Facile Hydrothermal and Solvothermal Synthesis and Characterization of Nitrogen-Doped Carbon Dots from Palm Kernel Shell Precursor
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Characterization Instruments
2.3. Preparation of Palm Kernel Shell
2.4. Carbonization of Palm Kernel Shell
2.5. Synthesis of Carbon Dots (CDs) Using Hydrothermal Method
2.6. Measurement of Quantum Yields
3. Results and Discussion
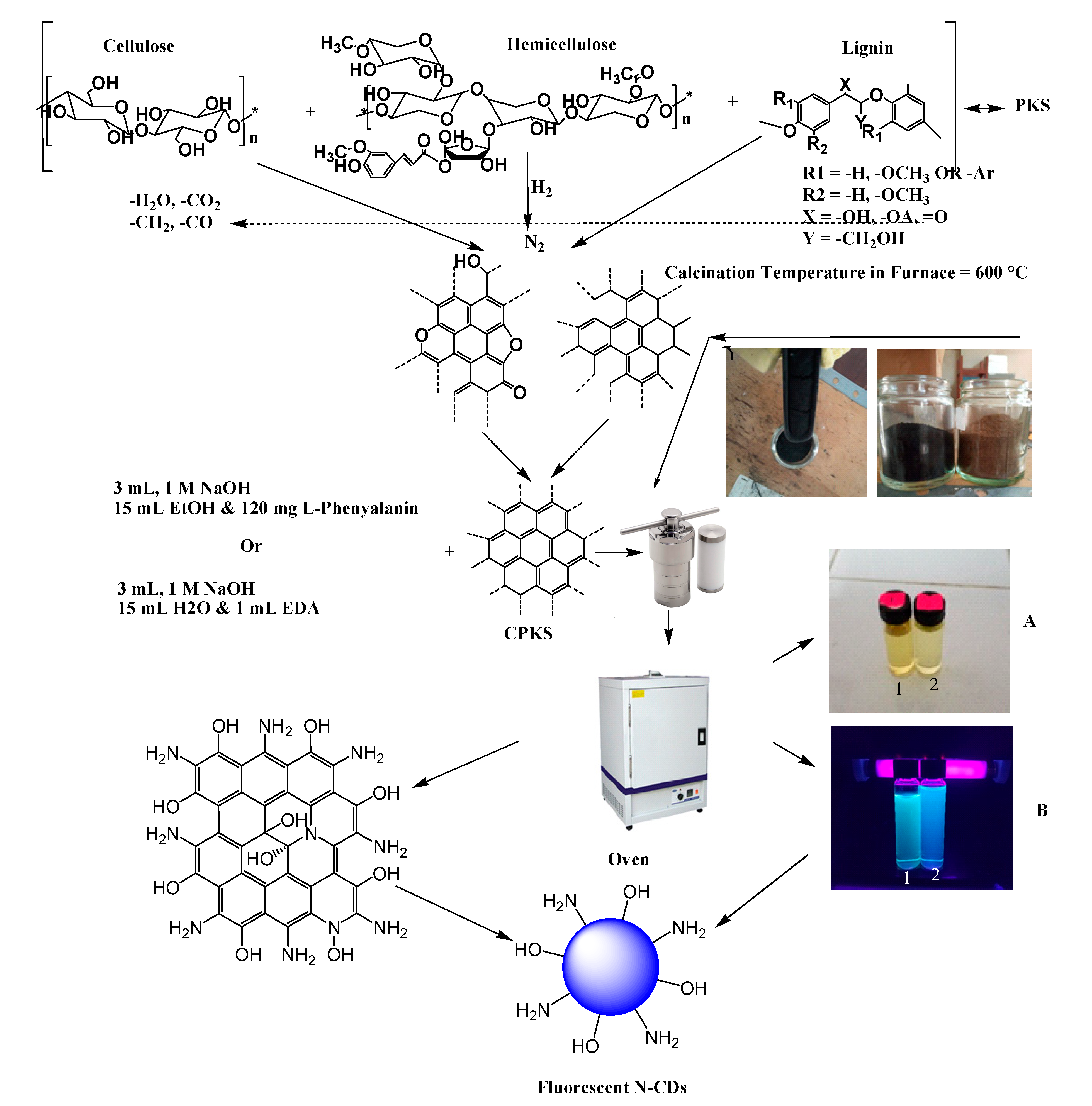
3.1. Carbonization of Palm Kernel Shell (PKS)
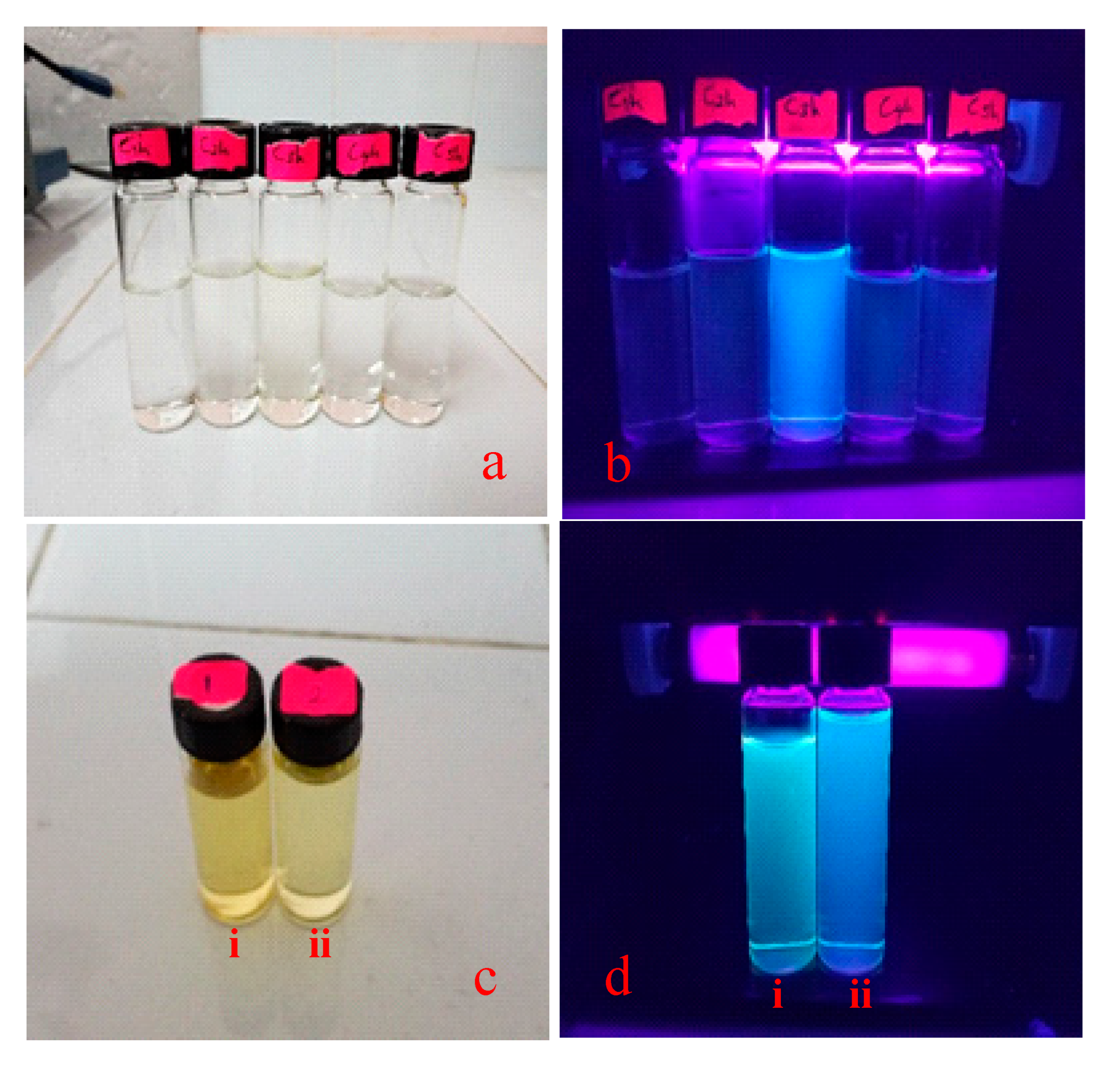
3.2. Synthesis and Characterization of CDs
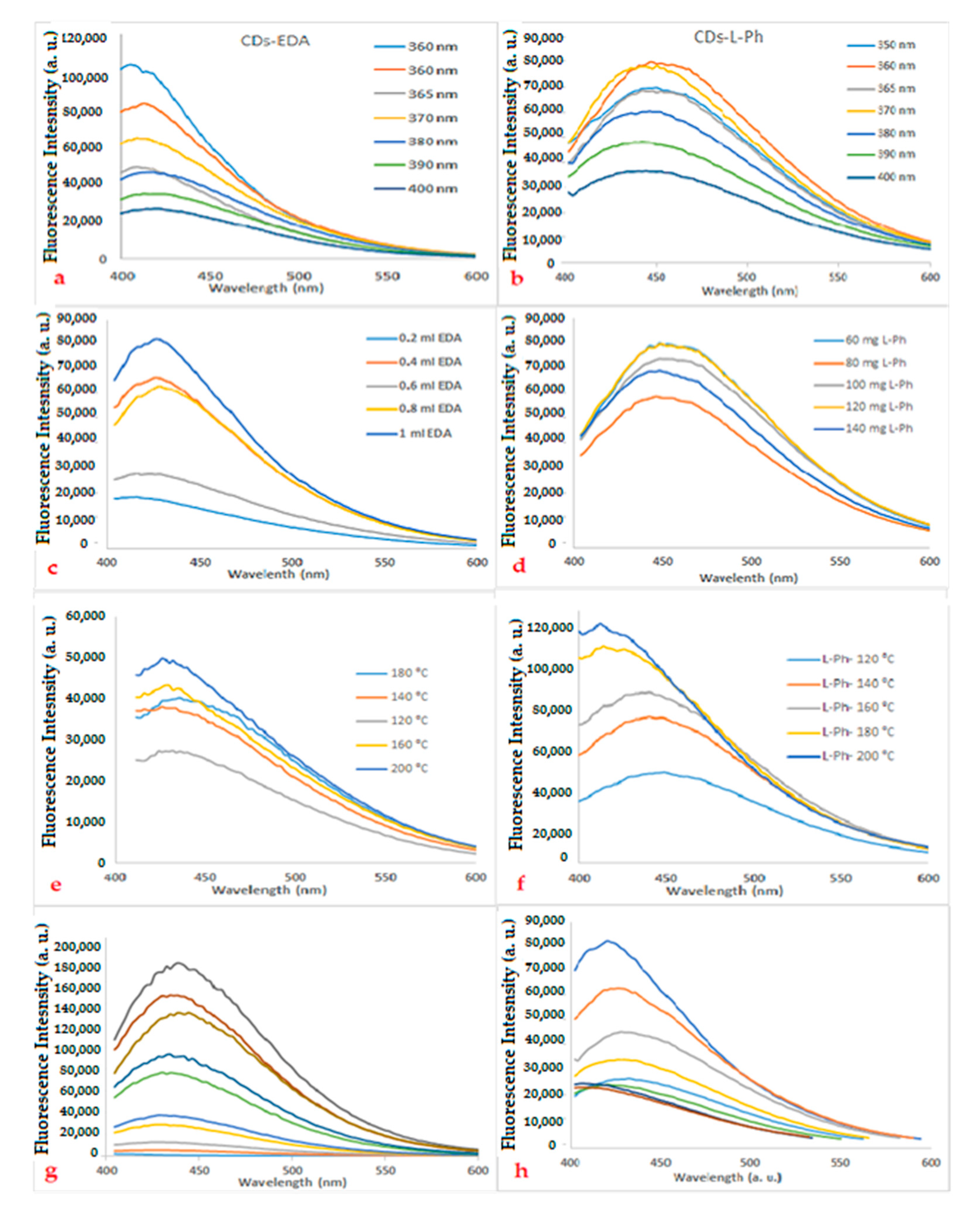
3.3. Optimization Parameter for the Synthesis of Carbon Dots (CDs)
3.3.1. Effect of Amount of Dopant
3.3.2. Effect of Temperature of Synthesis on CDs
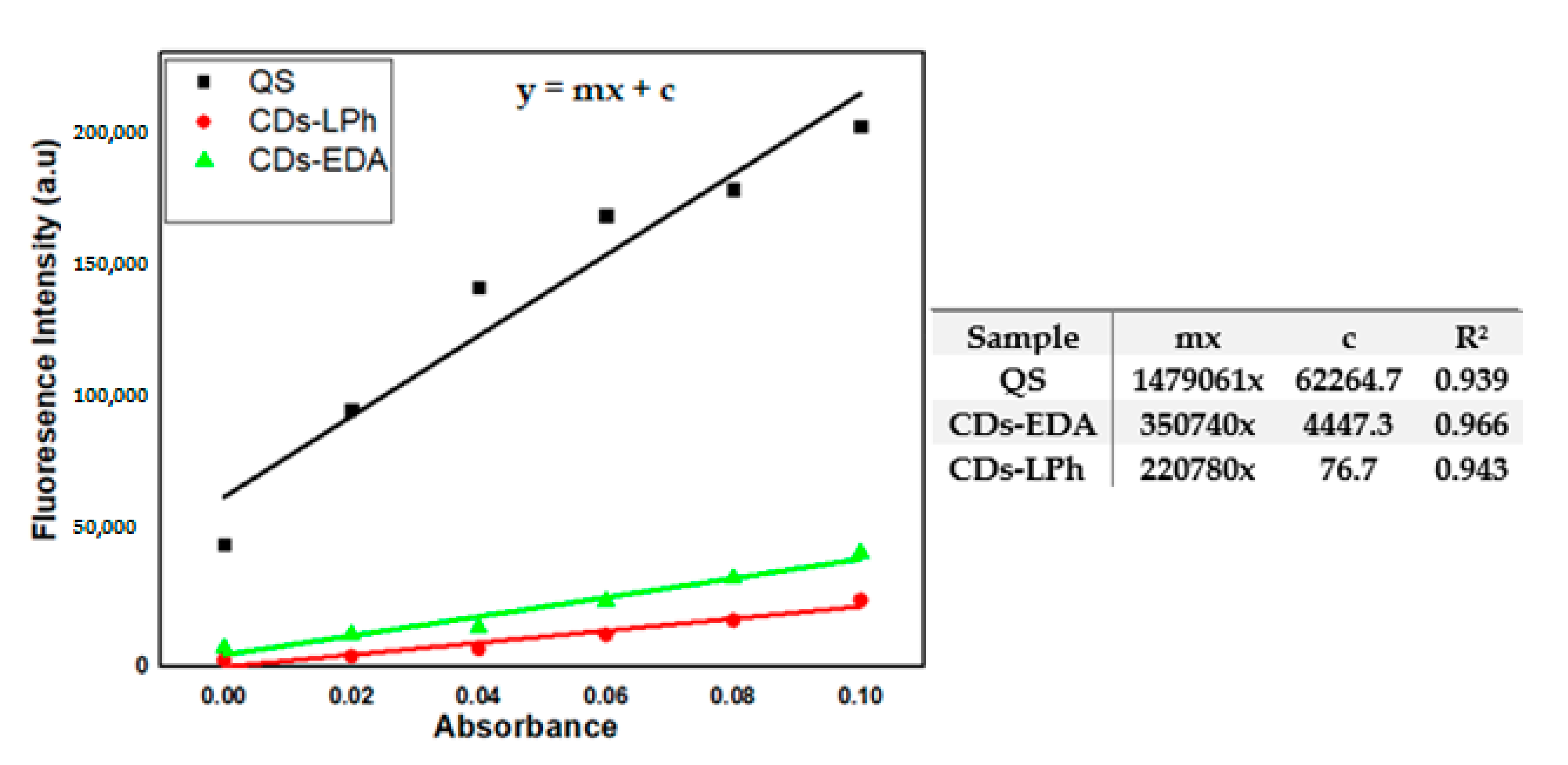
3.4. Determination of Photoluminescence Quantum Yield
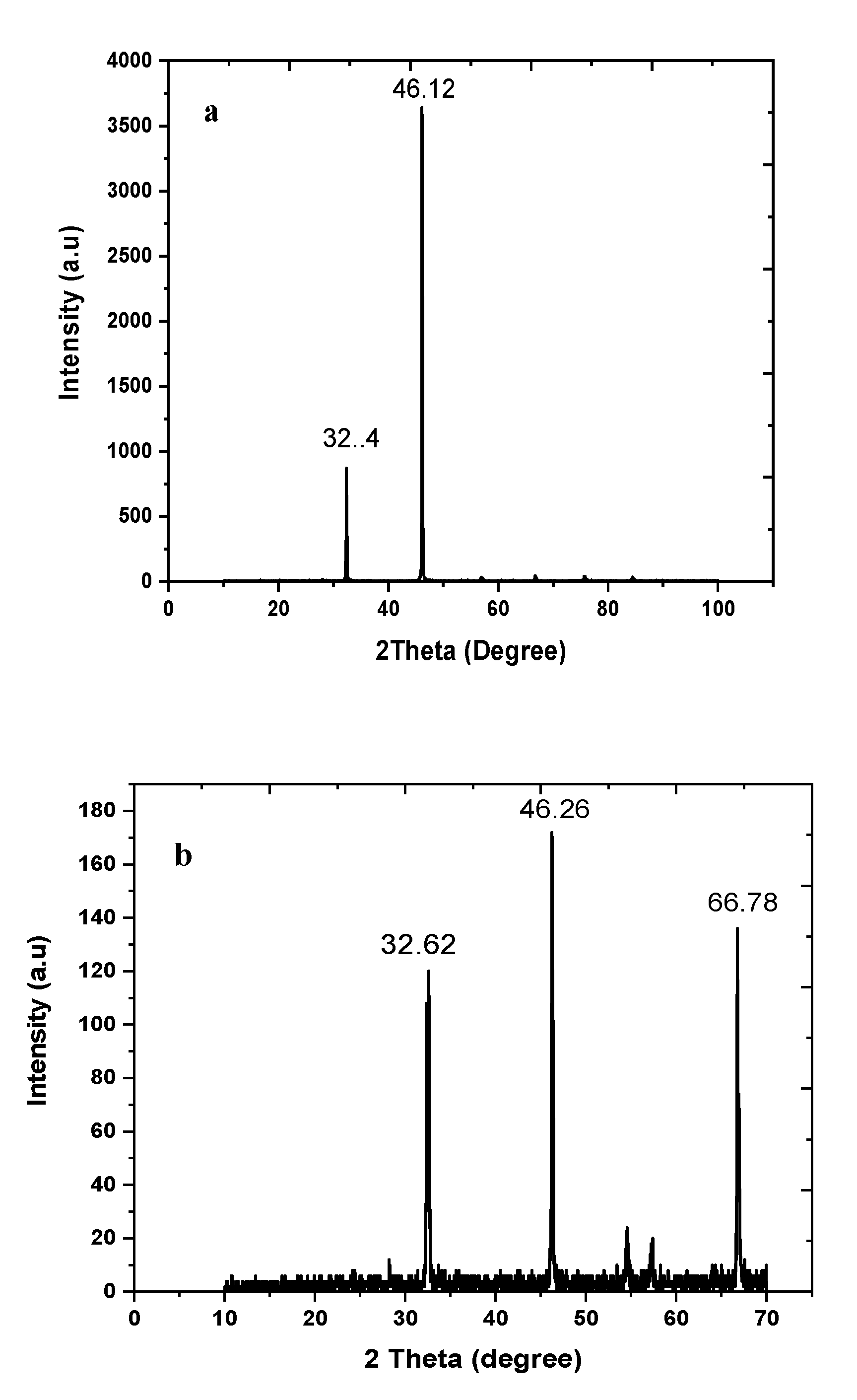
3.5. Transmission Electron Microscopy (TEM) and X-ray Diffraction Spectroscopy (XRD)
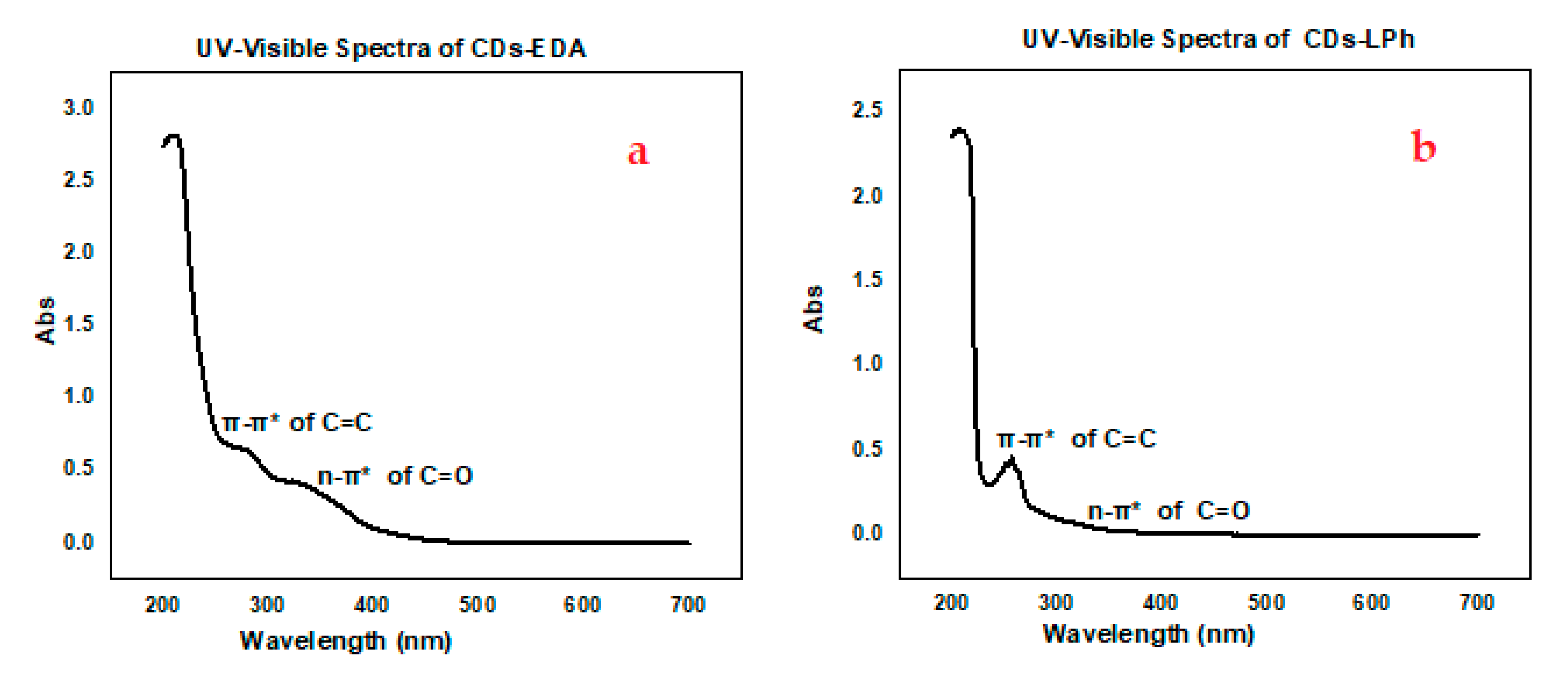
3.6. UV-Visible Spectroscopy
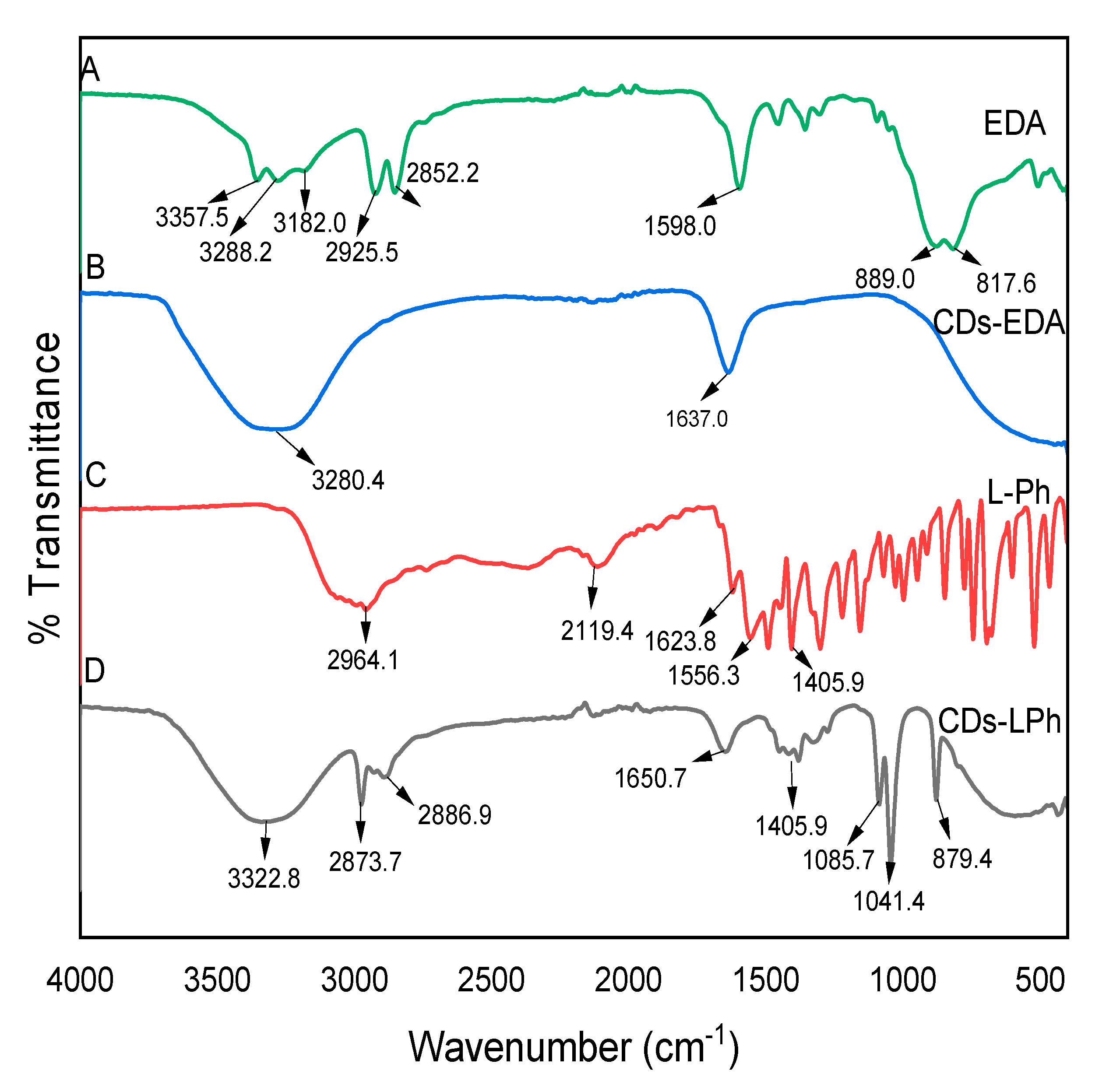
3.7. Fourier-Transform Infra-Red (FTIR) Spectroscopy
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Pudza, M.Y.; Abidin, Z.Z.; Abdul-Rashid, S.; Yassin, F.M.; Noor, A.S.M.; Abdullah, M. Synthesis and Characterization of Fluorescent Carbon Dots from Tapioca. ChemistrySelect 2019, 4, 4140–4146. [Google Scholar] [CrossRef]
- Xu, X.; Ray, R.; Gu, Y.; Ploehn, H.J.; Gearheart, L.; Raker, K.; Scrivens, W.A. Electrophoretic Analysis and Purification of Fluorescent Single-Walled Carbon Nanotube Fragments. J. Am. Chem. Soc. 2004, 126, 12736–12737. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Desserre, A.; Sharma, S.K.; Li, S.; Marksberry, M.H.; Chusuei, C.C.; Blackwelder, P.L.; Zhou, Y. Gel-like Carbon Dots: Characterization and their Potential Applications. ChemPhysChem 2017, 18, 890–897. [Google Scholar] [CrossRef] [PubMed]
- Shi, H.; Wei, J.; Qiang, L.; Chen, X.; Meng, X. Fluorescent Carbon Dots for Bioimaging and Biosensing Applications. J. Biomed. Nanotechnol. 2014, 10, 2677–2699. [Google Scholar] [CrossRef] [PubMed]
- Martins, N.C.; Ângelo, J.; Girão, A.V.; Trindade, T.; Andrade, L.; Mendes, A. N-doped carbon quantum dots/TiO2 composite with improved photocatalytic activity. Appl. Catal. B Environ. 2016, 193, 67–74. [Google Scholar] [CrossRef]
- Pal, T.; Mohiyuddin, S.; Packirisamy, G. Facile and Green Synthesis of Multicolor Fluorescence Carbon Dots from Curcumin: In Vitro and in Vivo Bioimaging and Other Applications. ACS Omega 2018, 3, 831–843. [Google Scholar] [CrossRef]
- Liu, M.L.; Bin Chen, B.; Li, C.M.; Huang, C.Z. Carbon dots: Synthesis, formation mechanism, fluorescence origin and sensing applications. Green Chem. 2019, 21, 449–471. [Google Scholar] [CrossRef]
- Cayuela, A.; Soriano, M.; Carrillocarrion, C.; Valcárcel, M. Semiconductor and carbon-based fluorescent nanodots: The need for consistency. Chem. Commun. 2016, 52, 1311–1326. [Google Scholar] [CrossRef]
