1. Introduction
Pectus excavatum (PE) is the most common congenital deformity of the anterior thoracic wall, with an estimated occurrence of 1 in 300–1000 live births (M:F = 3–5:1) [
1]. It can lead to a variety of clinical conditions, ranging from patients with cosmetic defects, which may cause psychological distress, to those with severe cardiopulmonary impairment, with exercise intolerance and dyspnea [
2]. In most patients, the chest deformity is already visible during the first year of life and typically progresses with pubertal growth [
3]. While in severe cases, surgical procedures like the Nuss minimally-invasive procedure (MIRPE) or other repairing techniques [
4] can be performed, in many cases, the degree of pectus deformity does not immediately warrant surgery. In 2005, E. Klobe, an engineer who had PE himself, described his first experience with Vacuum Bell (VB) therapy [
5], a conservative treatment that was first used more than 100 years ago [
6]. The Vacuum Bell is similar to a suction cup controlled by the patient with a hand pump, which creates a vacuum region in the affected area. The application, repeated over time, leads to a reduction of the malformation, and can allow the patient to avoid surgical correction. Several studies, in fact, reported an increasing number of patients treated with VB therapy [
7], and in 2011, the first systematic retrospective study summarized the results for 133 patients [
8]. Recent reports confirmed the efficacy of VB therapy in selected patients [
9,
10,
11,
12,
13], and larger studies were reported that considered both adult and pediatric patients [
14,
15].
Nevertheless, a study in a large cohort of pediatric patients treated with VB is still missing. Moreover, all the evaluation methods described in literature assess the progress of the malformation in a qualitative way by using, for instance, patient chest photos, satisfaction scoring scales, and manual/visual evaluation of the deepest point of the deformity [
8,
9,
16]. These aspects constitute an important limitation in literature studies about the conservative therapy of chest wall deformities.
In fact, the qualitative measurement of the conservative treatment for pectus excavatum by means of the Vacuum Bell implies several problems that entail the reliability of the measurement itself, as well as the monitoring and setting of the VB treatment (e.g., time of the treatment and pressure intensity). On the other hand, it is deemed necessary to draw attention to this device, which shows the possibility to effectively treating several cases of PE without resorting to invasive treatments; from this perspective, it was considered appropriate to develop tools for a quantitative and reliable evaluation of the progress of the pathology in order to assess VB effectiveness and to better adjust the treatment to each patient.
In this regard, in recent years the attention of the scientific community has shifted to measurement methods based on optical scanning rather than the more common CT measurements that are more invasive to the patient. Indeed, several state-of-the-art studies propose quantitative measurements based on more- or less-expensive optical scanners [
17,
18,
19] to assess chestwall deformities in order to facilitate treatment decision-making and post-treatment follow-up.
It is our opinion, however, that the realization of a device that is easily manageable in a pediatric outpatient clinic has not yet been achieved. In current literature, in fact, professional scanners, which require an important and cumbersome setup that not all hospital structures can provide, are used. By way of example, the recent and comprehensive study in [
20] proposed a scanning system requiring several seconds to acquire the chest anatomy, thus resulting in limited utility in a pediatric environment. Furthermore, such a system requires the input of manually inserted landmarks for extraction of severity indices and pathology monitoring.
With the aim of creating an easy-to-use instrument capable of quantitatively measuring chest deformation, in a previous work, we developed an optical acquisition system along with semiautomatic processing software capable of providing a rapid quantitative measurement of the chests of pectus patients [
21]. Such a novel 3D body scanner eliminated the need for any manual setup and reduced the acquisition time to a fraction of a second, allowing us to easily analyze pre- and post-treatment severity of the chest wall deformity. Furthermore, through several studies, external measurement parameters were defined to best describe the condition of each patient and to allow the monitoring of the progression of the pathology over time. For the definition of such chest indices, the state of the art was carefully evaluated with regard to both optical indices and measurements commonly extracted from tomographic images [
21,
22]. Despite the system devised in [
20] proving to be effective in providing an objective assessment of the PE severity, it had some drawbacks. In particular, the existing system is based on a device that is currently out of production, requires a structure that is still considered bulky for pediatric use (although it is much slimmer than others in the literature), and can be managed by the physician only with the support of expert engineers.
