Applications of Cold Atmospheric Pressure Plasma in Dentistry
Abstract
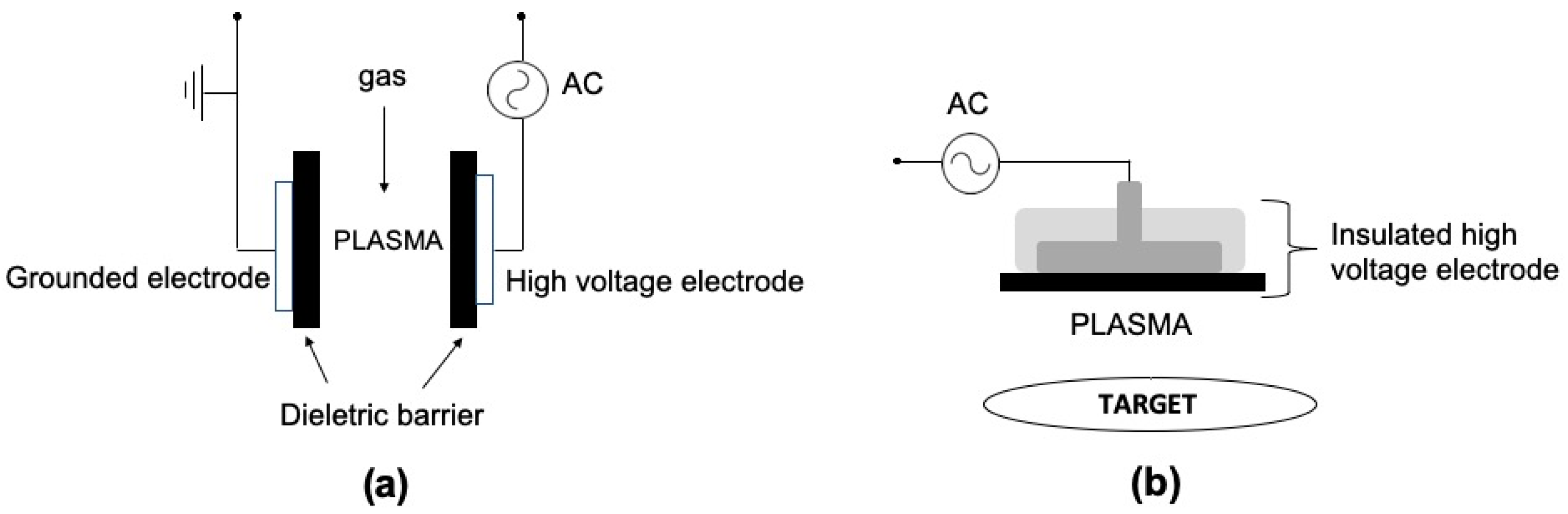
1. Introduction
2. CAPP Biological Activities
3. CAPP Application in Periodontology
4. CAPP Application in Endodontics
5. CAPP Application in Cariology
6. CAPP Application in Oral Oncology
7. CAPP for the Treatment of Oral Candidiasis
8. Conclusions
9. Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- von Keudell, A.; Schulz-von der Gathen, V. Foundations of low-temperature plasma physics—An introduction. Plasma Sources Sci. Technol. 2017, 26, 113001. [Google Scholar] [CrossRef]
- Bruggeman, P.J.; Iza, F.; Brandenburg, R. Foundations of atmospheric pressure non-equilibrium plasmas. Plasma Sources Sci. Technol. 2017, 26, 123002. [Google Scholar] [CrossRef]
- Gibalov, V.I.; Pietsch, G.J. The development of dielectric barrier discharges in gas gaps and on surfaces. J. Phys. D Appl. Phys. 2000, 33, 2618–2636. [Google Scholar] [CrossRef]
- Chirokov, A.; Gutsol, A.; Fridman, A. Atmospheric pressure plasma of dielectric barrier discharges. Pure Appl. Chem. 2005, 77, 487–495. [Google Scholar] [CrossRef]
- Hoffmann, C.; Berganza, C.; Zhang, J. Cold Atmospheric Plasma: Methods of production and application in dentistry and oncology. Medical Gas Res. 2013, 3, 21. [Google Scholar] [CrossRef]
- Winter, J.; Brandenburg, R.; Weltmann, K.D. Atmospheric pressure plasma jets: An overview of devices and new directions. Plasma Sources Sci. Technol. 2015, 24, 064001. [Google Scholar] [CrossRef]
- Lu, X.; Reuter, S.; Laroussi, M.; Liu, D. Nonequlibrium Atmospheric Pressure Plasma Jets. Fundamentals, Diagnostics and Medical Applications; CRC Press, Taylor & Francis Group: Boca Raton, FL, USA, 2019. [Google Scholar]
- Fanelli, F.; Fracassi, F. Atmospheric pressure non-equilibrium plasma jet technology: General features, specificities and applications in surface processing of materials. Surf. Coat. Technol. 2017, 322, 174–201. [Google Scholar] [CrossRef]
- Laroussi, M. Cold Plasma in Medicine and Healthcare: The New Frontier in Low Temperature Plasma Applications. Front. Phys. 2020, 8. [Google Scholar] [CrossRef]
- Kong, M.G.; Kroesen, G.; Morfill, G.; Nosenko, T.; Shimizu, T.; van Dijk, J.; Zimmermann, J.L. Plasma medicine: An introductory review. New J. Physi. 2009, 11, 115012. [Google Scholar] [CrossRef]
- Bourke, P.; Ziuzina, D.; Han, L.; Cullen, P.J.; Gilmore, B.F. Microbiological interactions with cold plasma. J. Appl. Microbiol. 2017, 123, 308–324. [Google Scholar] [CrossRef] [PubMed]
- Neyts, E.C.; Brault, P. Molecular Dynamics Simulations for Plasma-Surface Interactions. Plasma Process. Polym. 2017, 14, 1600145. [Google Scholar] [CrossRef]
- Nicol, M.J.; Brubaker, T.R.; Honish, B.J.; Simmons, A.N.; Kazemi, A.; Geissel, M.A.; Whalen, C.T.; Siedlecki, C.A.; Bilén, S.G.; Knecht, S.D.; et al. Antibacterial effects of low-temperature plasma generated by atmospheric-pressure plasma jet are mediated by reactive oxygen species. Sci. Rep. 2020, 10, 3066. [Google Scholar] [CrossRef]
- Kaushik, N.K.; Ghimire, B.; Li, Y.; Adhikari, M.; Veerana, M.; Kaushik, N.; Jha, N.; Adhikari, B.; Lee, S.J.; Masur, K.; et al. Biological and medical applications of plasma-activated media, water and solutions. Biol. Chem. 2018, 400, 39–62. [Google Scholar] [CrossRef] [PubMed]
- Bradu, C.; Kutasi, K.; Magureanu, M.; Puač, N.; Živković, S. Reactive nitrogen species in plasma-activated water: Generation, chemistry and application in agriculture. J. Phys. D Appl. Phys. 2020, 53, 223001. [Google Scholar] [CrossRef]
- Tanaka, H.; Nakamura, K.; Mizuno, M.; Ishikawa, K.; Takeda, K.; Kajiyama, H.; Utsumi, F.; Kikkawa, F.; Hori, M. Non-thermal atmospheric pressure plasma activates lactate in Ringer’s solution for anti-tumor effects. Sci. Rep. 2016, 6, 36282. [Google Scholar] [CrossRef]
- Laroussi, M. Sterilization of contaminated matter with an atmospheric pressure plasma. IEEE Trans. Plasma Sci. 1996, 24, 1188–1191. [Google Scholar] [CrossRef]
- Laroussi, M.; Mendis, D.A.; Rosenberg, M. Plasma interaction with microbes. New J. Phys. 2003, 5, 41. [Google Scholar] [CrossRef]
- Kostov, K.G.; Rocha, V.; Koga-Ito, C.Y.; Matos, B.M.; Algatti, M.A.; Honda, R.Y.; Kayama, M.E.; Mota, R.P. Bacterial sterilization by a dielectric barrier discharge (DBD) in air. Surf. Coat. Technol. 2010, 204, 2954–2959. [Google Scholar] [CrossRef]
