Imaging the Human Thyroid Using Three-Dimensional Diffuse Optical Tomography: A Preliminary Study
Abstract
:Featured Application
Abstract
1. Introduction
2. Subjects and Methods
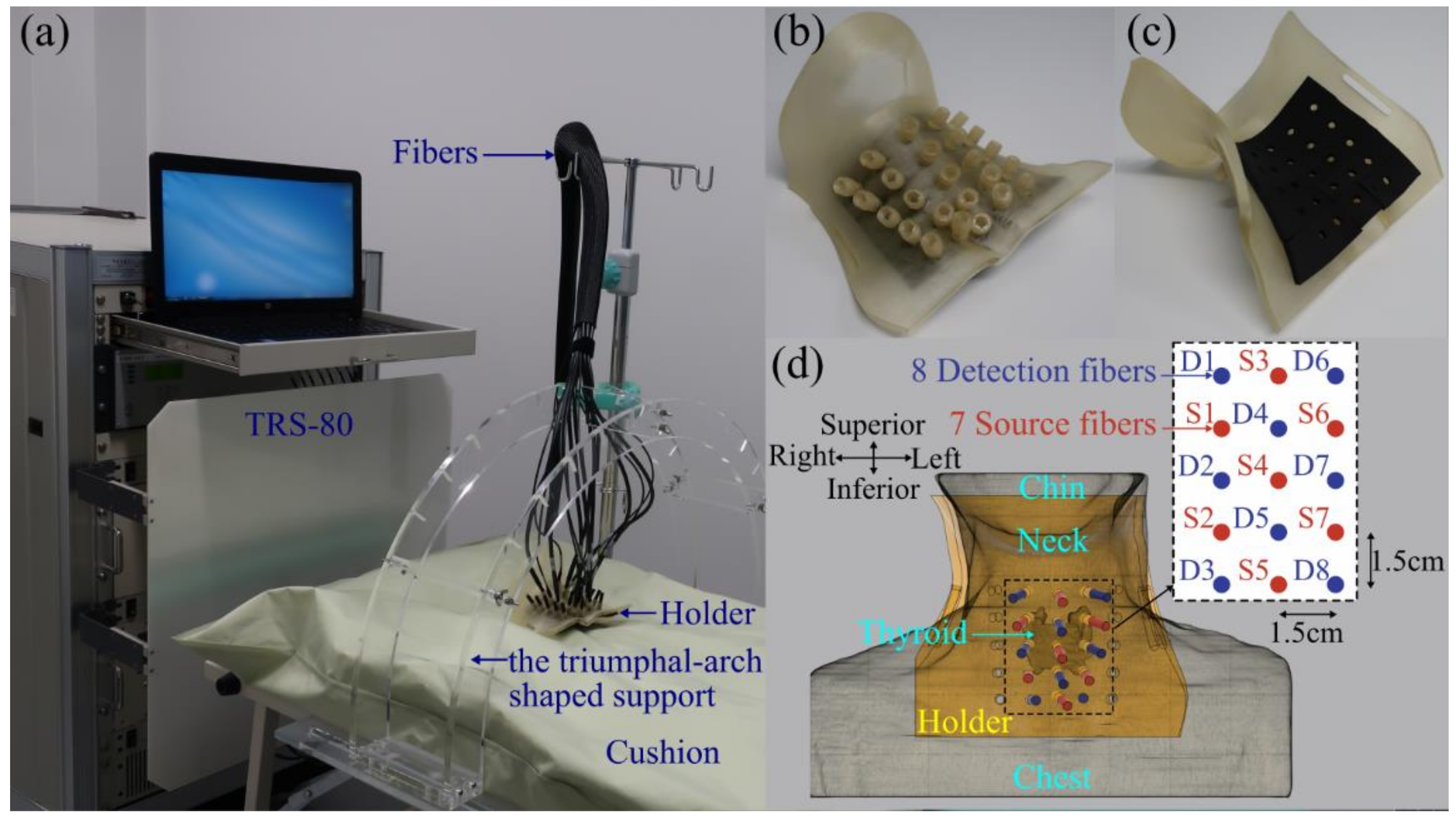
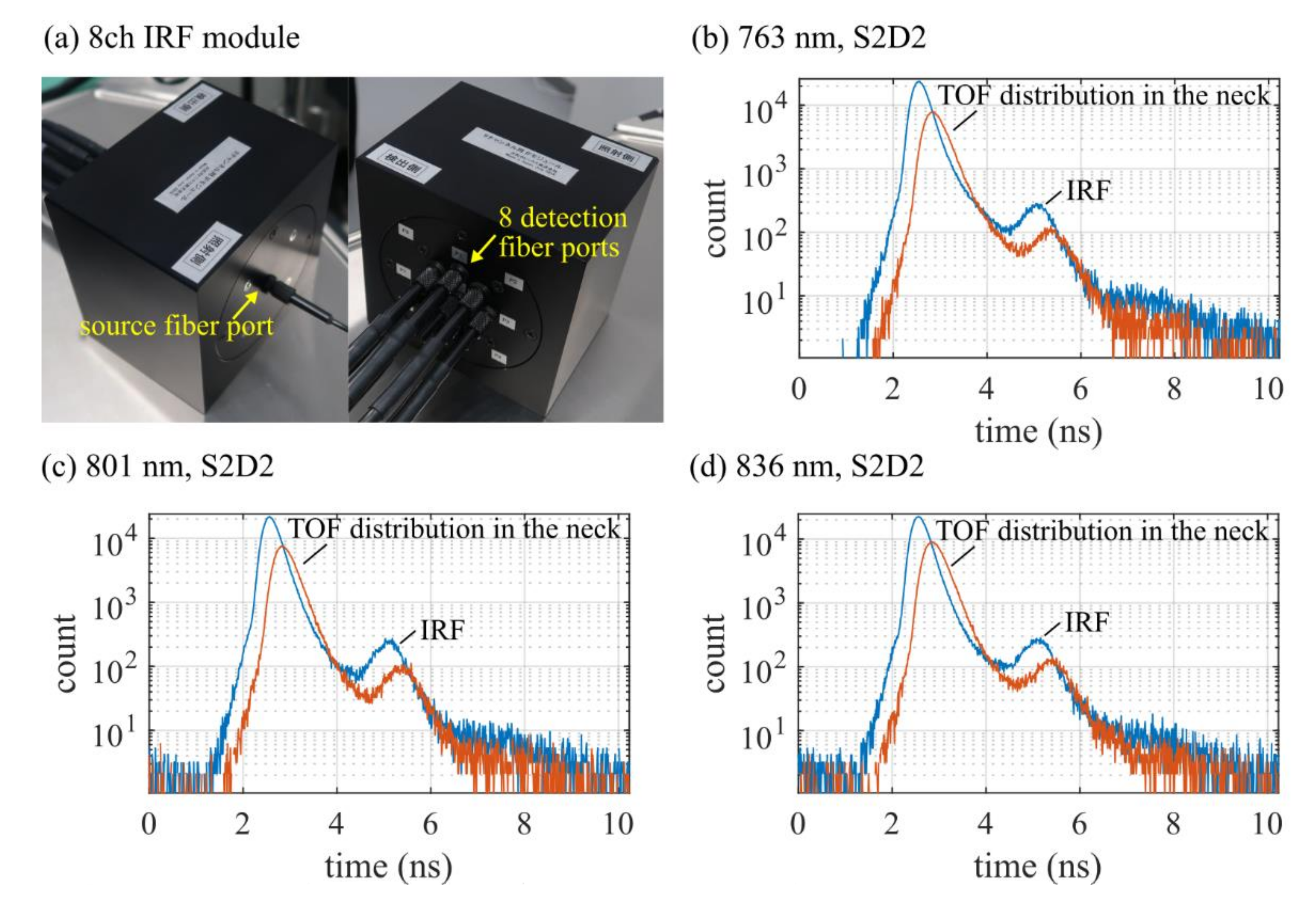
2.1. Instrumentation
2.2. Human Neck Measurements
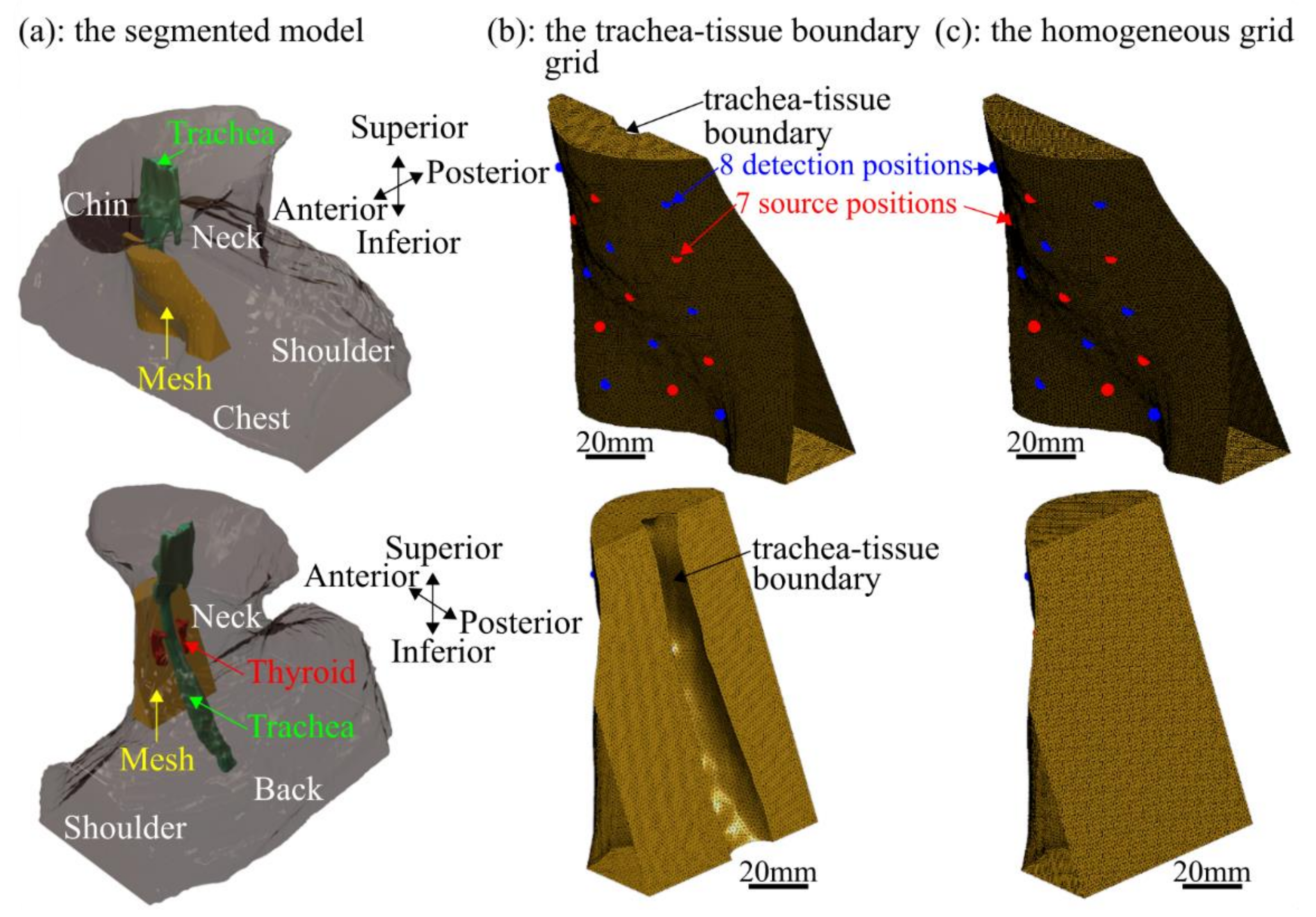
2.3. Grid Excluding the Trachea
2.4. Image Reconstruction
2.4.1. Light Transport Model
2.4.2. Finite Element Method
2.4.3. Inverse Problem
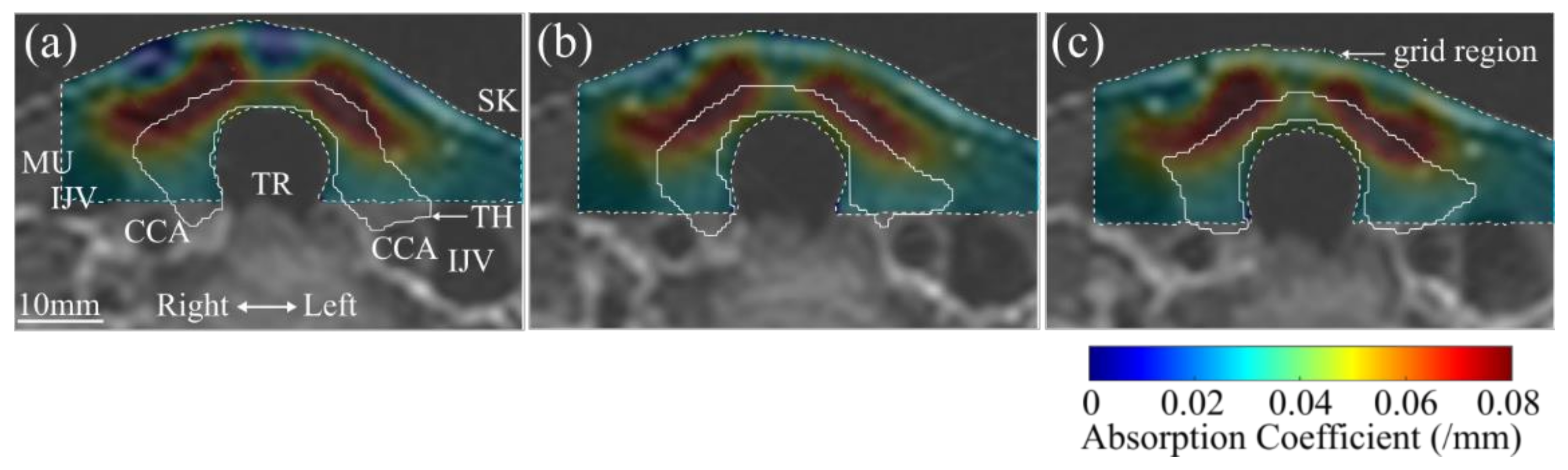
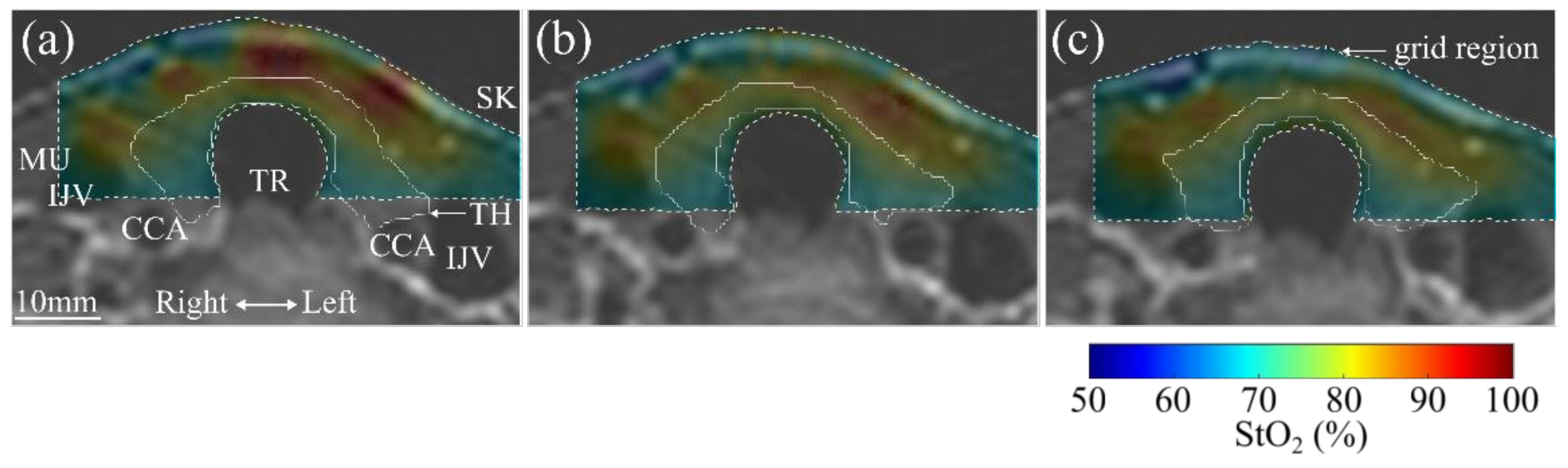
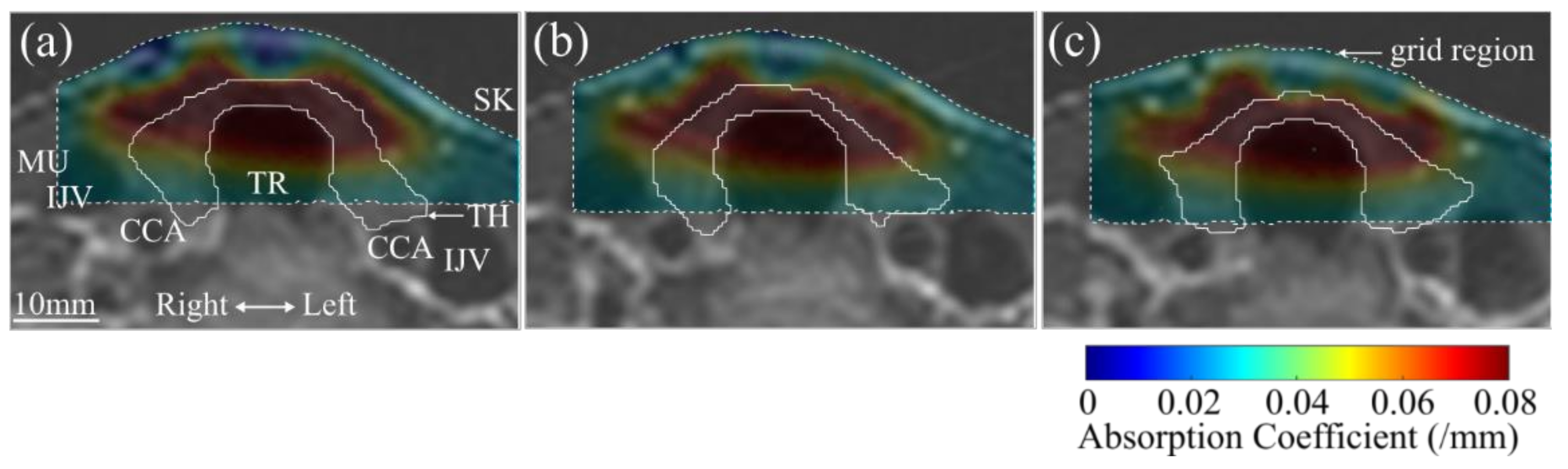
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wartofsky, L. Increasing world incidence of thyroid cancer: Increased detection or higher radiation exposure? Hormones 2010, 9, 103–108. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Deng, Y.J.; Li, H.T.; Wang, M.; Li, N.; Tian, T.; Wu, Y.; Xu, P.; Yang, S.; Zhai, Z.; Zhou, L.H.; et al. Global burden of thyroid cancer from 1990 to 2017. JAMA Netw. Open 2020, 3, e208759. [Google Scholar] [CrossRef] [PubMed]
- Davies, L.; Welch, H.G. Increasing incidence of thyroid cancer in the United States, 1973-2002. JAMA 2006, 295, 2164–2167. [Google Scholar] [CrossRef] [Green Version]
- Shah, J.P. Thyroid carcinoma: Epidemiology, histology, and diagnosis. Clin. Adv. Hematol. Oncol. 2015, 13, 3–6. [Google Scholar] [PubMed]
