Towards Phase Change Materials for Thermal Energy Storage: Classification, Improvements and Applications in the Building Sector
Abstract
1. Introduction
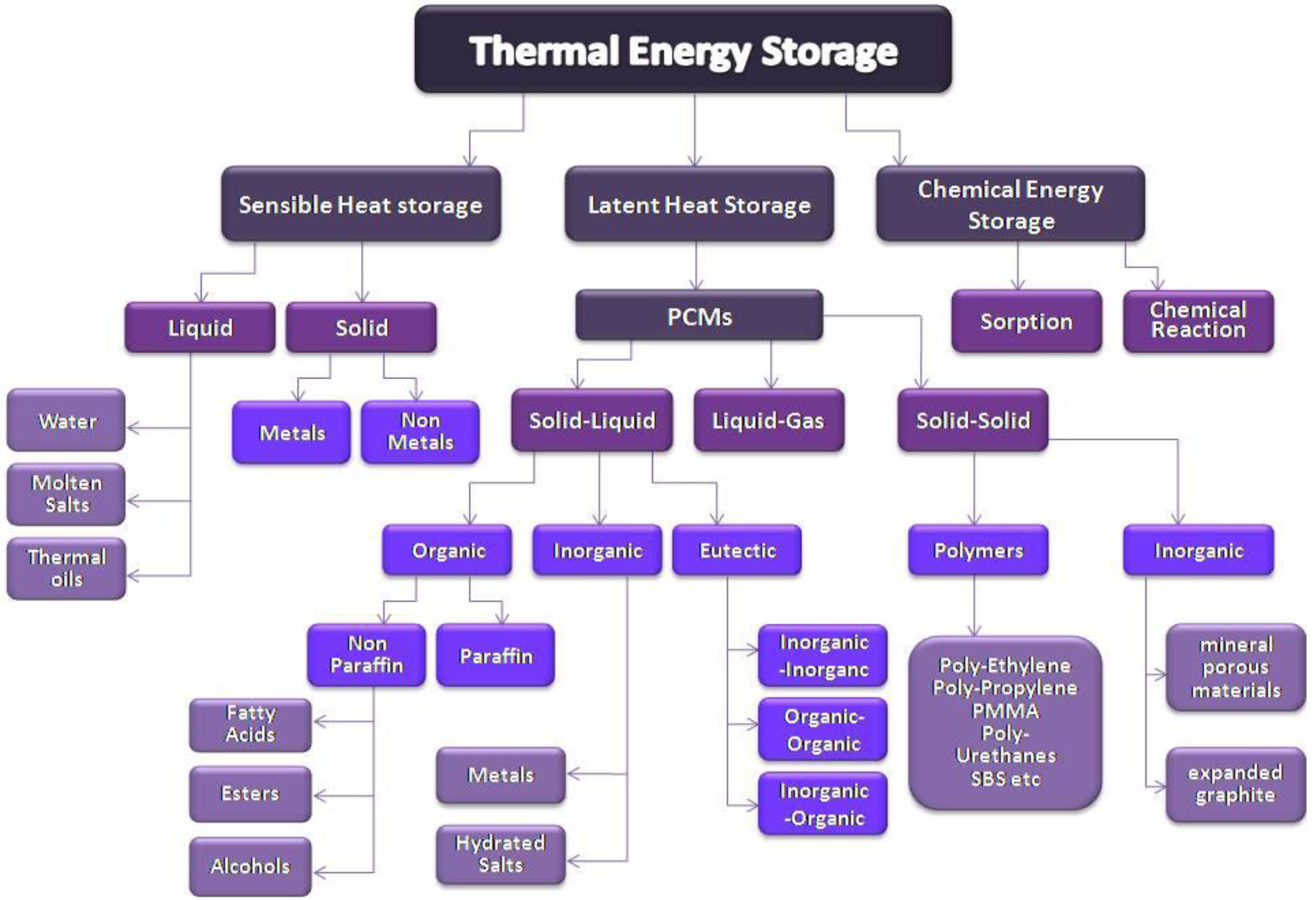
2. Thermal Energy Storage (TES)
2.1. Sensible Heat Storage (SHS)
2.2. Latent Heat Storage
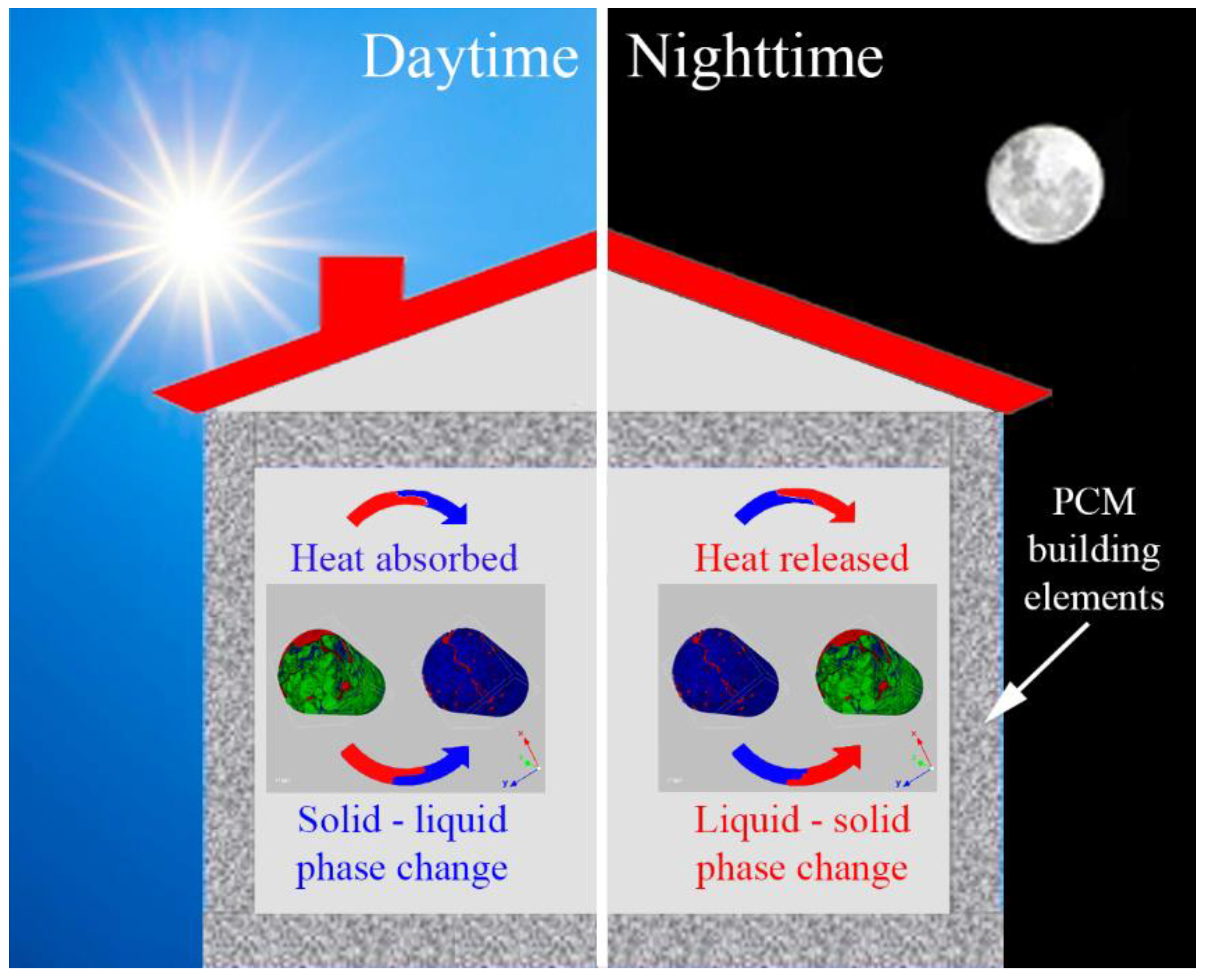
2.3. Thermal Energy Storage in Buildings
3. Phase Change Materials
3.1. Classification of PCMs
3.1.1. Organic Materials
3.1.2. Inorganic Materials
3.1.3. Eutectic Mixtures
3.2. PCM Properties
4. Improvement of Thermal Performance
4.1. Encapsulation
4.1.1. Microencapsulation
4.1.2. Methods of Microencapsulation
4.1.3. Nanoencapsulation
4.2. Form-stable PCMs
4.2.1. Polymer Form-stable PCMs
4.2.2. Inorganic Form-stable PCMs
4.3. Thermal Conductivity Enhancement Techniques
4.3.1. Enhancement of Thermal Conductivity by Encapsulation
4.3.2. Enhancement of Thermal Conductivity with Nanoparticle Additives
4.3.3. Enhancement of Thermal Conductivity with Metallic Foams and Expanded Graphite
5. Applications of PCMs in Buildings
5.1. PCM Passive Application Systems in Building
5.1.1. PCMs in Wall
Mortars and Plasters
Gypsum Boards
Concrete Blocks and Bricks
PCM Panels and Wallboards
5.1.2. Floor Applications
5.1.3. Ceiling Applications
5.1.4. Windows and Glazed Applications
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sarbu, I.; Sebarchievici, C. A Comprehensive Review of Thermal Energy Storage. Sustainability 2018, 10, 191. [Google Scholar] [CrossRef]
- Shchukina, E.M.; Graham, M.; Zheng, Z.; Shchukin, D.G. Nanoencapsulation of phase change materials for advanced thermal energy storage systems. Chem. Soc. Rev. 2018, 47, 4156–4175. [Google Scholar] [CrossRef]
- Drissi, S.; Ling, T.-C.; Mo, K.H.; Eddhahak, A. A review of microencapsulated and composite phase change materials: Alteration of strength and thermal properties of cement-based materials. Renew. Sustain. Energy Rev. 2019, 110, 467–484. [Google Scholar] [CrossRef]
- Cui, Y.; Xie, J.; Liu, J.; Wang, J.; Chen, S. A review on phase change material application in building. Adv. Mech. Eng. 2017, 9. [Google Scholar] [CrossRef]
- Zhu, N.; Li, S.; Hu, P.; Wei, S.; Deng, R.; Lei, F. A review on applications of shape-stabilized phase change materials embedded in building enclosure in recent ten years. Sustain. Cities Soc. 2018, 43, 251–264. [Google Scholar] [CrossRef]
- Nazir, H.; Batool, M.; Osorio, F.J.B.; Isaza-Ruiz, M.; Xu, X.; Vignarooban, K.; Phelan, P.; Kannan, A.M. Recent developments in phase change materials for energy storage applications: A review. Int. J. Heat Mass Transf. 2019, 129, 491–523. [Google Scholar] [CrossRef]
- de Gracia, A.; Cabeza, L.F. Phase change materials and thermal energy storage for buildings. Energy Build. 2015, 103, 414–419. [Google Scholar] [CrossRef]
- Sharma, A.; Tyagi, V.V.; Chen, C.R.; Buddhi, D. Review on thermal energy storage with phase change materials and applications. Renew. Sustain. Energy Rev. 2009, 13, 318–345. [Google Scholar] [CrossRef]
- Keshteli, A.N.; Sheikholeslami, M. Nanoparticle enhanced PCM applications for intensification of thermal performance in building: A review. J. Mol. Liq. 2019, 274, 516–533. [Google Scholar] [CrossRef]
- Drissi, S.; Ling, T.-C.; Mo, K.H. Thermal efficiency and durability performances of paraffinic phase change materials with enhanced thermal conductivity—A review. Thermochim. Acta 2019, 673, 198–210. [Google Scholar] [CrossRef]
- Leong, K.Y.; Rahman, M.R.A.; Gurunathan, B.A. Nano-enhanced phase change materials: A review of thermo-physical properties, applications and challenges. J. Energy Storage 2019, 21, 18–31. [Google Scholar] [CrossRef]
- Aziz, N.A.; Amin, N.A.M.; Majid, M.S.A.; Zaman, I. Thermal energy storage (TES) technology for active and passive cooling in buildings: A Review. Matec Web Conf. 2018, 225, 03022. [Google Scholar] [CrossRef]
- Khadiran, T.; Hussein, M.Z.; Zainal, Z.; Rusli, R. Advanced energy storage materials for building applications and their thermal performance characterization: A review. Renew. Sustain. Energy Rev. 2016, 57, 916–928. [Google Scholar] [CrossRef]
- da Cunha, S.R.L.; de Aguiar, J.L.B. Phase change materials and energy efficiency of buildings: A review of knowledge. J. Energy Storage 2020, 27, 101083. [Google Scholar] [CrossRef]
- Kahwaji, S.; White, M.A. Edible Oils as Practical Phase Change Materials for Thermal Energy Storage. Appl. Sci. 2019, 9, 1627. [Google Scholar] [CrossRef]
