Phosphogypsum Recycling: A Review of Environmental Issues, Current Trends, and Prospects
Abstract
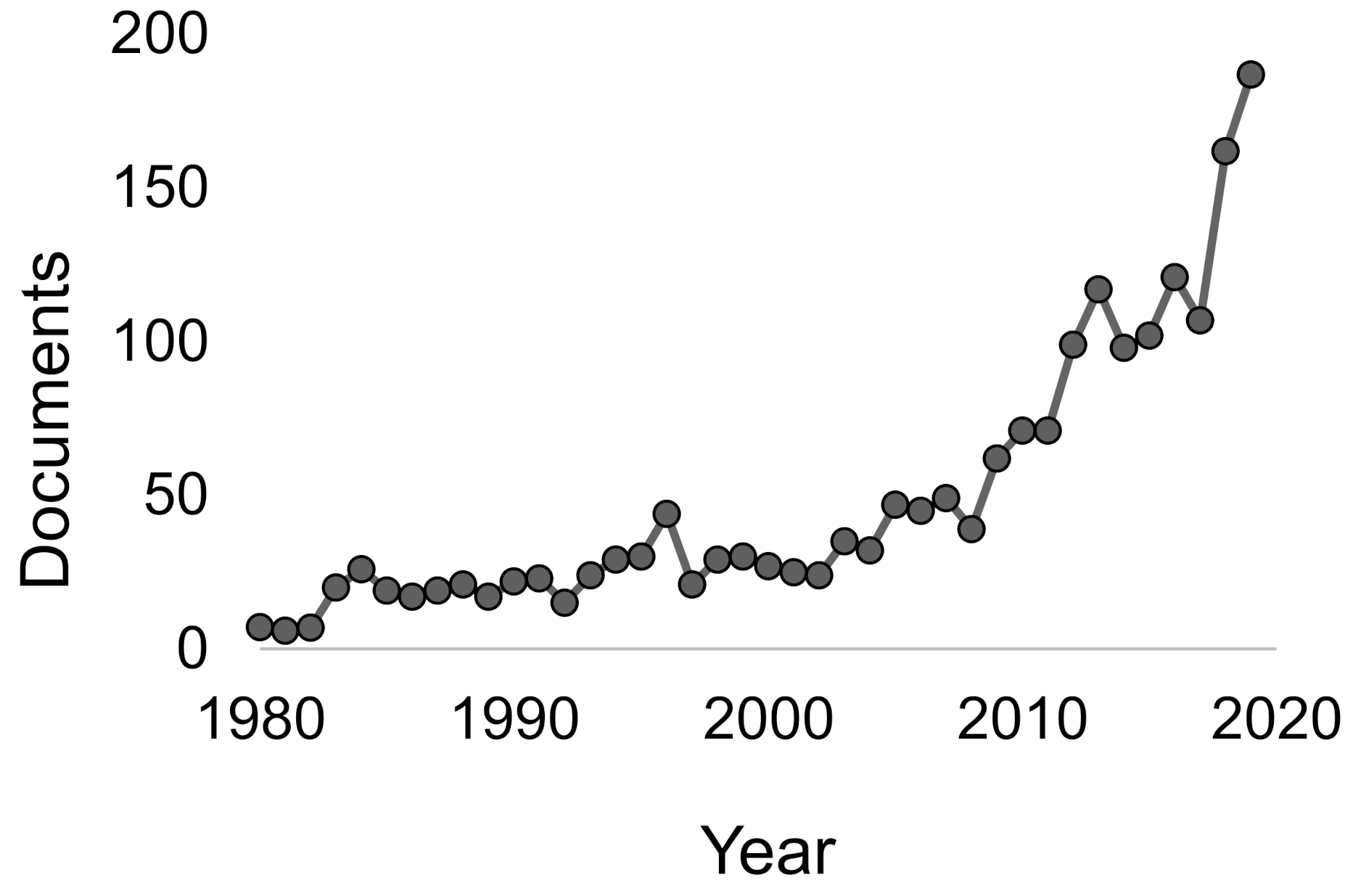
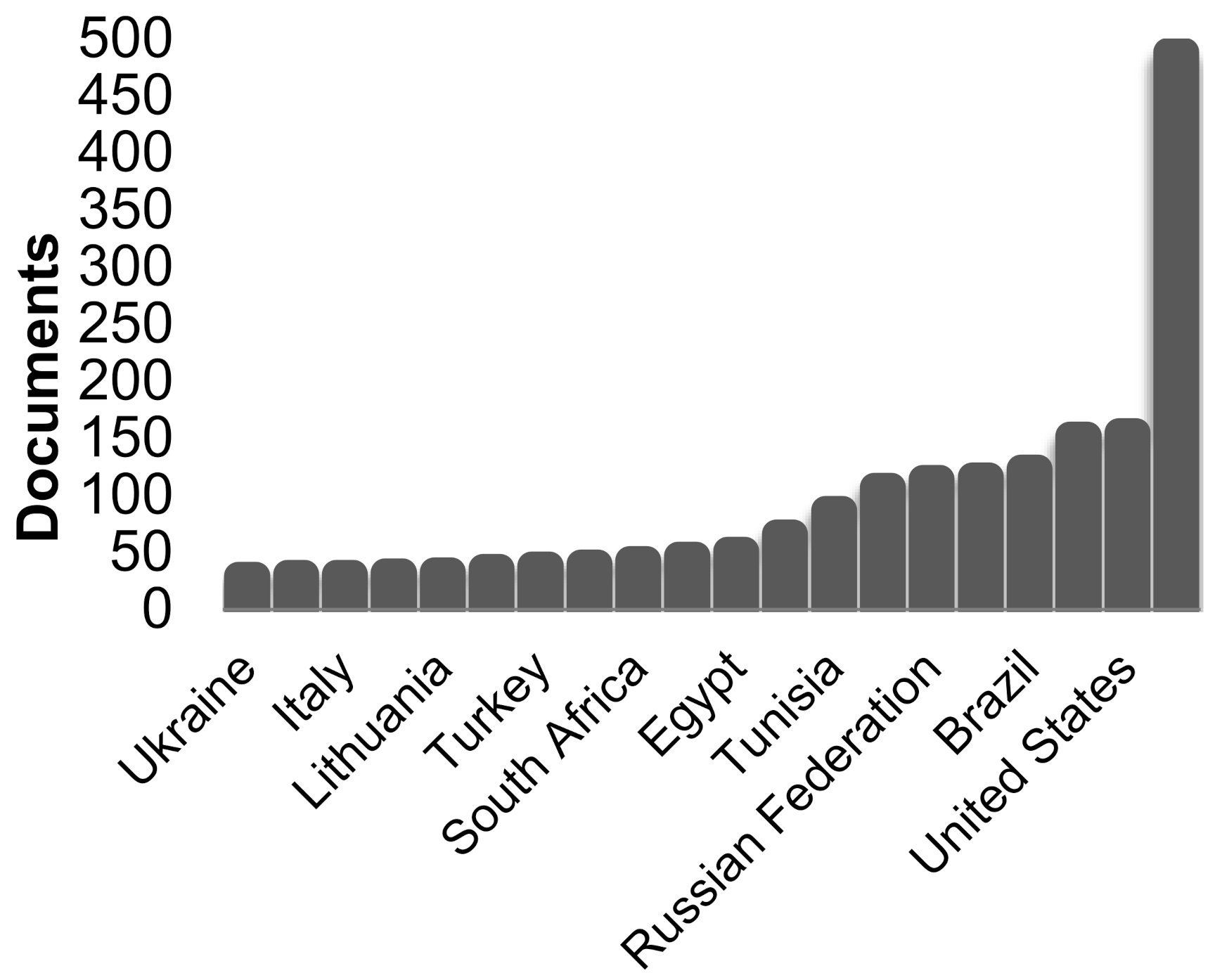
1. Introduction
Overview of Studies on the Environmental Impact of Phosphogypsum Accumulation and Storage
- the production capacity;
- the amount of phosphogypsum that must be removed;
- the remoteness of the extraction components from the phosphogypsum storage site;
- the availability of storage land (unsuitable for other uses);
- the dump topography;
- the climatic conditions;
- the geological and hydrogeological conditions at the phosphogypsum storage site.