- Klämpfl, T.G.; Isbary, G.; Shimizu, T.; Li, Y.F.; Zimmermann, J.L.; Stolz, W.; Schlegel, J.; Morfill, G.E.; Schmidt, H.U. Cold atmospheric air plasma sterilization against spores and other microorganisms of clinical interest. Appl. Environ. Microbiol. 2012, 78, 5077–5082. [Google Scholar] [CrossRef] [PubMed]
- Nishime, T.M.C.; Borges, A.C.; Koga-Ito, C.Y.; Machida, M.; Hein, L.R.O.; Kostov, K.G. Non-thermal atmospheric pressure plasma jet applied to inactivation of different microorganisms. Surf. Coat. Technol. 2017, 312, 19–24. [Google Scholar] [CrossRef]
- Liao, X.; Muhammad, A.I.; Chen, S.; Hu, Y.; Ye, X.; Liu, D.; Ding, T. Bacterial spore inactivation induced by cold plasma. Crit. Rev. Food Sci. Nutr. 2019, 59, 2562–2572. [Google Scholar] [CrossRef]
- Borges, A.C.; Nishime, T.M.C.; de Moura Rovetta, S.; Lima, G.M.G.; Kostov, K.G.; Thim, G.P.; de Menezes, B.R.C.; Machado, J.P.B.; Koga-Ito, C.Y. Cold Atmospheric Pressure Plasma Jet Reduces Trichophyton rubrum Adherence and Infection Capacity. Mycopathologia 2019, 184, 585–595. [Google Scholar] [CrossRef] [PubMed]
- Borges, A.C.; Castaldelli Nishime, T.M.; Kostov, K.G.; de Morais Gouvêa Lima, G.; Lacerda Gontijo, A.V.; de Carvalho, J.N.M.M.; Yzumi Honda, R.; Yumi Koga-Ito, C. Cold atmospheric pressure plasma jet modulates Candida albicans virulence traits. Clin. Plasma Med. 2017, 7, 9–15. [Google Scholar] [CrossRef]
- Rao, Y.; Shang, W.; Yang, Y.; Zhou, R.; Rao, X. Fighting Mixed-Species Microbial Biofilms With Cold Atmospheric Plasma. Front. Microbiol. 2020, 11, 1000. [Google Scholar] [CrossRef]
- Jiang, C.; Schaudinn, C.; Jaramillo, D.E.; Webster, P.; Costerton, J.W. In Vitro Antimicrobial Effect of a Cold Plasma Jet against Enterococcus faecalis Biofilms. ISRN Dent. 2012, 2012, 295736. [Google Scholar] [CrossRef] [PubMed]
- Idlibi, A.N.; Al-Marrawi, F.; Hannig, M.; Lehmann, A.; Rueppell, A.; Schindler, A.; Jentsch, H.; Rupf, S. Destruction of oral biofilms formed in situ on machined titanium (Ti) surfaces by cold atmospheric plasma. Biofouling 2013, 29, 369–379. [Google Scholar] [CrossRef]
- Delben, J.A.; Zago, C.E.; Tyhovych, N.; Duarte, S.; Vergani, C.E. Effect of Atmospheric-Pressure Cold Plasma on Pathogenic Oral Biofilms and In Vitro Reconstituted Oral Epithelium. PLoS ONE 2016, 11, e0155427. [Google Scholar] [CrossRef] [PubMed]
- Rutkowski, R.; Schuster, M.; Unger, J.; Seebauer, C.; Metelmann, H.R.; Woedtke, T.V.; Weltmann, K.D.; Daeschlein, G. Hyperspectral imaging for in vivo monitoring of cold atmospheric plasma effects on microcirculation in treatment of head and neck cancer and wound healing. Clin. Plasma Med. 2017, 7–8, 52–57. [Google Scholar] [CrossRef]
- Shome, D.; von Woedtke, T.; Riedel, K.; Masur, K. The HIPPO Transducer YAP and Its Targets CTGF and Cyr61 Drive a Paracrine Signalling in Cold Atmospheric Plasma-Mediated Wound Healing. Oxid. Med. Cell Longev. 2020, 2020, 4910280. [Google Scholar] [CrossRef] [PubMed]
- Arndt, S.; Unger, P.; Wacker, E.; Shimizu, T.; Heinlin, J.; Li, Y.F.; Thomas, H.M.; Morfill, G.E.; Zimmermann, J.L.; Bosserhoff, A.K.; et al. Cold atmospheric plasma (CAP) changes gene expression of key molecules of the wound healing machinery and improves wound healing in vitro and in vivo. PLoS ONE 2013, 8, e79325. [Google Scholar] [CrossRef]
- Xu, D.; Wang, S.; Li, B.; Qi, M.; Feng, R.; Li, Q.; Zhang, H.; Chen, H.; Kong, M.G. Effects of Plasma-Activated Water on Skin Wound Healing in Mice. Microorganisms 2020, 8, 1091. [Google Scholar] [CrossRef] [PubMed]
- Brun, P.; Pathak, S.; Castagliuolo, I.; Palù, G.; Zuin, M.; Cavazzana, R.; Martines, E. Helium generated cold plasma finely regulates activation of human fibroblast-like primary cells. PLoS ONE 2014, 9, e104397. [Google Scholar] [CrossRef]
- Bourdens, M.; Jeanson, Y.; Taurand, M.; Juin, N.; Carrière, A.; Clément, F.; Casteilla, L.; Bulteau, A.-L.; Planat-Bénard, V. Short exposure to cold atmospheric plasma induces senescence in human skin fibroblasts and adipose mesenchymal stromal cells. Sci. Rep. 2019, 9, 8671. [Google Scholar] [CrossRef] [PubMed]
