New Evolution of Robotic Radical Prostatectomy: A Single Center Experience with PERUSIA Technique
Abstract
1. Introduction
2. Materials and Methods
3. Main Steps of PERUSIA Technique
- A “U” bladder neck incision with the “Bladder neck sparing” technique;
- Approach perpendicular to the medial side of the seminal vesicles which are mobilized from their lodge always from the medial to the lateral side in order to minimize the manipulation of the neurovascular bundles;
- After incising the Denonvillier’s fascia, an intrafascial antegrade dissection is performed, always proceeding from the medial to the lateral aspect; in this way, the preservation of the bundles is maximized, which in fact remain outside the dissection plane;
- Preservation of the anterior periprostatic tissue and “no-touch” approach of the dorsal vascular complex, developing an anterior avascular plane; this allows us to:Reduce urethral retraction and maximize the functional length of the urethra, which are factors that are essential for the early recovery of urinary continence;Reduce bleeding;Preserve the anterior periprostatic nervous and vascular structures, which seem to have a role in the conservation of sexual potency and urinary continence;Preserve that part of the urethral sphincter that is lined anterolaterally by the dorsal vascular complex;
- Urethrovesical anastomosis with bidirectional self-locking suture (Quill ®).
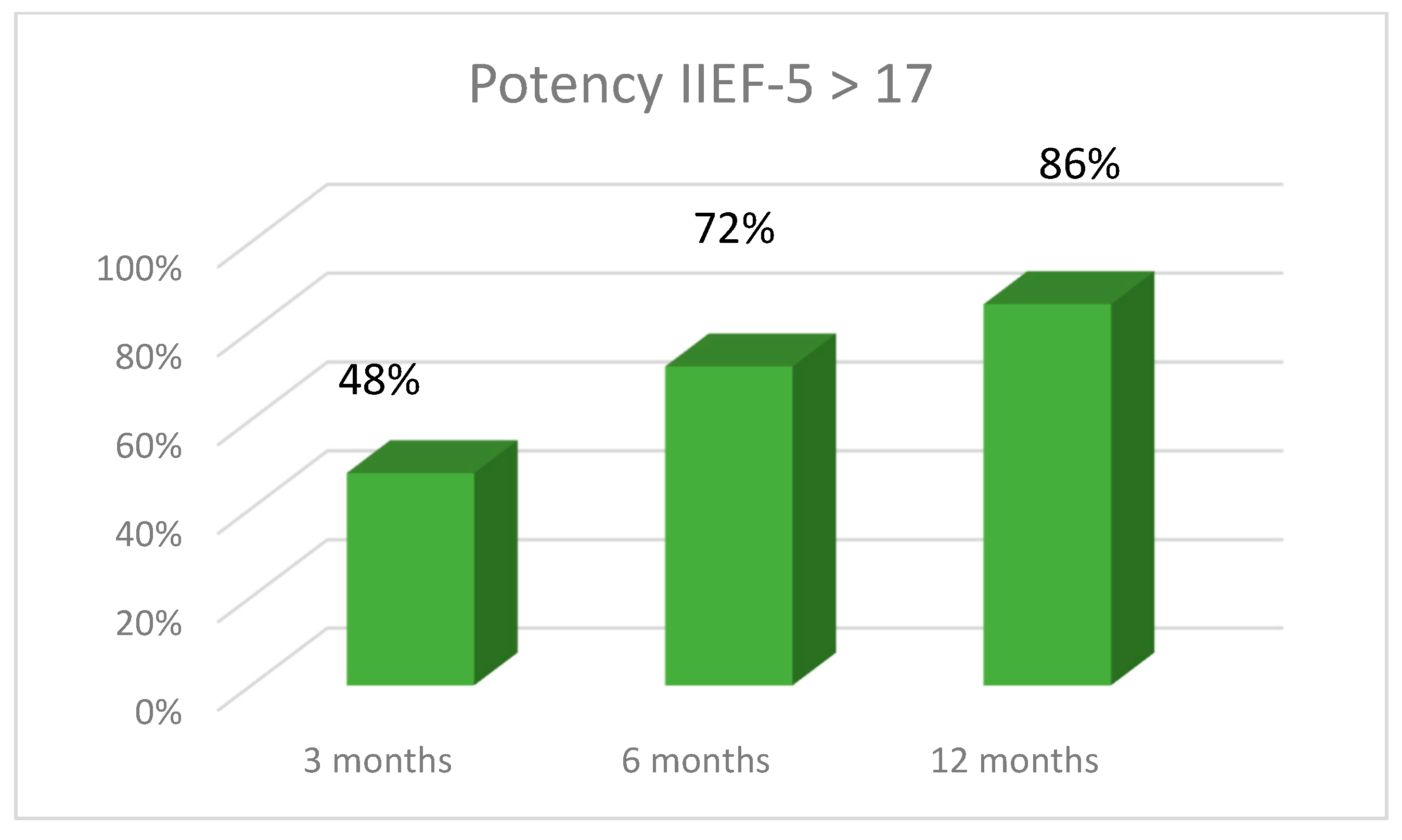
4. Results
5. Discussion
5.1. Why Robotics in Radical Prostatectomy?
5.2. PERUSIA Technique
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cochetti, G.; De Vermandois, J.A.R.; Maulà, V.; Giulietti, M.; Cecati, M.; Del Zingaro, M.; Cagnani, R.; Suvieri, C.; Paladini, A.; Mearini, E. Role of miRNAs in prostate cancer: Do we really know everything? Urol. Oncol. Semin. Orig. Investig. 2020, 38, 623–635. Available online: https://linkinghub.elsevier.com/retrieve/pii/S1078143920300910 (accessed on 10 April 2020). [CrossRef]
- Miller, K.D.; Nogueira, L.; Mariotto, A.B.; Rowland, J.H.; Yabroff, K.R.; Alfano, C.M.; Jemal, A.; Kramer, J.L.; Siegel, R.L. Cancer treatment and survivorship statis-tics, 2019. CA Cancer J. Clin. 2019, 69, 11. [Google Scholar] [CrossRef]
- Heidenreich, A.; Bellmunt, J.; Bolla, M.; Joniau, S.; Mason, M.; Matveev, V.; Mottet, N.; Schmid, H.; van der Kwast, T.; Wiegel, T.; et al. EAU Guidelines on Prostate Cancer. Part 1: Screening, Diagnosis, and Treatment of Clinically Localised Disease. Eur. Urol. 2011, 59, 61–71. [Google Scholar] [CrossRef]
- Schuessler, W.W.; Schulam, P.G.; Clayman, R.V.; Kavoussi, L.R. Laparoscopic radical prostatectomy: Initial short-term experience. Urology 1997, 50, 854–857. [Google Scholar] [CrossRef]
- Guillonneau, B.; Vallancien, G. Laparoscopic radical prostatectomy: The montsouris experience. J. Urol. 2000, 163, 418–422. [Google Scholar] [CrossRef]
- Binder, J.; Kramer, W. Robotically-assisted laparoscopic radical prostatectomy. BJU Int. 2001, 87, 408–410. [Google Scholar] [CrossRef]
