Featured Application
In endodontic treatment of teeth with thin root canal wall, we suggest to use thicker mineral trioxide aggregate (MTA) to overcome reduced mechanical properties of MTA’s interfacial layer.
Abstract
Setting of mineral trioxide aggregate (MTA) is affected by various factors. The purpose of this in vitro study was to evaluate the influence of root canal wall thickness on mechanical properties of MTA along the whole apical plug. Bovine bone mold tubes with internal diameter of 2 mm, height of 5 mm, and wall thickness of 0.8 mm, 1.2 mm, and 1.6 mm were filled with 3 mm ProRoot MTA and were kept in 37 °C and relative humidity of 100% for 7 days. The indentation hardness and reduced modulus of elasticity were measured in a large overview matrix and detailed matrix placed 1.5 mm from simulated apical foramen in order to obtain particularized information about gradient of altering mechanical properties. The uppermost layer of material in contact with simulated apical foramen had reduced mechanical properties irrespective of root canal wall thickness. The most distinct decrease of microhardness (32%) and reduced modulus of elasticity (27%) in interfacial layer were present in specimen with thinnest root canal wall. This effect could be observed in detailed measurement up to 190 µm in material. The interfacial layer of MTA, which was in contact during setting with root canal wall thinner than 1.2 mm, had reduced mechanical properties.
1. Introduction
Mineral trioxide aggregate (MTA) is an essential part of today’s armamentarium for both surgical and nonsurgical endodontic procedures. It is hydraulic calcium silicate cement which sets in the presence of moisture, so it is advantageous to use it in situations, such as root perforation repair, root-end retrofill, and apical plug of immature teeth, and some authors even recommend it for complete root canal obturation [1,2]. Because of hydraulic nature of the material, the manufacturer of MTA recommends that a wet cotton pellet should be placed against the intracanal surface of the MTA [3] in order to improve material setting.
Although various powder-to-water ratios of MTA do not influence expansion of the material [4] or dimensional stability [5], adding excessive amount of water over the suggested powder-to-water ratio during mixing increases porosity, solubility [6], setting time, calcium release [7], decreases bond strength [8], and diminishes compressive strength [9]. On the other hand, this undesirable scenario can occur if the wet curing of MTA is used, and the cotton pellet is too wet and placed on the material too early [10].
Several studies have assessed the effect of presence of intracanal moisture, the influence of intrinsic moisture source [11] or different thickness of applied MTA on its quality and properties [12]. There are indices that moist cotton pellet placement may not be crucial for MTA setting in apexification procedures [11] or in cases where thickness of the material does not exceed 4 mm in total [13]. But there is reported only marginal interest on the influence of the physiological moisture in dentin or dentinal permeability on setting of MTA. This could be more noticeable in immature teeth and teeth with extensive retrograde cavity where limited amount of dentin or wide dentinal tubules are present and could lead to improper setting of MTA and failure of treatment. There are studies which describe setting of dry MTA by moisture absorbed through the root [14], through the apical foramen [15], or in the presence of interstitial fluid alone [11]. However, the limitations of these studies are the use of Vickers indentation (four-sided pyramid) and measurement on horizontal sections which has only limited information about setting in different parts of material. Only a few studies have studied mechanical properties of MTA on longitudinal sections [16]. Another limitation is standardization of root canal wall thickness. The information about sizes of roots and their shape is usually missing, and usually only apical preparation is standardized; the inter-study comparison of results, thus, remains almost impossible. The last limitation is that, in many studies, the size of used specimen is out of clinical relevance.
Besides, investigation of the mechanical properties of contemporary dental materials at relevant scale and environment is necessary for understanding their performance, application limits and of course, for optimization of their application protocols. It should be noted that mechanical properties of dental restorative materials may exhibit an important size-dependent character [17] as was proved in the case of calcium silicate cements [16]. One of the most commonly mentioned mechanical properties of MTA related to setting is microhardness. It is considered as a crucial material’s parameter characterizing its mechanical strength and, in turn, also the quality of setting, as well. Microhardness testing is based on the evaluation of resistance of material against plastic/elastic deformation [18]. Conventionally, the hardness test is based on the measurement of the diagonal’s dimensions of the residual plastic impression created during the indentation process, where the hard indenter is pressed into the sample’s surface [18]. Vickers diamond indenter, four-sided pyramid, is used in most studies [16]. This approach relies on the visual observation of residual indentation and also on the assumption that only limited elastic recovery of the residual indent takes place. Generally, the measurement of the actual size of the indent becomes difficult with the decrease of its dimensions and increase of surface roughness, which can lead to inaccurate or incorrect conclusions after the evaluation [18]. On the contrary, the advanced approach of hardness testing, also called depth sensing indentation, based on the continuous recording of the force-displacement data during the whole test can be employed. This approach also allows study of material properties through exploration of the longitudinal cross-section of the specimen. To our knowledge, no study on the effects of thickness of root canal wall on setting of MTA has been reported in literature so far.
The purpose of this study was to examine the role of root canal wall thickness on microhardness and elastic modulus of MTA along the whole apical plug after setting in simulated root canal environment. The null hypothesis tested was that there are no differences in the microhardness and reduced elastic modulus of MTA within entire specimens.
2. Materials and Methods
2.1. Study Design
In this in-vitro experimental study, mold tubes from bovine compact bone, which simulated the root canal environment, were sealed with ProRoot MTA (Dentsply Tulsa Dental, Tulsa, OK, USA). Bovine tubes with internal diameter of 2 mm, approximate height of 5 mm, and wall thickness of 0.8 mm (further referred as “Thin”), 1.2 mm (“Medium”), and 1.6 mm (“Thick”) were used. Seven specimens were analyzed in each of these three experimental groups. Two mechanical properties of the studied material were measured for each specimen, namely the indentation hardness (gigapascal, GPa) and reduced modulus of elasticity (gigapascal, GPa) [18]. These properties were measured in two steps.
Firstly, a large overview (Study-I) matrix consisting of three 7 × 3 matrices was placed at the apical, middle, and coronal part of simulated root canal cross section (see red rectangles in Figure 1). The main aim was to evaluate the effect of the distance from simulated apical foramen and root canal wall thickness on mechanical properties of MTA. Secondly, to obtain particularized information about gradient of altering mechanical properties, the denser 7 × 14 matrix (Study-II) was standardly placed approximately 1.5 mm from simulated apical foramen on 2 areas in the central part of simulated root canal cross section and 2 interfacial areas (see yellow rectangles in Figure 1). The main feature of Study-2 is its significantly higher spatial resolution.