- Baloch, Z.W.; LiVolsi, V.A. Follicular-patterned lesions of the thyroid: The bane of the pathologist. Am. J. Clin. Pathol. 2002, 117, 143–150. [Google Scholar] [CrossRef] [Green Version]
- Cipriani, N.A.; Nagar, S.; Kaplan, S.P.; White, M.G.; Antic, T.; Sadow, P.M.; Aschebrook-Kilfoy, B.; Angelos, P.; Kaplan, E.L.; Grogan, R.H. Follicular thyroid carcinoma: How have histologic diagnoses changed in the last half-century and what are the prognostic implications? Thyroid 2015, 25, 1209–1216. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- King, A.D. Imaging for staging and management of thyroid cancer. Cancer Imaging 2008, 8, 57–69. [Google Scholar] [CrossRef] [Green Version]
- Marcus, C.; Whitworth, P.W.; Surasi, D.S.; Pai, S.I.; Subramaniam, R.M. PET/CT in the management of thyroid cancers. Am. J. Roentgenol. 2014, 202, 1316–1329. [Google Scholar] [CrossRef] [Green Version]
- Sprindzuk, M.V. Angiogenesis in malignant thyroid tumors. World J. Oncol. 2010, 1, 221–231. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cho Mar, K.; Eimoto, T.; Tateyama, H.; Arai, Y.; Fujiyoshi, Y.; Hamaguchi, M. Expression of matrix metalloproteinases in benign and malignant follicular thyroid lesions. Histopathology 2006, 48, 286–294. [Google Scholar] [CrossRef]
