Evaluation of Keratin/Bacterial Cellulose Based Scaffolds as Potential Burned Wound Dressing
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Bacterial Cellulose (BC) Production
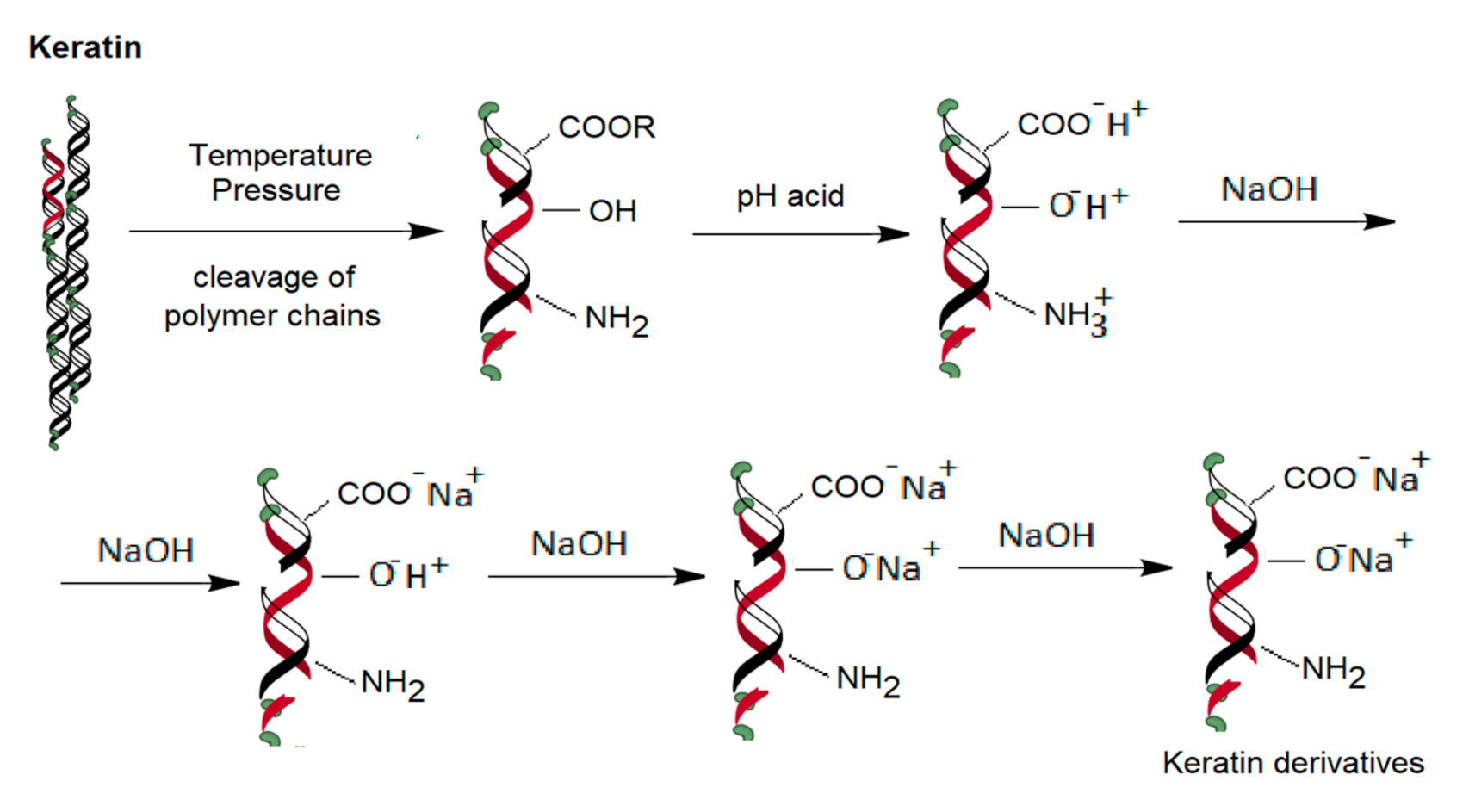
2.3. Obtaining the Keratin Hydrogel
2.4. Obtaining of Keratose-Bacterial Cellulose Scaffold
2.5. Scaffolds Characterization
2.6. Cytocompatibility Tests and Cells Seeding
2.7. In Vivo Tests
2.8. Histopathological Analysis
3. Results and Disscusion
3.1. BC and Keratose Preparation
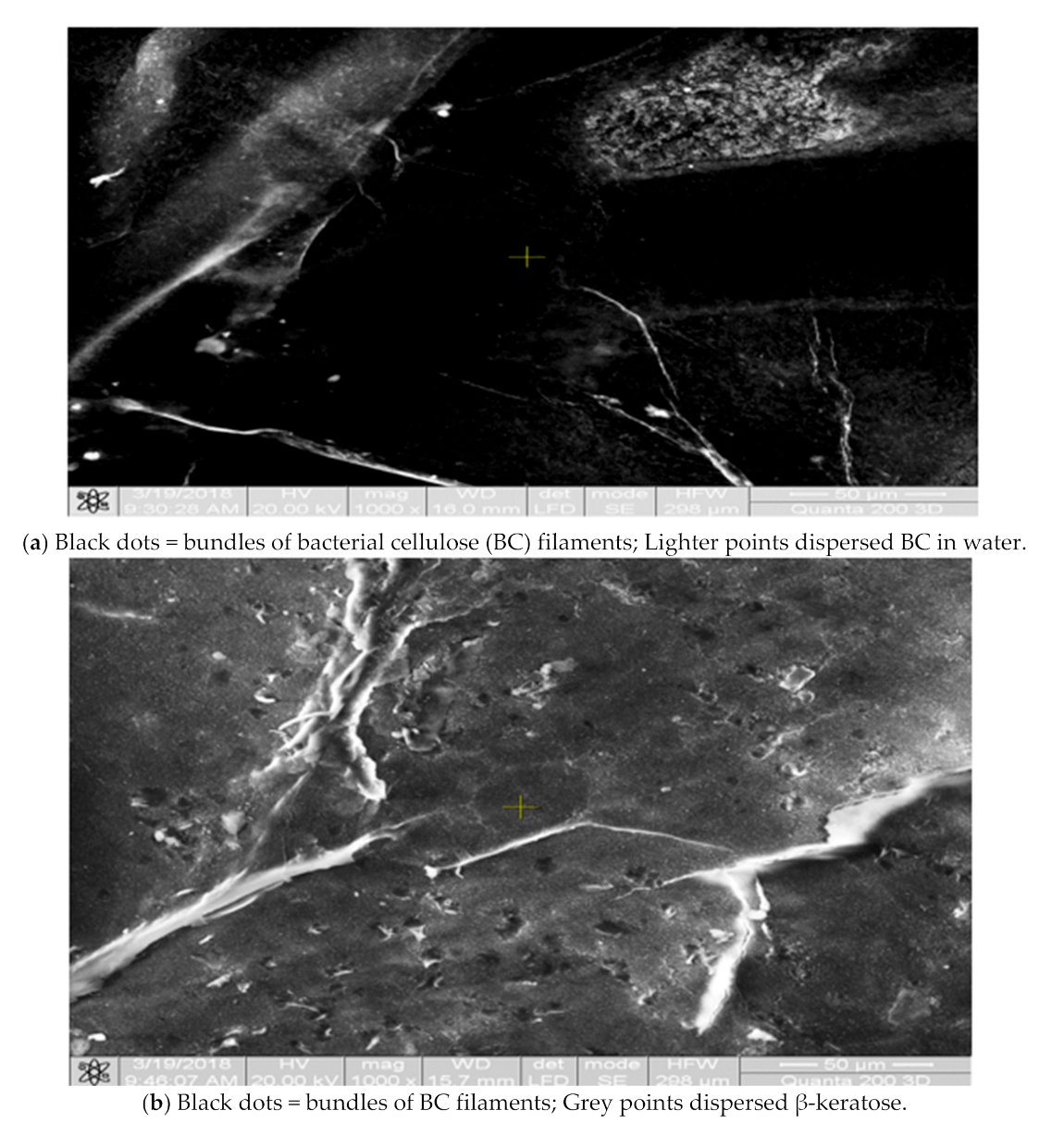
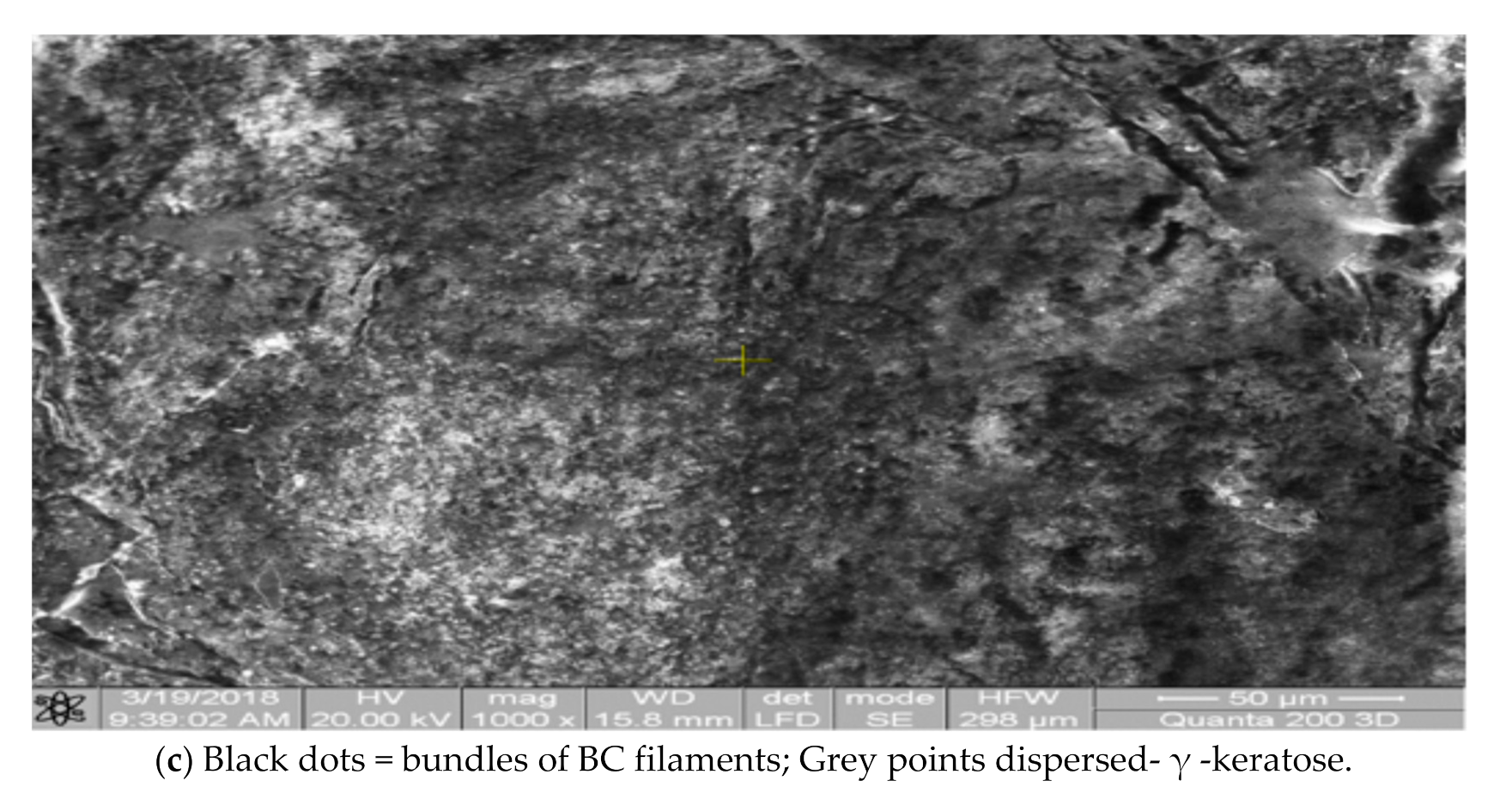
3.2. Scaffolds Morphology
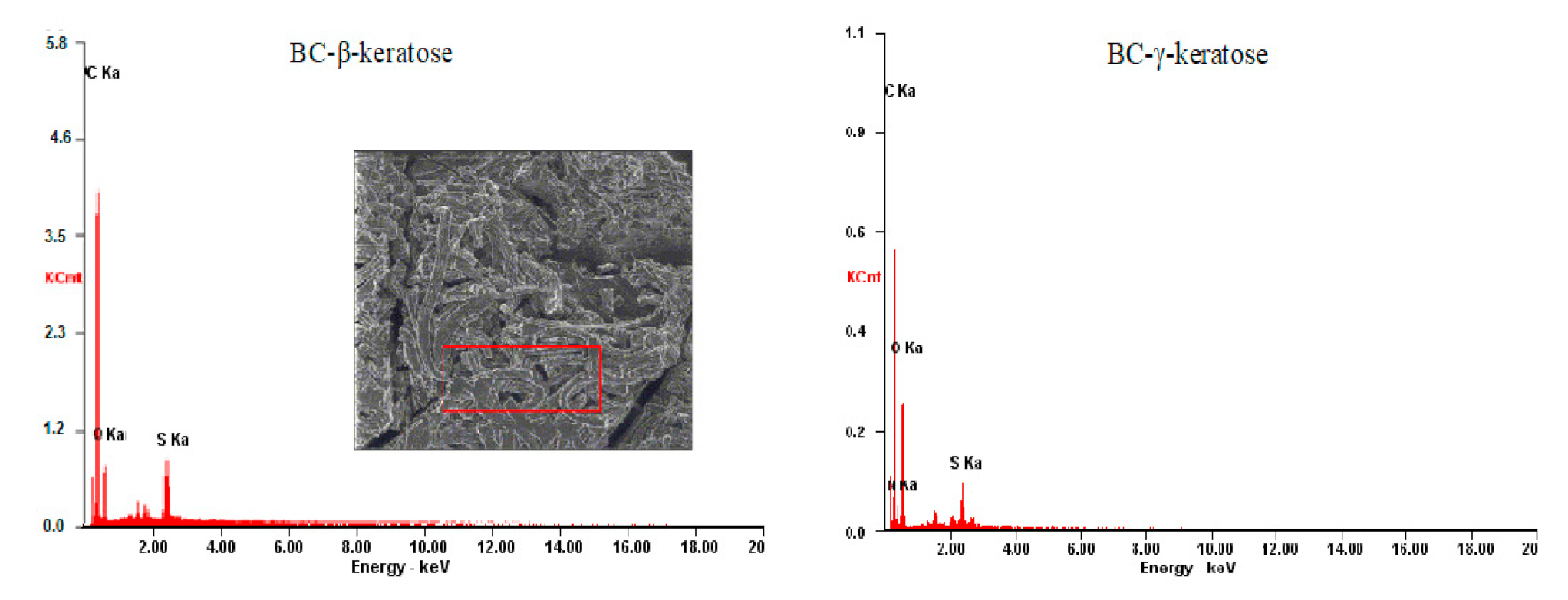
3.3. EDAX Elemental Analysis
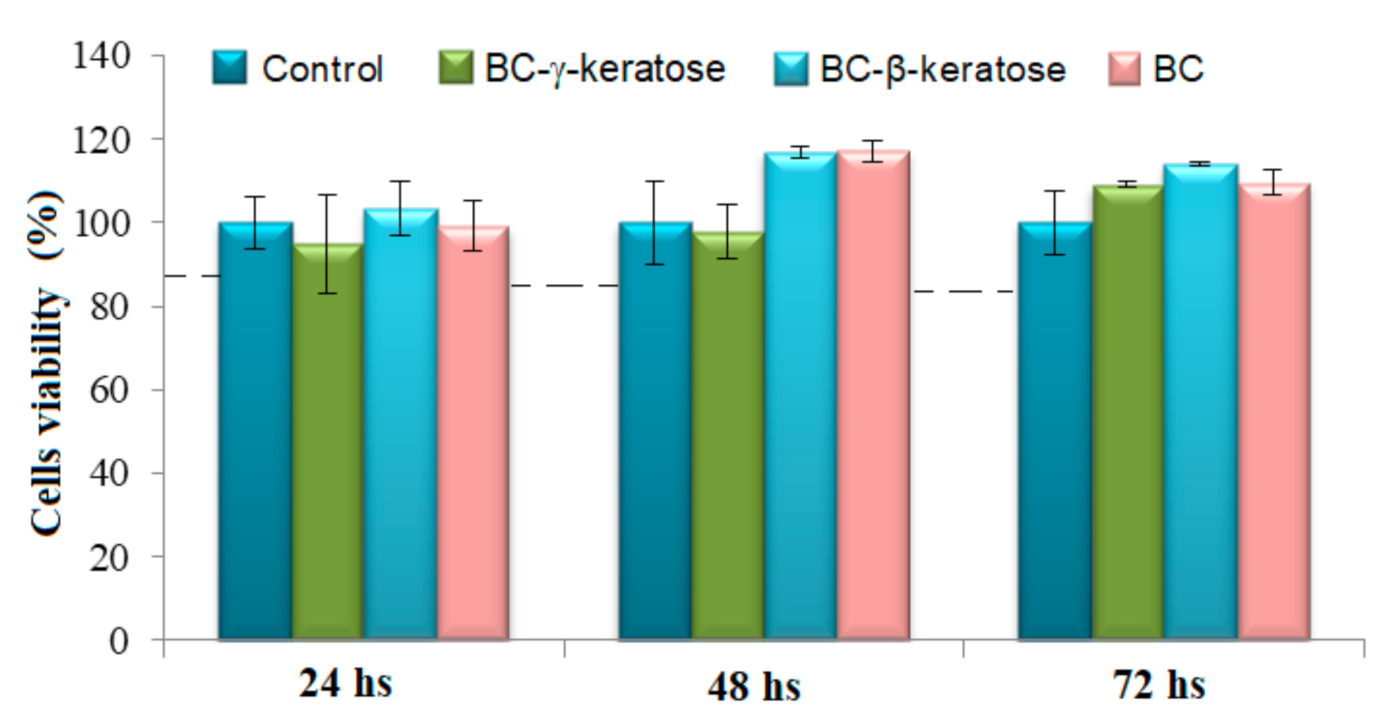
3.4. Cytocompatibility Tests
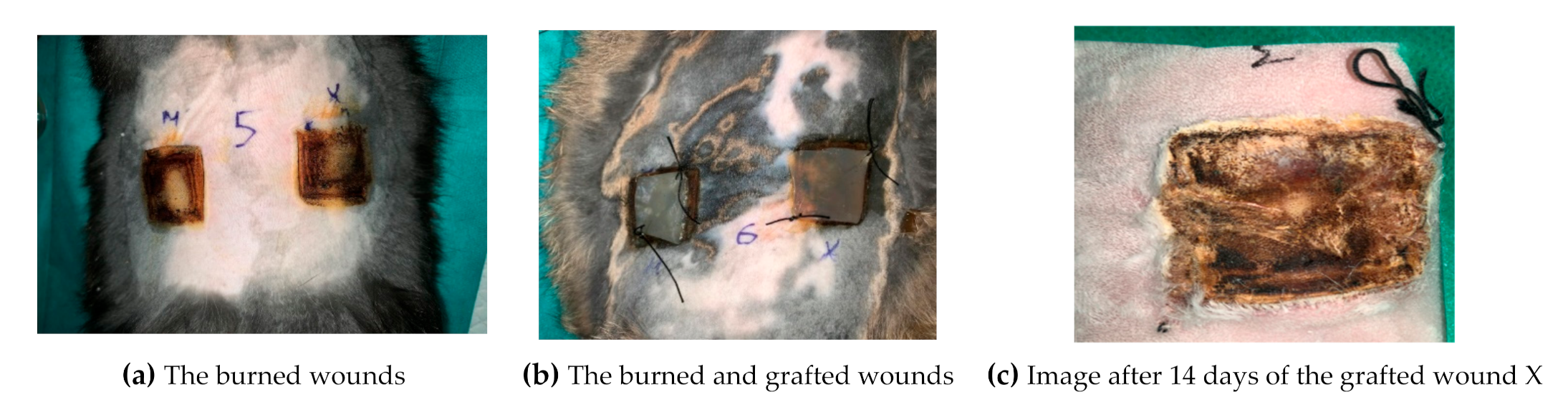
3.5. In Vivo Tests
3.6. Histopathological Results Interpretation
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Arbuthnot, M.K.; Garcia, A.V. Early resuscitation and management of severe pediatric burns. Semin. Pediatr. Surg. 2019, 28, 73–78. [Google Scholar] [CrossRef] [PubMed]
- McLaughlin, E.S.; Paterson, A.O. (Eds). Burns: Prevention, Causes and Treatment; First Chapter; Nova Publishers: Hauppauge, NY, USA, 2012; pp. 31–37. [Google Scholar]
- Clark, A.; Imran, J.; Madni, T.; Wolf, S.E. Nutrition and metabolism in burn patients. Burn. Trauma 2017, 5, 11. [Google Scholar] [CrossRef] [PubMed]
- Barrett, L.W.; Fear, V.S.; Waithman, J.C.; Wood, F.M.; Fear, M.W. Understanding acute burn injury as a chronic disease. Burn. Trauma 2019, 7, 23. [Google Scholar] [CrossRef] [PubMed]
- Singh, B.; Sharma, S.; Dhiman, A. Design of antibiotic containing hydrogel wound dressings: Biomedical properties and histological study of wound healing. Int. J. Pharm. 2013, 457, 82–91. [Google Scholar] [CrossRef]
- Sorg, H.; Tilkorn, D.J.; Hager, S.; Hauser, J.; Mirastschijski, U. Skin Wound Healing: An Update on the Current Knowledge and Concepts. Eur. Surg. Res. 2017, 58, 81–94. [Google Scholar] [CrossRef]