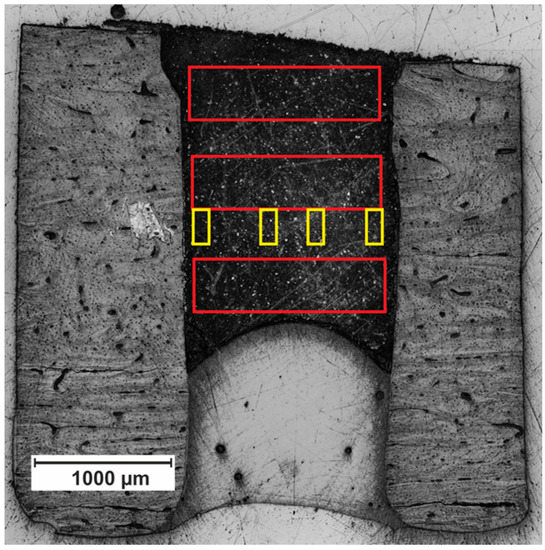
Figure 1.
Specimen cross-section. Red rectangle marked position of matrices used in Study-I. Yellow rectangle marked position of detailed matrices used in Study-II.
2.2. Specimen Preparation
Cortical parts of mature bovine femur diaphysis were obtained from a local abattoir. The specimens were washed after manual removal of soft tissues and cut longitudinally into rectangular bone segments using surgical burr after hip and knee heads were removed at two anatomical marks, the lesser trochanter and the nutrient foramen. The average segment dimensions were 40 × 8 × 5 mm. From the compact bone of each slice, cylindric molds were prepared with an internal diameter of 2.0 ± 0.2 mm and external diameter of 3.6 ± 0.2 mm (“Thin“ root canal wall), 4.4 ± 0.2 mm (“Medium“ root canal wall), and 5.2 ± 0.2 mm (“Thick“ root canal wall). Subsequently, the cylinders were reduced to a height of 5.0 ± 0.2 mm. The end of each tube was sealed with self-etch adhesive system (Singlebond Universal, 3M ESPE, Maplewood, MN, USA) with an approximately 2-mm thick layer of flowable resin composite (Filtek Ultimate flow, 3M ESPE, Maplewood, MN, USA), and, after light curing, tooth-colored ProRoot MTA (Dentsply Tulsa Dental, Tulsa, OK, USA) was mixed with sterile distilled water according to the manufacturer’s instructions and incrementally delivered and vertically compacted with #4 Machtou plugger (Dentsply Tulsa Dental, Tulsa, OK, USA) up to the end of simulated apical foramen which was in direct contact with simulated physiological environment. The specimens were kept in 37 °C and relative humidity of 100% for 7 days in order to simulate physiological conditions.
After that, the cylindrical samples were fixed into the acrylic resin Dentacryl (Spofadental, Jičín, Czech Republic) and grid by sequential procedure with 600 and 800-grit silicon carbide and subsequently polished using 0.25 µm diamond suspension. All the preparation procedures were performed under the conditions of continuous water cooling in order to prevent the sample from overheating. At the end of this procedure, the cross-sectional surface of MTA with the sufficiently low surface roughness was acquired.
2.3. Nanoindentation Measurement of Whole Specimen
Nanoindentation experiments were carried out using a fully calibrated NanoTest instrument in load-controlled mode at room temperature. During the test, the normal load of 40 mN was applied on the diamond Berkovich indenter (three-sided pyramid) at loading rate of 2 mN/s. The loading force was chosen to keep the local character of the measurements, on the one hand, and to avoid the negative effect of surface roughness, on the other hand. The corresponding penetration depths were typically about 1 µm.
2.3.1. Study-I Nanoindentation Mapping of Whole Specimen
In order to obtain the complete picture of the effect of the distance from simulated apical foramen and root canal wall thickness on the mechanical properties of MTA, three areas in the apical, middle, and coronal part of the root canal were chosen for three 7 × 3 matrices. The distance of the indents in the matrix was 200 µm in the vertical plane and approximately 250 µm in the horizontal plane (depending on the actual width of a specific root canal). The distance of the indent’s matrix from simulated apical foramen and root canal wall inner surface varied around 70 µm, depending on matrix position and local shape irregularities of the root canal (Figure 1). Indentation hardness and reduced modulus of elasticity were calculated using the standard procedure based on the analysis of the load-displacement record [19].
2.3.2. Study-II Nanoindentation Mapping of Periphery and the Center of Specimen
To obtain more detailed data about marginal parts of MTA, which are in contact with root canal wall surface, one representative specimen was randomly chosen from each experimental group for detailed measurement. For each specimen, four matrices of 7 × 14 indents were located in the central part of the specimen, in the approximate distance of 1.5 mm from simulated apical foramen (Figure 1). Two matrices were located on the periphery of MTA, and two were located at the center of the MTA. The distance of the indents was 20 µm in both the horizontal and in the vertical plane. The distance of the marginal indents from root canal wall inner surface was approximately 70 µm, depending on the shape of the edge of root canal.
2.4. Statistical Analysis
The effects of the wall thickness, distance from simulated apical foramen, and distance from root canal wall surface on the mechanical properties of MTA are described by the linear model. Both characteristics—the indentation hardness, as well as the modulus of elasticity—were log-transformed prior to the analysis since their distribution is skewed.
In Study-I, the general patterns were studied. The analysis was based on a set of measurements of 21 specimens. Measurements taken on a particular specimen are inherently mutually dependent and, therefore, are modeled as one cluster of observations. In order to suppress the effect of possible differences between specimens, the ID number of the specimen was treated as a random effect in the linear mixed effect model [20]. The effects of wall thickness, as well as of distances from the apical foramen and the root canal, were considered as fixed. Significance of the fixed effects is assessed according to p-values, which are approximated using the Satterthwaites’s method [21].
In Study-II, where a more detailed understanding of the effect of the distance from root canal was of the interest, a standard linear regression model was applied. This analysis was based on three randomly chosen specimens (one from each experimental group), for which detailed measurement were taken.
In both cases, the optimal models were chosen according to value of the Akaike information criterion.
The statistical analysis was performed in the statistical software R [22], using the packages lmer4 [23] and lmerTest [24].
3. Results
In order to study the effect of thickness of root canal walls on MTA setting, the local mechanical characterization approach featuring high spatial resolution was used. “Study-I” investigated the overall distribution of hardness and modulus of elasticity (representative mechanical characteristics) considering distance from simulated apical foramen and distance from inner surface of root canal wall. Furthermore, “Study-II” was employed to explore the detailed distribution of mechanical properties of MTA in contact with the inner surface of root canal wall (the range of influence of root canal wall thickness).
3.1. Representative Mechanical Characteristics
3.1.1. Hardness of Whole Specimen
According to the optimal model, summarized in Appendix A and Table A1, vary the estimated values of hardness around 1.57 GPa (median) with medians for individual specimens from 1.49 GPa to 1.66 GPa.
