UKF-Based State Estimation for Electrolytic Oxygen Generation System of Space Station
Abstract
1. Introduction
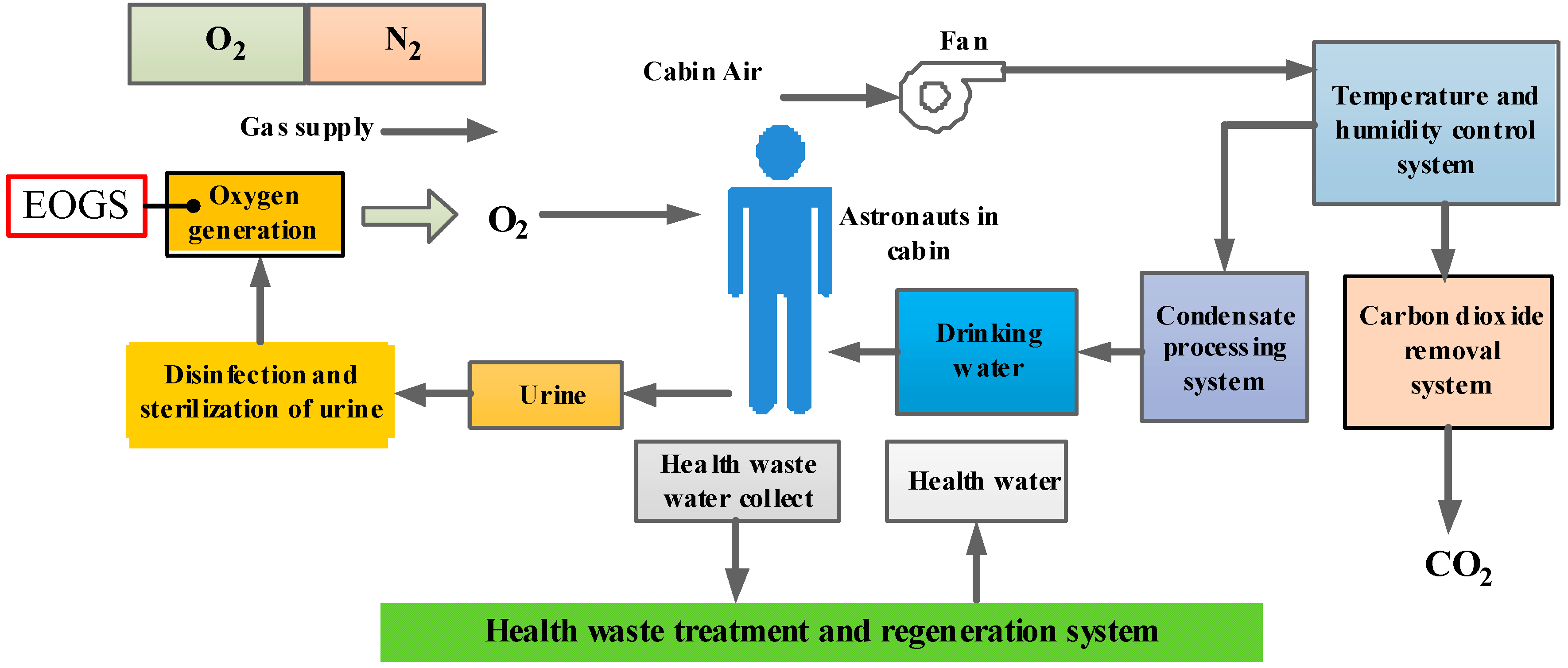
2. Operation Analysis and Working Principle of EOGS
2.1. Operation Analysis of EOGS