- The storage of phosphogypsum on the territory of the enterprise deteriorates the sanitary condition of the site and the adjacent territory;
- The transportation and storage of phosphogypsum in dumps are connected with rather high costs—about 18% of the cost of construction of phosphoric acid production itself—and they significantly increase during the transition to more reliable hydrotransport for phosphogypsum. Operating costs are approximately 12% of the cost of raw material processing [21];
- The need to alienate large areas to create dumps. These areas may exceed the size of industrial sites of enterprises;
- The exploitation of the dumps poses a potential threat to the environment and residential landscapes adjacent to the dump.
- The analysis of phosphogypsum processing methods and highlighting key trends;
- The visualization of the network system by phosphogypsum research areas and consideration of alternative solutions for the disposal of phosphogypsum.
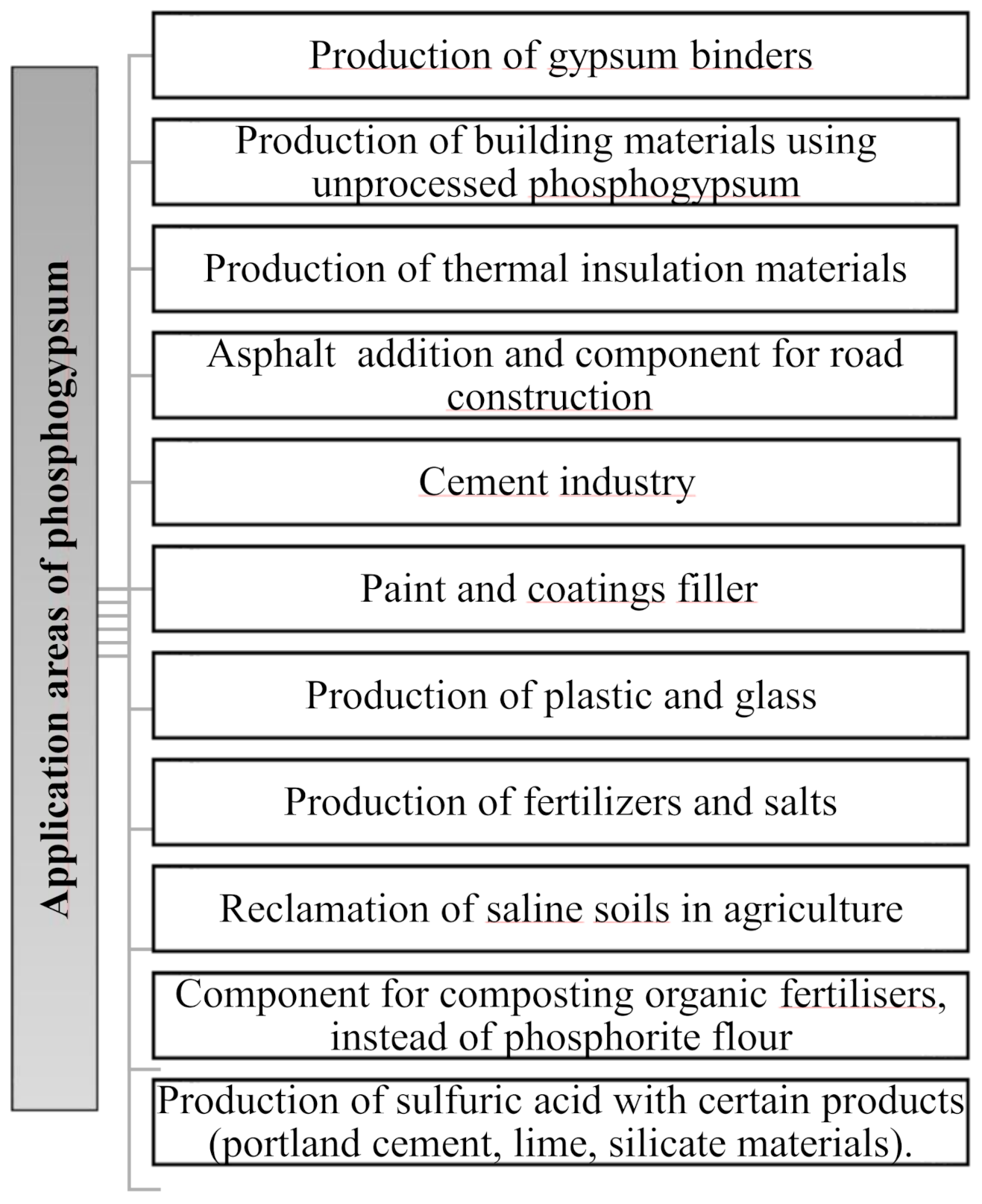
2. Analysis of Trends in Phosphogypsum Use
2.1. Phosphogypsum Recycling in Various Industry Fields
- washing of phosphogypsum with water;
- washing in combination with neutralization and deposition of impurities in water suspension;
- the method of thermal decomposition of impurities;
- the introduction of additives that neutralize, mineralize, and regulate crystallization before and after firing.
- a complicated method of obtaining a hemihydrate binder that requires high-energy devices used for mechanical and chemical activation of raw materials;
- the need to use fresh phosphogypsum with stable humidity and the difficulty of calculating the required amount of calcium sulfate hemihydrate to ensure the humidity needed for pressing;
- the need for high-energy grinding and the use of superplasticizers, which significantly increases the cost of the binder.