- Augustine, R.; Kalarikkal, N.; Thomas, S. Advancement of wound care from grafts to bioengineered smart skin substitutes. Prog. Biomater. 2014, 3, 103–113. [Google Scholar] [CrossRef]
- Mir, M.; Ali, M.N.; Barakullah, A.; Gulzar, A.; Arshad, M.; Fatima, S.; Asad, M. Synthetic polymeric biomaterials for wound healing: A review. Prog. Biomater. 2018, 7, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Chouhan, D.; Mandal, B.B. Silk biomaterials in wound healing and skin regeneration therapeutics: From bench to bedside. Acta Biomater. 2020, 103, 24–51. [Google Scholar] [CrossRef] [PubMed]
- Murray, R.Z.; West, Z.E.; Cowin, A.J.; Farrugia, B.L. Development and use of biomaterials as wound healing therapies. Burn. Trauma 2019, 7, 2. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Li, F.; Wang, X.; Yu, J.; Wu, D. Hyaluronic Acid and Polyethylene Glycol Hybrid Hydrogel Encapsulating Nanogel with Hemostasis and Sustainable Antibacterial Property for Wound Healing. ACS Appl. Mater. Interfaces 2018, 10, 13304–13316. [Google Scholar] [CrossRef] [PubMed]
- Liyang, S.; Yannan, Z.; Qifan, X.; Caixia, F.; Jöns, H.; Jianwu, D. Moldable hyaluronan hydrogel enabled by dynamic metal–bisphosphonate coordination chemistry for wound healing. Adv. Healthc. Mater. 2018, 7, 1700973. [Google Scholar]
- Neuman, M.G.; Nanau, R.M.; Oruña-Sanchez, L.; Coto, G. Hyaluronic Acid and Wound Healing. J. Pharm. Pharm. Sci. 2015, 18, 53–60. [Google Scholar] [CrossRef] [PubMed]
- Longinotti, C. The use of hyaluronic acid based dressings to treat burns: A review. Burn. Trauma 2014, 2, 162–168. [Google Scholar] [CrossRef] [PubMed]
- Botnariu, G.; Popa, A.; Mitrea, G.; Manole, M.; Pacurar, M.; Anghele, M.; Curis, C.; Teodorescu, E. Correlation of Glycemic and Lipid Control Parameters with Cognitive Dysfunction Scores, in Type 2 Diabetic Persons Results from a cross- sectional study. Rev. Chim. 2019, 69, 3486–3489. [Google Scholar] [CrossRef]
- Botezatu, C.; Duceac, L.D.; Mastalier, B.; Stafie, L.; Jitareanu, C.M.; Luca, A.C.; Tarca, E.; Mitrea, G.; Iordache, A.C.; Patrascu, T. Hepatic Cystic Echinococcosis Studied in A Family Group. IJMD 2018, 22, 346–350. [Google Scholar]