- Haralambiev, L.; Bandyophadyay, A.; Suchy, B.; Weiss, M.; Kramer, A.; Bekeschus, S.; Ekkernkamp, A.; Mustea, A.; Kaderali, L.; Stope, M.B. Determination of Immediate. Anticancer Res. 2020, 40, 3743–3749. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, A.; Dietrich, S.; Steuer, A.; Weltmann, K.D.; von Woedtke, T.; Masur, K.; Wende, K. Non-thermal plasma activates human keratinocytes by stimulation of antioxidant and phase II pathways. J. Biol. Chem. 2015, 290, 6731–6750. [Google Scholar] [CrossRef] [PubMed]
- Kisch, T.; Helmke, A.; Schleusser, S.; Song, J.; Liodaki, E.; Stang, F.H.; Mailaender, P.; Kraemer, R. Improvement of cutaneous microcirculation by cold atmospheric plasma (CAP): Results of a controlled, prospective cohort study. Microvasc. Res. 2016, 104, 55–62. [Google Scholar] [CrossRef]
- Busco, G.; Robert, E.; Chettouh-Hammas, N.; Pouvesle, J.M.; Grillon, C. The emerging potential of cold atmospheric plasma in skin biology. Free Radic. Biol. Med. 2020, 161, 290–304. [Google Scholar] [CrossRef] [PubMed]
- Dobrynin, D.; Fridman, G.; Friedman, G.; Fridman, A. Physical and biological mechanisms of direct plasma interaction with living tissue. New J. Phys. 2009, 11, 115020. [Google Scholar] [CrossRef]
- Weltmann, K.D.; von Woedtke, T. Plasma medicine—Current state of research and medical application. Plasma Phys. Control. Fusion 2016, 59, 014031. [Google Scholar] [CrossRef]
- Kumar, N.; Attri, P.; Yadav, D.K.; Choi, J.; Choi, E.H.; Uhm, H.S. Induced apoptosis in melanocytes cancer cell and oxidation in biomolecules through deuterium oxide generated from atmospheric pressure non-thermal plasma jet. Sci. Rep. 2014, 4, 7589. [Google Scholar] [CrossRef]
- Xu, D.; Liu, D.; Wang, B.; Chen, C.; Chen, Z.; Li, D.; Yang, Y.; Chen, H.; Kong, M.G. In Situ OH Generation from O2- and H2O2 Plays a Critical Role in Plasma-Induced Cell Death. PLoS ONE 2015, 10, e0128205. [Google Scholar] [CrossRef]
- Lin, A.; Truong, B.; Patel, S.; Kaushik, N.; Choi, E.H.; Fridman, G.; Fridman, A.; Miller, V. Nanosecond-Pulsed DBD Plasma-Generated Reactive Oxygen Species Trigger Immunogenic Cell Death in A549 Lung Carcinoma Cells through Intracellular Oxidative Stress. Int. J. Mol. Sci. 2017, 18, 966. [Google Scholar] [CrossRef] [PubMed]
- Lunov, O.; Zablotskii, V.; Churpita, O.; Lunova, M.; Jirsa, M.; Dejneka, A.; Kubinová, Š. Chemically different non-thermal plasmas target distinct cell death pathways. Sci. Rep. 2017, 7, 600. [Google Scholar] [CrossRef] [PubMed]
- Alkawareek, M.Y.; Algwari, Q.T.; Gorman, S.P.; Graham, W.G.; O’Connell, D.; Gilmore, B.F. Application of atmospheric pressure nonthermal plasma for the in vitro eradication of bacterial biofilms. FEMS Immunol. Med. Microbiol. 2012, 65, 381–384. [Google Scholar] [CrossRef] [PubMed]
- Puligundla, P.; Mok, C. Potential applications of nonthermal plasmas against biofilm-associated micro-organisms in vitro. J. Appl. Microbiol. 2017, 122, 1134–1148. [Google Scholar] [CrossRef]
- Borges, A.C.; Lima, G.M.G.; Nishime, T.M.C.; Gontijo, A.V.L.; Kostov, K.G.; Koga-Ito, C.Y. Amplitude-modulated cold atmospheric pressure plasma jet for treatment of oral candidiasis: In vivo study. PLoS ONE 2018, 13, e0199832. [Google Scholar] [CrossRef] [PubMed]
- Boehm, D.; Bourke, P. Safety implications of plasma-induced effects in living cells—A review of in vitro and in vivo findings. Biol. Chem. 2018, 400, 3–17. [Google Scholar] [CrossRef]
- Han, X.; Kapaldo, J.; Liu, Y.; Stack, M.S.; Alizadeh, E.; Ptasinska, S. Large-Scale Image Analysis for Investigating Spatio-Temporal Changes in Nuclear DNA Damage Caused by Nitrogen Atmospheric Pressure Plasma Jets. Int. J. Mol. Sci. 2020, 21, 4127. [Google Scholar] [CrossRef] [PubMed]
- Van der Paal, J.; Hong, S.-H.; Yusupov, M.; Gaur, N.; Oh, J.-S.; Short, R.D.; Szili, E.J.; Bogaerts, A. How membrane lipids influence plasma delivery of reactive oxygen species into cells and subsequent DNA damage: An experimental and computational study. Phys. Chem. Chem. Phys. 2019, 21, 19327–19341. [Google Scholar] [CrossRef]
- Bengtson, C.; Bogaerts, A. On the Anti-Cancer Effect of Cold Atmospheric Plasma and the Possible Role of Catalase-Dependent Apoptotic Pathways. Cells 2020, 9, 2330. [Google Scholar] [CrossRef]