- Burrows, N.; Resch, J.; Cowen, R.L.; Von Wasielewski, R.; Hoang-Vu, C.; West, C.M.; Williams, K.J.; Brabant, G. Expression of hypoxia-inducible factor 1α in thyroid carcinomas. Endocr. Relat. Cancer 2010, 17, 61–72. [Google Scholar] [CrossRef]
- Yang, M.; Zhao, L.; He, X.; Su, N.; Zhao, C.; Tang, H.; Hong, T.; Li, W.; Yang, F.; Lin, L.; et al. Photoacoustic/ultrasound dual imaging of human thyroid cancers: An initial clinical study. Biomed. Opt. Express 2017, 8, 3449–3457. [Google Scholar] [CrossRef] [Green Version]
- Levi, J.; Kothapalli, S.R.; Bohndiek, S.; Yoon, J.K.; Dragulescu-Andrasi, A.; Nielsen, C.; Tisma, A.; Bodapati, S.; Gowrishankar, G.; Yan, X.; et al. Molecular photoacoustic imaging of follicular thyroid carcinoma. Clin. Cancer Res. 2013, 19, 1494–1502. [Google Scholar] [CrossRef] [Green Version]
- Lindner, C.; Mora, M.; Farzam, P.; Squarcia, M.; Johansson, J.; Weigel, U.M.; Halperin, I.; Hanzu, F.A.; Durduran, T. Diffuse optical characterization of the healthy human thyroid tissue and two pathological case studies. PLoS ONE 2016, 11, e0147851. [Google Scholar] [CrossRef] [PubMed]
- Mahkamova, K.; Latar, N.; Aspinall, S.; Meeson, A. Hypoxia increases thyroid cancer stem cell-enriched side population. World J. Surg. 2018, 42, 350–357. [Google Scholar] [CrossRef] [Green Version]
