications
As a result of the calculated LoD and LoQ values, the Au/pRuTt/Tyr biosensor here reported does not seem suitable for the determination of EP in biological fluids, where EP is in the nmol dm−3 concentration level [22]. On the other hand, the proposed biosensor can be used for pharmacological samples. For this purpose, recovery tests were performed on commercially available injectable solutions containing EP 1 mg/1 cm3 (5.5 mmol dm−3) that were properly diluted to 25.0 and 50.0 µmol dm−3. Results reported in Table 1 were calculated as the percent ratio of the detected EP concentration to the real value and evidence a good accuracy in detecting the analyte. The RSD% (percent Relative Standard Deviation) values were calculated on five replicated measurements for each sample.

Table 1.
Recovery tests results for Au/pRuTt/Tyr (n = 5).
The affinity of the enzyme to a substrate, i.e., the so-called cooperative ligand binding, is usually described by the Hill coefficient (nH). This is a dimensionless parameter obtained from a curve log[I/(Imax − I)] against log[C] (I = anodic current, Imax = steady-state current, [C] = EP concentration (µmol dm−3). In particular, when nH = 1, the affinity of the enzyme for the substrate does not depend on other substrate molecules, whether bounded or not, and the kinetics of the process fit a Michaelis–Menten-type mechanism. On the other hand, when nH > 1, the affinity for the substrate increases once a molecule of the substrate is bound. nH values higher than 1.15 refer to a strong cooperative effect between the occupied active centers, and the Michaelis–Menten mechanism is no more adequate to describe the kinetics of the enzymatic process [23,24]. Hence, in order to understand the kinetics of the mechanism of the biosensors studied, nH values were calculated and found to be 1.06 ± 0.02 from DPV and 1.28 ± 0.01 from CA tests, respectively. The significant difference between nH values calculated from the DPV and CA experiments can be reasonably ascribed to the different performing mode of these techniques: in DPV tests, the potential is continuously scanned, while in CA, a constant potential is applied to the biosensor during the entire test, causing a difference in the behavior of the biosensor as seen above in the case of the determination of LoD and LoQ. Therefore, the apparent Michaelis–Menten constant (KM) can be calculated according to the adapted Lineweaver–Burk Equation (3) [24,25] only from the DPV experiments.
1/I = 1/Imax + KM/(Imax[C])
The KM value was calculated as 46 µmol dm−3, suggesting a strong affinity of the DPV biosensor towards the analyte. On the other hand, the relatively low value of Imax (8.3 × 10−7 A) confirms the relatively low sensitivity of the biosensor.
3.5. Comparison between the Au/pRuTt/Tyr and GC/pRuTt/Tyr Biosensors
The analogue GC/pRuTt/Tyr was prepared, and its performances were compared to Au/pRuTt/Tyr (Table 2).

Table 2.
Analytical parameters of the calibration curves for EP determination at the Au/pRuTt/Tyr and glassy carbon (GC)/pRuTt/Tyr biosensors.
In particular, LoD values appear higher in the case of the GC-based biosensor, but comparable to (or better than) analogue devices reported in literature (Table 3).

Table 3.
Comparison of amperometric biosensors for determination of EP.
The recovery test results performed on 25.0 µmol dm−3 of EP solutions are comparable to Au/pRuTt/Tyr biosensor (Table 4). According to the decrease in recovery as the EP concentration increased, which was observed at the Au/pRuTt/Tyr device, as well as to the less satisfactory performances of the GC-based biosensor, recovery in 100 µmol dm−3 EP solutions is lower than 100%.

Table 4.
Recovery tests results for GC/RuTt/Tyr.
The effect of interference by AA, UA, Trp, and L-cys (Figure 7) at GC-biosensors can be compared to the Au-based arrangement. In particular, at the EP:interferent ratio 1:1, the only interference in the GC-based biosensor seems to be L-cys, which causes an underestimation of 14%. Moreover, with increasing concentrations of interferents, the GC arrangement demonstrates a lower effect than that of the Au. In particular, an over- or underestimation from 0% to −20% and from +9% to −52% was observed for the EP:interferent 1:5 and 1:10 ratios, respectively, for the GC-biosensor, with ranges of +7% ÷ −49% and +7% ÷ −66% for the Au-biosensor in the same conditions.