- Ichim, D.L.; Duceac, L.D.; Marcu, C.; Iordache, A.C.; Ciomaga, I.M.; Luca, A.C.; Goroftei, E.R.B.; Mitrea, G.; Damir, D.; Stafie, L. Synthesis and Characterization of Colistin Loaded Nanoparticles Used to Combat Multi-drug Resistant Microorganisms. Rev. Chim. 2019, 70, 3734–3737. [Google Scholar] [CrossRef]
- Luca, A.C.; Eva, L.; Iordache, A.C.; Duceac, L.D.; Mitrea, G.; Marcu, C.; Stafie, L.; Ciuhodaru, M.I.; Ciomaga, I.M.; Goroftei, E.R.B.; et al. Drug Encapsulated Nanomaterials as Carriers Used in Cardiology Field. Rev. Chim. 2020, 71, 413–417. [Google Scholar] [CrossRef]
- Aderibigbe, B.A.; Buyana, B. Alginate in Wound Dressings. Pharmaceutics 2018, 10, 42. [Google Scholar] [CrossRef]
- Wang, T.; Gu, Q.; Zhao, J.; Mei, J.; Shao, M.; Pan, Y.; Zhang, J.; Wu, H.; Zhang, Z.; Liu, F. Calcium alginate enhances wound healing by up-regulating the ratio of collagen types I/III in diabetic rats. Int. J. Clin. Exp. Pathol. 2015, 8, 6636–6645. [Google Scholar]
- Liu, H.; Wang, C.; Li, C.; Qin, Y.; Wang, Z.; Yang, F.; Li, Z.; Wang, J. A functional chitosan-based hydrogel as a wound dressing and drug delivery system in the treatment of wound healing. RSC Adv. 2018, 8, 7533–7549. [Google Scholar] [CrossRef]
- Ahmed, S.; Ikram, S. Chitosan Based Scaffolds and Their Applications in Wound Healing. Achiev. Life Sci. 2016, 10, 27–37. [Google Scholar] [CrossRef]
- Patrulea, V.; Ostafe, V.; Borchard, G.; Jordan, O. Chitosan as a starting material for wound healing applications. Eur. J. Pharm. Biopharm. 2015, 97, 417–426. [Google Scholar] [CrossRef] [PubMed]
- Acevedo, C.A.; Sánchez, E.; Orellana, N.; Morales, P.; Olguín, Y.; Brown, D.I.; Enrione, J. Re-Epithelialization Appraisal of Skin Wound in a Porcine Model Using a Salmon-Gelatin Based Biomaterial as Wound Dressing. Pharmaceutics 2019, 11, 196. [Google Scholar] [CrossRef] [PubMed]