- Li, L.; Dong, T. Photoluminescence tuning in carbon dots: Surface passivation or/and functionalization, heteroatom doping. J. Mater. Chem. C 2018, 6, 7944–7970. [Google Scholar] [CrossRef]
- Nekoueian, K.; Amiri, M.; Sillanpää, M.; Marken, F.; Boukherroub, R.; Szunerits, S. Carbon-based quantum particles: An electroanalytical and biomedical perspective. Chem. Soc. Rev. 2019, 48, 4281–4316. [Google Scholar] [CrossRef]
- Deng, J.; Lu, Q.; Mi, N.; Li, H.; Liu, M.; Xu, M.; Tan, L.; Xie, Q.; Zhang, Y.; Yao, S. Electrochemical Synthesis of Carbon Nanodots Directly from Alcohols. Chem. A Eur. J. 2014, 20, 4993–4999. [Google Scholar] [CrossRef]
- Arcudi, F.; Ðorđević, L.; Prato, M. Design, Synthesis, and Functionalization Strategies of Tailored Carbon Nanodots. Acc. Chem. Res. 2019, 52, 2070–2079. [Google Scholar] [CrossRef]
- Azmi, N.E.; Ramli, N.I.; Abdullah, J.; Hamid, M.A.A.; Sidek, H.; Rahman, S.A.; Ariffin, N.; Yusof, N.A. A simple and sensitive fluorescence based biosensor for the determination of uric acid using H2O2-sensitive quantum dots/dual enzymes. Biosens. Bioelectron. 2015, 67, 129–133. [Google Scholar] [CrossRef]
- Mousavi, S.M.; Hashemi, S.A.; Zarei, M.; Amani, A.M.; Babapoor, A. Nanosensors for Chemical and Biological and Medical Applications. Med. Chem. 2018, 8, 205–217. [Google Scholar] [CrossRef]
- Patra, J.K.; Das, G.; Fraceto, L.F.; Campos, E.V.R.; del Rodriguez-Torres, M.P.; Acosta-Torres, L.S.; Diaz-Torres, L.A.; Grillo, R.; Swamy, M.K.; Sharma, S.; et al. Nano based drug delivery systems: Recent developments and future prospects 10 Technology 1007 Nanotechnology 03 Chemical Sciences 0306 Physical Chemistry (incl. Structural) 03 Chemical Sciences 0303 Macromolecular and Materials Chemistry 11 Medical and He. J. Nanobiotechnol. 2018, 16, 1–33. [Google Scholar] [CrossRef]
- Pengju, L.G.; Sahu, S.; Yang, S.T.; Sonkar, S.K.; Wang, J.; Wang, H. Carbon “quantum” dots for optical bioimaging. J. Mater. Chem. B 2013, 1, 2116–2127. [Google Scholar]
- Yang, S.; Sun, X.; Wang, Z.; Wang, X.; Guo, G.; Pu, Q. Anomalous enhancement of fluorescence of carbon dots through lanthanum doping and potential application in intracellular imaging of ferric ion. Nano Res. 2018, 11, 1369–1378. [Google Scholar] [CrossRef]
- Chu, K.-W.; Lee, S.L.; Chang, C.-J.; Liu, L. Recent Progress of Carbon Dot Precursors and Photocatalysis Applications. Polymer 2019, 11, 689. [Google Scholar] [CrossRef] [PubMed]
- Raquin, V.; Wannagat, M.; Zouache, K.; Legras-Lachuer, C.; Moro, C.V.; Mavingui, P. Detection of dengue group viruses by fluorescence in situ hybridization. Parasites Vectors 2012, 5, 243. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Pang, S.; Wang, L.; Li, Q.; Kreiter, M.; Liu, C. One-Step Synthesis of Highly Luminescent Carbon Dots in Noncoordinating Solvents. Chem. Mater. 2010, 22, 4528–4530. [Google Scholar] [CrossRef]