- Cochetti, G.; Cocca, D.; Maddonni, S.; Paladini, A.; Sarti, E.; Stivalini, D.; Mearini, E. Combined Robotic Surgery for Double Renal Masses and Prostate Cancer: Myth or Reality? Medicina 2020, 56, 26. [Google Scholar] [CrossRef] [PubMed]
- Boni, A.; Cochetti, G.; Del Zingaro, M.; Paladini, A.; Turco, M.; De Vermandois, J.A.R.; Mearini, E. Uroflow stop test with electromyography: A novel index of urinary continence recovery after RARP. Int. Urol. Nephrol. 2019, 51, 609–615. Available online: http://link.springer.com/10.1007/s11255-019-02107-3 (accessed on 23 February 2019). [CrossRef]
- De Carvalho, P.A.; Barbosa, J.A.B.A.; Guglielmetti, G.B.; Cordeiro, M.D.; Rocco, B.; Nahas, W.C.; Patel, V.; Coelho, R.F. Retrograde Release of the Neuro-vascular Bundle with Preservation of Dorsal Venous Complex During Robot-assisted Radical Prostatectomy: Optimizing Functional Outcomes. Eur. Urol. 2020, 77, 628–635. Available online: https://linkinghub.elsevier.com/retrieve/pii/S0302283818304810 (accessed on 21 July 2018). [CrossRef]
- Cochetti, G.; Boni, A.; Barillaro, F.; Pohja, S.; Cirocchi, R.; Mearini, E. Full Neurovascular Sparing Extraperitoneal Robotic Radical Prostatectomy: Our Experience with PERUSIA Technique. J. Endourol. 2017, 31, 32–37. [Google Scholar] [CrossRef] [PubMed]
- Mitropoulos, D.; Artibani, W.; Graefen, M.; Remzi, M.; Rouprêt, M.; Truss, M. Reporting and Grading of Complications after Uro-logic Surgical Procedures: An ad hoc EAU Guidelines Panel Assessment and Recommendations. Eur. Urol. 2012, 61, 341–349. [Google Scholar] [CrossRef]
- Wei, J.T.; Dunn, R.L.; Litwin, M.S.; Sandler, H.M.; Sanda, M.G. Development and validation of the expanded prostate cancer index composite (EPIC) for comprehensive assessment of health-related quality of life in men with prostate cancer. Urology 2000, 56, 899–905. [Google Scholar] [CrossRef]
- Novara, G.; Ficarra, V.; Rosen, R.C.; Artibani, W.; Costello, A.; Eastham, J.A.; Graefen, M.; Guazzoni, G.; Shariat, S.F.; Stolzenburg, J.E.; et al. Systematic Review and Meta-analysis of Periop-erative Outcomes and Complications After Robot-assisted Radical Prostatectomy. Eur. Urol. 2012, 62. [Google Scholar] [CrossRef]
- Dindo, D.; Demartines, N.; Clavien, P.-A. Classification of Surgical Complications. Ann. Surg. 2004, 240, 205. [Google Scholar] [CrossRef]
- Boorjian, S.A.; Eastham, J.A.; Graefen, M.; Guillonneau, B.; Karnes, R.J.; Moul, J.W.; Schaeffer, E.M.; Stief, C.; Zorn, K.C. A Critical Analysis of the Long-Term Impact of Radical Prostatectomy on Cancer Control and Function Outcomes. Eur. Urol. 2012, 61, 664–675. [Google Scholar] [CrossRef]
- Wilson, T.; Torrey, R. Open versus robotic-assisted radical prostatectomy: Which is better? Curr. Opin. Urol. 2011, 21, 200–205. [Google Scholar] [CrossRef]
- Patel, V.; Coelho, R.F.; Rocco, B.; Orvieto, M.; Sivaraman, A.; Palmer, K.J.; Kameh, D.; Santoro, L.; Coughlin, G.D.; Liss, M.; et al. Positive Surgical Margins After Robotic Assisted Radical Prostatectomy: A Multi-Institutional Study. J. Urol. 2011, 186, 511–517. [Google Scholar] [CrossRef] [PubMed]
