Abstract
In this study, we maximized the reactivity of phospholipids hydrolysis with immobilized industrial-class phospholipase A1 (PLA1) at the desired water content in the water-in-oil (W/O) microemulsion phase. The optimal hydrophobic-hydrophilic condition of the reaction media in a hydrophobic enzyme reaction is critical to realize the maximum yields of enzyme activity of phospholipase A1. It was attributed to enzymes disliking hydrophobic surroundings as a special molecular structure for reactivity. Immobilization of PLA1 was successfully achieved with the aid of a hydrophobic carrier (Accurel MP100) combination with the treatment using glutaraldehyde. The immobilized yield was over 90% based on simple adsorption. The hydrolysis reaction was kinetically investigated through the effect of glutaraldehyde treatment of carrier and water content in the W/O microemulsion phase. The initial reaction rate increased linearly with an increasing glutaraldehyde concentration and then leveled off over a 6% glutaraldehyde concentration. The initial reaction rate, which was predominantly driven by the water content in the organic phase, changed according to a typical bell-shaped curve with respect to the molar ratio of water to phospholipid. It behaved in a similar way with different glutaraldehyde concentrations. After 10 cycles of repeated use, the reactivity was well sustained at 40% of the initial reaction rate and the creation of the final product. Accumulated yield after 10 times repetition was sufficient for industrial applications. Immobilized PLA1 has demonstrated potential as a biocatalyst for the production of phospholipid biochemicals.
1. Introduction
Phospholipids are major constituents of cell membranes and play a critical in the metabolic activity of cells [1]. They have been beneficial used as biocompatible amphiphilic materials for foods, cosmetics and pharmaceutical components. Egg yolks and plant seeds are commonly known as major bioresources of phospholipids [2,3].
Phospholipase A1 (PLA1) is one subgroup of phospholipases with 1-acyl hydrolytic activity. PLA1, particularly, hydrolyzes the ester bond at the sn−1 position of a phospholipid [4,5], and therefore it is commonly employed to modify the fatty acid ester bond at the sn−1 position. Fatty acid esters act as excellent biocompatible emulsifiers for the food and pharmaceutical industries such as polyglycerol esters of fatty acids or sugar ester [6,7]. In the industrial applications for biotransformation, enzyme immobilization is a key technique that ensures the recycling of the biocatalyst [8,9,10]. However, the arising challenge is to assess the industrial applications of hydrophobic reactions. Particularly, accurate selection of lipase carrier which has good compatibility with hydrophobic solvent, requires special attention. The enhanced reproducibility and repeatability allow for the continuous easy separation of the product and reduces the cost of the biochemical processing [11,12,13].
Hydrophobic enzyme reactions normally contain an antinomy. A fatty acid substrate needs a hydrophobic solvent [14,15,16]. However, enzymes dislike hydrophobic surroundings as they require a certain amount of water molecules to form a special molecular structure for reactivity. Therefore, the optimal hydrophobic-hydrophilic condition of the reaction media in a hydrophobic enzyme reaction is critical to realize the maximum yields of lipase-aided biochemical reactions. The optimal water content in water-in-oil (W/O) microemulsion systems has been the focus of previous investigations [17,18]. However, the relationship with the water content in the immobilized enzyme reaction system has not been reported in previous study in this field. Therefore, the investigation of the water content under the immobilized enzyme reaction should be considered and optimized.
Stable reactivity by repeated use of immobilized lipases is a dominant factor to determine suitable carriers. Previously, hydrophilic gels (such as silica gel) and solid porous carriers have been employed for reactions with hydrophobic substrates. In the case of lipases, hydrolysis, synthesis, and esterification should be considered for industrial applications. Table 1 summarizes previously studied hydrolysis, synthesis and esterification reactions using immobilized lipases and phospholipases.

Table 1.
Previous hydrophobic substrate reactions using immobilized lipases and phospholipases.
The suitable immobilization of an enzyme allows for its long-term repeated use and higher purity of the product without any by-product separation. The physicochemical characteristics of the surface of the carrier and the morphological structure of the carrier particles determine the lifetime of the immobilized enzymes in the bioreactor. Immobilized enzymes are more tolerant to organic solvents, heat, and shearing forces and are much easier to recover than free lipases. Thus, these enzymes are more suitable for industrial applications. However, although a reaction component can be produced, there is a demand for higher reaction activity and reuse. Therefore, it is important to find the optimized carrier of PLA1 for immobilization. Among the carriers used for immobilization of enzymes, Accurel has been widely used. This polymer has a highly hydrophobic microporous structure and has been used in this study.