2.2. Phosphogypsum Recycling in Agriculture
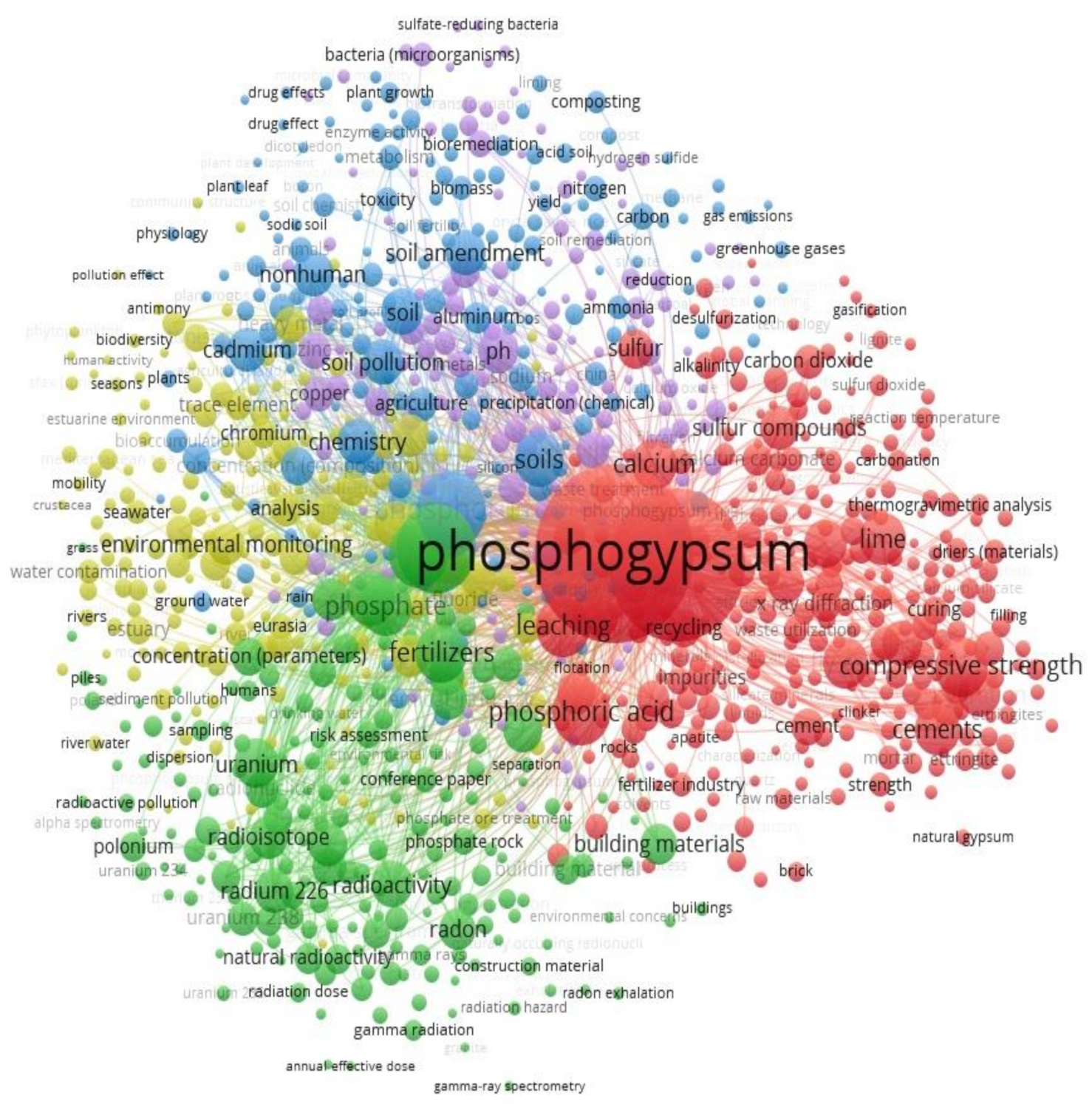
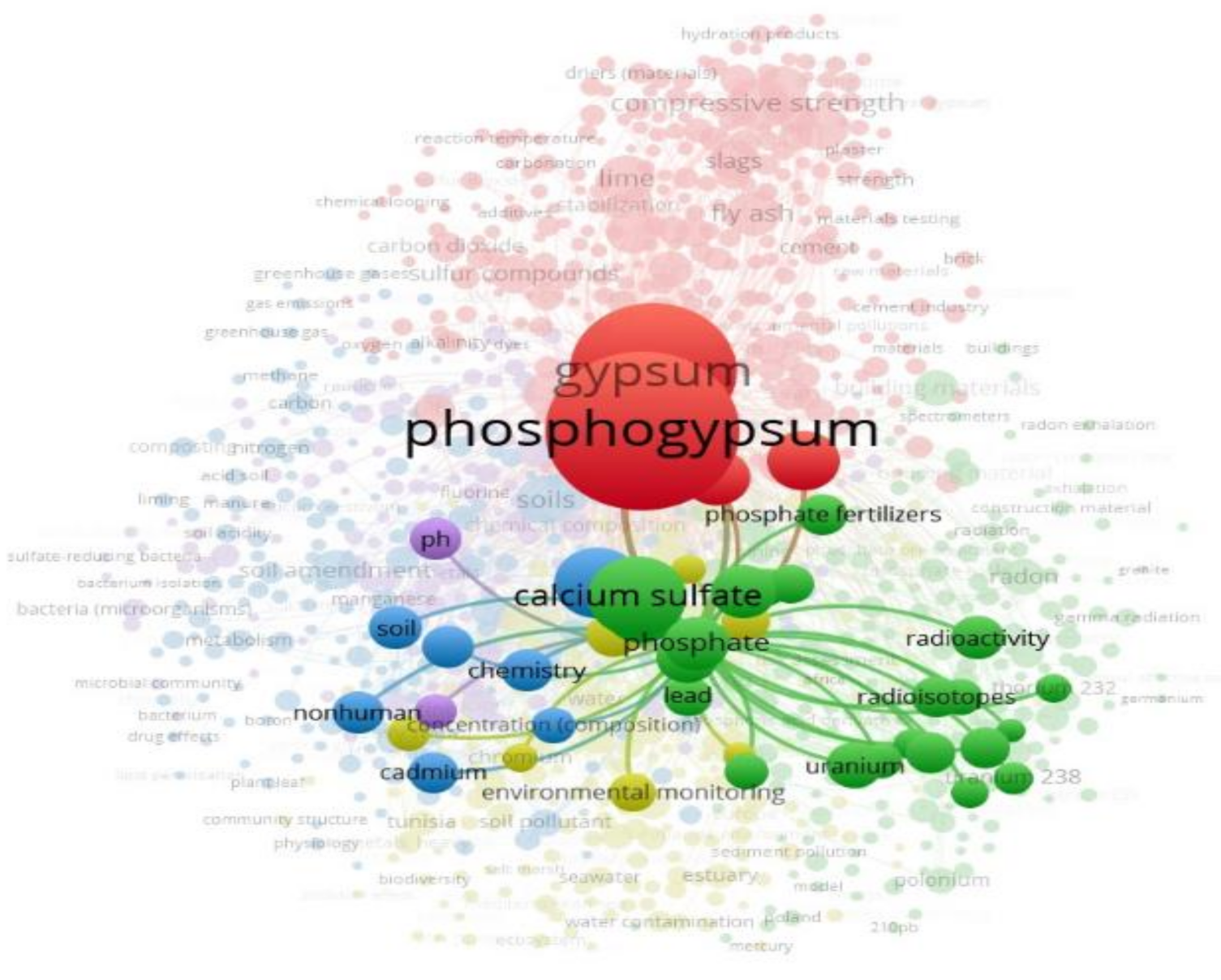
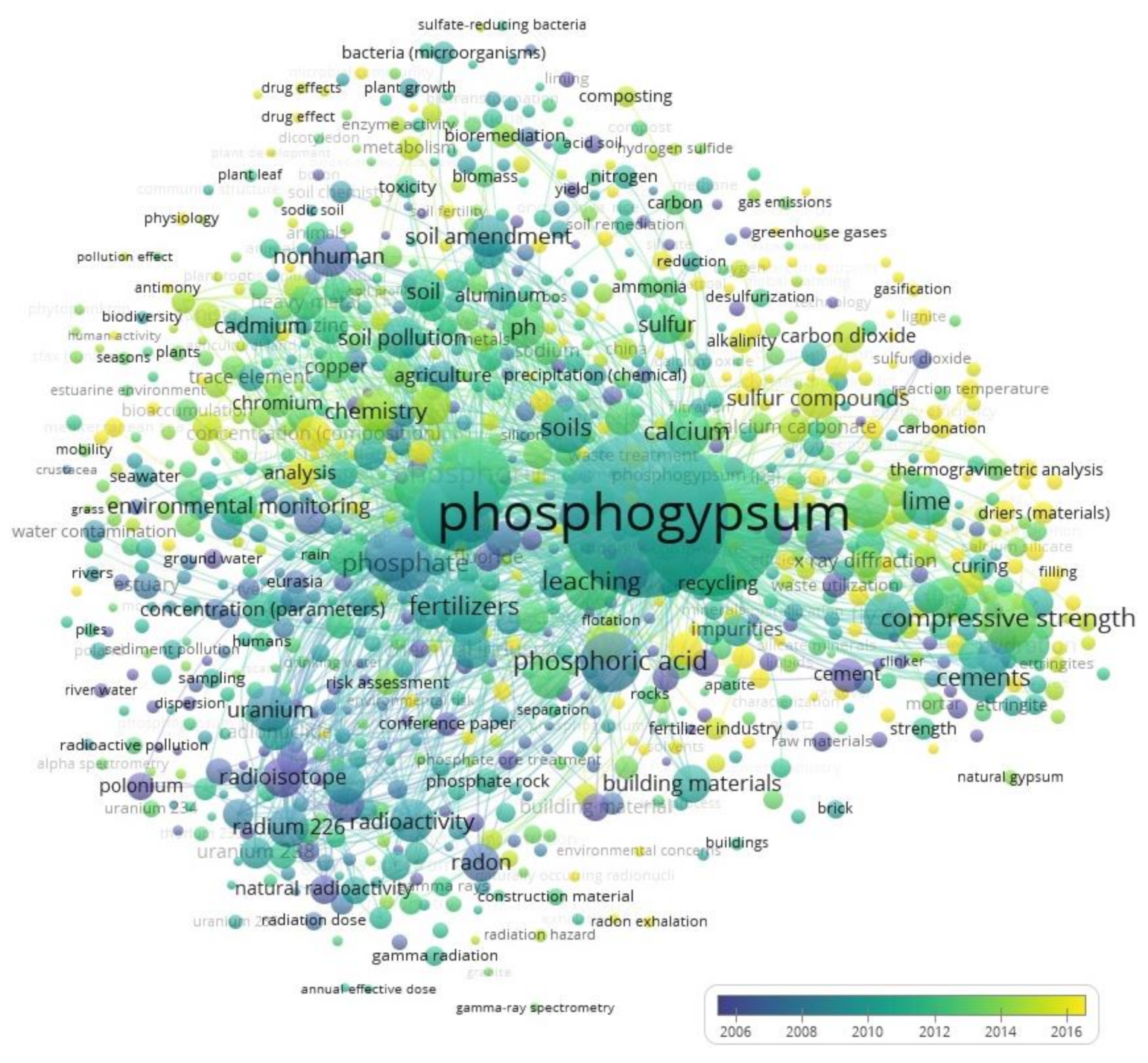
3. Visualization of the Areas of Phosphogypsum Research and Identification of Alternative Solutions for Its Use
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Malik, N.Y.; Malovanyi, M.S.; Malyk, O.V. Two-Stage chemical processing of phosphogypsum into ammonium nitrate. Chem. Technol. Subst. Appl. 2005, 536, 207–211. (In Ukrainian) [Google Scholar]
- Malanchuk, Z.R.; Vasylchuk, O.Y.; Oksenіuk, R.R. Current trends of technogenic phosphogypsum fields use and recycling. Bull. NUWEE Tech. Sci. 2016, 2, 133–139. (In Ukrainian) [Google Scholar]
- The State Department of Agro-Industrial Development, Environment Protection and Natural Resources in Vinnytsia Region. The Report on the State of the Environment in Vinnytsia Region, 2018 Year; The State Department of Agro-Industrial Development, Environment Protection and Natural Resources in Vinnytsia Region: Vinnytsia, Ukraine, 2019. (In Ukrainian)
- Florida Industrial and Phosphate Research Institute. Potential Phosphogypsum Uses; Florida Industrial and Phosphate Research Institute: Bartow, FL, USA, 2020. Available online: http://www.fipr.state.fl.us/about-us/phosphate-primer/potential-phosphogypsum-use/ (accessed on 29 July 2020).