- VON Woedtke, T.; Schmidt, A.; Bekeschus, S.; Wende, K.; Weltmann, K.D. Plasma Medicine: A Field of Applied Redox Biology. In Vivo 2019, 33, 1011–1026. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.; Xiong, Z.; Mao, X.; Meng, D.; Lei, Q.; Li, Y.; Deng, P.; Chen, M.; Tu, M.; Lu, X.; et al. Atmospheric pressure room temperature plasma jets facilitate oxidative and nitrative stress and lead to endoplasmic reticulum stress dependent apoptosis in HepG2 cells. PLoS ONE 2013, 8, e73665. [Google Scholar] [CrossRef]
- Zhao, J.; Nie, L. Five gaseous reactive oxygen and nitrogen species (RONS) density generated by microwave plasma jet. Phys. Plasmas 2019, 26, 073503. [Google Scholar] [CrossRef]
- Kim, S.J.; Chung, T.H. Cold atmospheric plasma jet-generated RONS and their selective effects on normal and carcinoma cells. Sci. Rep. 2016, 6, 20332. [Google Scholar] [CrossRef]
- Kurita, H.; Haruta, N.; Uchihashi, Y.; Seto, T.; Takashima, K. Strand breaks and chemical modification of intracellular DNA induced by cold atmospheric pressure plasma irradiation. PLoS ONE 2020, 15, e0232724. [Google Scholar] [CrossRef] [PubMed]
- Bartosz, G. Reactive oxygen species: Destroyers or messengers? Biochem. Pharmacol. 2009, 77, 1303–1315. [Google Scholar] [CrossRef]
- Brieger, K.; Schiavone, S.; Miller, F.J.; Krause, K.H. Reactive oxygen species: From health to disease. Swiss Med. Wkly 2012, 142, w13659. [Google Scholar] [CrossRef] [PubMed]
- Imlay, J.A. Pathways of oxidative damage. Annu. Rev. Microbiol. 2003, 57, 395–418. [Google Scholar] [CrossRef]
- Finkel, T.; Holbrook, N.J. Oxidants, oxidative stress and the biology of ageing. Nature 2000, 408, 239–247. [Google Scholar] [CrossRef]
- Bogdan, C. Nitric oxide and the immune response. Nat. Immunol. 2001, 2, 907–916. [Google Scholar] [CrossRef] [PubMed]
- Pacher, P.; Beckman, J.S.; Liaudet, L. Nitric oxide and peroxynitrite in health and disease. Physiol. Rev. 2007, 87, 315–424. [Google Scholar] [CrossRef]
- Mai-Prochnow, A.; Clauson, M.; Hong, J.; Murphy, A.B. Gram positive and Gram negative bacteria differ in their sensitivity to cold plasma. Sci. Rep. 2016, 6, 38610. [Google Scholar] [CrossRef]
- Laroussi, M.; Leipold, F. Evaluation of the roles of reactive species, heat, and UV radiation in the inactivation of bacterial cells by air plasmas at atmospheric pressure. Int. J. Mass Spectrom. 2004, 233, 81–86. [Google Scholar] [CrossRef]
- Laroussi, M. Plasma Medicine: A Brief Introduction. Plasma 2018, 1, 5. [Google Scholar] [CrossRef]
- Brelles-Mariño, G. Challenges in biofilm inactivation: The use of cold plasma as a new approach. J. Bioprocess. Biotech. 2012, 2, 4. [Google Scholar] [CrossRef]
- Bostanci, N.; Bao, K.; Wahlander, A.; Grossmann, J.; Thurnheer, T.; Belibasakis, G.N. Secretome of gingival epithelium in response to subgingival biofilms. Mol. Oral Microbiol. 2015, 30, 323–335. [Google Scholar] [CrossRef] [PubMed]
- Mahasneh, A.; Darby, M.; Tolle, S.L.; Hynes, W.; Laroussi, M.; Karakas, E. Inactivation of Porphyromonas gingivalis by Low-Temperature Atmospheric Pressure Plasma. Plasma Medicine 2011, 1, 191–204. [Google Scholar] [CrossRef]
- Liu, D.; Xiong, Z.; Du, T.; Zhou, X.; Cao, Y.; Lu, X. Bacterial-killing effect of atmospheric pressure non-equilibrium plasma jet and oral mucosa response. J. Huazhong Univ. Sci. Technol. 2011, 31, 852–856. [Google Scholar] [CrossRef]
- Küçük, D.; Savran, L.; Ercan, U.K.; Yarali, Z.B.; Karaman, O.; Kantarci, A.; Sağlam, M.; Köseoğlu, S. Evaluation of efficacy of non-thermal atmospheric pressure plasma in treatment of periodontitis: A randomized controlled clinical trial. Clin. Oral Investig. 2020, 24, 3133–3145. [Google Scholar] [CrossRef]
- Kwon, J.S.; Kim, Y.H.; Choi, E.H.; Kim, C.K.; Kim, K.N.; Kim, K.M. Non-thermal atmospheric pressure plasma increased mRNA expression of growth factors in human gingival fibroblasts. Clin. Oral Investig. 2016, 20, 1801–1808. [Google Scholar] [CrossRef] [PubMed]
- Eggers, B.; Marciniak, J.; Memmert, S.; Kramer, F.J.; Deschner, J.; Nokhbehsaim, M. The beneficial effect of cold atmospheric plasma on parameters of molecules and cell function involved in wound healing in human osteoblast-like cells in vitro. Odontology 2020, 108, 607–616. [Google Scholar] [CrossRef] [PubMed]
- Shi, Q.; Song, K.; Zhou, X.; Xiong, Z.; Du, T.; Lu, X.; Cao, Y. Effects of non-equilibrium plasma in the treatment of ligature-induced peri-implantitis. J. Clin. Periodontol. 2015, 42, 478–487. [Google Scholar] [CrossRef] [PubMed]