With the purpose of overcoming the above-mentioned issues, the present work proposes a highly portable low-cost device that can be used in common clinical practice and that simplifies the interaction of the physician with the instrument as much as possible. This allows independence in the use of these innovative tools, which often need the supervision of experts. It is believed that an instrument with these characteristics, combined with corrective treatment with VB, can be used to monitor the course of the pathology and to define personalized treatments for the patient, thus achieving greater effectiveness of the corrective device. Moreover, in the systemupgrade process, a possible use within a telemedicine framework can also be considered. In this regard, although an optical sensor makes it possible to evaluate the patient’s situation in a short time, today it is still necessary for the patient to attend a clinic to scan the three-dimensional geometry and to be examined. This is therefore a situation in which, even for a brief examination, the patient is required to physically travel to the outpatient clinic to get information on the course of their pathology and assess any worsening of their condition or benefits of the treatment. It is hence impossible to perform any routine check-ups if the patient is unable to reach the outpatient clinic. This concern is even more severe in these days due to the COVID-19 pandemic, which has created further limitations to the mobility of patients to be monitored.
These issues can be solved by creating an easy-to-use, handheld and reliable system that can be managed by doctors within the outpatient clinic but also directly by the patient during self-use. This would allow the creation of telemedicine and telemonitoring practices to manage, as mentioned above, the treatment adjustment to the current state of the patient and to different response times.
The new system proposed in this work can be considered a novel handheld version of the body scanner proposed in [
21], redesigned to facilitate data collection and analysis, to provide nonexpert users with state-of-the-art 3D modeling systems. In detail, the hardware component has been redesigned and revalorized to allow for smoother use in the outpatient clinic environment, and the software component has been updated to be more user-oriented and evaluated in terms of usability for both medical staff and domestic use. This new scanner, named Thor 2.0, can allow a remote diagnostic approach, offering the patient the possibility to communicate with experienced physicians and perform routine check-ups telematically. This is considered to be a first step toward the implementation of a telemedicine framework.
In the next section, the hardware and software aspects of the new scanner are presented, and the chosen usability tests are detailed. In
Section 3, the results of the clinical usage of the device and the results of the usability tests are discussed. Finally, in
Section 4, we present our conclusions.
2. Materials and Methods
2.1. System Architecture
In order to develop an accurate and handheld chest scanner, a remodeling process of the body scanner presented by the authors in [
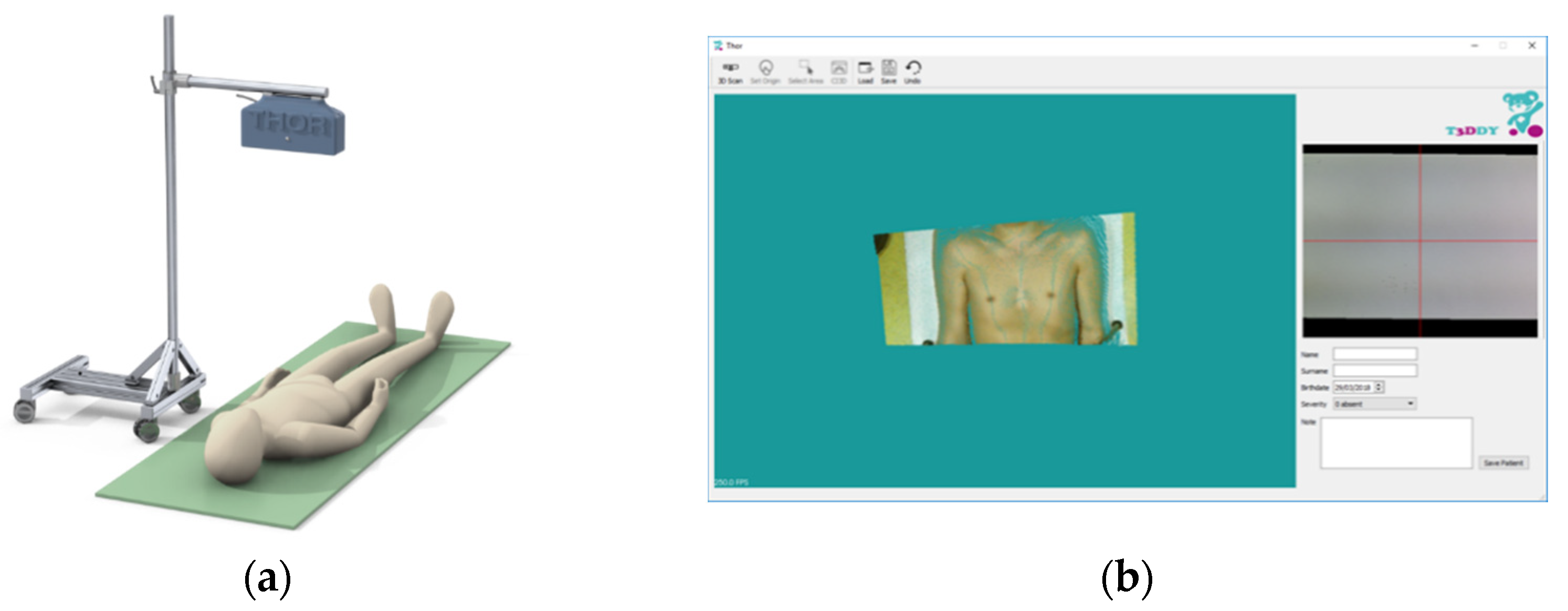
21] was carried out by T3Ddy lab., a joint research center between Meyer’s Children Hospital (Italy) and the Department of Industrial Engineering of Florence (Italy). The previous scanner (
Figure 1) consists of an RGB-D device coupled with control software. The device, mounted on a free-standing support, was maintained perpendicular to a semirigid mattress on which the patient was lying. The use of a single device made it possible to overcome some limits imposed by state-of-the-art scanning systems that require long acquisition times, including the maintenance of a steady position for an unsuitable amount of time, as well as procedures to set up the scanning environment.