- Chuan, L.M.; Zheng, H.G.; Zhao, J.J.; Wang, A.L.; Sun, S.F. Phosphogypsum production and utilization in China. IOP Conf. Ser. Mater. Sci. Eng. 2018, 382, 022099. [Google Scholar] [CrossRef]
- Kulczycka, J.; Kowalski, Z.; Smol, M.; Wirth, H. Evaluation of the recovery of rare earth elements (REE) from phosphogypsum Waste—Case study of the WIZÓW Chemical Plant (Poland). J. Clean. Prod. 2016, 113, 345–354. [Google Scholar] [CrossRef]
- Manzhina, S.A.; Denisov, V.V.; Denisova, I.A. Usingof large-scale waste phosphogypsum to reduce emis-sions of SO2-containing coal power plant. Eng. J. Don 2014, 28, 77–87. (In Russian) [Google Scholar]
- El Kateb, A.; Stalder, C.; Rüggeberg, A.; Neururer, C.; Spangenberg, J.E.; Spezzaferri, S. Impact of industrial phosphate waste discharge on the marine environment in the Gulf of Gabes (Tunisia). PLoS ONE 2018, 13, e0197731. [Google Scholar] [CrossRef] [PubMed]
- Gaudry, A.; Zeroual, S.; Gaie-Levrel, F.; Moskura, M.; Boujrhal, F.-Z.; El Moursil, R.C.; Guessous, A.; Mouradi, T.; Givernaud, T.; Delmas, R. Heavy metals pollution of the Atlantic marine environment by the Moroccan phosphate industry, as observed through their bioaccumulation in Ulva lactuca. Water Air Soil Pollut. 2007, 178, 267–285. [Google Scholar] [CrossRef]
- El Zrelli, R.; Rabaoui, L.; Abda, H.; Daghbouj, N.; Perez-Lopez, R.; Castet, S.; Aigouy, T.; Bejaoui, N.; Courjault-Rade, P. Characterization of the role of phosphogypsum foam in the transport of metals and radionuclides in the Southern Mediterranean Sea. J. Hazard. Mater. 2019, 363, 258–267. [Google Scholar] [CrossRef] [PubMed]
- Kutepova, N.A.; Korobanova, T.N. Features of deformation development in phosphogypsum dumps near the Balakovo town in the Saratov region. Min. Inf. Anal. Bull. 2017, 10, 132–140. (In Russian) [Google Scholar] [CrossRef]
- Villa, M.; Mosqueda, F.; Hurtado, S.; Mantero, J.; Manjon, G.; Perianez, R.; Vaca, F.; Garcia-Tenorio, R. Contamination and restoration of an estuary affected by phosphogypsum releases. Sci. Total Environ. 2009, 408, 69–77. [Google Scholar] [CrossRef] [PubMed]
- Nadymova, M.A. Apatite concentrate as a promising source of rare-earth metals in complex processing. In “The North and the Arctic in the New Global Development Paradigm. Luzin Readings”, Proceedings of the International Scientific and Practical Conference, Apatity, Russia, 14–16 April 2016; Luzin Institute for Economic Studies: Saint Petersburg, Russia, 2016; pp. 674–679. (In Russian) [Google Scholar]
- Tovazhnyansky, L.L.; Meshalkin, V.P.; Kapustenko, P.O.; Bukhkalo, S.I.; Arsenyeva, O.P.; Perevertaylenko, O.Y. Energy efficiency of complex technologies of phosphogypsum conversion. Theor. Found. Chem. Eng. 2013, 3, 225–230. [Google Scholar] [CrossRef]
- Petrenko, D.V. Effect of Phosphate Fertilizer on the Strontium Content of the Landscapes. Ph.D. Dissertation, Kuban State Agrarian University, Moscow, Russia, 2014. (In Russian). [Google Scholar]
- Torres-Sanchez, R.; Sanchez-Rodas, D.; Sanchez de la Campa, A.M.; de la Rosa, J.D. Hydrogen fluoride concentrations in ambient air of an urban area based on the emissions of a major phosphogypsum deposit (SW, Europe). Sci. Total Environ. 2020, 714, 136891. [Google Scholar] [CrossRef] [PubMed]
- Kasimov, A.M.; Leonova, O.E.; Kononov, Y.A. Utilization of phosphogypsum with available materials for the production of gypsum binders. In Cooperation in Solving Waste Problems, Proceedings of the 4th International Conference, Kharkov, Ukraine, 31 January–1 February 2007; EcoInform: Kharkov, Ukraine, 2007; pp. 120–122. (In Russian) [Google Scholar]
- Sharipov, T.V. Processing Karate Phosphorites into Sodium Hexafluorosilicate. Dissertation, Bashkir State University, Kazan, Russia, 2014. (In Russian). [Google Scholar]
- Donskikh, I.V. The influence of fluorine and its compounds on people’s health (literature review). Acta Biomed. Sci. 2013, 3, 179–185. (In Russian) [Google Scholar]
- Chernysh, Y.; Plyatsuk, L. Environmentally friendly concept of phosphogypsum recycling on the basis of the biotechnological approach. In International Business, Trade and Institutional Sustainability; World Sustainability Series; Leal Filho, W., Borges de Brito, P., Frankenberger, F., Eds.; Springer: Cham, Switzerland, 2020; pp. 167–182. [Google Scholar]
- Yunusova, S.S. Composite Wall Materials and Products Based on Phosphogypsum, Obtained by Semi-Dry Pressing. Dissertation, Samara State Architecture Academy, Samara, Russia, 2004. (In Russian). [Google Scholar]
- Maazoun, H.; Bouassida, M. Phosphogypsum management perspectives. Massive valorization or massive storage? ACTA Sci. Agric. 2019, 8, 184–189. [Google Scholar] [CrossRef]
- Dvorkin, L.Y.; Shestakov, V.L.; Myronenko, A.V. A Method for Producing Binding Material of Phosphogypsum-Dihydrate. Ukraine Patent UA4226U, 17 January 2005. [Google Scholar]