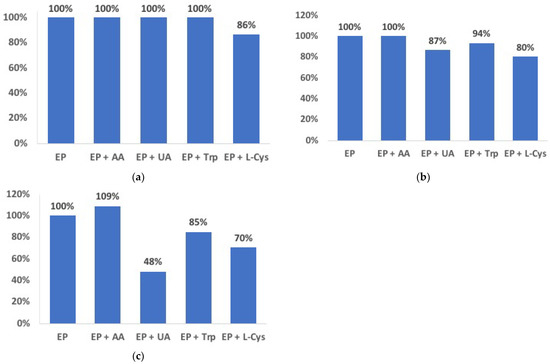
Figure 7.
Effect of AA, UA, Trp, and L-cys in a 10 µmol dm−3 EP solution at EP:interferent ratios = 1:1 (a), 1:5 (b), 1:10 (c) in 0.1 mol dm−3 phosphate buffer at the GC/pRuTt/Tyr biosensor.
4. Conclusions
In this study, the behavior of a Ru(II)-based conducting polymer in the development of two amperometric biosensors for epinephrine was investigated. A Ru(II) complex coordinated by two different terpyridine (terpy) ligands was used as polymerizable specie. The first terpy was functionalized by a terthiophene unit and was able to polymerize on a proper electrode surface, whereas the second terpy ligand bore a −COOH functionalization in the para position in each nitrogen ring. The anchoring of the enzyme was achieved by cross-linking with glutaraldehyde. A CP film was obtained either on a gold or on a glassy carbon electrode, and tyrosinase was immobilized on the modified electrode. The comparison, in terms of the limit of detection, the limit of quantification, the linear range, and sensitivity, between the Au- and the GC-based biosensors showed more satisfactory analytical performances for the Au-based one. Furthermore, the Au/pRuTt/Tyr biosensor showed a good affinity towards epinephrine.
On the other hand, the GC/pRuTt/Tyr biosensor seemed to be less affected by the presence of some typical interfering substances than Au/pRuTt/Tyr, even at a EP/interfering ratio lower than 1.
Because of the features reported herein, the [Ru(terpy)2]2+ unit can be considered a potential tool as a central component in the development of amperometric biosensors for pharmaceutical formulations, acting as an efficient transducer as well as an immobilizing agent for enzymatic receptors.
Supplementary Materials
The following are available online at https://www.mdpi.com/2076-3417/11/5/2065/s1, Figure S1: CV characterization in CH3CN/0.1 mol dm−3 TEAPF6 solvent system of a pRuTt film grown by scanning the potential in the range 0 ÷ 1.3 V on a gold disk electrode. The two anodic peaks at 0.98 V and 1.2 V are ascribable to the polaron and bipolaron form, respectively, according to ref. [14], Figure S2: CV responses in absence (blue line) and presence (red line) of EP (500 µM) at Au/pRuTt electrode (on the left), and at Au/pRuTt/Tyr sensor (on the right), Figure S3: DPV responses of EP between 1 and 100 µmol dm−3 in 0.1 M phosphate buffer (pH 7.0) at Au/pRuTt sensor, Figure S4: DPV curves of solutions containing EP:AA 1:10 (top left), EP:UA 1:10 (top right), EP:Trp 1:10 (bottom left) in 0.1 mol dm−3 phosphate buffer at Au/pRuTt/Tyr biosensor. Concentration of interfering analytes as in Figure 5.
Author Contributions
Conceptualization, F.M. and M.I.P.; methodology, M.I.P. and A.Z.; validation, M.I.P. and A.Z.; formal analysis, F.M.; investigation, F.M.; writing—original draft preparation, M.I.P.; writing—review and editing, F.M., G.S., N.S., A.Z.; supervision, M.I.P. and A.Z.; funding acquisition, M.I.P., G.S., N.S., A.Z. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Università degli Studi di Sassari (“Fondo di Ateneo per la ricerca 2019”).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available in www.mdpi.com/xxx/s1.
Acknowledgments
Authors gratefully acknowledge Università degli Studi di Sassari for financial support (“Fondo di Ateneo per la ricerca 2019”).