The hardness was significantly (p < 0.0001) lower at the uppermost part (the row closest to the surface, approximately 70 µm). The decrease was approximately by 27% in comparison to measurements from the lower layers. This holds regardless of the wall thickness.
The hardness was significantly (p < 0.0001) lower in locations closest to the root canal wall for the thinnest root canal wall (0.8 mm). The decrease was by about 32%. This effect was not observed in experimental groups with thicker root canal walls. This feature is clearly demonstrated in Figure 2.
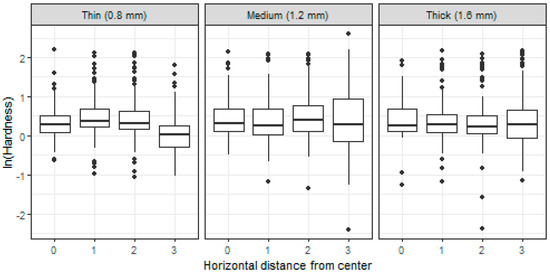
Figure 2.
Boxplots of (log-)hardness measured on specimens with different root canal wall thickness (0.8, 1.2, and 1.6 mm) and in different distance from the center of a specimen (0–center, 3–periphery).
3.1.2. Modulus of Elasticity of Whole Specimen
Based on the fitted model, summarized in Appendix B and Table A2, vary the estimated values of modulus of elasticity around the overall median value 47.22 GPa. More specifically, the model-based median value of the modulus of elasticity in a particular specimen is between 42.26 and 51.08 GPa. This range reflects variability among specimens.
Similarly, as in the case of the hardness, the value of modulus of elasticity was also significantly (p < 0.0001) lower at the uppermost part of the samples (the row closest to the surface, approximately 70 µm). Regardless the thickness of the root canal wall, this decrease was approximately by 24%.
The value of modulus of elasticity was significantly (p < 0.0001) lower in locations closest to the root canal wall for the thinnest root canal wall (0.8 mm). The decrease was with respect to the value in the inner part of the sample by 27%. Unlike the case of the hardness, a slight effect of the root canal wall proximity was also significant for the thicknesses 1.2 and 1.6 mm. The decrease of the modulus of elasticity value was approximately by 10%, compared to the center of the sample. These features are also visible in Figure 3.
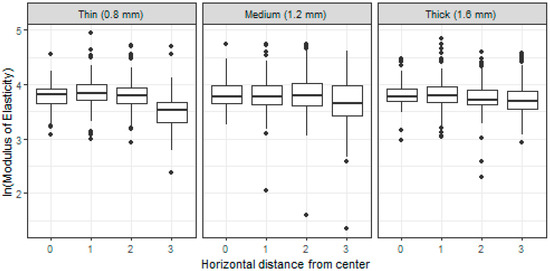
Figure 3.
Boxplots of (log-)modulus of elasticity measured on specimens with different root canal wall thickness (0.8, 1.2, and 1.6 mm) and in different distance from the center of a specimen (0–center, 3–periphery).
3.2. The Range of Influence of Root Canal Wall Thickness on Mechanical Properties of MTA—Effect of Wall Thickness on Hardness Spatial Dependence
At the second stage of the research, the effect of distance from the internal surface of the root canal wall was of the main interest. For a dataset, based on more detailed measurements of three samples with wall thicknesses 0.8 mm (“Thin”), 1.2 mm (“Medium”), and 1.6 mm (“Thick”), a simple linear model was employed for a detailed interpretation. Introduction of the proper model allowed to (statistically) estimate the appropriated values of median for hardness and reduced modulus.
Based on the estimated model, summarized in Appendix C and Table A3, the hardness measured in the center part of the sample with thick root canal wall was systematically about 28% higher than hardness in the center of samples with thin and medium root canal wall. Moreover, the values of hardness in the center of the latter two samples were comparable. More specifically, the estimated median values were at the uppermost layer of the samples 1.11 GPa and 0.87 GPa for the thick and thin or medium walls, respectively.
While the hardness did not change significantly with changing proximity to the wall in the case of the thick root canal wall (boxplots C/0–C/13 in Figure 4), an effect of the root canal wall proximity was present for the thinner canal walls. In both cases of the 0.8 mm and 1.2 mm wall, the hardness remained constant within the central matrices, when, in the peripheral ones, it decreased with the horizontal distance from the center of the sample towards the root canal wall. For the 1.2 mm wall, the decrease was observable within the whole peripheral matrices (i.e., the last seven indentation points B/7–B/13 in Figure 4, approximately 190 µm), when, with each 20 µm, the hardness decreased approximately by 13%. By the 0.8 mm wall was the decrease significant for the last five of the most peripheral indentation points A/9–A/13 in Figure 4 (approximately 150 µm) and was more dramatic, while each 20 µm decreases the hardness approximately by 21%. Finally, the hardness slightly changed with the vertical distance from the surface. Despite the wall thickness and the horizontal location of the measurement, the value decreased by approximately 2% with each 20 µm of depth. These effects quantified with the model are also clearly visible in Figure 4.
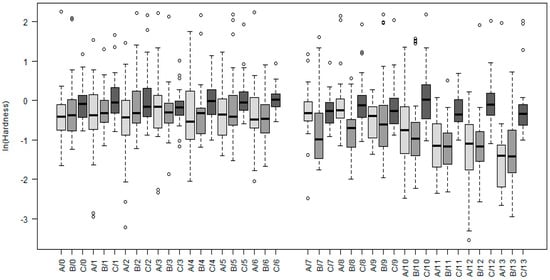
Figure 4.
Boxplots of hardness measured on three samples differing in thickness of the root canal wall (A–0.8 mm, B–1.2 mm, C–1.6 mm). The measurements were taken on different locations, where 0 denotes measurements in the center of a sample and 13 on the periphery. Indexes 0–6, therefore, represent measurements in the central matrices and 7–13 measurements in the peripheral ones.
Similar features hold also for the modulus of elasticity, as is clearly visible in Figure 5. The estimated value of the modulus of elasticity at the uppermost layer equaled for the sample with the thick root canal wall 43.32 GPa and was about 8% higher than its value in the center of the samples with thinner walls, which was estimated as 40.25 GPa.
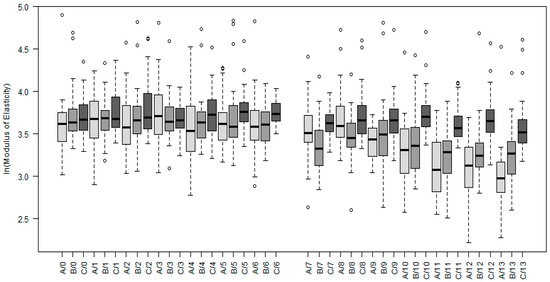
Figure 5.