- Dvorkin, L.Y.; Myronenko, A.V.; Shestakov, V.L.; Hryb, Y.S.; Vovk, O.O.; Karpenko, H.V. Method for Preparation of Phosphogypsum Binding Material. Ukraine Patent UA28055U, 26 November 2007. (In Ukrainian). [Google Scholar]
- Dvorkin, L.Y.; Shestakov, V.L.; Ischuk, O.O.; Hryb, Y.S.; Vovk, O.O.; Karpovets, V.P. Method for Manufacturing of Phosphogypsum Binding Agent. Ukraine Patent UA32645U, 26 May 2008. (In Ukrainian). [Google Scholar]
- Dvorkin, L.Y.; Zhytkovskyi, V.V.; Marchuk, V.V. Method for the Production of Gypsum Articles of Phosphogypsum. Ukraine Patent UA102561U, 10 November 2005. (In Ukrainian). [Google Scholar]
- Ivashchenko, Yu.G.; Evstigneev, S.A.; Strakhov, A.V. Obtaining a composite binder based on technogenic raw materials. Sci. Rev. 2015, 8, 177–180. (In Russian) [Google Scholar]
- Karpovych, E.O.; Vakal, S.V.; Zolotariov, O.Y.; Sharapov, S.V.; Sokolovych, S.O. Process for the Preparation of Gypsum Binder from Phosphogypsum. Ukraine Patent UA36150U, 10 October 2008. (In Ukrainian). [Google Scholar]
- Marakhovska, O.Y.; Pavlenko, O.V.; Vaziiev, Y.H.; Akulenko, V.L.; Pepeliaev, I.O. Process for Processing of Phosphogypsum to Gypsum Binder. Ukraine Patent UA68540U, 26 March 2012. (In Ukrainian). [Google Scholar]
- Shepliakov, Y.O.; Movsesian, V.A.; Titkov, A.B.; Shepliakov, O.Y. Method for Manufacture of Construction Articles of Phosphogypsum. Ukraine Patent UA29230U, 10 January 2008. (In Ukrainian). [Google Scholar]
- Vinnychenko, V.I.; Ivaschenko, T.H. Process for Phosphogypsum Treatment to the Gypsum Binder. Ukraine Patent UA39919U, 25 March 2009. (In Ukrainian). [Google Scholar]
- Vinnichenko, V.I.; Krot, O.Y.; Supryaga, N.M.; Supryaga, D.V. A Method of Manufacturing Construction Products from Phosphogypsum. Ukraine Patent UA112942C2, 10 November 2006. (In Ukrainian). [Google Scholar]
- Ivashchenko, T.G.; Ince, D. Ecological aspects of phosphogypsum utilization technologies. Visnyk ChSTU 2014, 2, 223–228. (In Russian) [Google Scholar]
- Trunova, I.A.; Sidorenko, R.V.; Vakal, S.V. Analysis of the main directions of a phosphogypsum utilization-a waste of phosphoric acid production. Environ. Saf. 2010, 2, 31–35. (In Russian) [Google Scholar]
- Dvorkin, L.I.; Dvorkin, O.I. Building Materials from Industrial Wastes; Phoenix: Rostov-on-Don, Russia, 2007. (In Russian) [Google Scholar]
- Derevyanko, V.N.; Tel’yanov, V.A. Technologies for the production of gypsum binders from phosphogypsum. Bull. PSACEA 2010, 2–3, 68–73. (In Russian) [Google Scholar]
- Malik, N.Y.; Malovanyi, M.S.; Malyk, O.V. Environmental safety achievements of deep conversion of phosphogypsum into sulfuric acid and ammonium nitrate. Newsl. LNPU 2004, 497, 122–124. (In Ukrainian) [Google Scholar]
- Tovazhnyansky, L.L.; Kapustenko, P.L.; Khavin, G.L. Complex processing of phosphogypsum with the extraction of rare earth elements. Integr. Ind. Technol. 2008, 2, 73–81. (In Russian) [Google Scholar]
- Lokshin, E.P.; Kalinnikov, V.T.; Ivlev, K.G.; Levin, B.V.; Pogrebnjak, O.S. Method of Recovering Rare-Earth Elements from Phosphogypsum. Russian. Federation Patent RU2293781C1, 20 February 2007. (In Russian). [Google Scholar]
- Lokshin, E.P.; Tareeva, O.A.; Kalinnikov, V.T. Method of Phosphogypsum Processing for Manufacture of Concentrate of Rare-Earth Elements and Gypsum. Russian Federation Patent RU2458999C1, 20 August 2012. (In Russian). [Google Scholar]
- Vlasian, S.V.; Voloshyn, M.D.; Shestozub, A.B.; Mukhachev, A.P. Process for the Extraction of Rare-Earth Elements from Phosphogypsum. Ukraine Patent UA88658U, 25 March 2014. (In Ukrainian). [Google Scholar]
- Fokin, K.S.; Nesterova, E.O. Method of Extracting Rare-Earth Metals from Phosphogypsum. Russian Patent RU2491362C1, 27 August 2013. (In Russian). [Google Scholar]
- Bashlykova, T.V.; Zhivaeva, A.D.; Ashirbaeva, E.A.; Danil’chenko, L.M. Method of Processing Phosphogypsum with Extraction of Rare-Earth Elements and Phosphorus. Russian Patent RU2457267C2, 27 July 2012. (In Russian). [Google Scholar]
- Zyk, V.V. Method for Extracting Rare Earth Elements from Phosphogypsum. Belarus Patent BY6905C1, 30 March 2005. (In Russian). [Google Scholar]
- Markov, S.G. Material-Energy-Saving technology of drywall production. Innov. Sci. 2015, 11, 245–247. (In Russian) [Google Scholar]
- Zhou, J.; Li, X.; Zhao, Y.; Shu, Z.; Wang, Y.; Zhang, Y.; Shen, X. Preparation of paper-free and fiber-free plasterboard with high strength using phosphogypsum. Constr. Build. Mater. 2020, 243, 118091. [Google Scholar] [CrossRef]
- Gong, X.; Liu, J.; Sun, Z.; Li, F. Effects of phosphogypsum and calcined phosphogypsum content on the basic physical and mechanical properties of Portland cement mortar. J. Test. Eval. 2020, 48, 3539–3549. [Google Scholar] [CrossRef]
- Borosenko, J.G.; Jashin, S.O.; Soldatov, A.A. Asphalt Mineral Mixture. Russian Patent RU2436819C1, 20 December 2001. (In Russian). [Google Scholar]
- Degirmenci, N.; Okucu, A.; Turabi, A. Application of phosphogypsum in soil stabilization. Build. Environ. 2007, 42, 3393–3398. [Google Scholar] [CrossRef]