- Atabaev, T.S. Doped Carbon Dots for Sensing and Bioimaging Applications: A Minireview. Nanomaterial 2018, 8, 342. [Google Scholar] [CrossRef] [PubMed]
- Sharma, V.; Tiwari, P.; Mobin, S.M. Sustainable carbon-dots: Recent advances in green carbon dots for sensing and bioimaging. J. Mater. Chem. B 2017, 5, 8904–8924. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Hu, A. Carbon quantum dots: Synthesis, properties and applications. J. Mater. Chem. C 2014, 2, 6921–6939. [Google Scholar] [CrossRef]
- Sharma, A.; Das, J. Small molecules derived carbon dots: Synthesis and applications in sensing, catalysis, imaging, and biomedicine. J. Nanobiotechnol. 2019, 17, 1–24. [Google Scholar] [CrossRef] [PubMed]
- Grumezescu, A.M. Fullerens, Graphenes and Nanotubes: A Pharmaceutical Approach Pharmaceutical Nanotechnology; Grumezescu, A.M., Ed.; Elsevier Science: Amsterdam, The Netherlands, 2018; Volume 2018, pp. 1–724. [Google Scholar]
- Jhonsi, M.A. Carbon Quantum Dots for Bioimaging; NPG Asia Materials; Intech Open.: London, UK, 2018; p. 64. [Google Scholar] [CrossRef]
- Dager, A.; Uchida, T.; Maekawa, T.; Tachibana, M. Synthesis and characterization of Mono-disperse Carbon Quantum Dots from Fennel Seeds: Photoluminescence analysis using Machine Learning. Sci. Rep. 2019, 9, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Han, M.Y.; Oezyilmaz, B.; Zhang, Y.; Kim, P. Energy Band-Gap Engineering of Graphene Nanoribbons. Phys. Rev. Lett. 2007, 98, 206805. [Google Scholar] [CrossRef]
- Peng, H.; Travas-Sejdic, J. Simple Aqueous Solution Route to Luminescent Carbogenic Dots from Carbohydrates. Chem. Mater. 2009, 21, 5563–5565. [Google Scholar] [CrossRef]
- Qiao, Z.-A.; Wang, Y.; Gao, Y.; Li, H.; Dai, T.; Liu, Y.; Huo, Q. Commercially activated carbon as the source for producing multicolor photoluminescent carbon dots by chemical oxidation. Chem. Commun. Suppl. Inf. 2010, 46, 8812–8814. [Google Scholar] [CrossRef]
- Wang, J.; Sahu, S.; Sonkar, S.K.; Ii, K.N.T.; Sun, K.W.; Liu, Y.; Maimaiti, H.; Anilkumar, P.; Sun, Y.-P. Versatility with carbon dots—From overcooked BBQ to brightly fluorescent agents and photocatalysts. RSC Adv. 2013, 3, 15604–15607. [Google Scholar] [CrossRef]
- Li, X.; Wang, H.; Shimizu, Y.; Pyatenko, A.; Kawaguchi, K.; Koshizaki, N. Preparation of carbon quantum dots with tunable photoluminescence by rapid laser passivation in ordinary organic solvents. Chem. Commun. 2011, 47, 932–934. [Google Scholar] [CrossRef]
- Zhou, J.; Booker, C.; Li, R.; Zhou, X.; Sham, T.-K.; Sun, X.; Ding, Z. An Electrochemical Avenue to Blue Luminescent Nanocrystals from Multiwalled Carbon Nanotubes (MWCNTs). J. Am. Chem. Soc. 2007, 129, 744–745. [Google Scholar] [CrossRef]
- Zhuo, S.; Shao, M.; Lee, S.-T. Upconversion and downconversion fluorescent graphene quantum dots: Ultrasonic preparation and photocatalysis. ACS Nano 2012, 6, 6530–6531. [Google Scholar] [CrossRef]
- Wang, Y.; Xia, Y. Bottom-Up and Top-Down Approaches to the Synthesis of Monodispersed Spherical Colloids of Low Melting-Point Metals. Nano Lett. 2004, 4, 2047–2050. [Google Scholar] [CrossRef]