- Souayfane, F.; Fardoun, F.; Biwole, P.-H. Phase change materials (PCM) for cooling applications in buildings: A review. Energy Build. 2016, 129, 396–431. [Google Scholar] [CrossRef]
- Heier, J.; Bales, C.; Martin, V. Combining thermal energy storage with buildings—A review. Renew. Sustain. Energy Rev. 2015, 42, 1305–1325. [Google Scholar] [CrossRef]
- Ghadim, H.B.; Shahbaz, K.; Al-Shannaq, R.; Farid, M.M. Binary mixtures of fatty alcohols and fatty acid esters as novel solid-liquid phase change materials. Int. J. Energy Res. 2019. [Google Scholar] [CrossRef]
- Cabeza, L.F.; Castell, A.; Barreneche, C.; de Gracia, A.; Fernández, A.I. Materials used as PCM in thermal energy storage in buildings: A review. Renew. Sustain. Energy Rev. 2011, 15, 1675–1695. [Google Scholar] [CrossRef]
- Rodríguez-Cumplido, F.; Pabón-Gelves, E.; Chejne-Jana, F. Recent developments in the synthesis of microencapsulated and nanoencapsulated phase change materials. J. Energy Storage 2019, 24, 100821. [Google Scholar] [CrossRef]
- Milián, Y.E.; Gutiérrez, A.; Grágeda, M.; Ushak, S. A review on encapsulation techniques for inorganic phase change materials and the influence on their thermophysical properties. Renew. Sustain. Energy Rev. 2017, 73, 983–999. [Google Scholar] [CrossRef]
- Jamekhorshid, A.; Sadrameli, S.M.; Farid, M. A review of microencapsulation methods of phase change materials (PCMs) as a thermal energy storage (TES) medium. Renew. Sustain. Energy Rev. 2014, 31, 531–542. [Google Scholar] [CrossRef]
- Liu, H.; Wang, X.; Wu, D.; Ji, S. Morphology-controlled synthesis of microencapsulated phase change materials with TiO2 shell for thermal energy harvesting and temperature regulation. Energy 2019, 172, 599–617. [Google Scholar] [CrossRef]
- Sarı, A.; Bicer, A.; Alkan, C.; Özcan, A.N. Thermal energy storage characteristics of myristic acid-palmitic eutectic mixtures encapsulated in PMMA shell. Sol. Energy Mater. Sol. Cells 2019, 193, 1–6. [Google Scholar] [CrossRef]
- Sánchez, L.; Sánchez, P.; de Lucas, A.; Carmona, M.; Rodríguez, J.F. Microencapsulation of PCMs with a polystyrene shell. Colloid Polym. Sci. 2007, 285, 1377–1385. [Google Scholar] [CrossRef]
- Su, J.; Wang, L.; Ren, L. Fabrication and thermal properties of microPCMs: Used melamine-formaldehyde resin as shell material. J. Appl. Polym. Sci. 2006, 101, 1522–1528. [Google Scholar] [CrossRef]
- Döğüşcü, D.K.; Damlıoğlu, Y.; Alkan, C. Poly(styrene-co-divinylbenzene-co-acrylamide)/n-octadecane microencapsulated phase change materials for thermal energy storage. Sol. Energy Mater. Sol. Cells 2019, 198, 5–10. [Google Scholar] [CrossRef]
- Giro-Paloma, J.; Al-Shannaq, R.; Fernandez, A.I.; Farid, M.M. Preparation and Characterization of Microencapsulated Phase Change Materials for Use in Building Applications. Materials 2015, 9, 11. [Google Scholar] [CrossRef] [PubMed]
- Alva, G.; Lin, Y.; Liu, L.; Fang, G. Synthesis, characterization and applications of microencapsulated phase change materials in thermal energy storage: A review. Energy Build. 2017, 144, 276–294. [Google Scholar] [CrossRef]
- Onder, E.; Sarier, N.; Cimen, E. Encapsulation of phase change materials by complex coacervation to improve thermal performances of woven fabrics. Thermochim. Acta 2008, 467, 63–72. [Google Scholar] [CrossRef]
- Su, W.; Darkwa, J.; Kokogiannakis, G. Review of solid-liquid phase change materials and their encapsulation technologies. Renew. Sustain. Energy Rev. 2015, 48, 373–391. [Google Scholar] [CrossRef]
- Liu, C.; Rao, Z.; Zhao, J.; Huo, Y.; Li, Y. Review on nanoencapsulated phase change materials: Preparation, characterization and heat transfer enhancement. Nano Energy 2015, 13, 814–826. [Google Scholar] [CrossRef]
- Kenisarin, M.M.; Kenisarina, K.M. Form-stable phase change materials for thermal energy storage. Renew. Sustain. Energy Rev. 2012, 16, 1999–2040. [Google Scholar] [CrossRef]
- Alkan, C.; Sari, A. Fatty acid/poly(methyl methacrylate) (PMMA) blends as form-stable phase change materials for latent heat thermal energy storage. Sol. Energy 2008, 82, 118–124. [Google Scholar] [CrossRef]
- Sobolciak, P.; Karkri, M.; Al-Maadeed, M.A.; Krupa, I. Thermal characterization of phase change materials based on linear low-density polyethylene, paraffin wax and expanded graphite. Renew. Energy 2016, 88, 372–382. [Google Scholar] [CrossRef]
- Alkan, C.; Kaya, K.; Sari, A. Preparation, Thermal Properties and Thermal Reliability of Form-Stable Paraffin/Polypropylene Composite for Thermal Energy Storage. J. Polym. Environ. 2009, 17, 254–258. [Google Scholar] [CrossRef]