As already stated, this scanner was revised to obtain a better portability, thus allowing a simpler use within the outpatient clinic environment and envisioning a telemedicine framework. Moreover, the graphical user interface (GUI) was redesigned to allow the clinicians to carry out the monitoring of the PE in a simple way (i.e., without the assistance of an engineer). Finally, a simplified version of the software, which allows only a few tasks of the monitoring procedure, was devised for self-use by the patient.
From the hardware point of view (thus to improve the portability), a new optical device from the Intel RealSense family of depth sensors was selected—the D415 model (Intel Corporation, Santa Clara, CA, USA). RealSense technology basically consists of vision processors, depth and tracking modules, and depth cameras, supported by an open-source multiplatform software development kit (SDK) called librealsense [
23].
The Intel RealSense D415 device is equipped with a very compact camera (dimensions: 99 × 20 × 23 mm3, weight: 72 g) that can be incorporated into computers and mobile devices or used as a standalone device. It is equipped with a color camera, an IR projector, and two IR cameras. The infrared projectors boost the ability of the camera system to assess depth by projecting a static infrared pattern on the scene. The left and right cameras capture the scene and send the raw data to the vision processor, which calculates the depth values for each pixel of the image by correlating the points on the left with those on the right image. The depth frame is generated from pixel depth values. This active stereo depth calculation makes this depth camera suitable for both indoor and outdoor acquisitions with reasonable illumination.
The device was chosen for this task after extensive characterization to ensure its accuracy [
24]; as indicated in [
24], the raw data are filtered after the acquisition to obtain a less noisy result.
These characteristics allowed the realization of the handheld chest scanning system shown in
Figure 2. Specifically, in the new version of the scanner, the depth sensor is attached to a Microsoft
® Surface Laptop Go (Microsoft Corporation, Redmond, WA, USA) [
25] with a customized back cover.
Like the previously presented 3D chest scanner, the user must place the camera approximately perpendicular to a semirigid mattress on which the patient is asked to lie down. The correct position of the patient was studied in [
18], and requires the patient to hold their arms along their body, so as not to alter the shape of their chest, and exhale completely, to best expose the defect. In the previous version, the system was used for the acquisition and processing of a single frontal scan, rather than a complete reconstruction of the patient’s torso, to avoid small body movements that could compromise the results. In order to position the scanner correctly, and overcome errors introduced by the user in the positioning of the device during the acquisition, the program automatically detects the plane corresponding to the semirigid mattress by detecting the largest plane in the point cloud, and the normal vector of the plane is brought to coincide with the scanning point of view. To ensure correct detection of the plane, the camera must be positioned about 120 cm from the mattress; this way, the scan will include both the entire chest geometry and the portion of the mattress required to estimate the plane for the alignment. Since the program automatically orients the model, perpendicularity of the camera during the acquisition may be not accurate, i.e., an error of approximately 15° for both pitch or roll.