Moreover, in the preparation of immobilized enzymes, attention has also been paid to the low inactivation rate of enzymes and the long-term retention rate. However, since there are many types of enzymes, the optimum combination of carrier, enzyme, and reaction conditions should be altered individually. In this study, we adopted a composite method, in which an enzyme was adsorbed on a carrier and then crosslinked with glutaraldehyde (GA) treatment. Since the stabilization by GA treatment depending on the type of carrier and enzyme, the stability of immobilization due to the difference in GA concentration between Accurel MP 100 and Phospholipase A1 was investigated.
The objectives of the study were to examine the effects of the optimized reaction conditions on hydrolysis between immobilized PLA1. Particularly, we used Accurel as a carrier to develop a higher immobilized enzyme. Moreover, we investigated phospholipid hydrolysis using an immobilized industrial-class PLA1 on Accurel MP100. The latter was made of a polypropylene-based hydrophobic granular support [25]. We solved this problem of the water content under the immobilized enzyme reaction and obtained optimized water content. Moreover, we solved the problem about the impact of the enzyme immobilization process and the water content in the organic phase on the kinetic data to achieve maximization on reactivity and repeated productivity of immobilized PLA1 for a long period of time.
2. Materials and Methods
2.1. Materials
Accurel MP100 was provided by Evonik Industries AG (Essen, Germany). PLA1 from Aspergillus oryzae was donated by Mitsubishi-Kagaku Foods Corporation (Tokyo, Japan). It was commercially distributed for industrial enzymatic process use. Lecithin from soybeans was purchased from FUJIFILM Wako Pure Chemical Corporation (Osaka, Japan). The purity of lecithin was assumed to be 60% phosphatidylcholine dipalmitoyl. The organic phase used 2,2,4-trimethylpentane (isooctane) as the main solvent and 1-butanol as the co-solvent. In this study, a volumetric ratio of 10% (v/v) of 1-butanol was employed during the organic phase. Phospholipid was adopted as a typical lipid substrate, and palmitic acid and lysolecithin assumed to be the main reaction products. Glutaraldehyde (GA) was employed as a cross-linker of PLA1 onto the outer and inner pore surface of the Accurel particle. Analytical grade GA was purchased from FUJIFILM Wako Pure Chemical Corporation (Osaka, Japan).
2.2. Hydrolysis of Phospholipids by Phospholipase A1 in the Water-in-Oil Microemulsion Phase
The enzymatic reactivity of PLA1 was determined by the hydrolysis of the phospholipids in W/O microemulsion. The PLA1 was solubilized into 0.2 M acetate buffer solution (pH 5.0). The enzyme buffer solution was prepared using 0.4 g of PLA1 powder and 10 mL of acetate buffer solution. The organic solution (50 mL) was prepared in 10% (v/v) 1-butaol/isooctane. The phospholipids were dissolved into the organic phase with a concentration of 6–18 mM, calculated based on phosphatidylcholine. The substrate organic solution was magnetically stirred in a thermostatic water bath (313 K). The enzymatic reaction was initiated by introducing PLA1 into the organic solution, which formed a W/O microemulsion. A supplemental amount of acetate buffer solution was then added to the W/O microemulsion to make the desired water content. The enzymatic reactivity was determined by measuring the increase in palmitic acid concentration according to the Lowry-Tinsley colorimetric method [26].
A mixture of cupric acetate aqueous solution (1 mL) and benzene (5 mL) was prepared in a vial. The pH of the cupric acetate aqueous solution was set at 6.0 using the additive pyridine. The stirring speed was 1300 min−1. A sample (1.0 mL) was taken from the reaction solution at the desired running time. The sample was introduced into the benzene phase of the benzene-cupric acetate mixture mentioned above. The solution was vigorously mixed for 1 min using a vortex-mixer (TTM-1, SIBATA, Tokyo, Japan).
After centrifugation at 3000 min−1 for 3 min, the transparent upper benzene phase was added mixed for 1 min using a vortex mixer to dehydrate the benzene phase. After centrifugation at 3000 min−1 for 3 min, the optical density of the transparent upper benzene phase was measured by UV spectrophotometer (V-6301RM, JASCO Corporation, Tokyo, Japan) at 715 nm.