- Tang, B.; Wang, L.; Xu, Y.; Xiu, J.; Zhang, S. Hexadecanol/phase change polyurethane composite as form-stable phase change material for thermal energy storage. Sol. Energy Mater. Sol. Cells 2016, 144, 1–6. [Google Scholar] [CrossRef]
- Wang, L.; Meng, D. Fatty acid eutectic/polymethyl methacrylate composite as form-stable phase change material for thermal energy storage. Appl. Energy 2010, 87, 2660–2665. [Google Scholar] [CrossRef]
- Jin, X.; Li, J.; Xue, P.; Jia, M. Preparation and characterization of PVC-based form-stable phase change materials. Sol. Energy Mater. Sol. Cells 2014, 130, 435–441. [Google Scholar] [CrossRef]
- Chen, P.; Gao, X.; Wang, Y.; Xu, T.; Fang, Y.; Zhang, Z. Metal foam embedded in SEBS/paraffin/HDPE form-stable PCMs for thermal energy storage. Sol. Energy Mater. Sol. Cells 2016, 149, 60–65. [Google Scholar] [CrossRef]
- Şentürk, S.B.; Kahraman, D.; Alkan, C.; Gökçe, İ. Biodegradable PEG/cellulose, PEG/agarose and PEG/chitosan blends as shape stabilized phase change materials for latent heat energy storage. Carbohydr. Polym. 2011, 84, 141–144. [Google Scholar] [CrossRef]
- Wu, B.; Zhao, Y.; Liu, Q.; Zhou, C.; Zhang, X.; Lei, J. Form-stable phase change materials based on castor oil and palmitic acid for renewable thermal energy storage. J. Therm. Anal. Calorim. 2019, 137, 1225–1232. [Google Scholar] [CrossRef]
- Lv, P.; Liu, C.; Rao, Z. Review on clay mineral-based form-stable phase change materials: Preparation, characterization and applications. Renew. Sustain. Energy Rev. 2017, 68, 707–726. [Google Scholar] [CrossRef]
- Umair, M.M.; Zhang, Y.; Iqbal, K.; Zhang, S.; Tang, B. Novel strategies and supporting materials applied to shape-stabilize organic phase change materials for thermal energy storage—A review. Appl. Energy 2019, 235, 846–873. [Google Scholar] [CrossRef]
- Qureshi, Z.A.; Ali, H.M.; Khushnood, S. Recent advances on thermal conductivity enhancement of phase change materials for energy storage system: A review. Int. J. Heat Mass Transf. 2018, 127, 838–856. [Google Scholar] [CrossRef]
- Salunkhe, P.B.; Shembekar, P.S. A review on effect of phase change material encapsulation on the thermal performance of a system. Renew. Sustain. Energy Rev. 2012, 16, 5603–5616. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, X.; Wu, D. Silica encapsulation of n-octadecane via sol-gel process: A novel microencapsulated phase-change material with enhanced thermal conductivity and performance. J. Colloid Interface Sci. 2010, 343, 246–255. [Google Scholar] [CrossRef]
- Harish, S.; Ishikawa, K.; Chiashi, S.; Shiomi, J.; Maruyama, S. Anomalous Thermal Conduction Characteristics of Phase Change Composites with Single-Walled Carbon Nanotube Inclusions. J. Phys. Chem. C 2013, 117, 15409–15413. [Google Scholar] [CrossRef]
- Lin, Y.; Zhu, C.; Fang, G. Synthesis and properties of microencapsulated stearic acid/silica composites with graphene oxide for improving thermal conductivity as novel solar thermal storage materials. Sol. Energy Mater. Sol. Cells 2019, 189, 197–205. [Google Scholar] [CrossRef]
- Lin, S.C.; Al-Kayiem, H.H. Evaluation of copper nanoparticles—Paraffin wax compositions for solar thermal energy storage. Sol. Energy 2016, 132, 267–278. [Google Scholar] [CrossRef]
- Mohamed, N.H.; Soliman, F.S.; El Maghraby, H.; Moustfa, Y.M. Thermal conductivity enhancement of treated petroleum waxes, as phase change material, by α nano alumina: Energy storage. Renew. Sustain. Energy Rev. 2017, 70, 1052–1058. [Google Scholar] [CrossRef]
- Huang, X.; Lin, Y.; Alva, G.; Fang, G. Thermal properties and thermal conductivity enhancement of composite phase change materials using myristyl alcohol/metal foam for solar thermal storage. Sol. Energy Mater. Sol. Cells 2017, 170, 68–76. [Google Scholar] [CrossRef]
- Li, C.; Zhang, B.; Xie, B.; Zhao, X.; Chen, J.; Chen, Z.; Long, Y. Stearic acid/expanded graphite as a composite phase change thermal energy storage material for tankless solar water heater. Sustain. Cities Soc. 2019, 44, 458–464. [Google Scholar] [CrossRef]
- Liu, Y.-D.; Zhou, Y.-G.; Tong, M.-W.; Zhou, X.-S. Experimental study of thermal conductivity and phase change performance of nanofluids PCMs. Microfluid. Nanofluidics 2009, 7, 579–584. [Google Scholar] [CrossRef]
- Akeiber, H.; Nejat, P.; Majid, M.Z.A.; Wahid, M.A.; Jomehzadeh, F.; Famileh, I.Z.; Calautit, J.K.; Hughes, B.R.; Zaki, S.A. A review on phase change material (PCM) for sustainable passive cooling in building envelopes. Renew. Sustain. Energy Rev. 2016, 60, 1470–1497. [Google Scholar] [CrossRef]