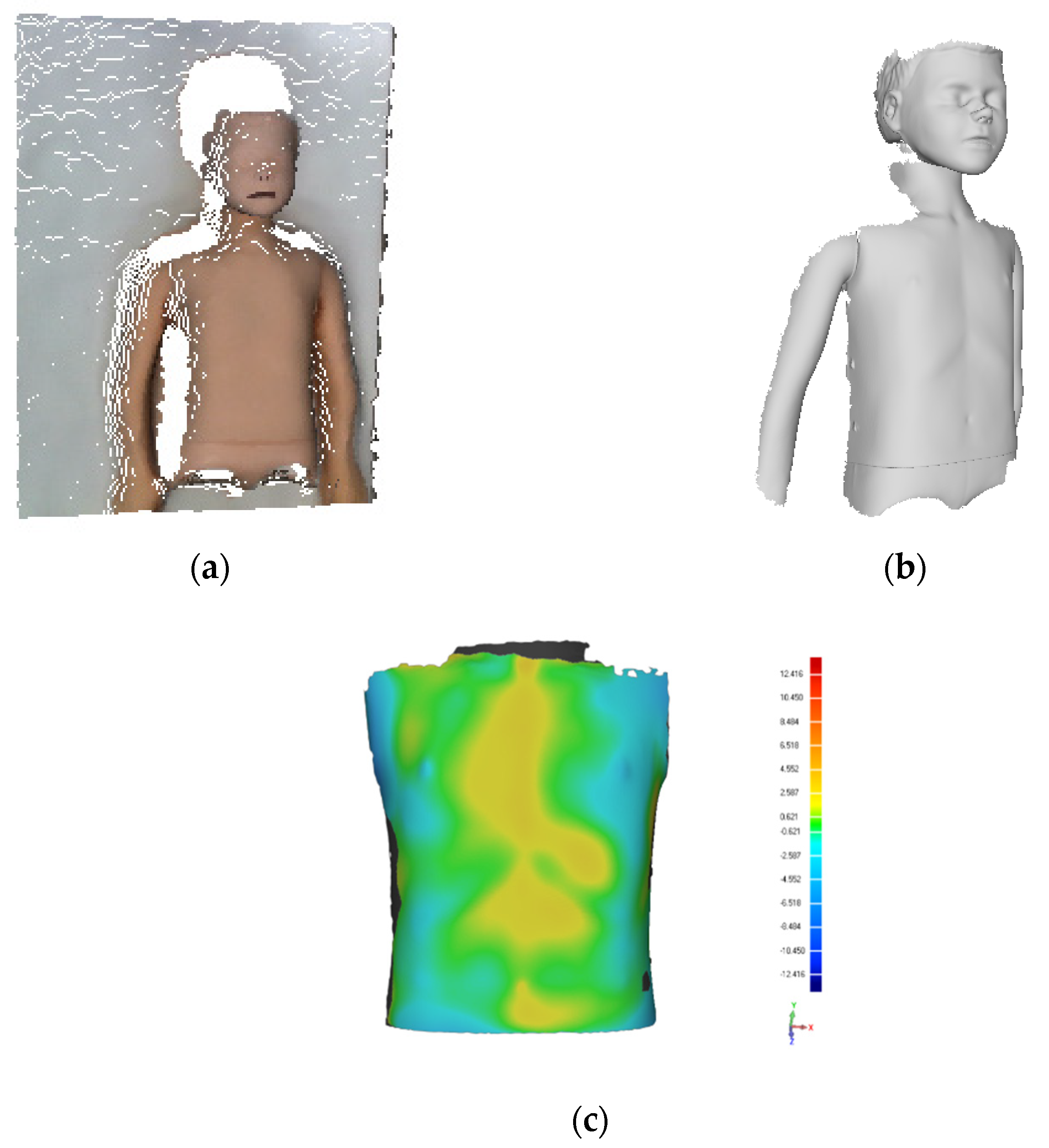
The scanner was primarily evaluated on a mock-up case to ensure a correct acquisition of the chest anatomy. To this aim, a mannequin representative of the age group of 6–9 years old was used. The mannequin was scanned both with a professional optical scanner, the Artec Eva [
26], and with Thor 2.0 by keeping the camera approximately perpendicular to the chest at a distance of approximately 120 cm, as indicated above.