2.3. Scanning Electron Microscopy (SEM)
Scanning electron microscopy (SEM) was employed to examine the surface of Accurel. The sample was coated with gold and viewed under a high vacuum. A surface of Accurel was conducted using a JSM-6701F scanning electron microscope (JEOL Ltd., Tokyo, Japan) at an acceleration voltage of 10.0 kV.
2.4. Phospholipase A1 Immobilization Method
In total, 30 mL ethanol (guaranteed reagent, 99.5 w/w%) was introduced into a 150 mL capacity glass vial with an inner diameter of 45 mm. Accurel particles (1 g) were immersed into ethanol and then irradiated using an ultrasonic water bath (UT-206, SHARP Corporation, Osaka, Japan) for 60 min under 200 W. Accurel MP100 was pretreated by ethanol for modification of surface hydrophobicity [25]. PLA1 was simply adsorbed onto the Accurel MP100 carrier before the crosslinked immobilization. The desired amount of PLA1 was added to the acetate buffer solution (pH 5.0). The concentration of PLA1 was set at 10 g/L. The Accurel carrier was placed into the PLA1 solution and was then shaken to adsorb PLA1 for at least 24 h at 298 K. The unwashed wetted carrier was moderately dried in a desiccator for 24 h at room temperature (298 ± 2 K). The amount of adsorbed PLA1 on the Accurel carrier was evaluated from the mass balance based on decreasing concentration of PLA1 in the buffer solution.
The dried Accurel carrier with the adsorbed PLA1 was then moderately shaken in a GA aqueous solution (CGA: 0%−10%) for 60 min at room temperature to immobilize the PLA1 on the Accurel pore surfaces. The amount of PLA1 desorbed in the GA solution was then measured using the Bradford method [27,28], with the aid of a Protein quantification Kit-Rapid (Dojindo Molecular Technologies, Inc., Tokyo, Japan). The effective amount of immobilized PLA1 was estimated from the difference between the adsorbed and desorbed amounts of PLA1. The immobilized PLA1 carrier was briefly washed in distilled water and moderately dried in the desiccators for 2 days at room temperature.
2.5. Hydrolysis of Phospholipids Using Immobilized Phospholipase A1 on an Accurel Carrier
The organic solvent was prepared with 10% (v/v) 1-butanol/isooctane. The initial concentration of the phospholipid was in the range of 6−18 mM, based on phosphatidylcholine. The substrate was stirred into the organic solution in a thermostatic water bath (313 K), and the aqueous buffer solution without PLA1 was added to the solution containing the substrate (phospholipid). It was stirred magnetically for 10 min to produce an optically transparent and mature W/O microemulsion phase. In this study, phospholipid acted not only as a substrate of hydrolysis but also as an amphiphilic component to prepare W/O microemulsion. The reaction was initiated when the immobilized PLA1 on the Accurel carrier was added to the W/O microemulsion phase. The concentration of palmitic acid in the sample after the initial reaction was measured using the same protocol as described in Section 2.2.
2.6. Statistical Analysis
The experiments were performed in triplicates and the data deviation was expressed by error bars. To clear presentation on data for readers, the error bars were eliminated reluctantly in Figures 2, 5, 7, 9, and 11. In this study, the statistical analysis was examined that was results valid statistically significant if p ≤ 0.05.
3. Results and Discussion
3.1. Progress of Hydrolysis Reaction in Water-in-Oil Microemulsion by Free Industrial-Class Phospholipase A1
Figure 1 presents a schematic illustration of the hydrolysis of phospholipid by non-immobilized PLA1 containing W/O microemulsion.
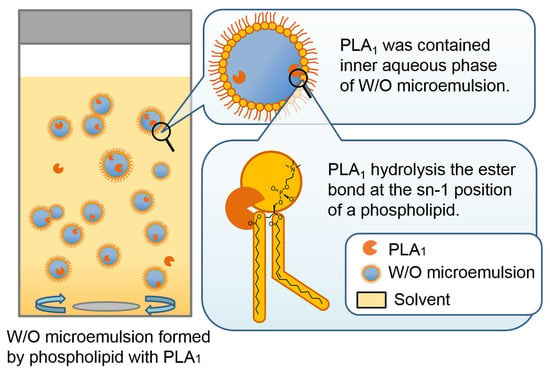
Figure 1.