- Xia, Y.; Cui, W.; Ji, R.; Huang, C.; Huang, Y.; Zhang, H.; Xu, F.; Huang, P.; Li, B.; Sun, L. Design and synthesis of novel microencapsulated phase change materials with enhancement of thermal conductivity and thermal stability: Self-assembled boron nitride into shell materials. Colloids Surf. A Physicochem. Eng. Asp. 2020, 586, 124225. [Google Scholar] [CrossRef]
- Zhou, Y.; Li, C.; Wu, H.; Guo, S. Construction of hybrid graphene oxide/graphene nanoplates shell in paraffin microencapsulated phase change materials to improve thermal conductivity for thermal energy storage. Colloids Surf. A Physicochem. Eng. Asp. 2020, 597, 124780. [Google Scholar] [CrossRef]
- Zhu, C.; Lin, Y.; Fang, G. Preparation and thermal properties of microencapsulated stearyl alcohol with silicon dioxide shell as thermal energy storage materials. Appl. Therm. Eng. 2020, 169, 114943. [Google Scholar] [CrossRef]
- Wang, Z.; Ma, W.; Hu, D.; Wu, L. Synthesis and characterization of microencapsulated methyl laurate with polyurethane shell materials via interfacial polymerization in Pickering emulsions. Colloids Surf. A Physicochem. Eng. Asp. 2020, 600, 124958. [Google Scholar] [CrossRef]
- Zhu, Y.; Qin, Y.; Liang, S.; Chen, K.; Tian, C.; Wang, J.; Luo, X.; Zhang, L. Graphene/SiO2/n-octadecane nanoencapsulated phase change material with flower like morphology, high thermal conductivity, and suppressed supercooling. Appl. Energy 2019, 250, 98–108. [Google Scholar] [CrossRef]
- Zhao, M.; Zhang, X.; Kong, X. Preparation and characterization of a novel composite phase change material with double phase change points based on nanocapsules. Renew. Energy 2020, 147, 374–383. [Google Scholar] [CrossRef]
- Zhu, Y.; Qin, Y.; Wei, C.; Liang, S.; Luo, X.; Wang, J.; Zhang, L. Nanoencapsulated phase change materials with polymer-SiO2 hybrid shell materials: Compositions, morphologies, and properties. Energy Convers. Manag. 2018, 164, 83–92. [Google Scholar] [CrossRef]
- Liao, H.; Chen, W.; Liu, Y.; Wang, Q. A phase change material encapsulated in a mechanically strong graphene aerogel with high thermal conductivity and excellent shape stability. Compos. Sci. Technol. 2020, 189, 108010. [Google Scholar] [CrossRef]
- Su, J.; Ren, L.; Wang, L. Preparation and mechanical properties of thermal energy storage microcapsules. Colloid Polym. Sci. 2005, 284, 224–228. [Google Scholar] [CrossRef]
- Song, Y.; Zhang, N.; Jing, Y.; Cao, X.; Yuan, Y.; Haghighat, F. Experimental and numerical investigation on dodecane/expanded graphite shape-stabilized phase change material for cold energy storage. Energy 2019, 189, 116175. [Google Scholar] [CrossRef]
- Zhang, W.; Zhang, X.; Zhang, X.; Yin, Z.; Liu, Y.; Fang, M.; Wu, X.; Min, X.; Huang, Z. Lauric-stearic acid eutectic mixture/carbonized biomass waste corn cob composite phase change materials: Preparation and thermal characterization. Thermochim. Acta 2019, 674, 21–27. [Google Scholar] [CrossRef]
- Song, S.; Qiu, F.; Zhu, W.; Guo, Y.; Zhang, Y.; Ju, Y.; Feng, R.; Liu, Y.; Chen, Z.; Zhou, J.; et al. Polyethylene glycol/halloysite@Ag nanocomposite PCM for thermal energy storage: Simultaneously high latent heat and enhanced thermal conductivity. Sol. Energy Mater. Sol. Cells 2019, 193, 237–245. [Google Scholar] [CrossRef]
- Tan, N.; Xie, T.; Feng, Y.; Hu, P.; Li, Q.; Jiang, L.-M.; Zeng, W.-B.; Zeng, J.-L. Preparation and characterization of erythritol/sepiolite/exfoliated graphite nanoplatelets form-stable phase change material with high thermal conductivity and suppressed supercooling. Sol. Energy Mater. Sol. Cells 2020, 217, 110726. [Google Scholar] [CrossRef]
- Wu, B.; Lao, D.; Fu, R.; Su, X.; Liu, H.; Jin, X. Novel PEG/EP form-stable phase change materials with high thermal conductivity enhanced by 3D ceramics network. Ceram. Int. 2020, 46, 25285–25292. [Google Scholar] [CrossRef]
- Tan, N.; Xie, T.; Hu, P.; Feng, Y.; Li, Q.; Zhao, S.; Zhou, H.-N.; Zeng, W.-B.; Zeng, J.-L. Preparation and characterization of capric-palmitic acids eutectics/silica xerogel/exfoliated graphite nanoplatelets form-stable phase change materials. J. Energy Storage 2021, 34, 102016. [Google Scholar] [CrossRef]
- Mishra, A.K.; Lahiri, B.B.; Philip, J. Carbon black nano particle loaded lauric acid-based form-stable phase change material with enhanced thermal conductivity and photo-thermal conversion for thermal energy storage. Energy 2020, 191, 116572. [Google Scholar] [CrossRef]