- Kasimov, A.M.; Reshta, E.E. Promising treatment processes and waste disposal of certain production of mineral fertilizers. East Eur. J. Enterp. Technol. 2011, 52, 66–70. (In Russian) [Google Scholar]
- Kraynyuk, O.V. Construction of Highways with Safe Use of Phosphogypsum and Ash Slag from Thermal Power Plants. Dissertation, Kharkiv National Automobile and Highway University, Kharkiv, Ukraine, 2004. (In Ukrainian). [Google Scholar]
- Amrani, M.; Taha, Y.; Kchikach, A.; Benzaazoua, M.; Hakkou, R. Phosphogypsum recycling: New horizons for a more sustainable road material application. J. Build. Eng. 2020, 30, 101267. [Google Scholar] [CrossRef]
- Kochetkov, A.V.; Shchegoleva, N.V.; Korotkovskiy, S.A.; Talalai, V.V.; Vasilyev, Y.E.; Shashkov, I.G. The device layers and transport facilities of hemihydrate phosphogypsum (waste byproduct of the production of nitrogen-phosphorus fertilizers). Russ. J. Transp. Eng. 2019, 1, 18SATS119, In Russian. [Google Scholar] [CrossRef]
- Soldatkin, S.I.; Hohlov, A.E. Problems of using phosphogypsum in road constrcation. Nedra Povolz’a Prikaspia 2019, 97, 58–61. (In Russian) [Google Scholar]
- Folek, S.; Walawska, B.; Wilczek, B.; Miśkiewicz, J. Use of phosphogypsum in road construction. Pol. J. Chem. Technol. 2011, 13, 18–22. [Google Scholar] [CrossRef]
- Shen, W.; Zhou, M.; Ma, W.; Hu, J.; Cai, Z. Investigation on the application of steel slag-fly ash-phosphogypsum solidified material as road base material. J. Hazard. Mater. 2009, 164, 99–104. [Google Scholar] [CrossRef] [PubMed]
- Cichy, B.; Kraszewski, C.; Rafalski, L. Geotechnical properties of phosphogypsum and its use in road engineering. In Springer Series in Geomechanics and Geoengineering, Proceedings of China-Europe Conference on Geotechnical Engineering; Vienna, Austria, 13–16 August 2016; Wu, W., Yu, H.S., Eds.; Springer: Cham, Germany, 2018; pp. 1664–1667. [Google Scholar]
- Kraynyuk, O.; Buts, Y.; Kobzin, V. To question of danger of wastes of industry in building of highways. Constr. Mater. Sci. Mech. Eng. 2013, 71, 153–157. (In Ukrainian) [Google Scholar]
- Lyubimova, I.; Tersin, V.; Troshin, M.; Gorobets, V.; Bogomolova, I. Assessing the impact of road construction using phosphogypsum on soil pollution. Dokuchaev Soil Bull. 2009, 63, 75–83. (In Russian) [Google Scholar]
- Belyuchenko, I.S.; Dobrydnev, E.P.; Muravev, E.I. Ecological features of phosphogypsum and appropriateness of its use in agriculture. In Proceedings of the II All-Russian Scientific Conference “Problems of Reclamation of Household, Industrial and Agricultural Production Wastes”, Krasnodar, Russia, 18–19 March 2010; pp. 13–22. (In Russian). [Google Scholar]
- Sheudghen, A.H.; Bondareva, T.N. Use of neutralized phosphogypsum as multicomponent fertilizer for rice crops. Sci. J. KubSAU 2015, 113, 1–27. (In Russian) [Google Scholar]
- Zielinska, S.; Radkowski, S. First insight into microbial community composition in a phosphogypsum waste heap soil. Acta Biochim. Pol. 2017, 4, 693–698. [Google Scholar] [CrossRef]
- Belyuchenko, I.S. Features of mineral waste and expediency of their use in the formation of complex composts. Sci. J. Kuban SAU 2014, 101, 1–21. (In Russian) [Google Scholar]
- Astrelin, I.M.; Krymets, H.V.; Fedorov, O.S.; Moliuha, A.I. Method of Phosphogypsum Processing in Complex Fertilizers with Production Semi Wet-Process Phosphoric Acid. Ukraine Patent UA93253U, 25 September 2014. (In Ukrainian). [Google Scholar]
- Erayzer, L.M.; Udovenko, O.H.; Smalii, M.I.; Horniev, V.O. A Method for the Complex Reprocessing Phosphogypsum into Fertilizers. Ukraine Patent UA75743C2, 15 May 2006. (In Ukrainian). [Google Scholar]
- Avramchuk, P.P. A method for Treatment of Manure with Phosphogypsum. Ukraine Patent UA32564C2, 15 March 2001. (In Ukrainian). [Google Scholar]
- Dmitrevsky, B.A.; Dremov, A.V.; Ivanova, N.Y.; Nifontova, T.K.; Treushchenko, N.N.; Yurieva, V.I. The Method of Obtaining Fodder Dicalcium Phosphate. Russian Patent RU2149828C1, 27 May 2000. (In Russian). [Google Scholar]
- Saueia, C.H.R.; Nisti, M.B.; Silva, P.S.C.D.; Oliveira, J.P.D.; Mazzilli, B.P. Lixiviation of rare earth elements in tropical soils amended with phosphogypsum. Int. J. Environ. Anal. Chem. 2019, 6, 675–685. [Google Scholar] [CrossRef]
- Trigub, V. Modern processes of migration and accumulation of fluorine in agrolandscapes of irrigation areas. ONU Geogr. Geol. 2009, 14, 362–368. (In Ukrainian) [Google Scholar]
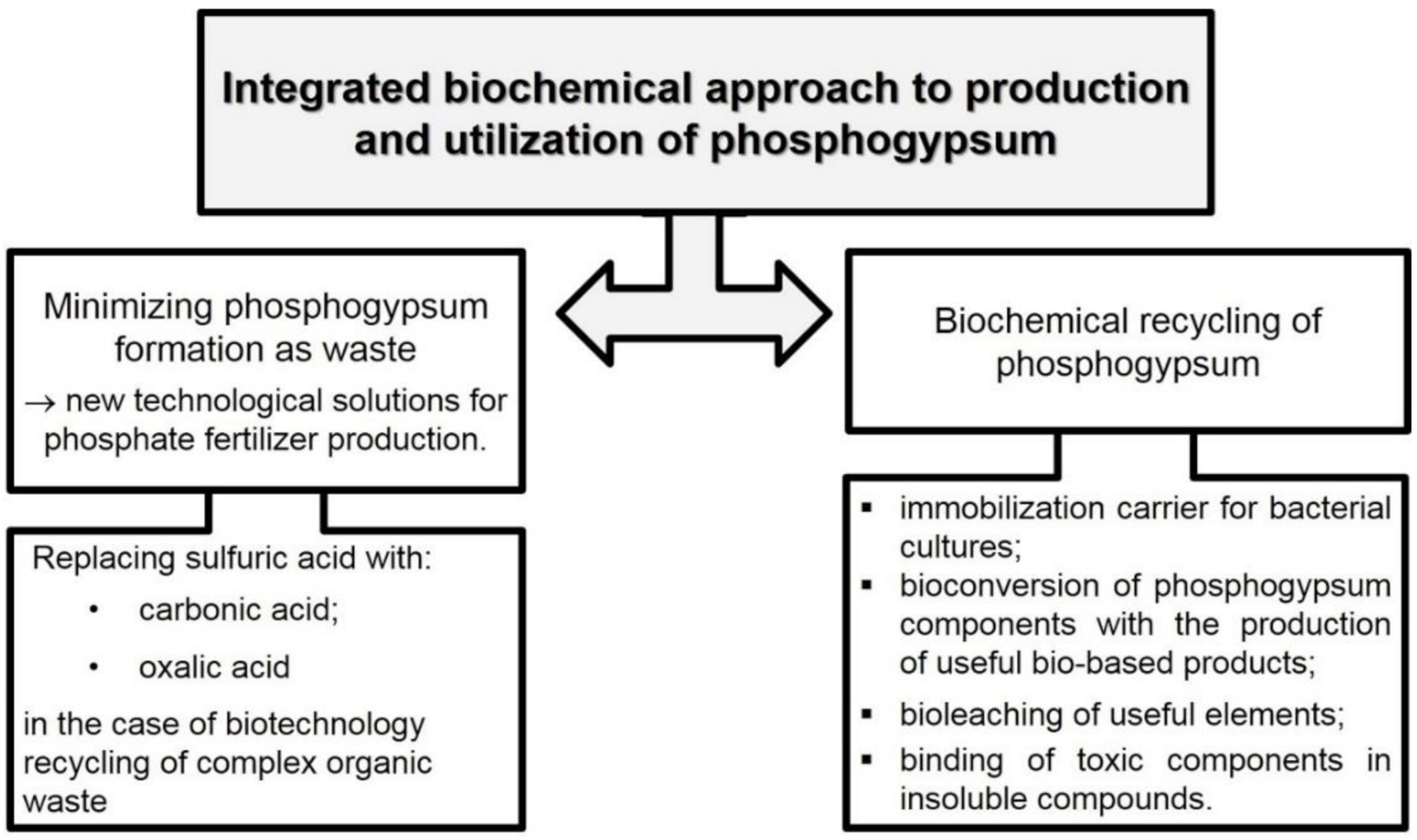
- Plyatsuk, L.; Balintova, M.; Chernysh, Y.; Ablieieva, I.; Ablieiev, O. The process of environmentally safe biochemical recycling of phosphogypsum. In Advances in Design, Simulation and Manufacturing. DSMIE 2019, LNME; Lecture Notes in Mechanical Engineering; Springer: Cham, Switzerland, 2020; pp. 843–852. [Google Scholar]
- Myrzakhmetov, B.; Sholak, A. Chemical composition and complex processing method of phosphogypsum. In Modern Trends in the Development of Science and Production, Proceedings of the West Siberian Scientific Center Conference: Kemerovo, Russia, 15–16 January 2015; pp. 67–71. (In Russian)