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
References
- Thévenot, D.R.; Toth, K.; Durst, R.A.; Wilson, G.S. Electrochemical biosensors: Recommended definitions and classification (technical report). Pure Appl. Chem. 1999, 71, 2333–2334. [Google Scholar] [CrossRef]
- Cosnier, S. Biosensors based on electropolymerized films: New trends. Anal. Bioanal. Chem. 2003, 377, 507–520. [Google Scholar] [CrossRef]
- Lupu, S.; Lete, C.; Balaure, P.C.; Caval, D.I.; Mihailciuc, C.; Lakard, B.; Hihn, J.-Y.; del Campo, F.J. Development of amperometric biosensors based on nanostructured tyrosinase-conducting polymer composite electrode. Sensors 2013, 13, 6759–6774. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Kuwahara, T.; Yamazaki, R.; Shimomura, M. Covalent immobilization of glucose oxidase on films prepared by electrochemical copolymerization of 3-methylthiophene and thiophene-3-acetic acid for amperometric sensing of glucose: Effects of polymerization conditions on sensing properties. Eur. Polym. J. 2007, 43, 3264–3276. [Google Scholar] [CrossRef]
- Pilo, M.I.; Farre, R.; Lachowicz, J.I.; Masolo, E.; Panzanelli, A.; Sanna, G.; Senes, N.; Sobral, A.; Spano, N. Design of amperometric biosensors for the detection of glucose prepared by immobilization of glucose oxidase on conducting (poly)thiophene films. J. Anal. Meth. Chem. 2018, 2018, 1849439. [Google Scholar] [CrossRef]
- Hiller, M.; Kranz, C.; Huber, J.; Bäuerle, P.; Schuhmann, W. Amperometric biosensors produced by immobilization of redox enzymes at polythiophene-modified electrode surfaces. Adv. Mater. 1996, 8, 219–222. [Google Scholar] [CrossRef]
- Cosnier, S. Biomolecule immobilization on electrode surfaces by entrapment or attachment to electrochemically polymerized films. A review. Biosens. Bioelectr. 1999, 14, 443–456. [Google Scholar] [CrossRef]
- Lu, X.; Li, Y.; Du, J.; Zhou, X.; Xue, Z.; Liu, X.; Wang, Z. A novel nanocomposites sensor for epinephrine detection in the presence of uric acids and ascorbic acids. Electrochim. Acta 2011, 56, 7261–7266. [Google Scholar] [CrossRef]
- Runsewe, D.; Betancourt, T.; Irvin, J.A. Biomedical Application of Electroactive Polymers in Electrochemical Sensors: A Review. Materials 2019, 12, 2629. [Google Scholar] [CrossRef]
- Prajapati, D.G.; Kandasubramanian, B. Progress in the Development of Intrinsically Conducting Polymer Composites as Biosensors. Macromol. Chem. Phys. 2019, 220, 1800561. [Google Scholar] [CrossRef]
- Kanteev, M.; Goldfeder, M.; Fishman, A. Structure–function correlations in tyrosinases. Protein Sci. 2015, 24, 1360–1369. [Google Scholar] [CrossRef] [PubMed]
- Nawaz, A.; Shafi, T.; Khaliq, A.; Mukhtar, H.; ul Haq, I. Tyrosinase: Sources, structure and applications. Int. J. Biotechnol. Bioeng. 2017, 3, 142–148. [Google Scholar] [CrossRef]
- Moon, J.-M.; Thapliyal, N.; Hussain, K.K.; Goyal, R.N.; Shim, Y.-B. Conducting polymer-based electrochemical biosensors for neurotransmitters: A review. Biosens. Bioelectr. 2018, 102, 540–552. [Google Scholar] [CrossRef] [PubMed]
- Manca, P.; Pilo, M.I.; Sanna, G.; Bergamini, G.; Ceroni, P.; Boaretto, R.; Caramori, S. Heteroleptic Ru(II)-terpyridine complex and its metal-containing conducting polymer: Synthesis and characterization. Synth. Met. 2015, 200, 109–116. [Google Scholar] [CrossRef]