- Chen, Y.; Cui, Z.; Ding, H.; Wan, Y.; Tang, Z.; Gao, J. Cost-Effective Biochar Produced from Agricultural Residues and Its Application for Preparation of High Performance Form-Stable Phase Change Material via Simple Method. Int. J. Mol. Sci. 2018, 19, 3055. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, K.; Tao, W.; Li, D. Preparation of microencapsulated phase change materials used graphene oxide to improve thermal stability and its incorporation in gypsum materials. Constr. Build. Mater. 2019, 224, 48–56. [Google Scholar] [CrossRef]
- Cheng, W.-L.; Li, W.-W.; Nian, Y.-L.; Xia, W.-d. Study of thermal conductive enhancement mechanism and selection criteria of carbon-additive for composite phase change materials. Int. J. Heat Mass Transf. 2018, 116, 507–511. [Google Scholar] [CrossRef]
- Sami, S.; Etesami, N. Improving thermal characteristics and stability of phase change material containing TiO2 nanoparticles after thermal cycles for energy storage. Appl. Therm. Eng. 2017, 124, 346–352. [Google Scholar] [CrossRef]
- Zheng, H.; Wang, C.; Liu, Q.; Tian, Z.; Fan, X. Thermal performance of copper foam/paraffin composite phase change material. Energy Convers. Manag. 2018, 157, 372–381. [Google Scholar] [CrossRef]
- Hussain, A.; Tso, C.Y.; Chao, C.Y.H. Experimental investigation of a passive thermal management system for high-powered lithium ion batteries using nickel foam-paraffin composite. Energy 2016, 115, 209–218. [Google Scholar] [CrossRef]
- Sedeh, M.M.; Khodadadi, J.M. Thermal conductivity improvement of phase change materials/graphite foam composites. Carbon 2013, 60, 117–128. [Google Scholar] [CrossRef]
- Sarı, A.; Karaipekli, A. Preparation, thermal properties and thermal reliability of palmitic acid/expanded graphite composite as form-stable PCM for thermal energy storage. Sol. Energy Mater. Sol. Cells 2009, 93, 571–576. [Google Scholar] [CrossRef]
- Tang, Y.; Lin, Y.; Jia, Y.; Fang, G. Improved thermal properties of stearyl alcohol/high density polyethylene/expanded graphite composite phase change materials for building thermal energy storage. Energy Build. 2017, 153, 41–49. [Google Scholar] [CrossRef]
- Gholamibozanjani, G.; Farid, M. A comparison between passive and active PCM systems applied to buildings. Renew. Energy 2020, 162, 112–123. [Google Scholar] [CrossRef]
- Meng, E.; Cai, R.; Sun, Z.; Yang, J.; Wang, J. Experimental study of the passive and active performance of real-scale composite PCM room in winter. Appl. Therm. Eng. 2021, 185, 116418. [Google Scholar] [CrossRef]
- Song, M.; Niu, F.; Mao, N.; Hu, Y.; Deng, S. Review on building energy performance improvement using phase change materials. Energy Build. 2018, 158, 776–793. [Google Scholar] [CrossRef]
- Cunha, S.; Lima, M.; Aguiar, J.B. Influence of adding phase change materials on the physical and mechanical properties of cement mortars. Constr. Build. Mater. 2016, 127, 1–10. [Google Scholar] [CrossRef]
- Cunha, S.; Leite, P.; Aguiar, J. Characterization of innovative mortars with direct incorporation of phase change materials. J. Energy Storage 2020, 30, 101439. [Google Scholar] [CrossRef]
- Guardia, C.; Barluenga, G.; Palomar, I.; Diarce, G. Thermal enhanced cement-lime mortars with phase change materials (PCM), lightweight aggregate and cellulose fibers. Constr. Build. Mater. 2019, 221, 586–594. [Google Scholar] [CrossRef]
- Hattan, H.A.; Madhkhan, M.; Marani, A. Thermal and mechanical properties of building external walls plastered with cement mortar incorporating shape-stabilized phase change materials (SSPCMs). Constr. Build. Mater. 2021, 270. [Google Scholar] [CrossRef]
- Srinivasaraonaik, B.; Singh, L.P.; Sinha, S.; Tyagi, I.; Rawat, A. Studies on the mechanical properties and thermal behavior of microencapsulated eutectic mixture in gypsum composite board for thermal regulation in the buildings. J. Build. Eng. 2020, 31, 101400. [Google Scholar] [CrossRef]
- Bravo, J.P.; Venegas, T.; Correa, E.; Álamos, A.; Sepúlveda, F.; Vasco, D.A.; Barreneche, C. Experimental and Computational Study of the Implementation of mPCM-Modified Gypsum Boards in a Test Enclosure. Buildings 2020, 10, 15. [Google Scholar] [CrossRef]
- Cao, V.D.; Pilehvar, S.; Salas-Bringas, C.; Szczotok, A.M.; Rodriguez, J.F.; Carmona, M.; Al-Manasir, N.; Kjøniksen, A.-L. Microencapsulated phase change materials for enhancing the thermal performance of Portland cement concrete and geopolymer concrete for passive building applications. Energy Convers. Manag. 2017, 133, 56–66. [Google Scholar] [CrossRef]