Boxplots of modulus of elasticity measured on three samples differing in thickness of the root canal wall (A–0.8 mm, B–1.2 mm, C–1.6 mm). The measurements were taken on different locations, where 0 denotes measurements in the center of a sample and 13 on the periphery. Indexes 0–6, therefore, represent measurements in the central matrices and 7–13 measurements in the peripheral ones.
The root canal wall proximity affects the mechanical properties in the case of thicknesses 0.8 and 1.2 mm. For these two settings, the value of modulus of elasticity remained fixed within the whole central matrices and in the peripheral ones decreased with the horizontal distance from the center of a sample. Similar to hardness was the decrease of modulus of elasticity observable for the 1.2 mm wall within the whole peripheral matrices (i.e., the last seven indentation points B/7–B/13), when, with each 20 µm, its value decreased approximately by 6%. By the 0.8 mm wall was the decrease significant for the last five of the most peripheral indentation points (A/9–A/13) and was more dramatic, while each 20 µm decreases the value approximately by 13%. In addition, the value of modulus of elasticity decreased with the vertical distance from the surface of the sample. Each 20 µm of depth was accompanied with the decrease by approximately 1%. The detailed description of the respective statistical model in given in Appendix C and Table A4.
3.3. Distribution of Clincker
The final research question was focused on the distribution of clinker grains and dependence of its appearance on the type of the root canal wall and distance from the center of a sample. Considering experimental data from detailed measurements (samples A, B, and C) under Study-II, locations with a measured hardness higher than 4 GPa were considered as a clinker grain. The distribution of all grains according to the type of the root canal wall and distance from the center of a sample is given in Table 1 and shows that no significant differences are present.

Table 1.
Clicker grain distribution according to the thickness of the root canal wall (0.8, 1.2, 1.6 mm) and location within a sample (LP–left peripheral, LC–left center, RC–right center, RP–right peripheral).
4. Discussion
To the best of our knowledge, in this study, the influence of root canal wall thickness and distance from root canal wall inner surface on the mechanical properties (indentation hardness and elastic modulus) of MTA was studied for the first time. The in vitro results demonstrated deteriorated mechanical properties of MTA in the contact with internal surface of root canal wall and with external environment. The analysis of hardness and elastic modulus values revealed deterioration of mechanical properties of MTA in the closest vicinity to the root canal wall only in the specimen with the thinnest root canal wall thickness (microhardness decrease of 32%). The decrease in modulus of elasticity was most prominent in the material located closest to root canal wall of “Thin” group (27%) but was detectable even in “Medium” and “Thick” groups (both 10%). During detailed nanoindentation mapping of randomly chosen specimen, we observed decreased microhardness in “Thin” and “Medium” specimen, which reached up to 190 µm from inner surface of root canal wall. This decrease was not present in the “Thick” specimen. A similar decrease pattern was observed for modulus of elasticity. The similar result can be observed in the material which is in contact with external environment. To sum it up, Study-I detected, in particular, a decrease of the modulus of elasticity in the locations in the proximity of root canal wall, while Study-II, based on more detailed measurement, specified this decrease as linear dependence on the distance from the center of the sample. Moreover, it turned out that the decrease gets more dramatic with thinner walls. Study-1 also clearly showed the effect of the contact with the external environment, which causes a significant decrease of mechanical properties of the material, compared to its values measured in the inner parts of the samples. This strong effect overcame the moderate effect of the vertical position within the samples. This property was uncovered in Study-II, in which measurements were located on a dense grid in the vertical center of the samples and which, therefore, allowed a more detailed insight in the structure of the samples.
The microhardness and modulus of elasticity are decreased most probably by excessive amount of moisture. When the mixed material is in contact with additional moisture in early phase of setting, the water-powder ratio can be changed [25]. The higher water-powder ratios influence several properties of MTA, such as decreasing its bond strength [8] and diminishing its compressive strength [26]. The increased hydration of portland cement leads to higher carbonation of hydrated cement with escalated shrinkage and raised production of calcium carbonate [27]. The disruption of MTA setting was more significant for the thinner root canal walls, and this effect can reach up to 190 µm in the material but is more prominent in the layers which are in close contact with inner surface of root canal wall. This effect is not so evident for the thickest root canal wall. In previous studies, it was mentioned that possible source of moisture can be deposition of water in dentine tubules. If the deposition of water in the root canal wall was the main source of additional moisture, the effect would be most distinct in the specimen with thicker root canal walls. In view of the fact that the decrease of microhardness and modulus of elasticity of material is similar in contact with external environment and with thinner root canal walls, we can assume that the water permeability of thinner root canal wall can be as high as it can have deleterious effect in early phase of MTA setting. In other words, water can more easily penetrate from surrounding tissue through the canal wall into the root canal and influence the setting process of MTA. It might be due to wash-out of material, altered water-powder ratios, or carbonation of hydrated MTA. From our study, we are not able to determine if it is due to different proportional representation of phases (such as elevated outer product in comparison to inner product of calcium silicate hydrate gel) or due to reduced mechanical properties of particular phases. To assess this influence, it would be necessary to couple measurement of microhardness with backscatter scanning electron microscope. From our study, we can conclude that the reduced mechanical properties of material are not caused by reduced frequency of clincker grain.
The variability in study designs of published papers makes the comparability of the results impossible. Despite endeavors for standardization of teeth specimen by sectioning and preparation of a root canal [8], it is highly unlikely to have a standardized root canal wall thickness because of their dissimilarity in shape and structure. In addition, human teeth are available only in a limited number. In this respect, the use of standardized samples can reduce the variability between specimens and provide a more accurate basis for comparison and, in turn, a more accurate insight into the factors which influence the setting dynamics. To achieve the best possible standardization of root canal wall thickness, the bovine compact bone was chosen as it was used to study bond strength of adhesives [28] or for standardized in vitro model of fracture resistance [29]. The permeability coefficient of human dentin is 2.7 × 10−4 cm/min and that of bovine compact bone is 1.1 × 10−5 cm/min [30,31]. The permeability of dentin is influenced by thickness of dentin, distance from dental pulp, localization of dentin [32], and by irrigation protocols used in endodontics, especially when ethylenediaminetetraacetic acid (EDTA) is used [33].