- Semenikhin, O.A.; Jiang, L.; Iyoda, T.; Hashimoto, K.; Fujishima, A. In situ AFM study of the electrochemical deposition of polybithiophene from propylene carbonate solution. Synth. Met. 2000, 110, 195–201. [Google Scholar] [CrossRef]
- Baluta, S.; Lesiak, A.; Cabaj, J. Graphene Quantum Dots-based Electrochemical Biosensor for Catecholamine Neurotransmitters Detection. Electroanalysis 2018, 30, 1781–1790. [Google Scholar] [CrossRef]
- Sassolas, A.; Blum, L.J.; Leca-Bouvier, B.D. Immobilization strategies to develop enzymatic biosensors. Biotechnol. Adv. 2012, 30, 489–511. [Google Scholar] [CrossRef]
- Hawley, M.D.; Tatawawadi, S.V.; Piekarski, S.; Adams, R.N. Electrochemical Studies of the Oxidation Pathways of Catecholamines. JACS 1967, 89, 447–450. [Google Scholar] [CrossRef]
- Zhang, H.-M.; Zhou, X.-L.; Hui, R.-T.; Li, N.-Q.; Liu, D.-P. Studies of the electrochemical behavior of epinephrine at a homocysteine self-assembled electrode. Talanta 2002, 56, 1081–1088. [Google Scholar] [CrossRef]
- Wang, S.; Du, D.; Zou, Q.-C. Electrochemical behavior of epinephrine at L-cysteine self-assembled monolayers modified gold electrode. Talanta 2002, 57, 687–692. [Google Scholar] [CrossRef]
- Desimoni, E.; Brunetti, B. Presenting Analytical Performances of Electrochemical Sensors. Some Suggestions. Electroanalysis 2013, 25, 1645–1651. [Google Scholar] [CrossRef]
- Alpat, Ş.; Özdemir, K.; Alpat, S.K. Voltammetric determination of epinephrine in pharmaceutical sample with a tyrosinase nanobiosensor. J. Sens. 2016, 2016, 5653975. [Google Scholar] [CrossRef]
- Hervás Pérez, J.P.; Sánchez-Paniagua López, M.; López-Cabarcos, E.; López-Ruiz, B. Amperometric tyrosinase biosensor based on polyacrylamide microgels. Biosens. Bioelectron. 2006, 22, 429–439. [Google Scholar] [CrossRef]
- Apetrei, I.M.; Apetrei, C. Biosensor based on tyrosinase immobilized on a single-walled carbon nanotube-modified glassy carbon electrode for detection of epinephrine. Int. J. Nanomed. 2013, 8, 4391–4398. [Google Scholar] [CrossRef]
- Alarcon-Angeles, G.; Alvarez-Romero, G.A.; Merkoçi, A. Electrochemical Biosensors: Enzyme Kinetics and Role of Nanomaterials. Encycl. Interfac. Chem. 2018, 140–155. [Google Scholar] [CrossRef]
- Brondani, D.; Scheeren, C.W.; Dupont, J.; Cruz Vieira, I. Biosensor based on platinum nanoparticles dispersed in ionic liquid and laccase for determination of adrenaline. Sens. Actuators B 2009, 140, 252–259. [Google Scholar] [CrossRef]
- Wierzbicka, E.; Szultka-Młyńska, M.; Buszewski, B.; Sulka, G.D. Epinephrine sensing at nanostructured Au electrode and determination its oxidative metabolism. Sens. Actuators B 2016, 237, 206–215. [Google Scholar] [CrossRef]
- Liu, X.; Ye, D.; Luo, L.; Ding, Y.; Wang, Y.; Chu, Y. Highly sensitive determination of epinephrine by a MnO2/Nafion modified glassy carbon electrode. J. Electroanal. Chem. 2012, 665, 1–5. [Google Scholar] [CrossRef]
- Thomas, T.; Mascarenhas, R.J.; Martis, P.; Mekhalif, Z.; Kumara Swamy, B.E. Multi-walled carbon nanotube modified carbon paste electrode as an electrochemical sensor for the determination of epinephrine in the presence of ascorbic acid and uric acid. Mater. Sci. Eng. C 2013, 33, 3294–3302. [Google Scholar] [CrossRef] [PubMed]
- Goyal, R.N.; Bishnoi, S. Simultaneous determination of epinephrine and norepinephrine in human blood plasma and urine samples using nanotubes modified edge plane pyrolytic graphite electrode. Talanta 2011, 84, 78–83. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).