Schematic illustration of hydrolysis reaction in water-in-oil (W/O) microemulsion of non-immobilized phospholipase A1 (PLA1).
Figure 2 shows the time course of the hydrolysis of phospholipid hydrolysis by PLA1 in the W/O microemulsion phase. The concentration of palmitic acid (Cp) was monitored as a typical product of the phospholipid hydrolysis, and the amounts of buffer solution containing PLA1 in the W/O microemulsion phase (Hb) were adjusted by the supplemental amount of buffer solution. In this study, the volume of the organic phase (butanol + isooctane) was set at 50 mL throughout the measurements. The reaction was initiated quickly with no reaction delay. The aqueous buffer solution was dispersed with the aid of W/O microemulsion. When the Hb was 0.3 mL, the concentration of palmitic acid was superior to other amounts of water.
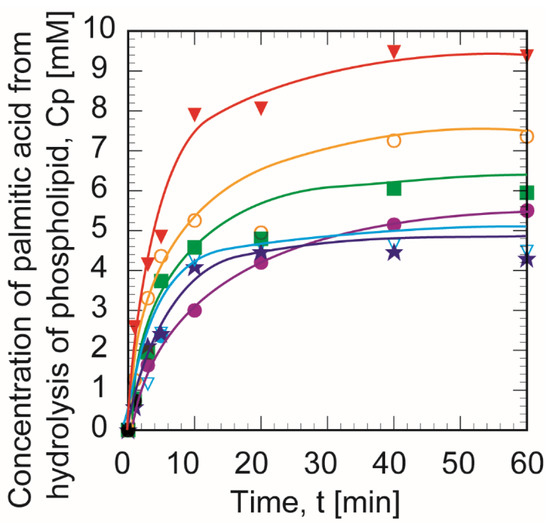
Figure 2.
Time course of produced palmitic acid concentration in W/O microemulsion. The amounts of buffer solution containing phospholipase A1 in the W/O microemulsion phase, Hb, were 0.2 (●), 0.225 (★), 0.25 (○), 0.3 (▼), 0.35 (▽), and 0.4 (■) mL. The experiments were each conducted a minimum of three times.
3.2. Immobilized Phospholipase A1 on Accurel Particle
Figure 3 depicts the adsorbed amount of PLA1 on the pretreated Accurel MP100 particles. The adsorbed amount increased with the increasing concentration of GA, and the maximum adsorbed amount appeared when the GA concentration was 6%.
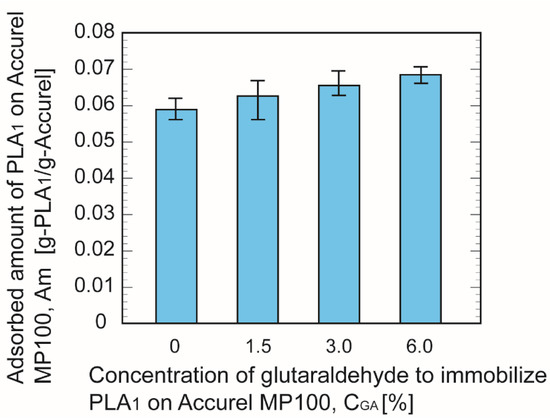
Figure 3.
Comparison of the adsorbed amount of phospholipase A1 per unit mass of Accurel MP100.
3.3. Reactivity of Immobilized Phospholipase A1
3.3.1. Effect of Glutaraldehyde as a Cross-Linker of Phospholipase A1
Figure 4a illustrated the schematic image of substrate-based W/O microemulsion association with immobilized PLA1. The W/O microemulsion droplet readily diffused into the inner pore channeled under the lower water content conditions. The pore size at the Accurel surface was found to be 1–5 μm (SEM images). Figure 4b demonstrates that substrate phospholipid molecules were located in the outer surrounding of the W/O microemulsion droplet. It is readily associated with immobilized PLA1. The hydrolysis reaction was quickly initiated, and a high reaction rate was realized.