- Basolo, F. Inorganic Chemistry. J. Am. Chem. Soc. 2005, 86, 311. [Google Scholar] [CrossRef]
- Zuo, J.; Jiang, T.; Zhao, X.; Xiong, X.; Xiao, S.; Zhu, Z. Preparation and Application of Fluorescent Carbon Dots. J. Nanomater. 2015, 2015, 1–13. [Google Scholar] [CrossRef]
- Soni, M.; Mehta, P.; Soni, A.; Goswami, G.K. Green Nanoparticles: Synthesis and Applications. IOSR J. Biotechnol. Biochem. 2018, 4, 78–83. [Google Scholar]
- Wang, J.; Zhang, P.; Huang, C.; Liu, G.; Leung, K.C.-F.; Wáng, Y.X.J. High Performance Photoluminescent Carbon Dots for In Vitro and In Vivo Bioimaging: Effect of Nitrogen Doping Ratios. Langmuir 2015, 31, 8063–8073. [Google Scholar] [CrossRef]
- Mehta, V.N.; Jha, S.; Basu, H.; Singhal, R.K.; Kailasa, S.K. One-step hydrothermal approach to fabricate carbon dots from apple juice for imaging of mycobacterium and fungal cells. Sensors Actuators B Chem. 2015, 213, 434–443. [Google Scholar] [CrossRef]
- Jeong, Y.; Moon, K.; Jeong, S.; Koh, W.-G.; Lee, K. Converting Waste Papers to Fluorescent Carbon Dots in the Recycling Process without Loss of Ionic Liquids and Bioimaging Applications. ACS Sustain. Chem. Eng. 2018, 6, 4510–4515. [Google Scholar] [CrossRef]
- Ren, X.; Zhang, F.; Guo, B.; Gao, N.; Zhang, X. Synthesis of N-Doped Micropore Carbon Quantum Dots with High Quantum Yield and Dual-Wavelength Photoluminescence Emission from Biomass for Cellular Imaging. Nanomaterial 2019, 9, 495. [Google Scholar] [CrossRef]
- De, B.; Karak, N. A green and facile approach for the synthesis of water soluble fluorescent carbon dots from banana juice. RSC Adv. 2013, 3, 8286–8290. [Google Scholar] [CrossRef]
- Qin, X.; Lu, W.; Asiri, A.M.; Al-Youbi, A.O.; Sun, X. Green, low-cost synthesis of photoluminescent carbon dots by hydrothermal treatment of willow bark and their application as an effective photocatalyst for fabricating Au nanoparticles–reduced graphene oxide nanocomposites for glucose detection. Catal. Sci. Technol. 2013, 3, 1027. [Google Scholar] [CrossRef]
- Zhu, C.; Zhai, J.; Dong, S. Bifunctional fluorescent carbon nanodots: Green synthesis via soy milk and application as metal-free electrocatalysts for oxygen reduction. Chem. Commun. 2012, 48, 9367–9369. [Google Scholar] [CrossRef]
- Hoan, B.T.; Tam, P.D.; Pham, V.-H. Green Synthesis of Highly Luminescent Carbon Quantum Dots from Lemon Juice. J. Nanotechnol. 2019, 2019, 1–9. [Google Scholar] [CrossRef]
- Li, L.; Li, L.; Chen, C.-P.; Cui, F. Green synthesis of nitrogen-doped carbon dots from ginkgo fruits and the application in cell imaging. Inorg. Chem. Commun. 2017, 86, 227–231. [Google Scholar] [CrossRef]
- Xue, M.; Zou, M.; Zhao, J.; Zhan, Z.; Zhao, S. Green preparation of fluorescent carbon dots from lychee seeds and their application for the selective detection of methylene blue and imaging in living cells. J. Mater. Chem. B 2015, 3, 6783–6789. [Google Scholar] [CrossRef]