- Arridge, S.R. Optical tomography in medical imaging. Inverse Probl. 1999, 15, R41–R93. [Google Scholar] [CrossRef] [Green Version]
- Hoshi, Y.; Hazeki, O.; Kakihana, Y.; Tamura, M. Redox behavior of cytochrome oxidase in the rat brain measured by near- infrared spectroscopy. J. Appl. Physiol. 1997, 83, 1842–1848. [Google Scholar] [CrossRef] [PubMed]
- Hoshi, Y.; Yamada, Y. Overview of diffuse optical tomography and its clinical applications. J. Biomed. Opt. 2016, 21, 091312. [Google Scholar] [CrossRef] [Green Version]
- Yoshizawa, N.; Ueda, Y.; Mimura, T.; Ohmae, E.; Yoshimoto, K.; Wada, H.; Ogura, H.; Sakahara, H. Factors affecting measurement of optic parameters by time-resolved near-infrared spectroscopy in breast cancer. J. Biomed. Opt. 2018, 23, 026010. [Google Scholar]
- Dehghani, H.; Arridge, S.R.; Schweiger, M.; Delpy, D.T. Optical tomography in the presence of void regions. J. Opt. Soc. Am. A Opt. Image Sci. Vis. 2000, 17, 1659–1670. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Riley, J.; Dehghani, H.; Schweiger, M.; Arridge, S.R.; Ripoll, J.; Nieto-Vesperinas, M. 3D optical tomography in the presence of void regions. Opt. Express 2000, 7, 462–467. [Google Scholar] [CrossRef]