- Jang, H.-J.; Kim, Y.-M.; Yoo, B.-Y.; Seo, Y.-K. Wound-healing effects of human dermal components with gelatin dressing. J. Biomater. Appl. 2018, 32, 716–724. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Liang, Y.; Zhang, D.; Sun, X.; Liang, L.; Li, J.; Liu, Y.N. Gelatin-Based Hydrogels Blended with Gellan as an Inject-able Wound Dressing. ACS Omega 2018, 3, 4766–4775. [Google Scholar] [CrossRef]
- Kiiskinen, J.; Merivaara, A.; Hakkarainen, T.; Kääriäinen, M.; Miettinen, S.; MarjoYliperttula, M.; Koivuniemi, R. Nano-fibrillar cellulose wound dressing supports the growth and characteristics of human mesenchymal stem/stromal cells without cell adhesion coatings. Stem. Cell Res. Ther. 2019, 10, 292. [Google Scholar] [CrossRef] [PubMed]
- Savitskaya, I.S.; Shokatayeva, D.H.; Kistaubayeva, A.S.; Ignatova, L.V.; Digel, I.E. Antimicrobial and wound healing proper-ties of a bacterial cellulose based material containing B. subtilis cells. Helion 2019, 5, e02592. [Google Scholar] [CrossRef] [PubMed]
- Gao, T.; Jiang, M.; Liu, X.; You, G.; Wang, W.; Sun, Z.; Ma, A.; Chen, J. Patterned Polyvinyl Alcohol Hydrogel Dressings with Stem Cells Seeded for Wound Healing. Polymers 2019, 11, 171. [Google Scholar] [CrossRef]
- Hikmawati, D.; Rohmadanik, A.R.; Putra, A.P.; Siswanto, A. The Effect of Aloe vera Extract Variation in Electrospun Polyvinyl Alcohol (PVA)-Aloe vera-Based Nanofiber Membrane. J. Phys. Conf. Ser. 2018, 1120, 012096. [Google Scholar] [CrossRef]
- Champeau, M.; Póvo, V.; Militão, L.; Cabrini, F.M.; Picheth, G.F.; Meneau, F.; Jara, C.P.; de Araujo, E.P.; de Oliveira, M.G. Supramolecular poly(acrylic acid)/F127 hydrogel with hydration-controlled nitric oxide release for enhancing wound heal-ing. Acta Biomater. 2018, 74, 312–325. [Google Scholar] [CrossRef]
- Nguyen, T.T.T.; Ghosh, C.; Hwang, S.-G.; Tran, L.D.; Park, J.S. Characteristics of curcumin-loaded poly (lactic acid) nanofibers for wound healing. J. Mater. Sci. 2013, 48, 7125–7133. [Google Scholar] [CrossRef]
- Foong, C.Y.; Hamzah, M.S.A.; Razak, S.I.A. Influence of Poly(lactic acid) Layer on the Physical and Antibacterial Prop-erties of Dry Bacterial Cellulose Sheet for Potential Acute Wound Healing Materials. Fibers Polym. 2018, 19, 263–271. [Google Scholar] [CrossRef]