- Bhattacharyya, D.; Sarswat, P.K.; Free, M.L. Quantum dots and carbon dots based fl uorescent sensors for TB biomarkers detection. Vacuum 2017, 146, 606–613. [Google Scholar] [CrossRef]
- Wang, C.; Shi, H.; Yang, M.; Yan, Y.; Liu, E.; Ji, Z.; Fan, J. Facile synthesis of novel carbon quantum dots from biomass waste for highly sensitive detection of iron ions. Mater. Res. Bull. 2020, 124, 110730. [Google Scholar] [CrossRef]
- Himaja, A.L.; Karthik, P.S.; Sreedhar, B.; Singh, S.P. Synthesis of Carbon Dots from Kitchen Waste: Conversion of Waste to Value Added Product. J. Fluoresc. 2014, 24, 1767–1773. [Google Scholar] [CrossRef]
- Liang, Z.; Zeng, L.; Cao, X.; Wang, Q.; Wang, X.; Sun, R. Sustainable carbon quantum dots from forestry and agricultural biomass with amplified photoluminescence by simple NH4OH passivation. J. Mater. Chem. C 2014, 2, 9760–9766. [Google Scholar] [CrossRef]
- Wang, J.; Qiu, J. A review of carbon dots in biological applications. J. Mater. Sci. 2016, 51, 4728–4738. [Google Scholar] [CrossRef]
- Laverdant, J.; De Marcillac, W.D.; Barthou, C.; Chinh, V.D.; Schwob, C.; Coolen, L.; Bénalloul, P.; Nga, P.T.; Maître, A. Experimental Determination of the Fluorescence Quantum Yield of Semiconductor Nanocrystals. Material 2011, 4, 1182–1193. [Google Scholar] [CrossRef]
- Pam, A.A. Innovative Activated Carbon Based on Deep Eutectic Solvents (DES) and H3PO4. C J. Carbon Res. 2019, 5, 43. [Google Scholar] [CrossRef]
- Waluyo, J.; Makertihartha, I.G.B.N.; Susanto, H. Pyrolysis with intermediate heating rate of palm kernel shells: Effect temperature and catalyst on product distribution. AIP Conf. Proc. 2018, 1977, 020026. [Google Scholar] [CrossRef]
- Nasir, S.; Hussein, M.Z.; Yusof, N.A.; Zainal, Z. Oil Palm Waste-Based Precursors as a Renewable and Economical Carbon Sources for the Preparation of Reduced Graphene Oxide from Graphene Oxide. Nanomaterial 2017, 7, 182. [Google Scholar] [CrossRef]
- Deng, J.; Xiong, T.; Wang, H.; Zheng, A.; Wang, Y. Effects of Cellulose, Hemicellulose, and Lignin on the Structure and Morphology of Porous Carbons. ACS Sustain. Chem. Eng. 2016, 4, 3750–3756. [Google Scholar] [CrossRef]
- Jiang, K.; Sun, S.; Zhang, L.; Lu, Y.; Wu, A.; Cai, C.; Lin, H. Red, Green, and Blue Luminescence by Carbon Dots: Full-Color Emission Tuning and Multicolor Cellular Imaging. Angew. Chem. Int. Ed. 2015, 54, 5360–5363. [Google Scholar] [CrossRef]
- Sarkar, N.; Sahoo, G.; Das, R.; Prusty, G.; Swain, S.K. Carbon quantum dot tailored calcium alginate hydrogel for pH-responsive controlled delivery of vancomycin. Eur. J. Pharm. Sci. 2017, 109, 359–371. [Google Scholar] [CrossRef] [PubMed]
- Adegoke, O.; Park, E.Y. Bright luminescent optically engineered core/alloyed shell quantum dots: An ultrasensitive signal transducer for dengue virus RNA via localized surface plasmon resonance-induced hairpin hybridization. J. Mater. Chem. B 2017, 5, 3047–3058. [Google Scholar] [CrossRef]