- Saxena, R.; Rakshit, D.; Kaushik, S.C. Phase change material (PCM) incorporated bricks for energy conservation in composite climate: A sustainable building solution. Sol. Energy 2019, 183, 276–284. [Google Scholar] [CrossRef]
- Mizan, M.H.; Ueda, T.; Matsumoto, K. Enhancement of the concrete-PCM interfacial bonding strength using silica fume. Constr. Build. Mater. 2020, 259, 119774. [Google Scholar] [CrossRef]
- El Omari, K.; Le Guer, Y.; Bruel, P. Analysis of micro-dispersed PCM-composite boards behavior in a building’s wall for different seasons. J. Build. Eng. 2016, 7, 361–371. [Google Scholar] [CrossRef]
- Zhu, N.; Liu, F.; Liu, P.; Hu, P.; Wu, M. Energy saving potential of a novel phase change material wallboard in typical climate regions of China. Energy Build. 2016, 128, 360–369. [Google Scholar] [CrossRef]
- Meng, E.; Yu, H.; Zhou, B. Study of the thermal behavior of the composite phase change material (PCM) room in summer and winter. Appl. Therm. Eng. 2017, 126, 212–225. [Google Scholar] [CrossRef]
- Yu, H.; Li, C.; Zhang, K.; Tang, Y.; Song, Y.; Wang, M. Preparation and thermophysical performance of diatomite-based composite PCM wallboard for thermal energy storage in buildings. J. Build. Eng. 2020, 32, 101753. [Google Scholar] [CrossRef]
- Gnanachelvam, S.; Ariyanayagam, A.; Mahendran, M. Fire resistance of LSF wall systems lined with different wallboards including bio-PCM mat. J. Build. Eng. 2020, 32, 101628. [Google Scholar] [CrossRef]
- Maleki, B.; Khadang, A.; Maddah, H.; Alizadeh, M.; Kazemian, A.; Ali, H.M. Development and thermal performance of nanoencapsulated PCM/ plaster wallboard for thermal energy storage in buildings. J. Build. Eng. 2020, 32, 101727. [Google Scholar] [CrossRef]
- Entrop, A.G.; Brouwers, H.J.H.; Reinders, A.H.M.E. Experimental research on the use of micro-encapsulated Phase Change Materials to store solar energy in concrete floors and to save energy in Dutch houses. Sol. Energy 2011, 85, 1007–1020. [Google Scholar] [CrossRef]
- Royon, L.; Karim, L.; Bontemps, A. Optimization of PCM embedded in a floor panel developed for thermal management of the lightweight envelope of buildings. Energy Build. 2014, 82, 385–390. [Google Scholar] [CrossRef]
- Sun, W.; Zhang, Y.; Ling, Z.; Fang, X.; Zhang, Z. Experimental investigation on the thermal performance of double-layer PCM radiant floor system containing two types of inorganic composite PCMs. Energy Build. 2020, 211, 109806. [Google Scholar] [CrossRef]
- Ansuini, R.; Larghetti, R.; Giretti, A.; Lemma, M. Radiant floors integrated with PCM for indoor temperature control. Energy Build. 2011, 43, 3019–3026. [Google Scholar] [CrossRef]
- Lu, S.; Xu, B.; Tang, X. Experimental study on double pipe PCM floor heating system under different operation strategies. Renew. Energy 2020, 145, 1280–1291. [Google Scholar] [CrossRef]
- Lin, K.; Zhang, Y.; Xu, X.; Di, H.; Yang, R.; Qin, P. Experimental study of under-floor electric heating system with shape-stabilized PCM plates. Energy Build. 2005, 37, 215–220. [Google Scholar] [CrossRef]
- Velasco-Carrasco, M.; Chen, Z.; Aguilar-Santana, J.L.; Riffat, S. Experimental Evaluation of Phase Change Material Blister Panels for Building Application. Future Cities Environ. 2020, 6. [Google Scholar] [CrossRef]
- Pasupathy, A.; Athanasius, L.; Velraj, R.; Seeniraj, R.V. Experimental investigation and numerical simulation analysis on the thermal performance of a building roof incorporating phase change material (PCM) for thermal management. Appl. Therm. Eng. 2008, 28, 556–565. [Google Scholar] [CrossRef]
- Griffiths, P.W.; Eames, P.C. Performance of chilled ceiling panels using phase change material slurries as the heat transport medium. Appl. Therm. Eng. 2007, 27, 1756–1760. [Google Scholar] [CrossRef]
- Lu, S.; Liang, B.; Li, X.; Kong, X.; Jia, W.; Wang, L. Performance Analysis of PCM Ceiling Coupling with Earth-Air Heat Exchanger for Building Cooling. Materials (Basel) 2020, 13, 2890. [Google Scholar] [CrossRef]
- Lim, H.; Kang, Y.-K.; Jeong, J.-W. Application of a phase change material to a thermoelectric ceiling radiant cooling panel as a heat storage layer. J. Build. Eng. 2020, 32, 101787. [Google Scholar] [CrossRef]
- Liu, C.; Wu, Y.; Zhu, Y.; Li, D.; Ma, L. Experimental investigation of optical and thermal performance of a PCM-glazed unit for building applications. Energy Build. 2018, 158, 794–800. [Google Scholar] [CrossRef]