The two acquisitions were then compared using Geomagic Design X software [
27] to assess the deviation. The acquisition performed with Thor 2.0 (
Figure 3a), the acquisition performed with the Artec Eva (
Figure 3b), and the deviation between the two (
Figure 3c) are shown below.
In detail, the mean distance is −0.25 mm with a standard deviation of 1.69 mm. Such values are considered sufficiently accurate to allow the use of the proposed scanner also in real-life environment.
2.2. Software Development
From the software-development point of view, two different GUIs were developed. The first one, to be used by the clinicians, allows the management of the entire process, from 3D scanning to PE index evaluation. The second one consists of a simplified version to be managed by the patient during self-use that only allows the tasks of 3D acquisition and data saving.
2.2.1. GUI for Outpatient Use
The novel GUI (
Figure 4) consists of a 3D navigator in which it is possible to navigate the chest scan, a command tab containing the actions required to scan the patient’s chest and to calculate the 3D optical indices, and a form that allows the storage of the patient’s information.
In detail, the command tab allows the user (i.e., the physician) to open the camera video stream (Open Camera button) to facilitate the correct positioning of the patient, to load a previously scanned cloud (Load button) and to make a new scan (Scan button). Once the scan has been performed (or loaded), the user can triangulate the point cloud (Triangulate button) to facilitate the computation of the optical indices. The computation requires a preset of the origin for which the user must manually click several points to correctly align the scan on the frontal plane (Set Origin button). Finally, the user selects the area containing the defect (Select Area button) and, after clicking the CI3D button, the Correction Index 3D is calculated (
Figure 4).
The decision to use this index derived from in-depth literature studies in which several external severity parameters were analyzed to identify the ones that best approximated and described the physician’s judgment [
28]. The CI3D variation, among the extracted measurements, proved to be the most significant index to measure the improvement/worsening of the patient’s condition, achieving an accuracy of ~90% when compared to the clinical severity score.
The CI3D is calculated by adapting the common correction index to the outer chest perimeter, as depicted in
Figure 5.
2.2.2. GUI for Self-Use
A second interface was created for the direct use of the device by the patient. To this end, the user should be able to perform the scan and send it to the doctor to receive the diagnosis. For this purpose, this second interface does not contain the buttons related to the index computation, but only the ones for opening the video stream, acquiring the 3D geometry, and loading (possibly present) previously performed scans. The simplified GUI (
Figure 6) includes an input form at the bottom to note any communications for the physician.
2.3. Usability Test Design
The application’s graphical interface was designed to be simple, clean, intuitive, and reliable. The result is a tool with fast access to the three main areas, which are the toolbar, the 3D navigator, and the patient-information form. The program was designed to be used independently by the medical staff and by the end user in a domestic setup; consequently, it was of crucial importance to assess the actual degree of acceptance and user-friendliness of the application by means of a usability test.
The tasks on which to conduct the usability tests were identified as follows:
Task 1: Chest acquisition. This involves the patient’s positioning on the semirigid mattress and the acquisition, after positioning the camera approximately perpendicular to the patient’s chest. Specifically, the doctor can open the video stream of the camera to help the correct positioning of the patient, after which they can perform the scan by using the proper button. The scan takes less than one second.
Task 2: Correction index 3D (CI3D), area of the defect, and width computation. This task is carried out when the scan is ready and requires the use of the software to measure severity indices. To pursue this task, the user is required to manually select the area of the chest containing the defect. Once the area has been selected, the program automatically measures the three indices by intersecting the scan with a parallel sheaf of planes to detect the region of maximum depression. Subsequently, it measures the percentage of depression (CI3D), the distance of the two most protuberant points (width), and the area subtended between the line connecting these two points and the chest profile (area). Once the indices have been calculated (in approximately 3 seconds) the program shows the results to the user within a message box.
The following metrics were chosen according to the ISO 9126 series of standards [
29] to assess the usability of the program:
Effectiveness, which is calculated by measuring the completion rate, and assigning a binary value of 1 if the test participant manages to complete a task, and a value of 0 otherwise.
Efficiency, which is measured in terms of task time, i.e., the time spent by the users to achieve the goals.
where
is the total number of tasks,
is the number of users,
is the result of task
by
jth user,
is the time spent by user
to complete task
i. Efficiency, which is the speed of work with the product, is typically compared with the expert efficiency, the highest theoretically possible speed of work.