- Shao, M.; Yao, M.; De Saeger, S.; Yan, L.; Song, S. Carbon Quantum Dots Encapsulated Molecularly Imprinted Fluorescence Quenching Particles for Sensitive Detection of Zearalenone in Corn Sample. Toxins 2018, 10, 438. [Google Scholar] [CrossRef] [PubMed]
- Saheeda, P.; Sabira, K.; Joseph, J.; Jayaleksmi, S. On the intriguing emission characteristics of size tunable carbon dots derived from functionalized multi-walled carbon nanotubes. Mater. Chem. Phys. 2019, 225, 8–15. [Google Scholar] [CrossRef]
- Nizamuddin, S.; Kumar, N.S.J.; Sahu, J.; Ganesan, P.; Bhutto, A.W.; Mubarak, N.M. Hydrothermal carbonization of oil palm shell. Korean J. Chem. Eng. 2015, 32, 1789–1797. [Google Scholar] [CrossRef]
- Yang, C.; Zhu, S.; Li, Z.; Li, Z.; Chenhui, Y.; Sun, L.; Tang, W.; Liu, R.; Sun, Y.; Yu, M. Nitrogen-doped carbon dots with excitation-independent long-wavelength emission produced by a room-temperature reaction. Chem. Commun. 2016, 52, 11912–11914. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Pang, J.; Xu, F.; Zhang, X. Simple Approach to Synthesize Amino-Functionalized Carbon Dots by Carbonization of Chitosan. Sci. Rep. 2016, 6, 31100. [Google Scholar] [CrossRef] [PubMed]
- Anisuzzaman, S.M.; Collin, J.; Daud, W.M.A.W.; Duduku, K.; Yee, H.S. Preparation and characterization of activated carbon from Typha orientalis leaves. Int. J. Ind. Chem. 2014, 6, 9–21. [Google Scholar] [CrossRef]
- Bhattacharya, D.; Mishra, M.K.; De, G. Carbon Dots from a Single Source Exhibiting Tunable Luminescent Colors through the Modification of Surface Functional Groups in ORMOSIL Films. J. Phys. Chem. C 2017, 121, 28106–28116. [Google Scholar] [CrossRef]
- Kandasamy, G. Recent Advancements in Doped/Co-Doped Carbon Quantum Dots for Multi-Potential Applications. C J. Carbon Res. 2019, 5, 24. [Google Scholar] [CrossRef]
- Mehrotra, P. Biosensors and their applications—A review. J. Oral Biol. Craniofacial Res. 2016, 6, 153–159. [Google Scholar] [CrossRef]
- Zheng, M.; Li, Y.; Zhang, Y.; Xie, Z. Solvatochromic fluorescent carbon dots as optic noses for sensing volatile organic compounds. RSC Adv. 2016, 6, 83501–83504. [Google Scholar] [CrossRef]
- Quraishi, S.; Plappert, S.F.; Ungerer, B.; Taupe, P.; Gindl-Altmutter, W.; Liebner, F. Preparation and Characterization of Bacterial Cellulose-Carbon Dot Hybrid Nanopaper for Potential Sensing Applications. Appl. Sci. 2018, 9, 107. [Google Scholar] [CrossRef]
- Edmund, C.O.; Christopher, M.S.; Pascal, D.K. Characterization of palm kernel shell for materials reinforcement and water treatment. J. Chem. Eng. Mater. Sci. 2014, 5, 1–6. [Google Scholar] [CrossRef]
- Wu, P.; Li, W.; Wu, Q.; Liu, Y.; Liu, S.-X. Hydrothermal synthesis of nitrogen-doped carbon quantum dots from microcrystalline cellulose for the detection of Fe3+ ions in an acidic environment. RSC Adv. 2017, 7, 44144–44153. [Google Scholar] [CrossRef]
- Li, J.; Ma, S.; Xiao, X.; Zhao, D. The One-Step Preparation of Green-Emissioned Carbon Dots through Hydrothermal Route and Its Application. J. Nanomater. 2019, 2019, 1–10. [Google Scholar] [CrossRef]