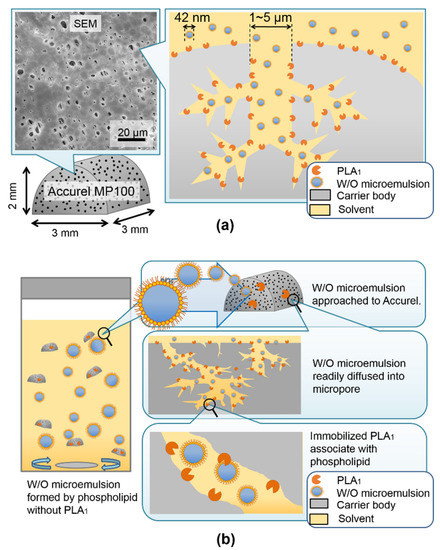
Figure 4.
(a) Schematic illustration of pore structure and W/O microemulsion droplets association with immobilized phospholipase A1 on the outer and inner surfaces of microspores in Accurel particles. (b) Phospholipid-based W/O microemulsion droplets readily diffused into the microspores of the PLA1 carrier.
To achieve the highest yield in phospholipids hydrolysis using immobilized PLA1, the Accurel MP100 with glutaraldehyde treatment and the amount of water content in W/O microemulsion were the key factors. These are especially important for the industrial production of enzymes.
Figure 5 shows the time course of concentration of palmitic acid using the immobilized PLA1 on Accurel with glutaraldehyde treatment. The enzyme reactions were quickly initiated regardless of the concentrations of GA. When the GA concentrations were 10% and 6%, the amounts of produced palmitic acid were the highest. The next highest production was for GA concentrations of 3%. There is no difference in the amount of production between 1.5% and 0% GA concentrations.
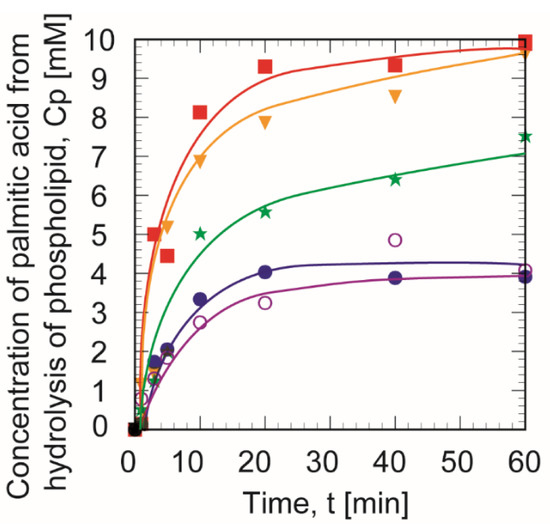
Figure 5.
Time course of palmitic acid production (Cp) using immobilized phospholipase A1 in various glutaraldehyde concentrations. The glutaraldehyde concentrations, CGA were 0% (○), 1.5% (●), 3% (★), 6% (▼), and 10% (■). The experiments were each conducted a minimum of three times.
Despite the small difference in the immobilized amount of PLA1 between GA concentrations of 0–10%, the amount of produced palmitic acid varied dramatically. Therefore, the GA concentration not only effectively immobilized the PLA1 but also acted as a hydrophobic modifier of the carrier surface.
Figure 6 shows the effect of the initial hydrolysis reaction rate for immobilized PLA1 on the GA treatment as a cross-linker of PLA1. The initial reaction rate increased with GA concentration and then dropped for GA concentrations exceeding 6%. According to the results, a GA concentration of 6% was optimal for effective immobilization of PLA1 and allowed to attain the required initiated reaction rate and reaction yield.
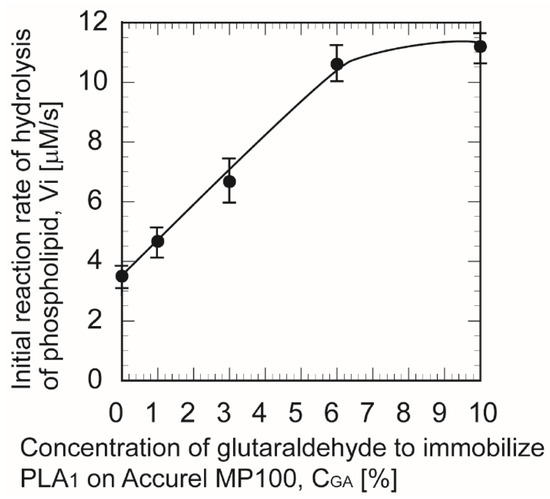
Figure 6.