- Preisser, F.; Coxilha, G.; Heinze, A.; Oh, S.; Chun, F.K.; Sauter, G.; Pompe, R.S.; Huland, H.; Graefen, M.; Tilki, D. Impact of positive surgical margin length and Gleason grade at the margin on biochemical recurrence in patients with organ-confined prostate cancer. Prostate 2019, 79, 1832–1836. [Google Scholar] [CrossRef] [PubMed]
- Coughlin, G.D.; Yaxley, J.W.; Chambers, S.K.; Occhipinti, S.; Samaratunga, H.; Zajdlewicz, L.; Teloken, P.; Dunglison, N.; Williams, S.; Lavin, M.F.; et al. Robot-assisted laparoscopic pros-tatectomy versus open radical retropubic prostatectomy: 24-month outcomes from a randomised controlled study. Lancet Oncol. 2018, 19, 1051–1060. [Google Scholar] [CrossRef]
- Voltolini, L.; Rapicetta, C.; Luzzi, L.; Paladini, P.; Ghiribelli, C.; Scolletta, S.; Fineschi, M.; Gotti, G. Lung resection for non-small cell lung cancer after prophylactic coronary angioplasty and stenting: Short- and long-term results. Minerva Chir. 2012, 67, 77–85. [Google Scholar]
- Marulli, G.; Breda, C.; Fontana, P.; Ratto, G.B.; Leoncini, G.; Alloisio, M.; Infante, M.; Luzzi, L.; Paladini, P.; Oliaro, A.; et al. Pleurectomy–decortication in malignant pleural mesothelioma: Are different surgical techniques associated with different outcomes? Results from a multicentre study. Eur. J. Cardio-Thorac. Surg. 2017, 52, 63–69. Available online: https://academic.oup.com/ejcts/article-lookup/doi/10.1093/ejcts/ezx079 (accessed on 1 July 2017). [CrossRef]
- Novara, G.; Ficarra, V.; Mocellin, S.; Ahlering, T.E.; Carroll, P.R.; Graefen, M.; Guazzoni, G.; Menon, M.; Patel, V.R.; Shariat, S.F.; et al. Systematic Review and Meta-analysis of Studies Reporting Oncologic Outcome After Robot-assisted Radical Prostatectomy. Eur. Urol. 2012, 62, 382–404. [Google Scholar] [CrossRef]
- Ficarra, V.; Novara, G.; Artibani, W.; Cestari, A.; Galfano, A.; Graefen, M.; Guazzoni, G.; Guillonneau, B.; Menon, M.; Montorsi, F.; et al. Retropubic, Laparoscopic, and Robot-Assisted Radical Prostatectomy: A Systematic Review and Cumulative Analysis of Comparative Studies. Eur. Urol. 2009, 55, 1037–1063. [Google Scholar] [CrossRef] [PubMed]
- Ficarra, V.; Novara, G.; Rosen, R.C.; Artibani, W.; Carroll, P.R.; Costello, A.J.; Menon, M.; Montorsi, F.; Patel, V.R.; Stolzenburg, J.-U.; et al. Systematic Review and Meta-analysis of Studies Reporting Urinary Continence Recovery After Robot-assisted Radical Prostatectomy. Eur. Urol. 2012, 62, 405–417. [Google Scholar] [CrossRef]
- Allan, C.; Ilic, D. Laparoscopic versus Robotic-Assisted Radical Prostatectomy for the Treatment of Localised Prostate Cancer: A Systematic Review. Urol. Int. 2016, 96. [Google Scholar] [CrossRef] [PubMed]
- Dubbelman, Y.D.; Dohle, G.R.; Schröder, F.H. Sexual Function Before and After Radical Retropubic Prostatectomy: A Systematic Review of Prognostic Indicators for a Successful Outcome. Eur. Urol. 2006, 50, 711–720. [Google Scholar] [CrossRef] [PubMed]
- Tewari, A.; Srivasatava, A.; Menon, M. A prospective comparison of radical retropubic and robot-assisted prostatectomy: Expe-rience in one institution. BJU Int. 2003, 92, 205–210. [Google Scholar] [CrossRef]