- Ismail, K.A.R.; Henríquez, J.R. Thermally effective windows with moving phase change material curtains. Appl. Therm. Eng. 2001, 21, 1909–1923. [Google Scholar] [CrossRef]
- Silva, T.; Vicente, R.; Amaral, C.; Figueiredo, A. Thermal performance of a window shutter containing PCM: Numerical validation and experimental analysis. Appl. Energy 2016, 179, 64–84. [Google Scholar] [CrossRef]
- Fokaides, P.A.; Kylili, A.; Kalogirou, S.A. Phase change materials (PCMs) integrated into transparent building elements: A review. Mater. Renew. Sustain. Energy 2015, 4, 1–13. [Google Scholar] [CrossRef]


| Material | Chemicalformula | Melting Temperature (°C) | Melting Enthalpy (J/g) | Thermal Conductivity (W/(m.K)) | Density (kg/m3) | Ref |
|---|---|---|---|---|---|---|
| n-Tetradecane | C14H30 | 5.5 | 228 | - | - | [8] |
| n-Pentadecane | C15H32 | 10 | 205 | - | - | [8] |
| n-Hexadecane | C16H34 | 18.0 | 210.0–238.0 | 0.2 (solid) | 760.0 (liquid, 20.0 °C) | [10] |
| n-Heptadecane | C17H36 | 19.0 | 240.0 | 0.2 | 776.0 (liquid, 20.0 °C) | [10] |
| n-Octadecane | C18H38 | 28.0 | 200.0–245.0 | 0.15 (liquid, 40.0 °C) 0.36 (solid, 25.0 °C) | 774.0 (liquid, 70.0 °C) 814.0 (solid, 20.0 °C) | [10] |
| 28.0 | 179 | 0.2 | 750.0 (liquid) 870.0 (solid) | [4] | ||
| n-Nonadecane | C19H40 | 32.0–33.0 | 222.0 | 0.18 (liquid, 60.0 °C) 0.26 (solid, 19.0 °C) | 780.0 | [10] |
| n- Eicosane | C20H42 | 36.0–37.0 | 247 | 0.15 (liquid) 0.42 (solid) | - | [10] |
| n-Heneicosane | C21H44 | 39.0–41.0 | 201.0 | - | - | [10] |
| n-Docosane | C22H46 | 44.0 | 249 | - | - | [8] |
| n-Tricosane | C23H48 | 47.5 | 232 | - | - | [8] |
| n-Tetracosane | C24H50 | 50.6 | 255 | - | - | [8] |
| Material | Melting Temperature (°C) | Melting Enthalpy (J/g) | Ref | |
|---|---|---|---|---|
| Fatty acids | Capric Acid | 30.2 | 142.7 | [4] |
| Capric Acid | 32 | 152.7 | [5] | |
| Lauric acid | 43.05 | 172.3 | [5] | |
| Myristic acid | 51.80 | 178.14 | [13] | |
| Palmitic acid | 60.42 | 233.24 | [13] | |
| Stearic acid | 54.29 | 188.28 | [5] | |
| Fatty acids esters | Butyl stearate | 19 | 140 | [16] |
| Propyl palmitate | 19 | 186 | [17] | |
| Methyl palmitate | 29 | 205 | [8] | |
| Methyl eicosanate | 45 | 230 | [8] | |
| Methyl behenate | 52 | 234 | [8] | |
| Alcohols | 1-dodecanol | 26 | 200 | [17] |
| Phenol | 41 | 120 | [8] | |
| Cetyl alcohol | 49.3 | 141 | [8] | |
| Polyethylene glycol (PEG) | PEG 400 | 3.2 | 91.4 | [13] |
| PEG 600 | 22.2 | 108.4 | [13] | |
| PEG800 | 25.39 | 133.6 | [5] | |
| PEG 1000 | 34.89 | 143.62 | [13] | |
| PEG 2000 | 52.63 | 180.70 | [13] | |
| PEG 4000 | 48.95 | 183.10 | [13] |
| Advantages | Disadvantages | |
|---|---|---|
| Organic PCMs | Wide phase change temperature range. Thermally stable—no degradation. Chemically inert. Noncorrosive. Congruent melting process—no phase segregation. High latent heat. Good nucleation properties. Low liquid phase sub-cooling capability. Minimal volume variation. Compatible with most of the construction materials. Recyclable. Low cost. | Low thermal conductivity. Low density. High flammability. |
| Inorganic PCMs | High thermal conductivity. High energy efficiency (high enthalpy). Low volume change during phase transition. Non-flammable. | Incongruent melting—phase segregation. Poor nucleating. Supercooling of the liquid phase. Corrosiveness. Toxicity. Limited compatibility with construction materials. Higher cost. |
| Inorganic Material | Melting Temperature (°C) | Melting Enthalpy (J/g) | Ref | Eutectic Mixtures | Melting Temperature (°C) | Melting Enthalpy (J/g) | Ref |
|---|---|---|---|---|---|---|---|
| CaCl2·12H2O | 29.8 | 174 | [8] | Capric Acid-Palmitic Acid | 26.2 | 177 | [4] |
| LiNO3· H2O | 30.0 | 296 | [8] | Capric Acid-Myristic Acid | 21.7 | 155 | [4] |
| LiNO3·3H2O | 30 | 189 | [8] | Capric Acid-Stearic Acid | 24.7 | 179 | [4] |
| LiNO3·3H2O | 30 | 296 | [13] | Capric Acid-Lauric Acid | 19.2–20.3 | 144–150 | [4] |
| KF·4H2O | 18.5 | 231 | [13] | Capric Acid-Lauric Acid | 19.09 | 141.5 | [5] |
| CaCl2·H2O | 29 | 190.8 | [13] | Butyl stearate-palmitate | 17–20 | 137.8 | [4] |
| Na2SO4· 10H2O | 32 | 251 | [13] | Palmitic Acid-Stearic Acid | 32.1 | 151.6 | [5] |
| Mn(NO3)2·6H2O | 25.8 | 125.9 | [13] | Capric Acid-Palmitic Acid-Stearic Acid | 19.93 | 129.4 | [4] |
| Mn(NO3)2·6H2O | 25.5 | 148 | [17] | Lauric Acid-Myristic Acid-Stearic acid | 29.29 | 140.9 | [5] |
| K2HPO4·4H2O | 18.5 | 231 | [17] | Capric Acid-1-dodecanol | 27 | 126.9 | [4] |