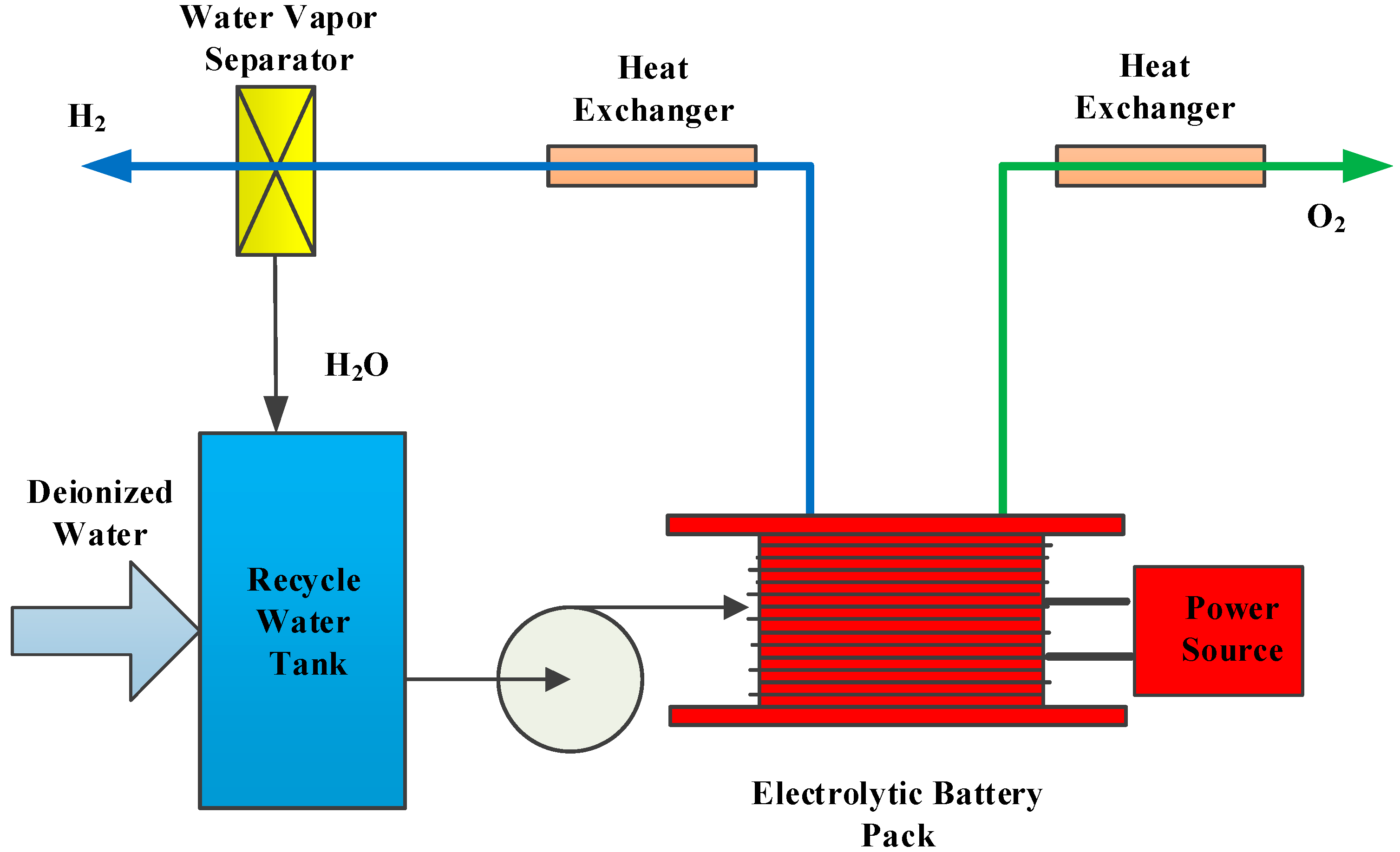
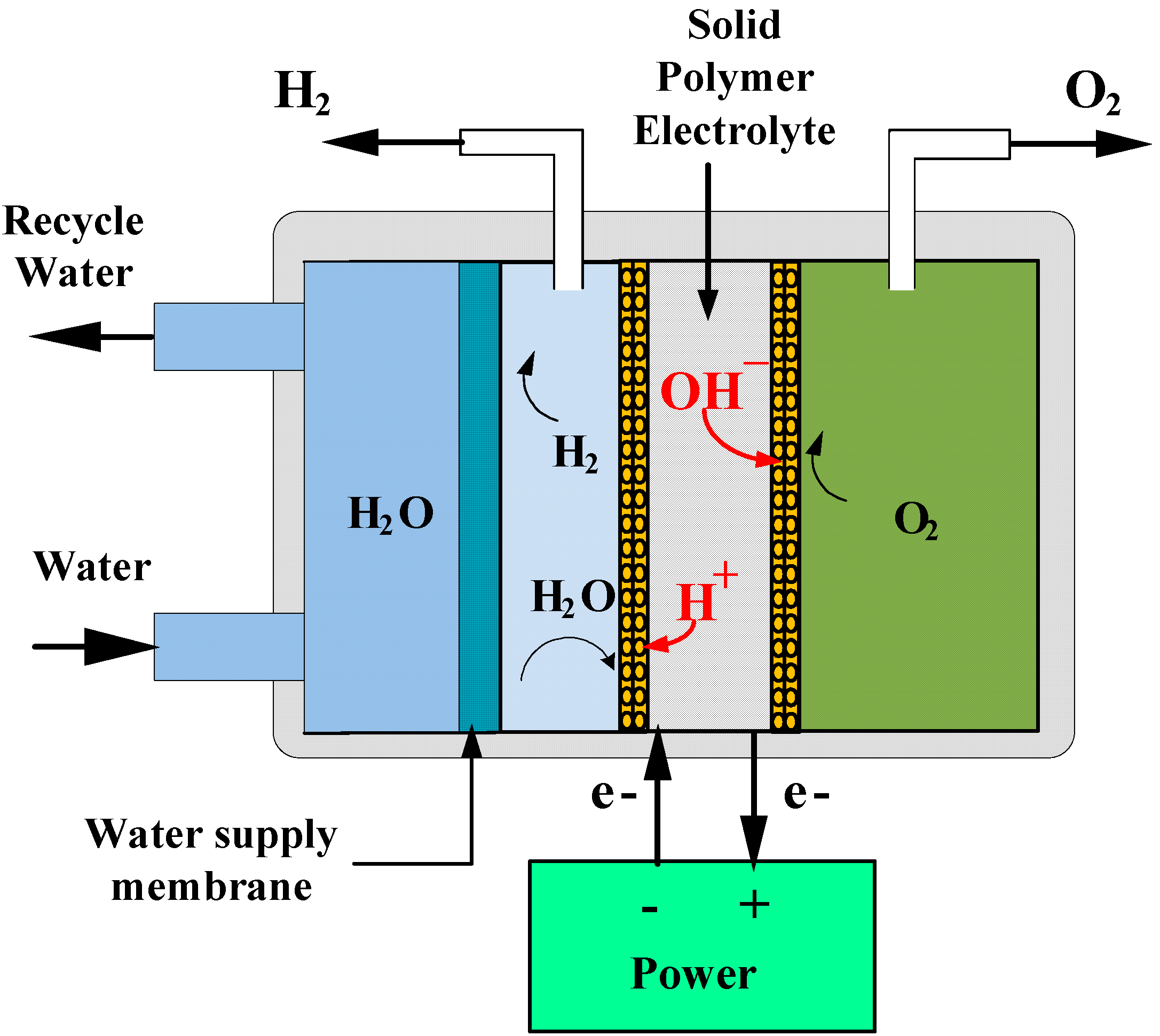
2.2. The Working Principle of EOGS
- The oxygen, hydrogen and water vapor in the core are all ideal gases, and the working pressure in the cell will not exceed 150 kPa;