It was shown that the use of EDTA during endodontic treatment increases the permeability of root canal wall because of dissolution of smear layer [33]. This increased permeability may have detrimental effect on sealability of MTA. It was shown that the use of EDTA and removal of smear layer during endodontic treatment leads to higher apical microleakage [34]. It must be emphasized that EDTA and other smear layer removal agents has detrimental effect on MTA [35,36]. Moreover, EDTA retained its calcium-complexing ability when mixed with sodium hypochlorite [37], so a copious amount of sodium hypochlorite has to be administered to wash out remnants of EDTA from the root canal system. The effect of increased permeability could be another explanation of increased leakage of apical plugs after orthograde root canal treatment in contrast to retrograde filling. Thicker layers of MTA (4 or 5 mm) provide a better seal compared to 2- or 3-mm layers [38,39], but there is no difference between 3- and 5-mm thick retrofill [40]. The better seal can be achieved not only by thicker layer of MTA but maybe even less deteriorated material in coronal part of material where the root canal wall is thicker. We must be cautious about assessing these studies because most of them used nail polish on the root surface during setting to avoid lateral microleakage, which affects the permeability of radicular dentin [39,40].
In recent studies, specimens were subjected to Vickers microhardness test only in horizontal plane and in specific distance from simulated apical foramen. Several studies suggest that dry or moist intracanal environment does not influence the setting of MTA up to 2 mm [12] or 4 mm [11,13]. Microhardness tests have been used for the evaluation of the quality and progression of the hydration process and as an indicator of the setting process [41,42]. Previous work marked on its capability to provide information on the effect of setting conditions and the strength of tested materials [43]. For accurate comparison with other materials, specimens should be polished and dimensions of at least 6 mm thick and 12 mm wide are required. These conditions are far from clinical endodontics. Thus, tests in this field are mainly comparative for the use within each study [44]. Unlike most of previous studies, we measured microhardness and modulus of elasticity of real size samples sectioned to longitudinal axis by depth sensing indentation.
Depth sensing indentation (nanoindentation) has been adopted as one of the most common techniques developed for the assessment of local mechanical properties at nano- and microscale. It is a contact-based method where the well-defined probe is pressed into the investigated surface at defined conditions. Nanoindentation is mostly used for assessment of hardness and elastic modulus. Microhardness testing is based on the evaluation of resistance of material against plastic/elastic deformation [18]. Conventionally, the hardness test is based on the measurement of the diagonal’s dimensions of the residual plastic impression created during the indentation process, where the hard indenter is pressed into the sample’s surface. Vickers indenter, four-sided pyramid, is used in most studies. This approach relies on the visual observation of residual indentation and also on the assumption that only very limited elastic recovery of the residual indent takes place. Generally, the measurement of the actual size of the indent becomes intricate with the decrease of its dimensions and increase of surface roughness. On the contrary, the advanced approach of hardness testing, also called depth sensing indentation, is based on the continuous recording of the force-displacement data during the whole test. This reduces the possible errors of the visual observation of the residual impression. The obvious advantage of depth sensing indentation is the ability to determine the elastic modulus of the specimen [19].
In order to take advantage of this advanced experimental technique, the prepared specimens had to be cross-sectioned and polished. This allowed precise local mechanical testing of the MTA material all along the longitudinal axis and not only on the surface [16], so we were able to assess indentation hardness and elastic modulus in vertical and horizontal direction. However, it should be noted that the polishing procedure itself can affect the chemical and structural composition of MTA if excessive cooling water is used during polishing procedure. Especially, dissolution and washout of MTA components (especially on the boundaries of material), as well as change of pH, can be considered as the main processes. Different polishing procedures are used in nanoindentation application to study hardened cement pastes [25,45]. Another important phenomenon altering properties of MTA is carbonation. It is a process based on a reaction between atmospheric CO2, and both calcium hydroxide and calcium silicate hydrate in cement are carbonated. Increased hydration time enhances the carbon dioxide uptake, which indicates that the calcium in the hydration products reacts more easily than the calcium in the clinker phase. In a humid CO2 atmosphere, the carbonation process is so pronounced that it decomposes calcium silicate hydrate into calcium carbonate and silica [26,46]. Furthermore, the carbonation is linked to shrinkage of material [47]. Humidity during exposure to carbon dioxide appears to be a major factor influencing the shrinkage directly produced by carbonation [27]. This could be another explanation of decreased mechanical properties on the periphery of MTA. This negative effect on the surface of MTA during specimen preparation can be reduced by early polishing and testing after setting of material.
In order to avoid and minimize the effect of MTA/bovine bone interface, the matrices of indents were always located approximately 70 µm from the border line and out of noticeable defects of material. This allows reliable evaluation of MTA only and to avoid the influence of any interfacial imperfection caused either to polishing procedure, improper bond between MTA and the bovine bone and/or spreading of the indentation stress field to the channel walls. Nevertheless, considering all these facts and the material specifics, all the samples were prepared and tested using the same protocol. That means that conclusions drawn within this study can be considered as sound and reliable.
The recommended water-to-powder ratio of MTA according manufacturer is about 0.33. In clinical practice, the cement pastes with a water-to-powder ratio higher than 0.33 are too fluid so the material cannot be manageable [6]. A very similar situation can occur if the wet curing of MTA is used, and the cotton pellet is too wet and placed on the material too early, and the water-to-powder ratio can change after compaction of material [10]. On the same basis, the water-to-powder ratio can be increased by higher permeability of thin root canal wall, and it can result in material on the boundary with higher water-to-powder ratio. The water-to-powder ratio 0.4 results in lower compressive strength values, regardless of the used MTA-based material [9] and decreased bond strength [8]. The sealing ability can be affected by water-to-powder ratio of MTA, as well. The packing with moist cotton pellet in lower water-to-powder ratios of MTA, as well as packing with a plugger in higher liquid-to-powder ratio, decreased apical dye leakage [48]. On the other hand, the lack of water during the preparation of MTA does not influence sealability of MTA on fluid filtration model [15].
5. Conclusions
The microhardness and elastic modulus mapping over the cross-section area along the longitudinal axis were employed in order to determine the possible effect of root canal wall thickness on MTA setting. Within limitations of this study, the interfacial layer of MTA which is in contact with root canal wall thinner than 1.2 mm has reduced mechanical properties, the same as in contact with simulated physiological conditions. This effect was observed up to 190 µm inwards from the root canal wall/MTA interface and leads to decrease in microhardness up to by 32% and for modulus of elasticity up to by 27%. The observed phenomenon can occur in clinical scenario during treatment of immature teeth, endodontic surgery with extensive preparation of retrograde cavity, or direct pulp capping with limited residual dentin thickness around perforation. It seems that, in these clinical situations, might be use of thicker layers of MTA beneficial, but clinical impact of deteriorated mechanical properties of MTA on the boundary is questionable, and further research is desirable. In further research of MTA and other calcium silicate cements, we recommend keeping in mind that permeability of root canal wall can affect the setting of material.