- Ortiz, S.N.C.; Ospino, E.M.; Cabanzo, R. Spectroscopy characterization and quantum yield determination of quantum dots. J. Physics: Conf. Ser. 2016, 687, 012097. [Google Scholar] [CrossRef]
- Yang, R.; Guo, X.; Jia, L.; Zhang, Y.; Zhao, Z.; Lonshakov, F. Green preparation of carbon dots with mangosteen pulp for the selective detection of Fe3+ ions and cell imaging. Appl. Surf. Sci. 2017, 423, 426–432. [Google Scholar] [CrossRef]
- Gedda, G.; Lee, C.-Y.; Lin, Y.-C.; Wu, H.-F. Green synthesis of carbon dots from prawn shells for highly selective and sensitive detection of copper ions. Sensors Actuators B Chem. 2016, 224, 396–403. [Google Scholar] [CrossRef]
- Bhamore, J.R.; Jha, S.; Singhal, R.K.; Park, T.J.; Kailasa, S.K. Facile green synthesis of carbon dots from Pyrus pyrifolia fruit for assaying of Al3+ ion via chelation enhanced fluorescence mechanism. J. Mol. Liq. 2018, 264, 9–16. [Google Scholar] [CrossRef]
- Yang, Z.-C.; Wang, M.; Yong, A.M.; Wong, S.Y.; Zhang, X.-H.; Tan, H.; Chang, A.Y.; Li, X.; Wang, J. Intrinsically fluorescent carbon dots with tunable emission derived from hydrothermal treatment of glucose in the presence of monopotassium phosphate. Chem. Commun. 2011, 47, 11615–11617. [Google Scholar] [CrossRef]
- Du, F.; Zhang, M.; Li, X.; Li, J.; Jiang, X.; Li, Z.; Hua, Y.; Shao, G.; Jin, J.; Shao, Q.; et al. Economical and green synthesis of bagasse-derived fluorescent carbon dots for biomedical applications. Nanotechnoloy 2014, 25, 315702. [Google Scholar] [CrossRef]
- Bhamore, J.R.; Jha, S.; Park, T.J.; Kailasa, S.K. Fluorescence sensing of Cu2+ ion and imaging of fungal cell by ultra-small fluorescent carbon dots derived from Acacia concinna seeds. Sens. Actuators B Chem. 2018, 277, 47–54. [Google Scholar] [CrossRef]
- Xu, D.; Lei, F.; Chen, H.; Yin, L.; Shi, Y.; Xie, J. One-step hydrothermal synthesis and optical properties of self-quenching-resistant carbon dots towards fluorescent ink and as nanosensors for Fe3+ detection. RSC Adv. 2019, 9, 8290–8299. [Google Scholar] [CrossRef]
- Das, T.; Saikia, B.K.; Dekaboruah, H.; Bordoloi, M.; Neog, D.; Bora, J.J.; Lahkar, J.; Narzary, B.; Roy, S.; Ramaiah, D. Blue-fluorescent and biocompatible carbon dots derived from abundant low-quality coals. J. Photochem. Photobiol. B Biol. 2019, 195, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Zhou, H.; Tang, J.; Deng, S.; Yan, F.; Li, W.; Qu, M. Carbon dots doped with heteroatoms for fluorescent bioimaging: A review. Microchim. Acta 2017, 184, 343–368. [Google Scholar] [CrossRef]
- Mailafiya, M.M.; Abubakar, K.; Danmaigoro, A.; Chiroma, S.M.; Rahim, E.B.A.; Moklas, M.A.M.; Zakaria, Z.A.B. Evaluation of in vitro release kinetics and mechanisms of curcumin-loaded cockle shell-derived calcium carbonate nanoparticles. Biomed. Res. Ther. 2019, 6, 3518–3540. [Google Scholar] [CrossRef]