- Carreiro, A.F.P.; Delben, J.A.; Guedes, S.; Silveira, E.J.D.; Janal, M.N.; Vergani, C.E.; Pushalkar, S.; Duarte, S. Low-temperature plasma on peri-implant-related biofilm and gingival tissue. J. Periodontol. 2019, 90, 507–515. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.Y.; Kim, K.H.; Park, S.Y.; Yoon, S.Y.; Kim, G.H.; Lee, Y.M.; Rhyu, I.C.; Seol, Y.J. The bactericidal effect of an atmospheric-pressure plasma jet on. J. Periodontal. Implant. Sci. 2019, 49, 319–329. [Google Scholar] [CrossRef] [PubMed]
- Arndt, S.; Landthaler, M.; Zimmermann, J.L.; Unger, P.; Wacker, E.; Shimizu, T.; Li, Y.F.; Morfill, G.E.; Bosserhoff, A.K.; Karrer, S. Effects of cold atmospheric plasma (CAP) on ß-defensins, inflammatory cytokines, and apoptosis-related molecules in keratinocytes in vitro and in vivo. PLoS ONE 2015, 10, e0120041. [Google Scholar] [CrossRef]
- Wang, P.; Duan, D.; Zhou, X.; Li, X.; Yang, J.; Deng, M.; Xu, Y. Relationship between expression of human gingival beta-defensins and levels of periodontopathogens in subgingival plaque. J. Periodontal. Res. 2015, 50, 113–122. [Google Scholar] [CrossRef] [PubMed]
- Brun, P.; Bernabè, G.; Marchiori, C.; Scarpa, M.; Zuin, M.; Cavazzana, R.; Zaniol, B.; Martines, E. Antibacterial efficacy and mechanisms of action of low power atmospheric pressure cold plasma: Membrane permeability, biofilm penetration and antimicrobial sensitization. J. Appl. Microbiol. 2018, 125, 398–408. [Google Scholar] [CrossRef]
- Jhajharia, K. Microbiology of endodontic diseases: A review article. Int. J. Appl. Dent. Sci. 2019, 5, 4. [Google Scholar]
- Neelakantan, P.; Romero, M.; Vera, J.; Daood, U.; Khan, A.U.; Yan, A.; Cheung, G.S.P. Biofilms in Endodontics-Current Status and Future Directions. Int. J. Mol. Sci. 2017, 18, 1748. [Google Scholar] [CrossRef] [PubMed]
- Prada, I.; Micó-Muñoz, P.; Giner-Lluesma, T.; Micó-Martínez, P.; Collado-Castellano, N.; Manzano-Saiz, A. Influence of microbiology on endodontic failure. Literature review. Med. Oral Patol. Oral Cir. Bucal. 2019, 24, e364–e372. [Google Scholar] [CrossRef] [PubMed]
- Borzini, L.; Condò, R.; De Dominicis, P.; Casaglia, A.; Cerroni, L. Root Canal Irrigation: Chemical Agents and Plant Extracts Against. Open Dent. J. 2016, 10, 692–703. [Google Scholar] [CrossRef]
- Chang, Y.T.; Chen, G. Oral bacterial inactivation using a novel low-temperature atmospheric-pressure plasma device. J. Dent. Sci. 2016, 11, 65–71. [Google Scholar] [CrossRef]
- Armand, A.; Khani, M.; Asnaashari, M.; AliAhmadi, A.; Shokri, B. Comparison study of root canal disinfection by cold plasma jet and photodynamic therapy. Photodiagnosis Photodyn. Ther. 2019, 26, 327–333. [Google Scholar] [CrossRef]
- Li, Y.; Sun, K.; Ye, G.; Liang, Y.; Pan, H.; Wang, G.; Zhao, Y.; Pan, J.; Zhang, J.; Fang, J. Evaluation of Cold Plasma Treatment and Safety in Disinfecting 3-week Root Canal Enterococcus faecalis Biofilm In Vitro. J. Endod. 2015, 41, 1325–1330. [Google Scholar] [CrossRef]
- Zhou, X.C.; Li, Y.L.; Liu, D.X.; Cao, Y.G.; Lu, X.P. Bactericidal effect of plasma jet with helium flowing through 3% hydrogen peroxide against. Exp. Ther. Med. 2016, 12, 3073–3077. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, K.; Ohshima, T.; Kitano, K.; Ikawa, S.; Yamazaki, H.; Maeda, N.; Hosoya, N. The efficacy of plasma-treated water as a root canal irrigant. Asian Pac. J. Dent. 2017, 17, 8. [Google Scholar]
- Ali, A.; Kim, Y.H.; Lee, J.Y.; Lee, S.; Uhm, H.S.; Cho, G.; Park, B.J.; Choi, E.H. Inactivation of Propionibacterium acnes and its biofilm by non-thermal plasma. Curr. Appl. Phys. 2014, 14, S142–S148. [Google Scholar] [CrossRef]
- Mackenzie, L.; Banerjee, A. Minimally invasive direct restorations: A practical guide. Br. Dent. J. 2017, 223, 163–171. [Google Scholar] [CrossRef] [PubMed]
- Frencken, J.E. Atraumatic restorative treatment and minimal intervention dentistry. Br. Dent. J. 2017, 223, 183–189. [Google Scholar] [CrossRef]
- Arrow, P. Restorative Outcomes of a Minimally Invasive Restorative Approach Based on Atraumatic Restorative Treatment to Manage Early Childhood Caries: A Randomised Controlled Trial. Caries Res. 2016, 50, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Duangthip, D.; Chen, K.J.; Gao, S.S.; Lo, E.C.M.; Chu, C.H. Managing Early Childhood Caries with Atraumatic Restorative Treatment and Topical Silver and Fluoride Agents. Int. J. Environ. Res. Public Health 2017, 14, 1204. [Google Scholar] [CrossRef]