Author Contributions
Conceptualization, R.Ž. and J.Š.; methodology, R.Ž., D.M., R.Č., J.T., and K.F.; software, R.Č., J.T., and K.F.; formal analysis, K.F. and O.V.; investigation, D.M., R.Č. and J.T.; writing—original draft preparation, R.Ž. and J.Š.; writing—review and editing, R.Ž., K.F., R.Č., J.T., O.V., and K.F. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by University of Palacký, grant number IGA_LF_2020_014; by the Operational Program Research, Development and Education of Ministry of Education, Youth and Sports of the Czech Republic, grant numbers CZ.02.1.01/0.0/0.0/17_049/0008422; CZ.02.1.01/0.0/0.0/16_019/0000754; CZ.1.05/2.1.00/19.0377.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data available in a publicly accessible repository that does not issue DOIs. Publicly available datasets were analyzed in this study. This data can be found here: https://drive.google.com/file/d/1VmO8kuVysvGUYe0pQbVIZvMdk4bsrC4a/view?usp=sharing.
Conflicts of Interest
The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Appendix A. Hardness of Whole Specimen—the Mixed Effect Regression Model
According to value of Akaike information criterion (AIC) simplifies the optimal linear mixed-effect model as follows:
ln(Hardness) = β0 + β1I1 + β2I2I3 + β3sampleID + error.
The regression coefficients β1 and β2, therefore, quantify the fixed effects of measurement at the uppermost layer of the sample and next to the surface of the 0.8 mm root canal wall, respectively. The random effect of the individual samples is covered with the coefficient β3. The estimates of fixed effects are summarized in Table A1.

Table A1.
Linear mixed effect model for hardness—summary statistics of the fixed effects. p-values are approximated using the Satterthwaites’s method [20], AIC = 2446.566, and standard deviation of the random effect equals 0.052.
Table A1.
Linear mixed effect model for hardness—summary statistics of the fixed effects. p-values are approximated using the Satterthwaites’s method [20], AIC = 2446.566, and standard deviation of the random effect equals 0.052.
| Parameter | Estimate | Std. Error | t Value | p-Value |
|---|---|---|---|---|
| β0 | 0.452 | 0.021 | 21.216 | <0.0001 |
| β1 | −0.313 | 0.051 | −6.131 | <0.0001 |
| β2 | 0.389 | 0.058 | −6.735 | <0.0001 |
Appendix B. Modulus od Elasticity of Whole Specimen—the Mixed Effect Regression Model
The optimal linear mixed-effect model was built according to value of the Akaike information criterion (AIC) and has the following structure:
where I1 indicates the measurement from the uppermost layer of the sample, I2 indicates the measurement from location next to the inner surface of root canal wall, and I3 indicates the measurement of a sample with the thinnest root canal wall (0.8 mm). Finally, sampleID stands for the random component of the model as it represents a unique identifier of the measured samples. In this model, the regression coefficients α0, α1, α2, α3 quantify the fixed effects, and coefficient α4 covers the random effect of the individual samples.
ln(Modulus of Elasticity) = α0 + α1I1 + α2I2 + α3I2I3 + α4sampleID + error,
The estimates of the regression coefficients are together with the respective t and p-values collected in Table A2.

Table A2.
Linear mixed effect model for modulus of elasticity—summary statistics of the fixed effects. p-values are approximated using the Satterthwaites’s method [20], AIC = 758.845, and standard deviation of the random effect equals 0.058.
Table A2.
Linear mixed effect model for modulus of elasticity—summary statistics of the fixed effects. p-values are approximated using the Satterthwaites’s method [20], AIC = 758.845, and standard deviation of the random effect equals 0.058.
| Parameter | Estimate | Std. Error | t Value | p-Value |
|---|---|---|---|---|
| α0 | 3.846 | 0.016 | 236.296 | <0.0001 |
| α1 | −0.270 | 0.027 | −10.003 | <0.0001 |
| α2 | −0.109 | 0.023 | −4.802 | <0.0001 |
| α3 | −0.206 | 0.038 | −5.406 | <0.0001 |
Appendix C. The Range of Influence of Root Canal Wall Thickness on Mechanical Properties of MTA—Effect of Wall Thickness on Hardness(and Modulus of Elasticity) Spatial Dependence—the Linear Model
Optimal models for hardness and modulus of elasticity have the similar structure, which is as follows:
and
where row reflects the vertical position of the measurement, and VA is an auxiliary variable, reflecting the distance of a measurement from the center of the “Thin” root canal wall sample. VA is zero for distance smaller than 8 indentation points and then grows linearly. Similarly, VB reflects distance from the center of the “Medium” root canal wall sample, when it equals zero for distances smaller than 6 indentation points, and then grows linearly. Dummy variable IC indicates a measurement at the sample with thick root canal wall. Estimates of the regression parameters are together with their sample characteristics collected in Table A3 and Table A4.
ln(Hardness) = γ0 + γ1row + γ2VA + γ3VB + γ4IC + error,
ln(Modulus of Elasticity) = δ0 + δ1row + δ2VA + δ3VB + δ4IC + error,

Table A3.
Summary statistics of the linear model for detailed measurements of hardness, with R2 = 0.230 and R2adj = 0.227, AIC = 2628.45.
Table A3.
Summary statistics of the linear model for detailed measurements of hardness, with R2 = 0.230 and R2adj = 0.227, AIC = 2628.45.
| Parameter | Estimate | Std. Error | t Value | p-Value |
|---|---|---|---|---|
| γ0 | −0.139 | 0.052 | −2.697 | 0.007 |
| γ1 | −0.023 | 0.005 | −4.219 | <0.0001 |
| γ2 | −0.240 | 0.021 | −11.550 | <0.0001 |
| γ3 | −0.142 | 0.013 | −10.575 | <0.0001 |
| γ4 | 0.246 | 0.045 | 4.980 | <0.0001 |

Table A4.
Summary statistics of the linear model for detailed measurements of modulus of elasticity, with R2 = 0.262 and R2adj = 0.260, AIC = 681.635.
Table A4.
Summary statistics of the linear model for detailed measurements of modulus of elasticity, with R2 = 0.262 and R2adj = 0.260, AIC = 681.635.
| Parameter | Estimate | Std. Error | t Value | p-Value |
|---|---|---|---|---|
| δ0 | 3.695 | 0.023 | 163.848 | <0.0001 |
| δ1 | −0.009 | 0.002 | −3.660 | 0.0003 |
| δ2 | −0.142 | 0.009 | −15.607 | <0.0001 |
| δ3 | −0.059 | 0.006 | −10.100 | <0.0001 |
| δ4 | 0.074 | 0.022 | 3.401 | 0.0007 |
References
- Torabinejad, M.; Parirokh, M.; Dummer, P.M.H. Mineral Trioxide Aggregate and Other Bioactive Endodontic Cements: An Updated Overview-Part II: Other Clinical Applications and Complications. Int. Endod. J. 2018, 51, 284–317. [Google Scholar] [CrossRef]
- Cervino, G.; Laino, L.; D’Amico, C.; Russo, D.; Nucci, L.; Amoroso, G.; Gorassini, F.; Tepedino, M.; Terranova, A.; Gambino, D.; et al. Mineral Troxide Aggregate Applications in Endodontics: A Review. Eur. J. Dent. 2020, 14, 683–691. [Google Scholar]
- ProRoot MTA Product Literature; Dentsply Tulsa Dental: Tulsa, OK, USA, 2019.