- Bajpai, S.K.; D’Souza, A.; Suhail, B. Blue light-emitting carbon dots (CDs) from a milk protein and their interaction with Spinacia oleracea leaf cells. Int. Nano Lett. 2019, 9, 203–212. [Google Scholar] [CrossRef]
- Stefanakis, D.; Philippidis, A.; Sygellou, L.; Filippidis, G.; Ghanotakis, D.F.; Anglos, D. Synthesis of fluorescent carbon dots by a microwave heating process: Structural characterization and cell imaging applications. J. Nanoparticle Res. 2014, 16, 1–10. [Google Scholar] [CrossRef]
- Coates, J. Interpretation of Infrared Spectra, a Practical Approach. In Encyclopedia of Analytical Chemistry; John Wiley & Sons Ltd.: Chichester, UK, 2000; pp. 10815–10837. [Google Scholar]
- Segal, L.; Eggerton, F.V. Infrared Spectra of Ethylenediamine and the Dimethylethylenediamines. Appl. Spectrosc. 1961, 15, 116–117. [Google Scholar] [CrossRef]
- Dong, W.; Li, H.; Dong, Y.; Wang, J.; Ge, X.; Sui, L. The preparation of ethylenediamine-modified fluorescent carbon dots and their use in imaging of cells. Luminescence 2015, 30, 867–871. [Google Scholar] [CrossRef]
- Yin, B.; Deng, J.; Peng, X.; Long, Q.; Zhao, J.; Lu, Q.; Chen, Q.; Li, H.; Tang, H.; Zhang, Y.; et al. Green synthesis of carbon dots with down- and up-conversion fluorescent properties for sensitive detection of hypochlorite with a dual-readout assay. Analyst 2013, 138, 6551–6557. [Google Scholar] [CrossRef]


| S/No | Mass of Boat + PKS Sample (g) (A) | Mass of Boat (g) (B) | Mass of PKS (g) (C) | Mass of Boat + CPKS Sample (g) (D) | Mass Yield of CPKS (g) (D − B) (E) | Percentage Yield of CPKS (%) (F) |
|---|---|---|---|---|---|---|
| 1. | 104.60 | 86.63 | 17.97 | 92.19 | 5.56 | 30.90 |
| 2. | 106.62 | 86.62 | 20.14 | 92.92 | 6.30 | 31.26 |
| 3. | 105.22 | 86.64 | 18.58 | 92.30 | 5.68 | 30.71 |
| Average Percentage Yield of CPKS (%) | 30.96 | |||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Newman Monday, Y.; Abdullah, J.; Yusof, N.A.; Abdul Rashid, S.; Shueb, R.H. Facile Hydrothermal and Solvothermal Synthesis and Characterization of Nitrogen-Doped Carbon Dots from Palm Kernel Shell Precursor. Appl. Sci. 2021, 11, 1630. https://doi.org/10.3390/app11041630
Newman Monday Y, Abdullah J, Yusof NA, Abdul Rashid S, Shueb RH. Facile Hydrothermal and Solvothermal Synthesis and Characterization of Nitrogen-Doped Carbon Dots from Palm Kernel Shell Precursor. Applied Sciences. 2021; 11(4):1630. https://doi.org/10.3390/app11041630
Chicago/Turabian StyleNewman Monday, Yakubu, Jaafar Abdullah, Nor Azah Yusof, Suraya Abdul Rashid, and Rafidah Hanim Shueb. 2021. "Facile Hydrothermal and Solvothermal Synthesis and Characterization of Nitrogen-Doped Carbon Dots from Palm Kernel Shell Precursor" Applied Sciences 11, no. 4: 1630. https://doi.org/10.3390/app11041630
APA StyleNewman Monday, Y., Abdullah, J., Yusof, N. A., Abdul Rashid, S., & Shueb, R. H. (2021). Facile Hydrothermal and Solvothermal Synthesis and Characterization of Nitrogen-Doped Carbon Dots from Palm Kernel Shell Precursor. Applied Sciences, 11(4), 1630. https://doi.org/10.3390/app11041630