- Napavichayanun, S.; Bonani, W.; YYang, Y.; Motta, A.; Aramwit, P. Fibroin and Polyvinyl Alcohol Hydrogel Wound Dress-ing Containing Silk Sericin Prepared Using High-Pressure Carbon Dioxide. Adv. Wound Care 2019, 8, 452–462. [Google Scholar] [CrossRef]
- Lin, S.-P.; Lo, K.-Y.; Tseng, T.-N.; Liu, J.-M.; Shih, T.-Y.; Cheng, K.-C. Evaluation of PVA/dextran/chitosan hydrogel for wound dressing. Cell. Polym. 2019, 38, 15–30. [Google Scholar] [CrossRef]
- Zhu, J.; Marchant, R. Design properties of hydrogel tissue-engineering scaffolds. Expert Rev. Med. Devices 2011, 8, 607–626. [Google Scholar] [CrossRef]
- Perez, R.A.; Kim, M.; Kim, T.H.; Kim, J.H.; Lee, J.H.; Park, J.H.; Knowles, J.C.; Kim, H.W. Utilizing core-shell fibrous colla-gen-alginate hydrogel cell delivery system for bone tissue engineering. Tissue Eng. 2014, 20, 103–114. [Google Scholar] [CrossRef]
- Imparato, G.; Urciuolo, F.; Casale, C.; Netti, P.A. The role of microscaffold properties in controlling the collagen assembly in 3D dermis equivalent using modular tissue engineering. Biomaterials 2013, 34, 7851–7861. [Google Scholar] [CrossRef]
- Jiang, T.; Deng, M.; Fattah, W.I.A.; Laurencin, C.T. Chitosan-Based Biopharmaceutical Scaffolds in Tissue Engineering and Regenerative Medicine. In Chitosan-Based Systems for Biopharmaceuticals; Wiley: Hoboken, NJ, USA, 2012; pp. 393–427. [Google Scholar]
- Kopp, J.; Wang, G.Y.; Horch, R.; Pallua, N.; Ge, S.D. Ancient traditional Chinese medicine in burn treatment: A historical review. Burns 2003, 29, 473–478. [Google Scholar] [CrossRef]
- Kim, S.Y.; Park, B.J.; Lee, Y.; Park, J.N.; Park, K.M.; Hwang, Y.S.; Park, K.D. Human hair keratin-based hydrogels as dynamic matrices for facilitating wound healing. J. Ind. Eng. Chem. 2019, 73, 142–151. [Google Scholar] [CrossRef]
- Lin, C.-W.; Chen, Y.-K.; Tang, K.-C.; Yang, K.-C.; Cheng, N.-C.; Yu, J. Keratin scaffolds with human adipose stem cells: Physical and biological effects toward wound healing. J. Tissue Eng. Regen. Med. 2019, 13, 1044–1058. [Google Scholar] [CrossRef] [PubMed]