- Hawley, M.; Webb, T.D.; Goodell, G.G. Effect of Varying Water-to-Powder Ratios on the Setting Expansion of White and Gray Mineral Trioxide Aggregate. J. Endod. 2010, 36, 1377–1379. [Google Scholar] [CrossRef]
- Bortoluzzi, E.A.; Cassel, A.T.; Néis, C.C.A.; Cássia, D.S.M.; Fonseca, R.G.L.; Dulcinéia, M.S.B.; Silveira, T.C. Effect of Different Water-to-Powder Ratios on the Dimensional Stability and Compressive Strength of Mineral Aggregate-Based Cements. Eur. Oral Res. 2019, 53, 94–98. [Google Scholar] [CrossRef] [PubMed]
- Fridland, M.; Rosado, R. Mineral Trioxide Aggregate (MTA) Solubility and Porosity with Different Water-to-Powder Ratios. J. Endod. 2003, 29, 814–817. [Google Scholar] [CrossRef] [PubMed]
- Cavenago, B.C.; Pereira, T.C.; Duarte, M.A.H.; Ordinola-Zapata, R.; Marciano, M.A.; Bramante, C.M.; Bernardineli, N. Influence of Powder-to-Water Ratio on Radiopacity, Setting Time, PH, Calcium Ion Release and a Micro-CT Volumetric Solubility of White Mineral Trioxide Aggregate. Int. Endod. J. 2014, 47, 120–126. [Google Scholar] [CrossRef]
- Türker, S.A.; Uzunoğlu, E. Effect of Powder-to-Water Ratio on the Push-out Bond Strength of White Mineral Trioxide Aggregate. Dent. Traumatol. 2016, 32, 153–155. [Google Scholar] [CrossRef]
- Basturk, F.B.; Nekoofar, M.H.; Gunday, M.; Dummer, P.M.H. Effect of Varying Water-to-Powder Ratios and Ultrasonic Placement on the Compressive Strength of Mineral Trioxide Aggregate. J. Endod. 2015, 41, 531–534. [Google Scholar] [CrossRef]
- Ha, W.N.; Kahler, B.; Walsh, L.J. Clinical Manipulation of Mineral Trioxide Aggregate: Lessons from the Construction Industry and Their Relevance to Clinical Practice. J. Can. Dent. Assoc. 2015, 81, f4. [Google Scholar]
- DeAngelis, L.; Chockalingam, R.; Hamidi-Ravari, A.; Hay, S.; Lum, V.; Sathorn, C.; Parashos, P. In Vitro Assessment of Mineral Trioxide Aggregate Setting in the Presence of Interstitial Fluid Alone. J. Endod. 2013, 39, 402–405. [Google Scholar] [CrossRef]
- Shokouhinejad, N.; Jafargholizadeh, L.; Khoshkhounejad, M.; Nekoofar, M.H.; Raoof, M. Surface Microhardness of Three Thicknesses of Mineral Trioxide Aggregate in Different Setting Conditions. Restor. Dent. Endod. 2014, 39, 253–257. [Google Scholar] [CrossRef]
- Caronna, V.; Himel, V.; Yu, Q.; Zhang, J.-F.; Sabey, K. Comparison of the Surface Hardness among 3 Materials Used in an Experimental Apexification Model under Moist and Dry Environments. J. Endod. 2014, 40, 986–989. [Google Scholar] [CrossRef]
- Budig, C.G.; Eleazer, P.D. In Vitro Comparison of the Setting of Dry ProRoot MTA by Moisture Absorbed through the Root. J. Endod. 2008, 34, 712–714. [Google Scholar] [CrossRef]
- Pelliccioni, G.A.; Vellani, C.P.; Gatto, M.R.A.; Gandolfi, M.G.; Marchetti, C.; Prati, C. Proroot Mineral Trioxide Aggregate Cement Used as a Retrograde Filling without Addition of Water: An in Vitro Evaluation of Its Microleakage. J. Endod. 2007, 33, 1082–1085. [Google Scholar] [CrossRef] [PubMed]
- Žižka, R.; Čtvrtlík, R.; Tomaštík, J.; Fačevicová, K.; Gregor, L.; Šedý, J. In Vitro Mechanical Properties of Mineral Trioxide Aggregate in Moist and Dry Intracanal Environments. Iran Endod. J. 2018, 13, 20. [Google Scholar]
- Qu, S.; Huang, Y.; Pharr, G.M.; Hwang, K.C. The Indentation Size Effect in the Spherical Indentation of Iridium: A Study via the Conventional Theory of Mechanism-Based Strain Gradient Plasticity. Int. J. Plast. 2006, 22, 1265–1286. [Google Scholar] [CrossRef]
- ISO 14577-1:2015. Metallic Materials—Instrumented Indentation Test for Hardness and Materials Parameters—Part 1: Test Method; British Standards Institution: London, UK, 2015. [Google Scholar]
- Oliver, W.C.; Pharr, G.M. An Improved Technique for Determining Hardness and Elastic Modulus Using Load and Displacement Sensing Indentation Experiments. J. Mater. Res. 1992, 7, 1564–1583. [Google Scholar] [CrossRef]
- Agresti, A. Foundations of Linear and Generalized Linear Models, 1st ed.; John Wiley & Sons: London, UK, 2015. [Google Scholar]
- Luke, S.G. Evaluating Significance in Linear Mixed-Effects Models in R. Behav. Res. Methods 2017, 49, 1494–1502. [Google Scholar] [CrossRef]
- R Core Team; R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing. 2019. Available online: https://www.R-project.org (accessed on 30 December 2020).