Effect of immobilized phospholipase A1 with glutaraldehyde treatment on the initial reaction rate.
3.3.2. Effect of Water Content on Production
Figure 7 shows the effect of the amount of buffer solution into the W/O microemulsion phase on the reactivity of immobilized PLA1. The productivity of the enzyme reaction was dependent on the supplemental aqueous volume in the W/O microemulsion phase. The palmitic acid concentration produced increased quickly with the reaction time and then dropped for over 40 min. When the Hb was 0.075 mL, the highest amount of palmitic acid was produced.
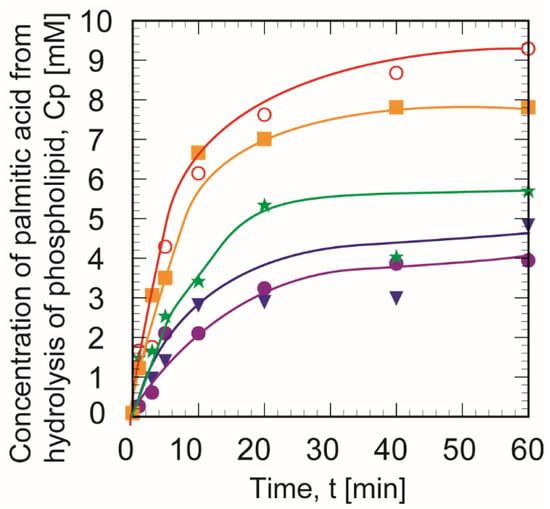
Figure 7.
Time course of palmitic acid concentration (Cp) produced with immobilized phospholipase A1 in various amounts of water. The volumetric amount of the supplemental acetate buffer solution, Hb was changed as follows; 0 (★), 0.05 (●), 0.075 (○), 0.1 (▼), and 0.2 (■) mL. The CGA was set at 6%. The experiments were each conducted a minimum of three times.
Figure 8 shows the change of the initial reaction rate with the concentration of lecithin using a soybean dissolved in the organic phase. The initial reaction rate in this figure was a recalculated value which was a per unit mass of PLA1. The reactivity of immobilized PLA1 indicated by the initial reaction rate per unit mass of PLA1 was well maintained, and the activity damage was fully suppressed. Industrial-class PLA1 was successfully immobilized onto the Accurel particles with the aid of GA aqueous solution.
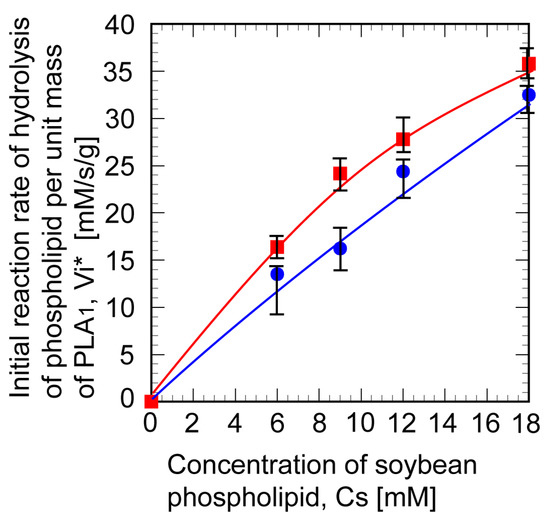
Figure 8.
Relationship between the substrate concentration and the initial reaction rate for non-immobilized phospholipase A1 in W/O microemulsion (■) at Hb = 0.3 mL, immobilized phospholipase A1 (●) at Hb = 0.075 mL. The concentration of glutaraldehyde for immobilization was 6%. Vi*: initial reaction rate of hydrolysis of phospholipid per unit mass of PLA1.
Figure 9 shows the relationship between the initial reaction rate and the molar ratio of water to lecithin, W. In this study, the W value was defined as the molar ratio of supplemental water moles to the phosphatidylcholine dipalmitoyl moles:
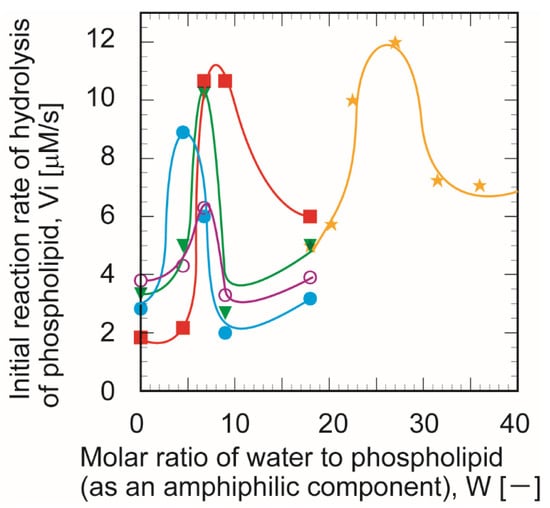
Figure 9.