- Fedorov, A.; Beichel, R.; Kalpathy-Cramer, J.; Finet, J.; Fillion-Robin, J.C.; Pujol, S.; Bauer, C.; Jennings, D.; Fennessy, F.; Sonka, M.; et al. 3D slicer as an image computing platform for the quantitative imaging network. Magn. Reson. Imaging 2012, 30, 1323–1341. [Google Scholar] [CrossRef] [Green Version]
- Fujii, H.; Yamada, Y.; Kobayashi, K.; Watanabe, M.; Hoshi, Y. Modeling of light propagation in the human neck for diagnoses of thyroid cancers by diffuse optical tomography. Int. J. Numer. Methods Biomed. Eng. 2017, 33, e2826. [Google Scholar] [CrossRef] [Green Version]
- Geuzaine, C.; Remacle, J.F. Gmsh: A 3-D finite element mesh generator with built-in pre- and post-processing facilities. Int. J. Numer. Methods Eng. 2009, 79, 1309–1331. [Google Scholar] [CrossRef]
- Arridge, S.R.; Schweiger, M. Direct calculation of the moments of the distribution of photon time of flight in tissue with a finite-element method. Appl. Opt. 1995, 34, 2683–2687. [Google Scholar] [CrossRef] [Green Version]
- Jorge, N.; Wright, S. Numerical Optimization, 2nd ed.; Springer: New York, NY, USA, 2006; pp. 121–124. [Google Scholar]
- Arridge, S.R.; Schweiger, M. A gradient-based optimisation scheme for optical tomography. Opt. Express 1998, 2, 213–226. [Google Scholar] [CrossRef] [PubMed]