- Bates, D.; Mächler, M.; Bolker, B.; Walker, S. Fitting Linear Mixed-Effects Models Using lme4. J. Stat. Softw. 2015, 67, 1–48. [Google Scholar] [CrossRef]
- Kuznetsova, A.; Brockhoff, P.B.; Christensen, R.H.B. lmerTest Package: Tests in Linear Mixed Effects Models. J. Stat. Softw. 2017, 82, 1–26. [Google Scholar] [CrossRef]
- Hu, C. Nanoindentation as a Tool to Measure and Map Mechanical Properties of Hardened Cement Pastes. MRS Commun. 2015, 5, 83. [Google Scholar] [CrossRef]
- Camilleri, J. Composition and setting reaction. In Mineral Trioxide Aggregate in Dentistry: From Preparation to Application; Camilleri, J., Ed.; Springer: New York, NY, USA, 2014; pp. 19–36. [Google Scholar]
- Verbeck, G. Carbonation of hydrated Portland cement. In Cement and Concrete; ASTM International: West Conshohocken, PA, USA, 1958; pp. 17–36. [Google Scholar]
- Jancar, J. Bond Strength of Five Dental Adhesives Using a Fracture Mechanics Approach. J. Mech. Behav. Biomed. Mater. 2011, 4, 245–254. [Google Scholar] [CrossRef]
- Cauwels, R.G.E.C.; Pieters, I.Y.; Martens, L.C.; Verbeeck, R.M.H. Fracture Resistance and Reinforcement of Immature Roots with Gutta Percha, Mineral Trioxide Aggregate and Calcium Phosphate Bone Cement: A Standardized in Vitro Model. Dent. Traumatol. 2010, 26, 137–142. [Google Scholar] [CrossRef]
- Lindberg, G.; Shokry, A.; Reheman, W.; Svensson, I. Determination of Diffusion Coefficients in Bovine Bone by Means of Conductivity Measurement. Int. J. Exp. Comput. Biomech. 2014, 2, 324–342. [Google Scholar] [CrossRef]
- Pashley, D.H.; Livingston, M.J. Effect of Molecular Size on Permeability Coefficients in Human Dentine. Arch. Oral Biol. 1978, 23, 391–395. [Google Scholar] [CrossRef]
- Fogel, H.M.; Marshall, F.J.; Pashley, D.H. Effects of Distance from the Pulp and Thickness on the Hydraulic Conductance of Human Radicular Dentin. J. Dent. Res. 1988, 67, 1381–1385. [Google Scholar] [CrossRef]
- Guignes, P.; Faure, J.; Maurette, A. Relationship between Endodontic Preparations and Human Dentin Permeability Measured in Situ. J. Endod. 1996, 22, 60–67. [Google Scholar] [CrossRef]
- Yildirim, T.; Oruçoğlu, H.; Cobankara, F.K. Long-Term Evaluation of the Influence of Smear Layer on the Apical Sealing Ability of MTA. J. Endod. 2008, 34, 1537–1540. [Google Scholar] [CrossRef]
- Ballal, N.V.; Sona, M.; Tay, F.R. Effects of Smear Layer Removal Agents on the Physical Properties and Microstructure of Mineral Trioxide Aggregate Cement. J. Dent. 2017, 66, 32–36. [Google Scholar] [CrossRef]
- Lee, Y.-L.; Lin, F.-H.; Wang, W.-H.; Ritchie, H.H.; Lan, W.-H.; Lin, C.-P. Effects of EDTA on the Hydration Mechanism of Mineral Trioxide Aggregate. J. Dent. Res. 2007, 86, 534–538. [Google Scholar] [CrossRef] [PubMed]
- Grawehr, M.; Sener, B.; Waltimo, T.; Zehnder, M. Interactions of Ethylenediamine Tetraacetic Acid with Sodium Hypochlorite in Aqueous Solutions. Int. Endod. J. 2003, 36, 411–417. [Google Scholar] [CrossRef]
- Lertmalapong, P.; Jantarat, J.; Srisatjaluk, R.L.; Komoltri, C. Bacterial Leakage and Marginal Adaptation of Various Bioceramics as Apical Plug in Open Apex Model. J. Investig. Clin. Dent. 2019, 10, e12371. [Google Scholar] [CrossRef]
- Al-Kahtani, A.; Shostad, S.; Schifferle, R.; Bhambhani, S. In-Vitro Evaluation of Microleakage of an Orthograde Apical Plug of Mineral Trioxide Aggregate in Permanent Teeth with Simulated Immature Apices. J. Endod. 2005, 31, 117–119. [Google Scholar] [CrossRef] [PubMed]
- Yildirim, T.; Er, K.; Taşdemir, T.; Tahan, E.; Buruk, K.; Serper, A. Effect of Smear Layer and Root-End Cavity Thickness on Apical Sealing Ability of MTA as a Root-End Filling Material: A Bacterial Leakage Study. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2010, 109, e67–e72. [Google Scholar] [CrossRef] [PubMed]
- Bolhari, B.; Nekoofar, M.H.; Sharifian, M.; Ghabrai, S.; Meraji, N.; Dummer, P.M.H. Acid and Microhardness of Mineral Trioxide Aggregate and Mineral Trioxide Aggregate–like Materials. J. Endod. 2014, 40, 432–435. [Google Scholar] [CrossRef]
- Lee, Y.-L.; Lee, B.-S.; Lin, F.-H.; Lin, A.Y.; Lan, W.-H.; Lin, C.-P. Effects of Physiological Environments on the Hydration Behavior of Mineral Trioxide Aggregate. Biomaterials 2004, 25, 787–793. [Google Scholar] [CrossRef]
- Namazikhah, M.S.; Nekoofar, M.H.; Sheykhrezae, M.S.; Salariyeh, S.; Hayes, S.J.; Bryant, S.T.; Mohammadi, M.M.; Dummer, P.M.H. The Effect of PH on Surface Hardness and Microstructure of Mineral Trioxide Aggregate. Int. Endod. J. 2008, 41, 108–116. [Google Scholar] [CrossRef]
- Caicedo, R.; Gettleman, L. Physical properties of MTA. In Mineral Trioxide Aggregate: Properties and Clinical Applications; Torabinejad, M., Ed.; John Wiley & Sons: Ames, IA, USA, 2014; pp. 37–71. [Google Scholar]
- Davydov, D.; Jirasek, M.; Kopecký, L. Critical Aspects of Nano-Indentation Technique in Application to Hardened Cement Paste. Cem. Concr. Res. 2011, 41, 20–29. [Google Scholar] [CrossRef]
- Ylmen, R.; Jäglid, U. Carbonation of Portland Cement Studied by Diffuse Reflection Fourier Transform Infrared Spectroscopy. Int. J. Concr. Struct. Mater. 2013, 7, 119–125. [Google Scholar] [CrossRef]
- Houst, Y.F. Carbonation Shrinkage of Hydrated Cement Paste. In Proceedings of the 4th CANMET/ACI International Conference on Durability of Concrete; CANMET: Ottawa, ON, Canada, 1997; pp. 481–491. [Google Scholar]
- Oraie, E.; Ghassemi, A.R.; Eliasifar, G.; Sadeghi, M.; Shahravan, A. Apical Sealing Ability of MTA in Different Liquid to Powder Ratios and Packing Methods. Iran. Endod. J. 2012, 7, 5. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).