- Vorobiev, A.E.; Danilova, Е.V. Audit of rare-earth apatite ore processing technology. RUDN J. Eng. Res. 2013, 4, 36–40. (In Russian) [Google Scholar]
- Samonov, A.E.; Vanshin, Y.V. Problems with apatite processing. Geol. Geogr. Glob. Energy Sci. Tech. J. 2007, 2, 7–9. (In Russian) [Google Scholar]
- Zhang, W.; Zhang, F.; Ma, L.; Ning, P.; Yang, J.; Wei, Y. An efficient methodology to use hydrolysate of phosphogypsum decomposition products for CO2 mineral sequestration and calcium carbonate production. J. Clean. Prod. 2020, 259, 120826. [Google Scholar] [CrossRef]
- Contreras, M.; Pérez-López, R.; Gázquez, M.J.; Morales-Flórez, V.; Santose, A.; Esquivias, L.; Bolívar, J.P. Fractionation and fluxes of metals and radionuclides during the recycling process of phosphogypsum wastes applied to mineral CO2 seques-tration. Waste Manag. 2015, 45, 412–419. [Google Scholar] [CrossRef]
- Bulat, A.F.; Ivanov, V.A.; Holov, K.S.; Mysovets, Y.V. Radio-Protective properties of phosphogypsum binding agent with rare-earth filler. Sci. Bull. Natl. Min. Univ. 2010, 5, 48–51. (In Ukrainian) [Google Scholar]
- Belyuchenko, I.S.; Dobrydnev, E.P.; Muravev, E.I.; Melnik, O.A.; Slavgorodskaya, D.A.; Tereshhenko, E.V. Use of phosphogypsum for reclamation of oil-polluted soils. Work. Kuban SAU 2008, 3, 72–77. (In Russian) [Google Scholar]
- Skipin, L.N.; Khramtsov, N.V.; Guzeeva, S.A.; Petukhova, V.S. Possibility of reclamation of drill cuttings and solonetzic soils using phosphogypsum. Agrar. Vestn. Ural. 2013, 6, 71–73. (In Russian) [Google Scholar]
- Kalinina, O.V. Application of phosphogypsum for reclamation of mazut-polluted soils. North Cauc. Ecol. Her. 2009, 5, 86–87. (In Russian) [Google Scholar]
- Trifi, H.; Najjari, A.; Achouak, W.; Barakat, M.; Ghedira, K.; Mrad, F.; Saidi, M.; Sghaier, H. Metataxonomics of Tunisian phosphogypsum based onfive bioinformaticspipelines: Insights for bioremediation. Genomics 2020, 112, 981–989. [Google Scholar] [CrossRef]
- Barakhnina, V.B.; Khafizova, A.A.; Kireev, I.R. The investigation of using of phosphogypsum in drilling discharged waters biopurification. Bashkir Chem. J. 2011, 18, 90–92. (In Russian) [Google Scholar]
- Chernysh, Y.Y. Sewage Sludge Utilization by Sulfidogenic Association of Microorganisms. Ph.D. Dissertation, Sumy State University, Sumy, Ukraine, 2014. (In Ukrainian). [Google Scholar]
- Plyatsuk, L.D.; Chernish, Y.Y. Intensification of the anaerobic microbiological degradation of sewage sludge under bio-sulfidogenic conditions. J. Solid Waste Technol. Manag. 2014, 40, 10–23. [Google Scholar] [CrossRef]
- Zouch, H.; Karray, F.; Armougom, F.; Chifflet, S.; Hirschler-Réa, A.; Kharrat, H.; Kamoun, L.; Hania, W.B.; Ollivier, B.; Sayadi, S.; et al. Microbial diversity in sulfate-reducing marine sediment enrichment cultures associated with anaerobic biotransformation of coastal stockpiled phosphogypsum (Sfax, Tunisia). Front. Microbiol. 2017, 8, 1583. [Google Scholar] [CrossRef] [PubMed]
- Plyatsuk, L.D.; Chernysh, Y.Y. The removal of hydrogen sulfide in the biodesulfurization system using granulated phosphogypsum. Eurasian Chem. Technol. J. 2016, 18, 47–54. [Google Scholar] [CrossRef]
- Denzanov, G.A.; Petruk, G.D. Resource-Saving technology of bioconversion of natural phosphates. Environ. Bull. 2006, 2, 25. (In Ukrainian) [Google Scholar]


| Phosphogypsum Sample | Origin of Raw Materials | ||
|---|---|---|---|
| Phosphorite | Apatite–Phosphorite | Apatite | |
| (Tatarstan) | (South African) | (Colsky Peninsula) | |
| Aktyubinsky | Apatite–Phosphorite | Apatite | |
| СаО | 24.4 | 30.0 | 32.4 |
| SO3 | 34.8 | 39.8 | 46.2 |
| Р2О5 (total) | 1.9 | 6.2 | 1.3 |
| Р2О5 (water soluble) | 1.1 | 3.3 | 0.7 |
| Fe2O3 | 0.9 | 0.7 | 0.1 |
| Al2O3 | 0.8 | 0.8 | 0.3 |
| F | 0.1 | 0.3 | 0.3 |
| Insoluble residue | 21.7 | 4.3 | 0.7 |
| Crystalline water | 15.6 | 17.4 | 18.6 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chernysh, Y.; Yakhnenko, O.; Chubur, V.; Roubík, H. Phosphogypsum Recycling: A Review of Environmental Issues, Current Trends, and Prospects. Appl. Sci. 2021, 11, 1575. https://doi.org/10.3390/app11041575
Chernysh Y, Yakhnenko O, Chubur V, Roubík H. Phosphogypsum Recycling: A Review of Environmental Issues, Current Trends, and Prospects. Applied Sciences. 2021; 11(4):1575. https://doi.org/10.3390/app11041575
Chicago/Turabian StyleChernysh, Yelizaveta, Olena Yakhnenko, Viktoriia Chubur, and Hynek Roubík. 2021. "Phosphogypsum Recycling: A Review of Environmental Issues, Current Trends, and Prospects" Applied Sciences 11, no. 4: 1575. https://doi.org/10.3390/app11041575
APA StyleChernysh, Y., Yakhnenko, O., Chubur, V., & Roubík, H. (2021). Phosphogypsum Recycling: A Review of Environmental Issues, Current Trends, and Prospects. Applied Sciences, 11(4), 1575. https://doi.org/10.3390/app11041575