- Schweiger, M.; Arridge, S.R. The Toast++ software suite for forward and inverse modeling in optical tomography. J. Biomed. Opt. 2014, 19, 040801. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Konugolu Venkata Sekar, S.; Farina, A.; Dalla Mora, A.; Lindner, C.; Pagliazzi, M.; Mora, M.; Aranda, G.; Dehghani, H.; Durduran, T.; Taroni, P.; et al. Broadband (550-1350 nm) diffuse optical characterization of thyroid chromophores. Sci. Rep. 2018, 8, 10015. [Google Scholar] [CrossRef] [Green Version]
- Matcher, S.J.; Elwell, C.E.; Cooper, C.E.; Cope, M.; Delpy, D.T. Performance comparison of several published tissue near-infrared spectroscopy algorithms. Anal. Biochem. 1995, 227, 54–68. [Google Scholar] [CrossRef]
- Kou, L.; Labrie, D.; Chylek, P. Refractive indices of water and ice in the 065- to 25-μm spectral range. Appl. Opt. 1993, 32, 3531–3540. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- White, D.R.; Woodard, H.Q.; Hammond, S.M. Average soft-tissue and bone models for use in radiation dosimetry. Br. J. Radiol. 1987, 60, 907–913. [Google Scholar] [CrossRef]
- Pogue, B.W.; McBride, T.O.; Prewitt, J.; Österberg, U.L.; Paulsen, K.D. Spatially variant regularization improves diffuse optical tomography. Appl. Opt. 1999, 38, 2950–2961. [Google Scholar] [CrossRef] [PubMed]
- Culver, J.P.; Durduran, T.; Furuya, D.; Cheung, C.; Greenberg, J.H.; Yodh, A.G. Diffuse optical tomography of cerebral blood flow, oxygenation, and metabolism in rat during focal ischemia. J. Cereb. Blood Flow Metab. 2003, 23, 911–924. [Google Scholar] [CrossRef] [Green Version]