Effect of the water content on the initial reaction rate (Vi) in immobilized phospholipase A1. W/O microemulsion system with non-immobilized phospholipase A1 was chosen as a reference (★). The glutaraldehyde concentration, CGA was 1.5% (●), 3% (○), 6% (▼), and 10% (■). The experiments were each conducted a minimum of three times.
The moles of water in the W/O microemulsion phase were calculated, assuming the supplemented aqueous volume with a density of 1.0 g/mL. The initial water content in the 1-butanol/isooctane mixed phase before the supplement of the aqueous phase was ignored. The remarkable decay of initial reaction rate for higher W values agreed with the change of hydrophobic character of the microwater pool. Previous studies of lipase reactivity in the W/O microemulsion phase have shown that water content is a dominant factor in regulating the reactivity of the lipase [17]. The size of the microemulsion is dependent on the water content. That correlates with the molar ratio of water to the amphiphilic component (W) [29]. Previously, the values of W have been often employed as a typical indicator of water content in the W/O microemulsion phase in other lipase [30,31] and phospholipase reactions [32].
The initial reaction rate indicated a bell-shaped curve with increasing water content in the W/O microemulsion phase. The maximum reactivity of immobilized PLA1 was obtained for W = 7. The maximum initial reaction rate in non-immobilized PLA1 was W = 27. When the W value was low, the W/O microemulsion size was very small [33]. The water molecules were strongly associated with each other and demonstrated hydrophobic behavior [34,35,36]. In contrast, for the larger values of W, more water molecules recovered their hydrophilic character. The latter was discussed in literature from the viewpoint of the structural formation of water molecules [37]. These results suggest that the local surroundings of the immobilized PLA1 in Accurel were more hydrophobic than that of the non-immobilized PLA1 in the W/O microemulsion phase. In a previous study, the maximum reactivity of immobilized lipase, W = 10, was achieved using AOT [38].
In general, the size of the W/O microemulsion droplet was dependent upon the molar ratio of water and amphiphiles. According to the AOT system used previously, the characteristic radius (RC) of the W/O microemulsion was linearly proportional to the molar ratio of water and amphiphile AOT. The correlation formula was previously reported as RC (Å) = 1.5 W [33]. The molecular size of lecithin was roughly assumed to be 10 times that of the AOT molecule. The outer diameter of the W/O microemulsion was estimated as 420 Å (42 nm) for W = 7.
3.4. Repeated Use of Immobilized Phospholipase A1 for High Yield Production
Figure 10 shows the concentration of palmitic acid after 60 min and the initial reaction rate in the repeated use of immobilized PLA1. The initial reaction rate and the production of palmitic acid until repeated five times were almost constant and then remained at 40% of the initial value after its sixth repetition. The results promise practical applications of immobilized industrial-class PLA1 onto Accurel particles for the prospective production of phospholipid chemicals. Repeated used was conducted 10 times in this study. Reactivity of immobilized PLA1 was regrettably decayed according to repeated number. Effective reactivation of immobilized PLA1 was main limitation was this application.
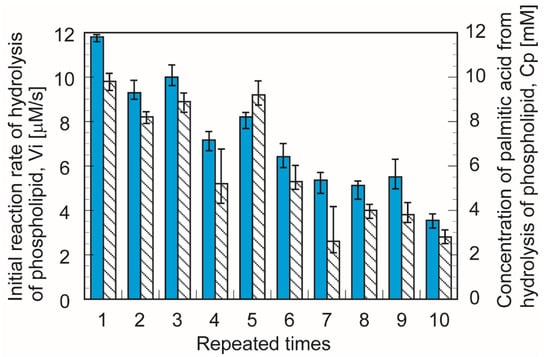
Figure 10.