- Ficarra, V.; Novara, G.; Ahlering, T.E.; Costello, A.; Eastham, J.A.; Graefen, M.; Guazzoni, G.; Menon, M.; Mottrie, A.; Patel, V.R.; et al. Systematic Review and Meta-analysis of Studies Reporting Potency Rates After Robot-assisted Radical Prostatectomy. Eur. Urol. 2012, 62, 418–430. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.C.; Song, C.; Kim, W.; Kang, T.; Park, J.; Jeong, I.G.; Lee, S.; Cho, Y.M.; Ahn, H. Factors Determining Functional Outcomes after Radical Prostatec-tomy: Robot-Assisted Versus Retropubic. Eur. Urol. 2011, 60, 413–419. [Google Scholar] [CrossRef]
- Asimakopoulos, A.D.; Fraga, C.T.P.; Annino, F.; Pasqualetti, P.; Calado, A.A.; Mugnier, C. Randomized Comparison between Laparoscopic and Robot-Assisted Nerve-Sparing Radical Prostatectomy. J. Sex. Med. 2011, 8, 1503–1512. [Google Scholar] [CrossRef]
- Ilic, D.; Evans, S.M.; Allan, C.A.; Jung, J.H.; Murphy, D.; Frydenberg, M. Laparoscopic and robotic-assisted versus open radical pros-tatectomy for the treatment of localised prostate cancer. Cochrane Database Syst. Rev. 2017, 9. [Google Scholar] [CrossRef]
- De Vermandois, J.A.R.; Cochetti, G.; Zingaro, M.; del Santoro, A.; Panciarola, M.; Boni, A.; Marsico, M.; Gaudio, G.; Paladini, A.; Guiggi, P.; et al. Evaluation of surgical site infection in mini-invasive urological surgery. Open Med. 2019, 14, 711–718. [Google Scholar] [CrossRef] [PubMed]
- Yaxley, J.W.; Coughlin, G.D.; Chambers, S.K.; Occhipinti, S.; Samaratunga, H.; Zajdlewicz, L.; Teloken, P.; Dunglison, N.; Williams, S.; Lavin, M.F.; et al. Robot-assisted laparoscopic pros-tatectomy versus open radical retropubic prostatectomy: Early outcomes from a randomised controlled phase 3 study. Lancet 2016, 388, 1057–1066. Available online: https://linkinghub.elsevier.com/retrieve/pii/S014067361630592X (accessed on 26 July 2016). [CrossRef]
- Tavukcu, H.H.; Aytac, O.; Atug, F. Nerve-sparing techniques and results in robot-assisted radical prostatectomy. Investig. Clin. Urol. 2016, 57, S172–S184. [Google Scholar] [CrossRef]
- Horovitz, D.; Feng, C.; Messing, E.M.; Joseph, J.V. Extraperitoneal vs Transperitoneal Robot-Assisted Radical Prostatectomy in the Setting of Prior Abdominal or Pelvic Surgery. J. Endourol. 2017, 31, 366–373. [Google Scholar] [CrossRef]
- Xylinas, E.; Ploussard, G.; Durand, X.; De La Taille, A. Robot-assisted extraperitoneal laparoscopic radical prostatectomy: A review of the current literature. Urol. Oncol. Semin. Orig. Investig. 2013, 31, 288–293. [Google Scholar] [CrossRef] [PubMed]
- Akand, M.; Erdogru, T.; Avci, E.; Ates, M. Transperitoneal versus extraperitoneal robot-assisted laparoscopic radical prostatec-tomy: A prospective single surgeon randomized comparative study. Int. J. Urol. 2015, 22, 916–921. [Google Scholar] [CrossRef]
- Shikanov, S.; Song, J.; Royce, C.; Al-Ahmadie, H.; Zorn, K.; Steinberg, G.; Zagaja, G.; Shalhav, A.; Eggener, S. Length of Positive Surgical Margin After Radical Prostatectomy as a Predictor of Biochemical Recurrence. J. Urol. 2009, 182, 139–144. [Google Scholar] [CrossRef]