- Water vapor and water can quickly saturate with oxygen and hydrogen in the mixed gas flow;
- Hydrogen and oxygen are insoluble in water;
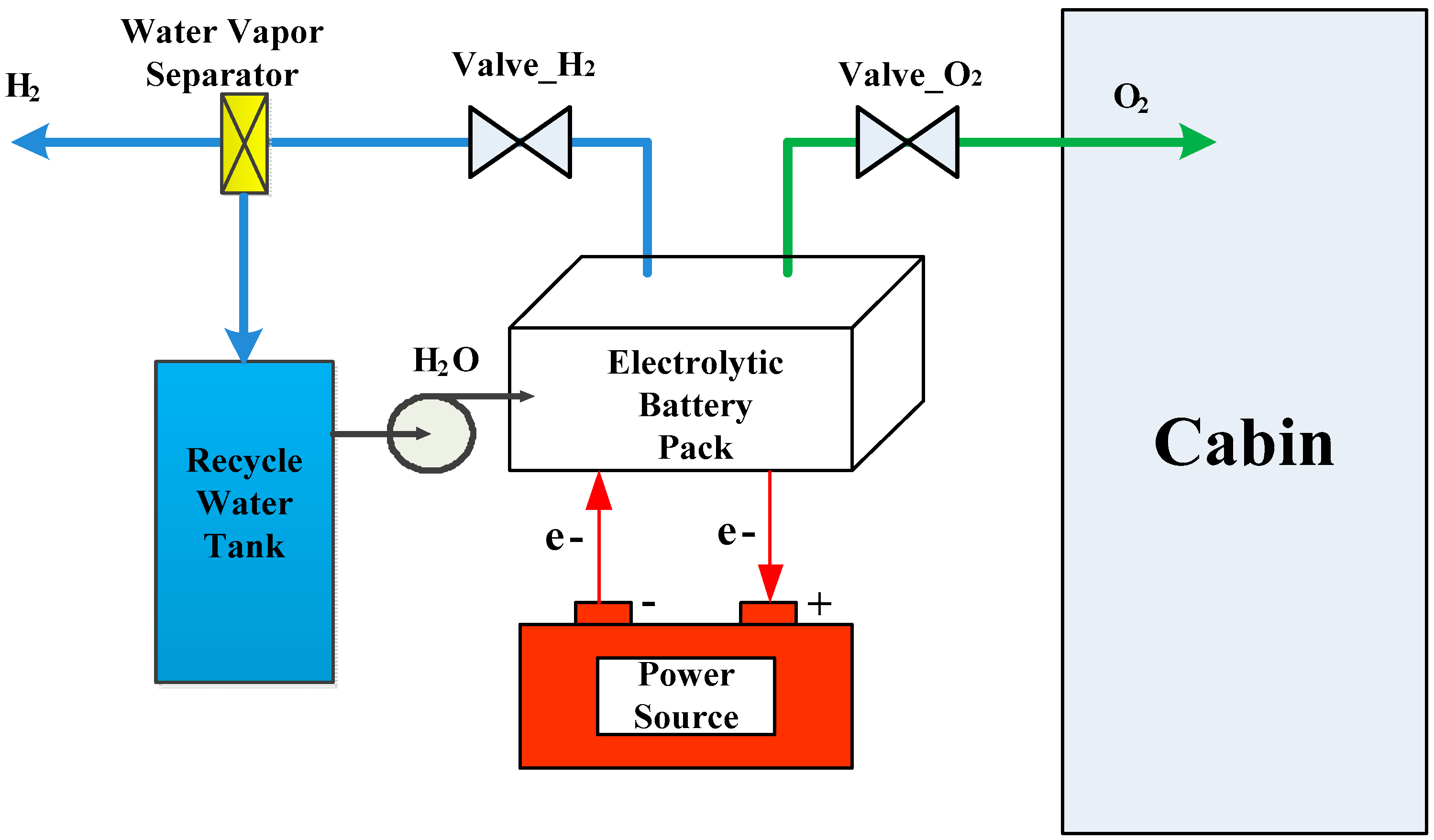
3. The Dynamic Model of EOGS
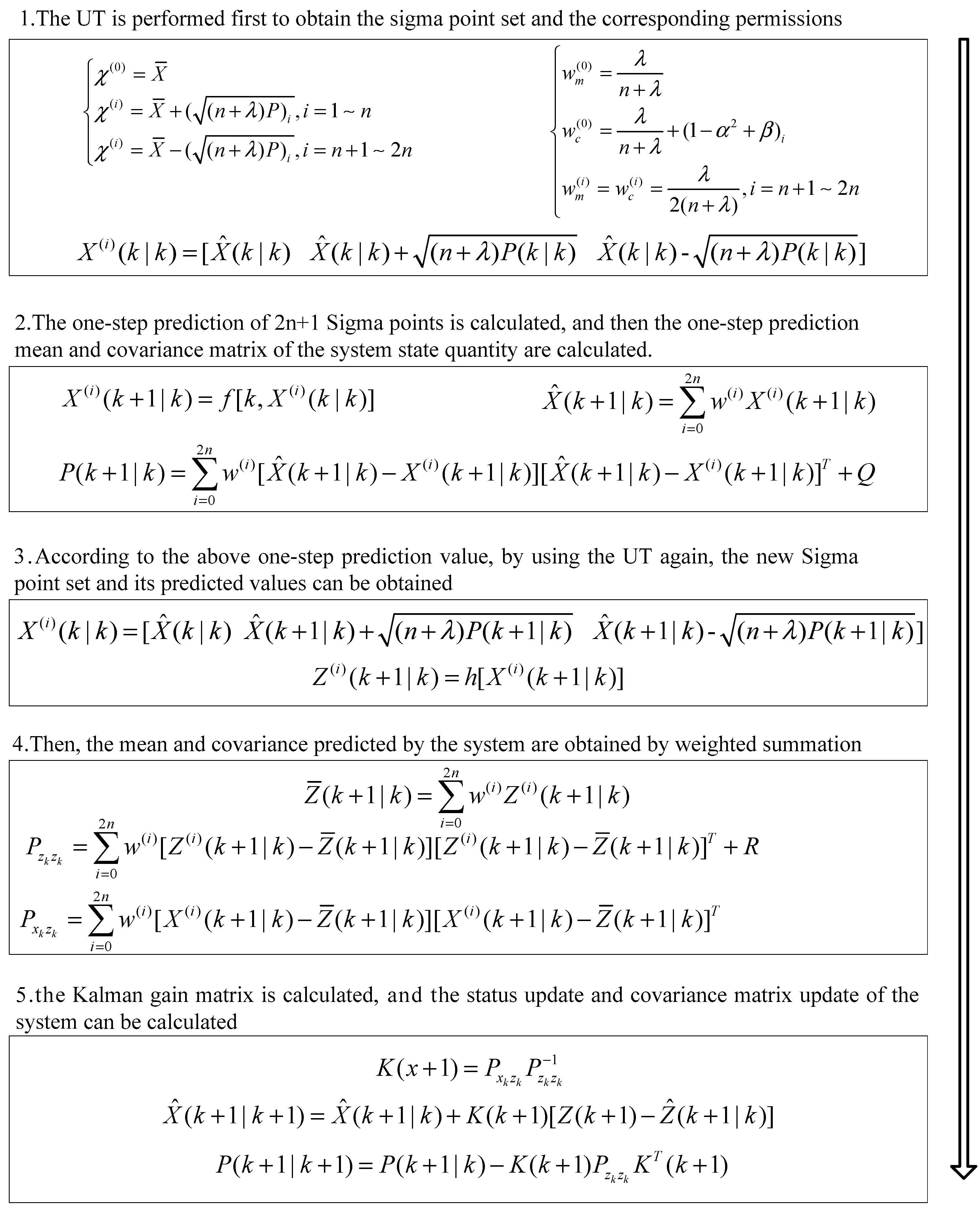