- Frencken, J.E.; Leal, S.C.; Navarro, M.F. Twenty-five-year atraumatic restorative treatment (ART) approach: A comprehensive overview. Clin. Oral Investig. 2012, 16, 1337–1346. [Google Scholar] [CrossRef]
- Cruz Gonzalez, A.C.; Marín Zuluaga, D.J. Clinical outcome of root caries restorations using ART and rotary techniques in institutionalized elders. Braz. Oral Res. 2016, 30. [Google Scholar] [CrossRef] [PubMed]
- Mohan, P.V.; Uloopi, K.S.; Vinay, C.; Rao, R.C. In vivo comparison of cavity disinfection efficacy with APF gel, Propolis, Diode Laser, and 2% chlorhexidine in primary teeth. Contemp. Clin. Dent. 2016, 7, 45–50. [Google Scholar] [CrossRef]
- Ranjan, R.; Krishnamraju, P.V.; Shankar, T.; Gowd, S. Nonthermal Plasma in Dentistry: An Update. J. Int. Soc. Prev. Community Dent. 2017, 7, 71–75. [Google Scholar] [CrossRef]
- Yang, B.; Chen, J.; Yu, Q.; Li, H.; Lin, M.; Mustapha, A.; Hong, L.; Wang, Y. Oral bacterial deactivation using a low-temperature atmospheric argon plasma brush. J. Dent. 2011, 39, 48–56. [Google Scholar] [CrossRef] [PubMed]
- Sladek, R.E.J.; Stoffels, E.; Walraven, R.; Tielbeek, P.J.A.; Koolhoven, R.A. Plasma treatment of dental cavities: A feasibility study. IEEE Trans. Plasma Sci. 2004, 32, 1540–1543. [Google Scholar] [CrossRef]
- Hirano, Y.; Hayashi, M.; Tamura, M.; Yoshino, F.; Yoshida, A.; Masubuchi, M.; Imai, K.; Ogiso, B. Singlet oxygen generated by a new nonthermal atmospheric pressure air plasma device exerts a bactericidal effect on oral pathogens. J. Oral Sci. 2019, 61, 521–525. [Google Scholar] [CrossRef] [PubMed]
- Park, S.R.; Lee, H.W.; Hong, J.W.; Lee, H.J.; Kim, J.Y.; Choi, B.B.; Kim, G.C.; Jeon, Y.C. Enhancement of the killing effect of low-temperature plasma on Streptococcus mutans by combined treatment with gold nanoparticles. J. Nanobiotechnol. 2014, 12, 29. [Google Scholar] [CrossRef]
- Blumhagen, A.; Singh, P.; Mustapha, A.; Chen, M.; Wang, Y.; Yu, Q. Plasma deactivation of oral bacteria seeded on hydroxyapatite disks as tooth enamel analogue. Am. J. Dent. 2014, 27, 84–90. [Google Scholar] [PubMed]
- Figueira, L.W.; Panariello, B.H.D.; Koga-Ito, C.Y.; Duarte, S. Low-Temperature Plasma as an Approach for Inhibiting a Multi-Species Cariogenic Biofilm. Appl. Sci. 2021, 11, 570. [Google Scholar] [CrossRef]
- Zhang, Y.; Yu, Q.; Wang, Y. Non-thermal atmospheric plasmas in dental restoration: Improved resin adhesive penetration. J. Dent. 2014, 42, 1033–1042. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Zhang, Y.; Yao, X.; Li, H.; Yu, Q.; Wang, Y. Effect of a non-thermal, atmospheric-pressure, plasma brush on conversion of model self-etch adhesive formulations compared to conventional photo-polymerization. Dent. Mater. 2012, 28, 1232–1239. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kim, J.H.; Han, G.J.; Kim, C.K.; Oh, K.H.; Chung, S.N.; Chun, B.H.; Cho, B.H. Promotion of adhesive penetration and resin bond strength to dentin using non-thermal atmospheric pressure plasma. Eur. J. Oral Sci. 2016, 124, 89–95. [Google Scholar] [CrossRef]
- Sarode, G.; Maniyar, N.; Sarode, S.C.; Jafer, M.; Patil, S.; Awan, K.H. Epidemiologic aspects of oral cancer. Dis. Mon. 2020, 66, 100988. [Google Scholar] [CrossRef]
- Pilleron, S.; Soto-Perez-de-Celis, E.; Vignat, J.; Ferlay, J.; Soerjomataram, I.; Bray, F.; Sarfati, D. Estimated global cancer incidence in the oldest adults in 2018 and projections to 2050. Int. J. Cancer 2021, 148, 601–608. [Google Scholar] [CrossRef] [PubMed]
- Speight, P.M.; Farthing, P.M. The pathology of oral cancer. Br. Dent. J. 2018, 225, 841–847. [Google Scholar] [CrossRef] [PubMed]
- Muzaffar, J.; Bari, S.; Kirtane, K.; Chung, C.H. Recent Advances and Future Directions in Clinical Management of Head and Neck Squamous Cell Carcinoma. Cancers 2021, 13, 338. [Google Scholar] [CrossRef]
- Iqbal, M.S.; West, N.; Richmond, N.; Kovarik, J.; Gray, I.; Willis, N.; Morgan, D.; Yazici, G.; Cengiz, M.; Paleri, V.; et al. A systematic review and practical considerations of stereotactic body radiotherapy in the treatment of head and neck cancer. Br. J. Radiol. 2021, 94, 20200332. [Google Scholar] [CrossRef]