| FeBr3·6H2O | 21 | 105 | [17] | Ca(NO3)·4H2O-Mg(NO3)3·6H2O | 30 | 136 | [17] |
| LiNO3·2H2O | 30 | 296 | [17] | CH3COONa·3H2O + NH2CONH2 | 30 | 200.5 | [17] |
| LiBO2·8H2O | 25.7 | 289 | [17] | CaCl2 + NaCl +KCl+H2O | 26–28 | 188 | [17] |
| CaCl2·6H2O | 29 | 191 | [17] | Na2SO4·10H2O-Na2HPO4·12H2O | 32.52 | 226.9 | [5] |
| Core Material | Shell Material | Encapsulation Method | Capsule Size | Thermal Conductivity of Pure PCM (W/(m.K)) | Thermal Conductivity of Encapsulated PCM (W/(m.K)) | Year | Ref |
|---|---|---|---|---|---|---|---|
| n-Eicosane | TiO2 | Interfacial polycondensation | Tubular: 1–5 μm length, 50–300 nm diameter Octahedral: 2–4 μm Spherical: 0.2–4 μm | 0.161 | 1.244 (tubular) 1.023 (octahedral) 0.724 (spherical) | 2019 | [23] |
| N-octadecane | Poly(styrene-co- divinylbenzene-co-acrylamide) | Miniemulsion polymerization | - | - | - | 2019 | [27] |
| Myristic acid- Palmitic acid Eutectic | PMMA | Emulsion polymerization | 0.1–70 µm | - | - | 2019 | [24] |
| Stearic Acid | Si02/GO | Sol-gel | 2 μm | 0.16 | 0.24 (Si02) 0.28 (Si02/GO) | 2018 | [49] |
n-Dodecane | CNTs reinforced Melamine−Formaldehyde resin | In situ polymerization | - | 0.14 | 0.297 0.219 | 2020 | [55] |
| n-Octadecane | Boron Nitride reinforced Melamine-Formaldehyde | In situ polymerization | 5–10 μm | 0.14 | 0.11 | 2020 | [56] |
| Paraffin | Graphene Oxide /Graphene nanoplatels | Self-assembly | - | 0.25 | 0.90 | 2020 | [57] |
| Stearyl Alcohol | SiO2 | Sol-gel | 5–12 μm | 0.14 | 0.15 | 2020 | [58] |
| Methyl Laurate-based | Polyurethane | Pickering emulsion interfacial polymerization | 8–10 μm | - | - | 2020 | [59] |
| n-Octadecane | SiO2/graphene | Miniemulsion polymerization | 256–473 nm | 0.6416 | 1.4941 | 2019 | [60] |
| Crosslinked Polystyrene | Miniemulsion polymerization | 136 nm 134 nm | 0.22 0.18 | 0.12 0.11 | 2020 | [61] |
| n-Octadecane | SiO2 | Miniemulsion polymerization | 335 nm | 0.15 | 0.38 | 2018 | [62] |
| n-Eicosane-Fe3O4 | SiO2/Cu | Pickering emulsion interfacial polymerization | 428–631 nm | 0.4716 | 1.3926 | 2020 | [63] |
| PCM | Carrier | Preparation Method | Thermal Conductivity of Pure PCM(W/(m.K)) | Thermal Conductivity of Encapsulated PCM (W/(m.K)) | Year | Refs |
|---|---|---|---|---|---|---|
| PEG 10000 | Graphene Aerogel/ Melamine Foam | Vacuum-assisted Impregnation | 0.32 | 1.32 | 2020 | [63] |
| Dodecane | Expanded graphite | Vacuum Infiltration | 0.14 | 2.2745 | 2019 | [65] |
| Lauric Acid Stearic Acid Eutectic | Carbonized Corn cob | Vacuum Impregnation | 0.228 | 0.441 | 2019 | [66] |
| PEG 1000 | Halloysite NanoTube reinforced with Ag nanoparticles | Vacuum Impregnation | 0.293 | 0.902 | 2019 | [67] |
| Castor Oil | Polyurethane-Acrylate Oligomer | In situ polymerization | - | - | 2019 | [42] |
| m-Erythritol | Sepiolite and Exfoliated Graphite nanoplatelets | Vacuum Infiltration | 0.372 | 0.756 | 2020 | [68] |
| PEG 6000 | Epoxy Resin porous Al2O3 ceramic | High-temperature blending and curing | 0.393 | 2.54 | 2020 | [69] |
| Capric Acid-Palmitic Acid Eutectic eutectic | Silica Xerogel/ Exfoliated Graphite nanoplatelets | Sol-gel | 0.22 | 0.70 | 2020 | [70] |
| Lauric Acid-based |
| Dispersion of nanoincusions in sonication bath | 0.042 0.033 | 0.268 0.024 | 2020 | [71] |
| PEG 4000 | Almond shell Biochar | Vacuum impregnation | 0.251 | 0.402 | 2018 | [72] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Podara, C.V.; Kartsonakis, I.A.; Charitidis, C.A. Towards Phase Change Materials for Thermal Energy Storage: Classification, Improvements and Applications in the Building Sector. Appl. Sci. 2021, 11, 1490. https://doi.org/10.3390/app11041490
Podara CV, Kartsonakis IA, Charitidis CA. Towards Phase Change Materials for Thermal Energy Storage: Classification, Improvements and Applications in the Building Sector. Applied Sciences. 2021; 11(4):1490. https://doi.org/10.3390/app11041490
Chicago/Turabian StylePodara, Christina V., Ioannis A. Kartsonakis, and Costas A. Charitidis. 2021. "Towards Phase Change Materials for Thermal Energy Storage: Classification, Improvements and Applications in the Building Sector" Applied Sciences 11, no. 4: 1490. https://doi.org/10.3390/app11041490
APA StylePodara, C. V., Kartsonakis, I. A., & Charitidis, C. A. (2021). Towards Phase Change Materials for Thermal Energy Storage: Classification, Improvements and Applications in the Building Sector. Applied Sciences, 11(4), 1490. https://doi.org/10.3390/app11041490