4. State Monitoring of EOGS Based on UKF
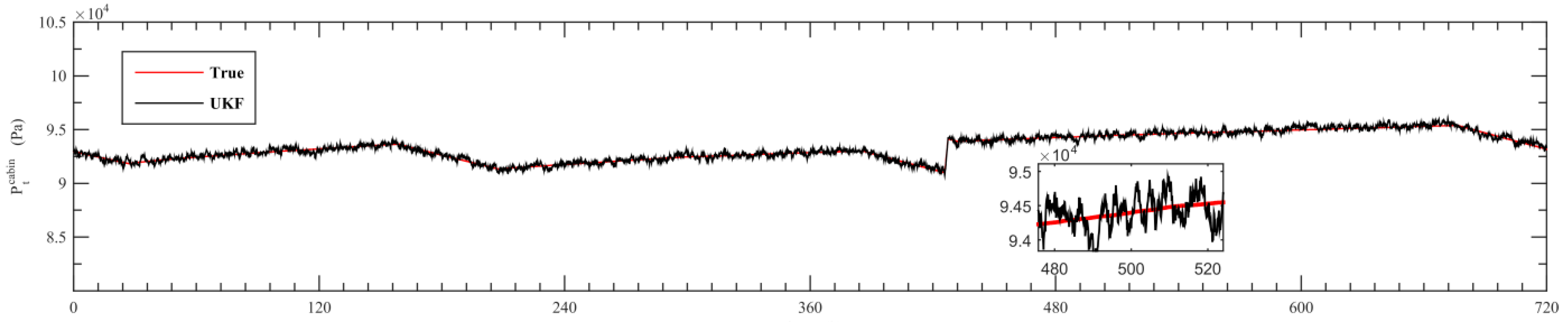
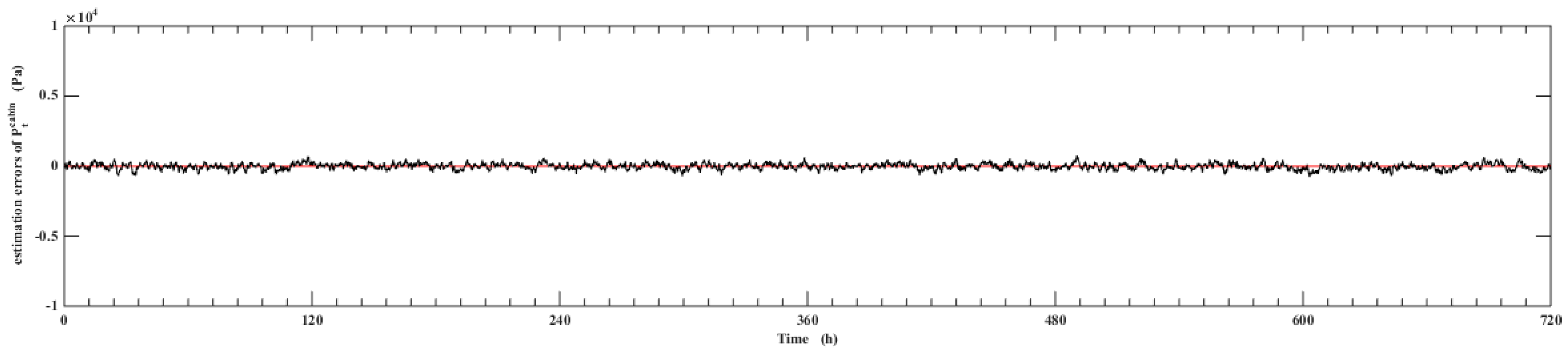
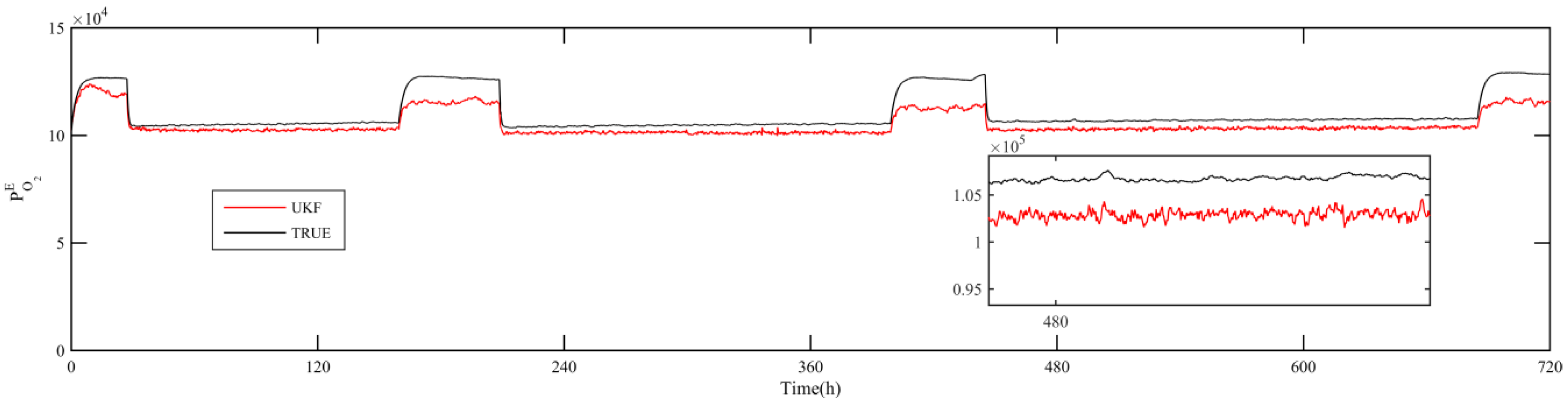
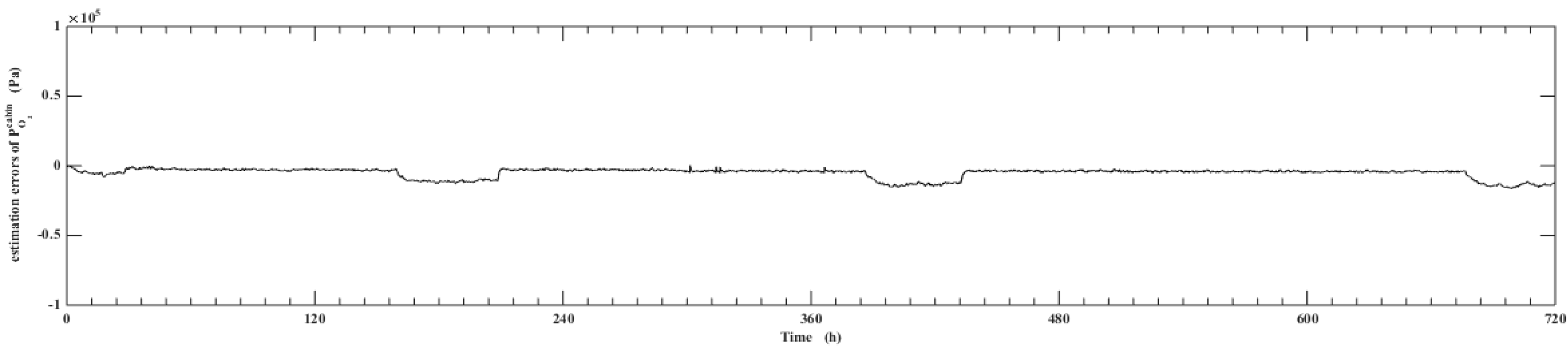
5. Simulation Results
6. Conclusions
- The established dynamic model of the system is relatively simple and accurate. It can be used for the simulation experiment of EOGS, avoiding the complex coupling relationship between the system and other systems caused by energy exchange.