- Eminaga, O.; Abbas, M.; Bettendorf, O.; Semjonow, A. Specific spatial distribution patterns of tumor foci are associated with a low risk of biochemical recurrence in pT2pN0R0 prostate cancer. World J. Urol. 2020, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Reeves, F.; Preece, P.; Kapoor, J.; Everaerts, W.; Murphy, D.G.; Corcoran, N.M.; Costello, A.J. Preservation of the Neurovascular Bundles Is Associated with Improved Time to Continence After Radical Prostatectomy but not Long-term Continence Rates: Results of a Systematic Review and Meta-analysis. Eur. Urol. 2015, 68, 692–704. [Google Scholar] [CrossRef]
- Steineck, G.; Bjartell, A.; Hugosson, J.; Axén, E.; Carlsson, S.; Stranne, J.; Wallerstedt, A.; Persson, J.; Wilderäng, U.; Thorsteinsdottir, T.; et al. Degree of Preservation of the Neurovascular Bundles During Radical Prostatectomy and Urinary Continence 1 Year after Surgery. Eur. Urol. 2015, 67, 559–568. [Google Scholar] [CrossRef] [PubMed]
- Ganzer, R.; Stolzenburg, J.-U.; Neuhaus, J.; Weber, F.; Burger, M.; Bründl, J. Is the Striated Urethral Sphincter at Risk by Standard Suture Ligation of the Dorsal Vascular Complex in Radical Prostatectomy? An Anatomic Study. Urology 2014, 84, 1453–1460. [Google Scholar] [CrossRef] [PubMed]
- Stevens, D.J.; Sooriakumaran, P. Oligometastatic Prostate Cancer. Curr. Treat. Options Oncol. 2016, 17, 1–8. [Google Scholar] [CrossRef] [PubMed]

| Values (Range) | |
|---|---|
| Time in console | 76 min (36–120) |
| Time of preparation the Retzius | 22 min (11–33) |
| Estimated blood loss | 128 mL (50–1000) |
| Conversions | 0% |
| Positive surgical margins | 8.1% |
| Biochemical recurrence | 8.6% |
| Complications | 16% |
| Grade I | 8% |
| Grade II | 5.3% |
| Grade III | 2.7% |
| Grade IV–V | 0% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cochetti, G.; Del Zingaro, M.; Ciarletti, S.; Paladini, A.; Felici, G.; Stivalini, D.; Cellini, V.; Mearini, E. New Evolution of Robotic Radical Prostatectomy: A Single Center Experience with PERUSIA Technique. Appl. Sci. 2021, 11, 1513. https://doi.org/10.3390/app11041513
Cochetti G, Del Zingaro M, Ciarletti S, Paladini A, Felici G, Stivalini D, Cellini V, Mearini E. New Evolution of Robotic Radical Prostatectomy: A Single Center Experience with PERUSIA Technique. Applied Sciences. 2021; 11(4):1513. https://doi.org/10.3390/app11041513
Chicago/Turabian StyleCochetti, Giovanni, Michele Del Zingaro, Sara Ciarletti, Alessio Paladini, Graziano Felici, Davide Stivalini, Valerio Cellini, and Ettore Mearini. 2021. "New Evolution of Robotic Radical Prostatectomy: A Single Center Experience with PERUSIA Technique" Applied Sciences 11, no. 4: 1513. https://doi.org/10.3390/app11041513
APA StyleCochetti, G., Del Zingaro, M., Ciarletti, S., Paladini, A., Felici, G., Stivalini, D., Cellini, V., & Mearini, E. (2021). New Evolution of Robotic Radical Prostatectomy: A Single Center Experience with PERUSIA Technique. Applied Sciences, 11(4), 1513. https://doi.org/10.3390/app11041513