At the end of each session, users were asked to compile a system usability scale (SUS) questionnaire to measure their impression of the overall ease of use of the system. The SUS provided a quick and reliable tool for measuring the usability. It consisted of a 10-item questionnaire with 5 response options, ranging from “strongly agree” to “strongly disagree.” To interpret the results of the questionnaire, the participant’s scores for each question were added together and then multiplied by 2.5 to convert the original scores of 0–40 to 0–100. Although the scores ranged from 0–100, they did not represent percentages, and should be considered only in terms of their percentile ranking: values below 51 were considered as strongly insufficient, 68 was considered to be the sufficiency threshold, and results above 80.3 were considered optimal.
3. Results
3.1. Clinical Experience
The new scanner was tested directly in an outpatient environment. The specialized clinic for thoracic deformities at the Meyer Children’s Hospital was provided with the new device to carry out a period of testing. Throughout the research, patient data were anonymized before being included in the study in accordance with common health-information practices. To perform a comparative study and test the efficacy and value of the scanner, all the collected data were investigated to select the patients scanned at least twice to compare data collected at different times during VB treatment.
The choice to monitor the effectiveness of VB resulted from the fact that, compared to surgery, VB therapy can be effectively applied to children from 5 to 6 years old, as the application is easy and the side effects are typically minor and self-limited. In fact, the vacuum bell allows, through the creation of an internal negative pressure, the lifting of the chest as long as the device remains applied. The response of the chest to this treatment varies significantly from patient to patient, and therefore it is currently not possible to define a standardized treatment.
The collected data consisted of 46 patients (40 males and 6 females) with an average age equal to 11.7 [±5.5] years old. After acquisition, each patient was analyzed with the new optical scanner to measure the above-mentioned Correction Index 3D.
Overall, the evaluations showed that VB treatment led to a clinically visible improvement of the chest wall defect.
For a visual evaluation, the CI3D trends of variation with the respective wearing times are shown below (
Figure 7). Blue and red lines indicate a CI3D value reduction or increase, respectively, during treatment.
In the present study, the acquired images led us to observe that the greatest improvement in chest wall deformity occurred during the first 12 months of treatment in patients who started VB treatment at the time of the first 3D body scan, whereas it remained stable or slightly improved in the following months. In a few patients, the 3D body scans showed only a slight deterioration or worsening of the defect, although globally there was a substantial thoracic deformity improvement.
3.2. Usability Test Results
As mentioned above, one of the main objectives of the study was to propose a new method of chest scanning that could facilitate the interaction of the physician with the procedures of data acquisition and processing when the system is used in the clinical center, or when used directly by the patient in telemedicine.
Therefore, two usability assessments were carried out. The first, dedicated to clinicians, evaluated how they were able to perform the entire PE assessment process safely, effectively, and efficiently. The second usability test was specifically for patients to assess how the newly devised simplified GUI could effectively help them in autonomously scanning the pathological area and provide 3D information to the clinicians without the need of physically attending the clinic.
Five specialized clinicians from the Meyer Children’s Hospital were involved in this testing process. Considering the low complexity of the tasks to be performed and the common practice of usability testing [
30,
31], five participants were deemed sufficient.
A preliminary meeting was held to explain the workflow and the tasks to be performed to the participants and give them the opportunity to familiarize themselves with the process. Following this preliminary process, participant assessments were arranged separately, and the tests were carried out without any support from the observers.
The results of the effectiveness test are reported in
Table 1, and the results of the efficiency tests are reported in
Table 2.
Figure 8 shows the results of the SUS questionnaire, in which values below the red dashed line were considered strongly insufficient, the orange line represents the sufficiency threshold, and results above the green line were considered optimal. The average SUS score was 89.
Assuming a domestic usage of the device within a telemedicine framework, it was essential to also test the simplicity of use by the patient. Since the system is meant to be used on pediatric patients, a third party must perform the scan (e.g., a parent); consequently, the panel group for the usability test consisted of 10 people aged – 55 years with basic knowledge of the use of mobile devices and PCs.
In this case, it was sufficient to evaluate the program only with regard to Task 1 (chest acquisition). Each participant was given an explanation of the task and was given time to familiarize themselves with the system, after which the tests took place in complete autonomy.