- Using UKF algorithm to design the observer the state of the system can achieve a high accuracy. The estimation error of the system state is within 2% by comparing the estimation value with the real value.
- The model and UKF algorithm built in this paper can monitor the state of the system, and diagnose the fault of the core components based on actual observation values simultaneously, which will lay a solid foundation for the subsequent comprehensive fault diagnosis of the EOGS.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Nomenclature
| amount of water supplied to the system | kg | |
| flow rate of water vapor in the mixed flow of hydrogen and water | kg | |
| hydrogen production | kg | |
| input power of the system and are the heat carried by oxygen and hydrogen | J | |
| heat carried by oxygen and hydrogen | J | |
| electrolysis cell | J | |
| heat dissipated to the environment by the oxygen heat exchanger, the hydrogen heat exchanger, and the water-gas separator, respectively | J | |
| heat carried by water vapor in the mixed flow of hydrogen and water | J | |
| theoretical power consumption of electrolyzed water | J | |
| heat taken away by the cold mass through the heat exchanger | J | |
| theoretical power consumption required for water electrolysis | J | |
| theoretical power consumption per unit mass of water electrolysis | J/kg | |
| mass of water being electrolyzed | kg | |
| mass of water entering the electrolytic core | kg | |
| mass of water discharged from the cathode | kg | |
| heat taken away from the outlet of the electrolytic cell | J | |
| heat taken away by the water discharged from the electrolytic core | J | |
| heat dissipation coefficient of the electrolytic cell to the environment | J/(m·℃) | |
| total heat dissipation length of the electrolyzer | m | |
| temperature of the electrolytic cell | ℃ | |
| ambient temperature | ℃ | |
| heat removed by oxygen | J | |
| heat removed by hydrogen respectively | J | |
| mass flow rate of water vapor carried by the cathode and anode respectively | kg/s | |
| inlet and outlet temperature of the electrolytic core respectively | ℃ | |
| heat taken away by anode water vapor and cathode water vapor | J | |
| specific heat capacities of water, hydrogen, and oxygen | J/(kg·°C | |
| latent heat of vaporization of water | J | |
| oxygen partial pressure | kPa | |
| total pressure | kPa | |
| molar mass of air | kg/s | |
| Ideal gas constant | \ | |
| temperature in cabin | ℃ | |
| total volume in cabin | m³ | |
| mass flow of oxygen into the cabin | kg/s | |
| the mass flow of nitrogen into the cabin | kg/s | |
| mass flow of carbon dioxide into the cabin | kg/s | |
| hydrogen partial pressure in cell | kPa | |
| oxygen partial pressure in cell | kPa | |
| molar mass of hydrogen | kPa | |
| temperature in the electrolysis cell | ℃ | |
| mass flow of hydrogen produced by the electrolysis cell | kg/s | |
| mass flow from electrolysis cell to cabin | kg/s | |
| volume of water vapor separator cavity | m³ | |
| volume of chamber in electrolysis cell | m³ | |
| mass flow of hydrogen produced by the electrolysis cell | kg/s | |
| Valve_O2’s flow coefficient and Valve_H2’s flow coefficient respectively | Kv | |
| Valve_O2’s valve area | m2 | |
| Valve_H2’s flow coefficient, valve area and opening respectively | \ | |
| electrolysis current | A | |
| number of single cells in the electrolysis system | \ | |
| Faraday constant | Ah/mol | |
| amount of charge | \ |
References
- Jerng, L.; Jeng, F. Modeling and Test Data Analysis Of The Life Support System Integration Facility Oxygen Generation Subsystem. SAE Tech. Paper Ser. 1995, 104, 1139–1152. [Google Scholar] [CrossRef]
- Grigoriev, G.Y.; Lagutin, A.S.; Nabiev, S.; Zuev, B.; Filonenko, V.; Legin, A.; Kirsanov, D. Water quality monitoring during interplanetary space flights. Acta Astronaut. 2019, 163, 126–132. [Google Scholar] [CrossRef]
- Anderson, M.; Sargusingh, M. Evolution of environmental control and life support system requirements and assumptions for future exploration missions. In Proceedings of the 47th International Conference on Environmental Systems, Charleston, South Carolina, 16–20 July 2017. [Google Scholar]