- Hossen, S.; Hossain, M.K.; Basher, M.K.; Mia, M.N.H.; Rahman, M.T.; Uddin, M.J. Smart nanocarrier-based drug delivery systems for cancer therapy and toxicity studies: A review. J. Adv. Res. 2019, 15, 1–18. [Google Scholar] [CrossRef]
- Lorscheider, M.; Gaudin, A.; Nakhlé, J.; Veiman, K.L.; Richard, J.; Chassaing, C. Challenges and opportunities in the delivery of cancer therapeutics: Update on recent progress. Ther. Deliv. 2021, 12, 55–76. [Google Scholar] [CrossRef]
- Liu, C.; Han, C.; Liu, J. The Role of Toll-Like Receptors in Oncotherapy. Oncol. Res. 2019, 27, 965–978. [Google Scholar] [CrossRef] [PubMed]
- Wijetunga, N.A.; Yu, Y.; Morris, L.G.; Lee, N.; Riaz, N. The head and neck cancer genome in the era of immunotherapy. Oral Oncol. 2021, 112, 105040. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, B.G.; Vit, F.F.; Carvalho, H.F.; Han, S.W.; de la Torre, L.G. Recent advances in co-delivery nanosystems for synergistic action in cancer treatment. J. Mater. Chem. B 2021, 9, 1208–1237. [Google Scholar] [CrossRef] [PubMed]
- DiStefano, M.J.; Kang, S.-Y.; Yehia, F.; Morales, C.; Anderson, G.F. Assessing the Added Therapeutic Benefit of Ultra-Expensive Drugs. Value Health 2021. [Google Scholar] [CrossRef]
- Vrdoljak, E.; Sekerija, M.; Plestina, S.; Belac Lovasic, I.; Katalinic Jankovic, V.; Garattini, L.; Bobinac, A.; Voncina, L. Is it too expensive to fight cancer? Analysis of incremental costs and benefits of the Croatian National Plan Against Cancer. Eur. J. Health Econ. 2021, 1–11. [Google Scholar] [CrossRef]
- Tringale, K.R.; Gennarelli, R.L.; Gillespie, E.F.; Mitchell, A.P.; Zelefsky, M.J. Association between Site-of-Care and the Cost and Modality of Radiotherapy for Prostate Cancer: Analysis of Medicare Beneficiaries from 2015 to 2017. Cancer Investig. 2021, 1–9. [Google Scholar] [CrossRef]
- Bauer, G.; Sersenová, D.; Graves, D.B.; Machala, Z. Cold Atmospheric Plasma and Plasma-Activated Medium Trigger RONS-Based Tumor Cell Apoptosis. Sci. Rep. 2019, 9, 14210. [Google Scholar] [CrossRef]
- Semmler, M.L.; Bekeschus, S.; Schäfer, M.; Bernhardt, T.; Fischer, T.; Witzke, K.; Seebauer, C.; Rebl, H.; Grambow, E.; Vollmar, B.; et al. Molecular Mechanisms of the Efficacy of Cold Atmospheric Pressure Plasma (CAP) in Cancer Treatment. Cancers 2020, 12, 269. [Google Scholar] [CrossRef]
- Malyavko, A.; Yan, D.; Wang, Q.; Klein, A.L.; Patel, K.C.; Sherman, J.H.; Keidar, M. Cold atmospheric plasma cancer treatment, direct versus indirect approaches. Mater. Adv. 2020, 1, 12. [Google Scholar] [CrossRef]
- Graves, D.B. Reactive Species from Cold Atmospheric Plasma: Implications for Cancer Therapy. Plasma Process. Polym. 2014, 11, 1120–1127. [Google Scholar] [CrossRef]
- Han, X.; Klas, M.; Liu, Y.; Sharon Stack, M.; Ptasinska, S. DNA damage in oral cancer cells induced by nitrogen atmospheric pressure plasma jets. Appl. Phys. Lett. 2013, 102, 5. [Google Scholar] [CrossRef]
- Guerrero-Preston, R.; Ogawa, T.; Uemura, M.; Shumulinsky, G.; Valle, B.L.; Pirini, F.; Ravi, R.; Sidransky, D.; Keidar, M.; Trink, B. Cold atmospheric plasma treatment selectively targets head and neck squamous cell carcinoma cells. Int. J. Mol. Med. 2014, 34, 941–946. [Google Scholar] [CrossRef] [PubMed]
- Seebauer, C.; Hasse, S.; Segebarth, M.; Bekeschus, S.; von Woedtke, T.; Weltmann, K.-D.; Schuster, M.; Rutkowski, R.; Metelmann, H.-R. Cold Atmospheric Plasma for the treatment of Oral Lichen Planus as intraoral precancerous lesion. Clin. Plasma Med. 2018, 9, 44–45. [Google Scholar] [CrossRef]
- Pereira, S.; Pinto, E.; Ribeiro, P.A.; Sério, S. Study of a Cold Atmospheric Pressure Plasma jet device for indirect treatment of Squamous Cell Carcinoma. Clin. Plasma Med. 2019, 13, 9–14. [Google Scholar] [CrossRef]
- Metelmann, H.-R.; Nedrelow, D.S.; Seebauer, C.; Schuster, M.; von Woedtke, T.; Weltmann, K.-D.; Kindler, S.; Metelmann, P.H.; Finkelstein, S.E.; Von Hoff, D.D.; et al. Head and neck cancer treatment and physical plasma. Clin. Plasma Med. 2015, 3, 17–23. [Google Scholar] [CrossRef]
- Metelmann, H.-R.; Seebauer, C.; Miller, V.; Fridman, A.; Bauer, G.; Graves, D.B.; Pouvesle, J.-M.; Rutkowski, R.; Schuster, M.; Bekeschus, S.; et al. Clinical experience with cold plasma in the treatment of locally advanced head and neck cancer. Clin. Plasma Med. 2018, 9, 6–13. [Google Scholar] [CrossRef]