The three metrics described above were evaluated. Specifically, these were the effectiveness, to measure if the task has been completed, the efficiency, to evaluate the execution time, and finally, the degree of user satisfaction.
Table 3 shows the results of the three metrics averaged over the 10 participants.
4. Discussion and Conclusions
In this work, a process of redesigning a body scanner previously developed for the acquisition and evaluation of the pectus excavatum was undertaken. The need to create a more manageable and user-friendly scanner arose from the lack state-of-the-art tools that can help doctors in a custom definition of the treatment. Pectus excavatum is treated, in cases that do not demand surgical intervention, with an external instrument called a vacuum bell.
Instruments like the one devised in this work, which allows for the evaluation of the severity of the pathology, are useful in adapting the parameters of use during the course of treatment based on the progression and response of the individual patient. These tools are even more necessary when considering the remote use of the device. A system easily accessible directly by the patient would open the doors of telemedicine in monitoring pectus excavatum.
The scanner presented in [
21] was modified to obtain a better portability and therefore a simpler use within outpatient clinics, and demonstrated an easier interaction with the scan acquisition, both in terms of portability and in terms of the graphical interface of the program, facilitating the clinical investigation. The new 3D body scanner evaluation can obtain a chest wall defect evaluation in less than one second, after which the patient is free to move, and the acquired data are processed in the background in few seconds. In this way, it is possible to inform parents about the progression of their child’s disease at the time of the outpatient visit, which helps in the consideration of any therapeutic changes at the same moment.
After an initial test that showed the accuracy of the device in acquiring the chest anatomy, the developed system was tested in terms of usability in both clinical and domestic usage. From the usability test performed with clinicians, the new improved scanner resulted in a high degree of appreciation, and obtained the maximum parameters with regard to effectiveness. The use times reached by the medical team were very close to the times reached by experienced technical users, resulting in high efficiency performances. Regarding the SUS questionnaire, the software tested obtained an average result of 89, and in general, all the scores were above the optimal line, indicating a high level of user satisfaction.
The usability tests conducted to evaluate the possibility of using this as a remote monitoring system confirmed excellent levels of effectiveness and efficiency in home usage. Although slightly lower results were obtained compared with the tests performed in the clinical environment, high satisfaction values were found using the SUS questionnaire. It should be noted that patients affected by PE were not the users to whom this second test has been dedicated, but rather third parties who were able to perform the scan.
The device is therefore suitable for both outpatient use and remote use by the patient, if properly supported by a short training process.
The clinical use of the device showed the possibility of tracing the course of the pathology and the effectiveness of the treatment. The measurements carried out were in accordance with the related literature, which showed that the pathology tended to have the first clear improvement in the first 12 months of use of the vacuum bell, while a general decrease or a lack of worsening of the pathological condition was achieved when assisted by this instrument. In a few patients, the measured PE indices demonstrated a slight worsening of the chest deformation. Some of these results could be explained by the fact that the use of a vacuum bell worsens the pectus excavatum pathology in subjects where the thoracic wall defect would have worsened in terms of severity during the pubertal age.
The clinical use demonstrated the possibilities of using the new device in clinical practice and of following the progression of the pathology. This would allow a physician to plan personalized interventions defined according to the responsive characteristics of the individual patient.
In conclusion, the proposed system is an alternative to common pectus excavatum tracking and measurement systems that are often invasive, time-consuming, or insufficiently accurate. The 3D scanner can be considered to be low budget, at least for clinics, as the chosen optical device, while achieving sufficient accuracy, belongs to the low-cost range of devices. In fact, it is possible to purchase the scanning system for the cost of a common personal computer. This cost is not significant when compared to the cost of performing tomographic scans for each patient, assuming monitoring disease progress with common chest indices, and is not comparable to the cost of professional device-based body scanners. This approach can pave the way toward a simpler interaction with scanning devices, which, if coupled with measuring software, can streamline procedures for patient monitoring and communication and make them more reliable.
To date, the realization of the scanner requires the availability of an Intel RealSense D415 camera and a Microsoft® Surface Laptop Go device; this could represent a limit from the end user’s point of view, in terms of the costs to obtain the instrumentation. For this reason, future developments will be addressed to adapt the system to tablets, which are more commonly present in the family environment. Furthermore, the realization of a web interface to manage the communication between the patient and the medical staff is planned for the near future.