- Stapleton, T.J.; Heldmann, M.; Schneider, S.; O’Neill, J.; Samplatsky, D.; White, K.; Corallo, R. Environmental Control and Life Support for Deep Space Travel. In Proceedings of the 46th International Conference on Environmental Systems, Vienna, Austria, 10–14 July 2016. [Google Scholar]
- Sharma, G.; Rai, R.N. Reliability modeling and analysis of environmental control and life support systems of space stations: A literature survey. Acta Astronaut. 2019, 155, 238–246. [Google Scholar] [CrossRef]
- Williams, D.E.; Dake, J.R.; Gentry, G.J. International Space Station Environmental Control and Life Support System Status: 2009–2010. In Proceedings of the 40th International Conference on Environmental Systems, Barcelona, Spain, 11–15 July 2010; pp. 2010–6180. [Google Scholar]
- Malin, J.; Kowing, J.; Schreckenghost, D.; Bonasso, P.; Nieten, J.; Graham, J.; Fleming, L.; MacMahon, M.; Thronesbery, C. Multi-agent diagnosis and control of an air revitalization system for life support in space. In Proceedings of the 2000 IEEE Aerospace Conference. Proceedings (Cat. No.00TH8484), Big Sky, MT, USA, 25 March 2002; Volume 6, pp. 309–326. [Google Scholar]
- Boscheri, G.; Lavagna, M.; Lamantea, M. Multidisciplinary Preliminary Sizing of Advanced Life Support Systems for Space. SAE Tech. Paper Ser. 2009. [Google Scholar] [CrossRef]
- Grigor’Iev, G.; Lagutin, A.S.; Nabiev, S.; Vasiliev, A.; Orlov, O.; Mukhamedieva, L.; Pakhomova, A.; Rodin, A.; Semenov, V.; Stavrovskii, D.; et al. Atmosphere composition control during long-duration space missions. Acta Astronaut. 2019, 163, 112–119. [Google Scholar] [CrossRef]
- Schneider, W.F.; Gatens, R.L.; Anderson, M.S.; Broyan, J.; Macatangay, A.; Shull, S.; Perry, J.; Toomarian, N. NASA environmental control and life support technology development and maturation for exploration. In Proceedings of the 46th International Conference on Environmental Systems, Vienna, Austria, 10–14 July 2016. [Google Scholar]
- Gentry, G.J. International Space Station (ISS) Environmental Control and Life Support (ECLS) System Overview of Events: 2015–2016. In Proceedings of the 46th International Conference on Environmental Systems, Vienna, Austria, 10–14 July 2016. [Google Scholar]
- Guo, R.; Li, Y.; Lv, M. Design of infinite sliding mode state observer with application to CO2 absorption system of space station. Acta Astronaut. 2019, 161, 579–587. [Google Scholar] [CrossRef]
- Brewster, H.S. International Space Station: Its history challenges and successes. In Proceedings of the 40th Aerospace Sciences Meeting & Exhibit, Reno, NV, USA, 6–9 January 2003. [Google Scholar]
- Popov, A.; Fink, W.; Hess, A. “PHM for Astronauts” Project to Run on the International Space Station: The Status and Plan Forward. In Proceedings of the 2019 IEEE Aerospace Conference, Big Sky, MT, USA, 2–9 March 2019; pp. 1–12. [Google Scholar]
- Hu, W.; Wu, Q.E.; Zou, J. Research on Automatic Fault Diagnosis of Environmental Control and Life Support Systems. J. Comput. Theor. Nanosci. 2014, 11, 1394–1402. [Google Scholar] [CrossRef]
- Li, Y.; Lv, M.; Ling, L.; Yang, L.; Li, D.; Dong, S.; Li, Y. Analysis of atmospheric circulation for physicochemical regenerative environment control and life support system in space station. In Proceedings of the 2016 IEEE International Conference on Advanced Intelligent Mechatronics (AIM), Banff, AB, Canada, 12–15 July 2016; pp. 1491–1496. [Google Scholar]
- Lv, M.; Li, Y.; Liu, W.; Guo, R.; Yang, L.; Pang, L.; Li, Y.; Wang, J. Development of simulation platform for physicochemical regenerative environment control and life support system in space station. In Proceedings of the 2016 IEEE 11th Conference on Industrial Electronics and Applications (ICIEA), Hefei, China, 5–7 June 2016; pp. 1145–1150. [Google Scholar]
- Samsonov, N.M.; Kurmazenko, E.A.; Gavrilov, L.I.; Farafonov, N.S.; Pavlova, N.V.; Pavlova, T.N.; Proshkin, V.J.; Romanov, S.J.; Rjabkin, A.M.; Guzenberg, A.S.; et al. Operation Results Onboard the International Space Station and Development Tendency of Atmosphere Revitalization and Monitoring System. SAE Tech. Paper Ser. 2004, 1. [Google Scholar] [CrossRef]