- Lombardi, A.; Ouanounou, A. Fungal infections in dentistry: Clinical presentations, diagnosis, and treatment alternatives. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2020, 130, 533–546. [Google Scholar] [CrossRef]
- Baumgardner, D.J. Oral Fungal Microbiota: To Thrush and Beyond. J. Patient Cent. Res. Rev. 2019, 6, 252–261. [Google Scholar] [CrossRef] [PubMed]
- StatPearls. Esophageal Candidiasis; NBK537268; StatPearls: Treasure Island, FL, USA, 2020. [Google Scholar]
- Bhattacharya, S.; Sae-Tia, S.; Fries, B.C. Candidiasis and Mechanisms of Antifungal Resistance. Antibiotics 2020, 9, 312. [Google Scholar] [CrossRef]
- Ahmad, A.; Molepo, J.; Patel, M. Challenges in the Development of Antifungal Agents Against Candida: Scope of Phytochemical Research. Curr. Pharm. Des. 2016, 22, 4135–4150. [Google Scholar] [CrossRef]
- Caldara, M.; Marmiroli, N. Known Antimicrobials Versus Nortriptyline in. Microorganisms 2020, 8, 742. [Google Scholar] [CrossRef] [PubMed]
- Yamazaki, H.; Ohshima, T.; Tsubota, Y.; Yamaguchi, H.; Jayawardena, J.A.; Nishimura, Y. Microbicidal activities of low frequency atmospheric pressure plasma jets on oral pathogens. Dent. Mater. J. 2011, 30, 384–391. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Yu, S.; Sun, P.; Wu, H.; Zhu, W.; Liu, W.; Zhang, J.; Fang, J.; Li, R. Inactivation of Candida biofilms by non-thermal plasma and its enhancement for fungistatic effect of antifungal drugs. PLoS ONE 2012, 7, e40629. [Google Scholar] [CrossRef] [PubMed]
- Kostov, K.G.; Borges, A.C.; Koga-Ito, C.Y.; Nishime, T.M.C.; Prysiazhnyi, V.; Honda, R.Y. Inactivation of Candida albicans by Cold Atmospheric Pressure Plasma Jet. IEEE Trans. Plasma Sci. 2015, 43, 770–775. [Google Scholar] [CrossRef]
- Rahimi-Verki, N.; Shapoorzadeh, A.; Razzaghi-Abyaneh, M.; Atyabi, S.M.; Shams-Ghahfarokhi, M.; Jahanshiri, Z.; Gholami-Shabani, M. Cold atmospheric plasma inhibits the growth of Candida albicans by affecting ergosterol biosynthesis and suppresses the fungal virulence factors in vitro. Photodiagnosis Photodyn. Ther. 2016, 13, 66–72. [Google Scholar] [CrossRef] [PubMed]
- Doria, A.C.O.C.; Sorge, C.D.P.C.; Santos, T.B.; Brandão, J.; Gonçalves, P.A.R.; Maciel, H.S.; Khouri, S.; Pessoa, R.S. Application of post-discharge region of atmospheric pressure argon and air plasma jet in the contamination control of Candida albicans biofilms. Res. Biomed. Eng. 2015, 31, 358–362. [Google Scholar] [CrossRef]
- Braný, D.; Dvorská, D.; Halašová, E.; Škovierová, H. Cold Atmospheric Plasma: A Powerful Tool for Modern Medicine. Int. J. Mol. Sci. 2020, 21, 2932. [Google Scholar] [CrossRef] [PubMed]
- Handorf, O.; Schnabel, U.; Bösel, A.; Weihe, T.; Bekeschus, S.; Graf, A.C.; Riedel, K.; Ehlbeck, J. Antimicrobial effects of microwave-induced plasma torch (MiniMIP) treatment on Candida albicans biofilms. Microb. Biotechnol. 2019, 12, 1034–1048. [Google Scholar] [CrossRef]
- Singh, S.; Halder, A.; Mohid, S.A.; Bagchi, D.; Sinha, O.; Banerjee, A.; Sarkar, P.K.; Bhunia, A.; Ghosh, S.K.; Mitra, A.; et al. Nonthermal Atmospheric Plasma-Induced Cellular Envelope Damage of Staphylococcus aureus and Candida albicans Biofilms: Spectroscopic and Biochemical Investigations. IEEE Trans. Plasma Sci. 2020, 48, 2768–2776. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Borges, A.C.; Kostov, K.G.; Pessoa, R.S.; de Abreu, G.M.A.; Lima, G.d.M.G.; Figueira, L.W.; Koga-Ito, C.Y. Applications of Cold Atmospheric Pressure Plasma in Dentistry. Appl. Sci. 2021, 11, 1975. https://doi.org/10.3390/app11051975
Borges AC, Kostov KG, Pessoa RS, de Abreu GMA, Lima GdMG, Figueira LW, Koga-Ito CY. Applications of Cold Atmospheric Pressure Plasma in Dentistry. Applied Sciences. 2021; 11(5):1975. https://doi.org/10.3390/app11051975
Chicago/Turabian StyleBorges, Aline C., Konstantin G. Kostov, Rodrigo S. Pessoa, Geraldo M.A. de Abreu, Gabriela de M.G. Lima, Leandro W. Figueira, and Cristiane Y. Koga-Ito. 2021. "Applications of Cold Atmospheric Pressure Plasma in Dentistry" Applied Sciences 11, no. 5: 1975. https://doi.org/10.3390/app11051975
APA StyleBorges, A. C., Kostov, K. G., Pessoa, R. S., de Abreu, G. M. A., Lima, G. d. M. G., Figueira, L. W., & Koga-Ito, C. Y. (2021). Applications of Cold Atmospheric Pressure Plasma in Dentistry. Applied Sciences, 11(5), 1975. https://doi.org/10.3390/app11051975