- Keskin, Z.; Urkmez, A.S.; Hames, E.E. Novel keratin modified bacterial cellulose nanocomposite production and character-ization for skin tissue engineering. Mater. Sci. Eng. C 2017, 75, 1144–1153. [Google Scholar] [CrossRef]
- Sulaeva, I.; Henniges, U.; Rosenau, T.; Potthast, A. Bacterial cellulose as a material for wound treatment: Properties and modifications. A review. Biotech. Adv. 2015, 333, 1547–1571. [Google Scholar] [CrossRef]
- Siller-Jackson, A.J.; Van Dyke, M.E.; Timmons, S.F.; Blanchard, C.R. Keratin-Based Powders and Hydrogel for Pharmaceuti-cal Applications. U.S. Patent US6544548B1, 8 April 2003. [Google Scholar]
- Blanchard, C.R. Keratin Based Hydrogel for Biomedical Applications. U.S. Patent US5932552, 3 August 1999. [Google Scholar]
- Rajwade, J.M.; Paknikar, K.M.; Kumbhar, J.V. Applications of bacterial cellulose and its composites in biomedicine. Appl. Microbiol. Biotechnol. 2015, 99, 2491–2511. [Google Scholar] [CrossRef]
- Bunnell, B.A.; Flaat, M.; Gagliardi, C.; Patel, B.; Ripoll, C. Adipose-derived stem expansion and differentiation. Methods 2008, 45, 115–120. [Google Scholar] [CrossRef]
- Schramm, M.; Hestrin, S. Factors affecting production of cellulose at the air/liquid interface of a culture of Acetobacter xy-linum. J. Gen. Microbiol. 1954, 11, 123–129. [Google Scholar] [CrossRef]
- Pavlov, K.F.; Romankov, P.G.; Noskov, A.A. Processes and Apparatus in Chemical Engineering, 8th ed.; Editura Tehnica: Bucharest, Romania, 1981; p. 529, Table LVII. (In Romanian) [Google Scholar]
- Zhang, Q.; Liebeck, B.M.; Yan, K.; Demco, D.E.; Körner, A.; Popescu, C. Alpha-Helix Self-Assembly of Oligopeptides Originated From Beta-Sheet Keratin. Macromol. Chem. Phys. 2012, 213, 2628–2638. [Google Scholar] [CrossRef]
- Liebeck, B.M.; Hidalgo, N.; Roth, G.; Popescu, C.; Böker, A. Synthesis and Characterization of Methyl Cellulose/Keratin Hydrolysate Composite Membranes. Polymers 2017, 9, 91. [Google Scholar] [CrossRef] [PubMed]
- Picheth, G.F.; Pirich, C.L.; Sierakowski, M.R.; Woehl, M.A.; Novak, S.C.; Souza, C.F.; Martin, A.A.; da Silva, R.; de Freitas, R.A. Bacterial cellulose in biomedical applications: A review. Int. J. Biol. Macromol. 2017, 104, 97–106. [Google Scholar] [CrossRef]