- Kurmazenko, E.A.; Samsonov, N.M.; Gavrilov, L.I.; Farafonov, N.S.; Belavencev, J.E.; Pavlova, N.V.; Proshkin, V.J.; Romanov, S.J.; Geleznyakov, A.G.; Ryabkin, A.M.; et al. Off-normal Situations Related to the Operation of the Electron-VM Oxygen Generation System aboard the International Space Station. In Proceedings of the International Conference on Environmental Systems, Rome, Italy, 11–14 July 2005. [Google Scholar]
- Wen, H. Tiangong-2 Space Laboratory achieved a number of “first” verifications. J. Aerospace. China 2016, 11, 4–7. (In Chinese) [Google Scholar]
- Bernier, M.C.; Alberici, R.M.; Keelor, J.D.; Dwivedi, P.; Zambrzycki, S.C.; Wallace, W.T.; Gazda, D.B.; Limero, T.F.; Symonds, J.M.; Orlando, T.M.; et al. Microplasma Ionization of Volatile Organics for Improving Air/Water Monitoring Systems On-Board the International Space Station. J. Am. Soc. Mass Spectrom. 2016, 27, 1203–1210. [Google Scholar] [CrossRef] [PubMed]
- Stuffler, T.; Hofmann, P.; Honne, A.; Witt, J. ANITA2 flight model development—A status report of the multicomponent ISS Air Analyser. In Proceedings of the 47th International Conference on Environmental Systems, Charleston, South Carolina, 16 July 2017. [Google Scholar]
- Gentry, G.J. International Space Station (ISS) Environmental Control and Life Support (ECLS) System Overview of Events: 2016–2017. In Proceedings of the 47th International Conference on Environmental Systems, Charleston, South Carolina, 16–20 July 2017. [Google Scholar]
- Xie, L.; Zhou, Z.; Zhao, L.; Wan, C.; Tang, H.; Xue, S. Parameter Identification for Structural Health Monitoring with Extended Kalman Filter Considering Integration and Noise Effect. Appl. Sci. 2018, 8, 2480. [Google Scholar] [CrossRef]
- Lv, M.-B.; Li, Y.-H.; Guo, R. Galerkin-based extended Kalman filter with application to CO2 removal system. J. Central South. Univ. 2020, 27, 1780–1789. [Google Scholar] [CrossRef]
- Han, H.; Wei, Y.; Ye, X.; Liu, W. Motion Planning and Coordinated Control of Underwater Vehicle-Manipulator Systems with Inertial Delay Control and Fuzzy Compensator. Appl. Sci. 2020, 10, 3944. [Google Scholar] [CrossRef]
- Yang, J.; Chang, Y.; Gao, T.; Wang, J. Failure Prediction of the Rotating Machinery Based on CEEMDAN-ApEn Feature and AR-UKF Model. Appl. Sci. 2020, 10, 2056. [Google Scholar] [CrossRef]
- Zhou, H.; Zhao, H.; Huang, H.; Zhao, X. A Cubature-Principle-Assisted IMM-Adaptive UKF Algorithm for Maneuvering Target Tracking Caused by Sensor Faults. Appl. Sci. 2017, 7, 1003. [Google Scholar] [CrossRef]
- Yang, L.; Fu, C.Y.; Li, Y.H.; Su, L.M. Survey and study on intelligent monitoring and health management for large civil structure. Int. J. Intell. Robotics Appl. 2019, 3, 239–254. [Google Scholar] [CrossRef]
- Sheng, W.; Liu, K.; Liu, Y.; Meng, X.; Li, Y.H. Optimal Placement and Sizing of Distributed Generation via an Improved Nondominated Sorting Genetic Algorithm II. IEEE Transactions on power delivery. 2015, 30, 569–578. [Google Scholar]
- Li, H.; Li, D.; Li, Y.H. A multi-index assessment method for evaluating coverage effectiveness of remote sensing satellite. Chinese J. Aeronautics. 2018, 31, 2023–2033. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lv, M.; Li, X.; Li, Y.; Zhang, W.; Guo, R. UKF-Based State Estimation for Electrolytic Oxygen Generation System of Space Station. Appl. Sci. 2021, 11, 2021. https://doi.org/10.3390/app11052021
Lv M, Li X, Li Y, Zhang W, Guo R. UKF-Based State Estimation for Electrolytic Oxygen Generation System of Space Station. Applied Sciences. 2021; 11(5):2021. https://doi.org/10.3390/app11052021
Chicago/Turabian StyleLv, Mingbo, Xiaopeng Li, Yunhua Li, Wei Zhang, and Rui Guo. 2021. "UKF-Based State Estimation for Electrolytic Oxygen Generation System of Space Station" Applied Sciences 11, no. 5: 2021. https://doi.org/10.3390/app11052021
APA StyleLv, M., Li, X., Li, Y., Zhang, W., & Guo, R. (2021). UKF-Based State Estimation for Electrolytic Oxygen Generation System of Space Station. Applied Sciences, 11(5), 2021. https://doi.org/10.3390/app11052021