- Gao, F.; Poulet, P.; Yamada, Y. Simultaneous mapping of absorption and scattering coefficients from a three-dimensional model of time-resolved optical tomography. Appl. Opt. 2000, 39, 5898–5910. [Google Scholar] [CrossRef] [PubMed]
- Schweiger, M.; Arridge, S.R. Application of temporal filters to time resolved data in optical tomography. Phys. Med. Biol. 1999, 44, 1699–1717. [Google Scholar] [CrossRef]
- Poudel, P.; Hansen, C.; Sprung, J.; Friebe, M. 3D segmentation of thyroid ultrasound images using active contours. Curr. Dir. Biomed. Eng. 2016, 2, 467–470. [Google Scholar] [CrossRef]
- Poudel, P.; Illanes, A.; Sheet, D.; Friebe, M. Evaluation of commonly used algorithms for thyroid ultrasound images segmentation and improvement using machine learning approaches. J. Healthc. Eng. 2018, 2018, 1–13. [Google Scholar] [CrossRef]
- Bernhard, P.; Jana, D.; Lars, D.; Andreas, B. Clinical Impact of the Tumor Therapy Manager. In Proceedings of the VisWeek 2010, Salt Lake City, UT, USA, 24–29 October 2010; IEEE: New York, NY, USA, 2010. [Google Scholar]
- Thiering, B.; Nagarajah, J.; Lipinski, H.G. Spatial Reconstruction of Human Thyroid based on Ultrasound and CT Image Data Fusion. Biomed. Tech. 2013, 58, 4284. [Google Scholar] [CrossRef]