- Aursulesei, V.; Vasincu, D.; Timofte, D.; Vrajitoriu, L.; Gatu, I.; Iacob, D.D.; Ghizdovat, V.; Buzea, C.; Agop, M. New mecha-nisms of vesicles migration. Gen. Physiol. Biophys. 2016, 35, 287–298. [Google Scholar] [CrossRef]
- Burlacu, A.; Siriopol, D.; Voroneanu, L.; Nistor, I.; Hogas, S.; Nicolae, A.; Nedelciuc, I.; Tinica, G.; Covic, A. Atherosclerotic Renal Artery Stenosis Prevalence and Correlations in Acute Myocardial Infarction Patients Undergoing Primary Percutaneous Coronary Interventions: Data from Nonrandomized Single-Center Study (REN-ACS)—A Single Center, Prospective, Observational Study. J. Am. Hear. Assoc. 2015, 4, e002379. [Google Scholar] [CrossRef]
- Hsieh, W.C.; Chen, P.C.; Corciova, F.C.; Tinica, G. Liver dysfunction as an important predicting risk factor in patients un-dergoing cardiac surgery: A systematic review and meta-analysis. Int. J. Clin. Exp. Med. 2015, 8, 20712–20721. [Google Scholar]
- Trofin, F.; Ciobica, A.; Honceriu, C. Modulatory effects of vitamin C on the relation between physical exercising and oxidative stress at young smokers. Rom. Biotechnol. Lett. 2017, 22, 12439–12447. [Google Scholar]
- Al-Tarrah, K.; Moiemen, N.; Lord, J.M. The influence of sex steroid hormones on the response to trauma and burn injury. Burn. Trauma 2017, 5, 29. [Google Scholar] [CrossRef]
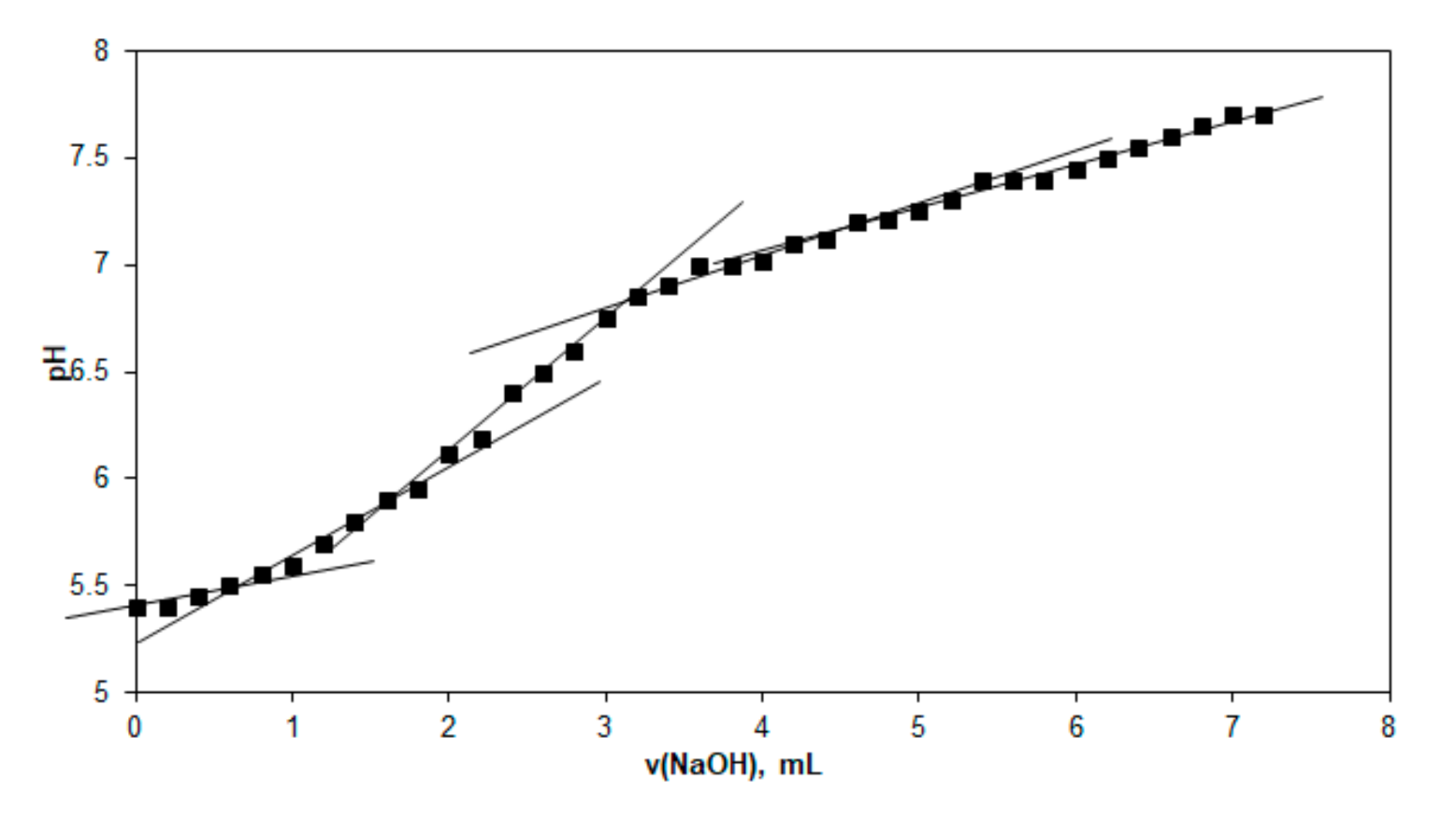



| No. | Domain | Equivalence Volume (mL) | Sample Volume (mL) | Equivalents/Liter |
|---|---|---|---|---|
| 1 | 1 | 0.75 | 10 | 0.0075 |
| 2 | 2 | 1.70 | 10 | 0.0170 |
| 3 | 3 | 3.20 | 10 | 0.0320 |
| 4 | 4 | 4.80 | 10 | 0.0480 |
| No. | BC-β-Keratose | BC-γ-Keratose | ||||
|---|---|---|---|---|---|---|
| Element | Wt % | At % | Element | Wt % | At % | |
| C | 65.01 | 73.29 | C | 50.75 | 58.78 | |
| N | 02.10 | 02.04 | N | 02.98 | 02.96 | |
| O | 25.70 | 21.65 | O | 41.75 | 36.30 | |
| S | 07.19 | 03.02 | S | 04.51 | 01.96 | |
| Matrix | Correction | ZAF | Matrix | Correction | ZAF | |