- Ciora, R.A.; Neamtu, B.; Şofariu, C.; Dospinescu, C.; Barbu, A.; Banciu, D. A Simple Method for 3D Thyroid Reconstruction from 2D Ultrasound Slices. In Proceedings of the 2019 E-Health and Bioengineering Conference (EHB), Iasi, Romania, 21–23 November 2019; IEEE: New York, NY, USA, 2019. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mimura, T.; Okawa, S.; Kawaguchi, H.; Tanikawa, Y.; Hoshi, Y. Imaging the Human Thyroid Using Three-Dimensional Diffuse Optical Tomography: A Preliminary Study. Appl. Sci. 2021, 11, 1670. https://doi.org/10.3390/app11041670
Mimura T, Okawa S, Kawaguchi H, Tanikawa Y, Hoshi Y. Imaging the Human Thyroid Using Three-Dimensional Diffuse Optical Tomography: A Preliminary Study. Applied Sciences. 2021; 11(4):1670. https://doi.org/10.3390/app11041670
Chicago/Turabian StyleMimura, Tetsuya, Shinpei Okawa, Hiroshi Kawaguchi, Yukari Tanikawa, and Yoko Hoshi. 2021. "Imaging the Human Thyroid Using Three-Dimensional Diffuse Optical Tomography: A Preliminary Study" Applied Sciences 11, no. 4: 1670. https://doi.org/10.3390/app11041670
APA StyleMimura, T., Okawa, S., Kawaguchi, H., Tanikawa, Y., & Hoshi, Y. (2021). Imaging the Human Thyroid Using Three-Dimensional Diffuse Optical Tomography: A Preliminary Study. Applied Sciences, 11(4), 1670. https://doi.org/10.3390/app11041670