| Healing Parameters | Control | BC-β-Keratose | BC-β-Keratose-Cells |
|---|---|---|---|
| 7 days post grafting | |||
| Inflammation | ++++ | ++++ | ++++ |
| Dermis regeneration (fibroblasts, connective tissue, vascular neogenesis) | - | - | - |
| Epidermal regeneration | - | - | - |
| 14 days post grafting | |||
| Inflammation | +++ | ++ | + |
| Dermis regeneration (fibroblasts, connective tissue, vascular neogenesis)–connective -vascular bud | + | ++ | +++ |
| Epidermis regeneration | - | - | - |
| 21 days post grafting | |||
| Inflammation | + | - | - |
| Dermis regeneration (fibroblasts, connective tissue, vascular neogenesis) | ++ | +++ | ++++ |
| Epidermis regeneration | + | ++ | +++ |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Radu, C.D.; Verestiuc, L.; Ulea, E.; Lipsa, F.D.; Vulpe, V.; Munteanu, C.; Bulgariu, L.; Pașca, S.; Tamas, C.; Ciuntu, B.M.; et al. Evaluation of Keratin/Bacterial Cellulose Based Scaffolds as Potential Burned Wound Dressing. Appl. Sci. 2021, 11, 1995. https://doi.org/10.3390/app11051995
Radu CD, Verestiuc L, Ulea E, Lipsa FD, Vulpe V, Munteanu C, Bulgariu L, Pașca S, Tamas C, Ciuntu BM, et al. Evaluation of Keratin/Bacterial Cellulose Based Scaffolds as Potential Burned Wound Dressing. Applied Sciences. 2021; 11(5):1995. https://doi.org/10.3390/app11051995
Chicago/Turabian StyleRadu, Cezar Doru, Liliana Verestiuc, Eugen Ulea, Florin Daniel Lipsa, Vasile Vulpe, Corneliu Munteanu, Laura Bulgariu, Sorin Pașca, Camelia Tamas, Bogdan Mihnea Ciuntu, and et al. 2021. "Evaluation of Keratin/Bacterial Cellulose Based Scaffolds as Potential Burned Wound Dressing" Applied Sciences 11, no. 5: 1995. https://doi.org/10.3390/app11051995
APA StyleRadu, C. D., Verestiuc, L., Ulea, E., Lipsa, F. D., Vulpe, V., Munteanu, C., Bulgariu, L., Pașca, S., Tamas, C., Ciuntu, B. M., Ciocan, M., Sîrbu, I., Gavrilas, E., Macarel, C. V., & Istrate, B. (2021). Evaluation of Keratin/Bacterial Cellulose Based Scaffolds as Potential Burned Wound Dressing. Applied Sciences, 11(5), 1995. https://doi.org/10.3390/app11051995