Comparison of the repeated initial reaction rate and reaction yields of hydrolysis with immobilized phospholipase A1 (Hb: 0.075 mL, CGA: 6%).  : Initial reaction rate, Vi.
: Initial reaction rate, Vi.  : Reaction product (palmitic acid) concentration, Cp.
: Reaction product (palmitic acid) concentration, Cp.
 : Initial reaction rate, Vi.
: Initial reaction rate, Vi.  : Reaction product (palmitic acid) concentration, Cp.
: Reaction product (palmitic acid) concentration, Cp.
Figure 11 indicates the accumulated yield of palmitic acid after 60 min using immobilized PLA1 throughout 10 cycle’s repetition. Accumulated production was 3.5-fold superior to that of a single use of non-immobilized PLA1. Immobilization of industrial-class PLA1 was successfully realized as a promising biocatalyst for production of the phospholipid biochemicals.
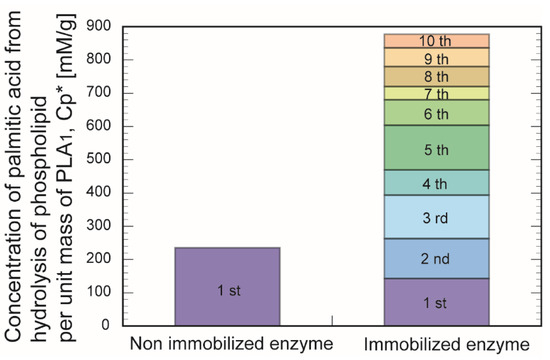
Figure 11.
Accumulated yield of palmitic acid using immobilized phospholipase A1 in W/O microemulsion system. It was compared with non-immobilized phospholipase A1 (non-immobilized phospholipase A1). Here, Hb was 0.075 mL, and CGA was 6%, respectively. The experiments were each conducted a minimum of three times. Cp*: concentration of palmitic acid from hydrolysis of phospholipid per unit mass of PLA1.
4. Conclusions
Maximization of immobilized industrial-class phospholipase A1 was successfully realized by regulation of water content of W/O microemulsion phase as a reaction media. Industrial-class PLA1 was successfully immobilized onto a polypropylene porous particle (Accurel MP100) with the aid of glutaraldehyde. The yield of immobilized PLA1 was over 90% based on simple adsorption. A hydrolysis reaction with the immobilized PLA1 was quickly initiated without any time lag. The minimization of activity loss of the PLA1 throughout the immobilization process was effectively accomplished using appropriate concentrations of glutaraldehyde with moderate shaking under room temperature. The initial reaction rate of the immobilized PLA1 increased with an increasing glutaraldehyde concentration. A glutaraldehyde concentration of 6% provided a sufficiently stable immobilization of PLA1 and the initial reaction rate. The initial reaction rate could be compared with that of the non-immobilized PLA1 in the W/O microemulsion system. It was strongly dependent on the water content, W (molar ratio of water and amphiphilic molecules (phospholipids)). In this study, the maximum reaction rate of 7 was achieved. The background of the maximum reactivity of immobilized PLA1. It was considered in terms of easy diffusion of substrate involved with the size of the W/O microemulsion and the inlet opening of pores on the surface of the Accurel particles. The productivity was examined after 10 repeated uses. Accumulated production was 3.5 times superior to that of a single use of non-immobilized PLA1 under the appropriate regulation of the water content in the W/O microemulsion phase. Industrial-class immobilized PLA1 on Accurel MP100 has been suggested as a promising biocatalyst to product phospholipid biochemicals.
Author Contributions
Conceptualization, R.N.; methodology, R.N.; formal analysis, Y.H.; investigation, Y.H.; writing—original draft preparation, Y.H.; writing—review and editing, Y.H., R.N. and M.I.; visualization, R.N., Y.H. and M.I.; supervision, N.N. All authors have read and agree to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
Phospholipase A1 was donated from Mitsubishi-Kagaku Foods Corporation (Tokyo, Japan). Accurel MP100 was provided by Evonik Industries AG (Essen, Germany). We sincerely appreciate their kind cooperation.
Conflicts of Interest
The authors declare no conflict of interest.
Nomenclature
| Am | the adsorbed amount of PLA1 on Accurel MP100 [g-PLA1/g-Accurel] |
| CGA | concentration of glutaraldehyde to immobilize PLA1 on Accurel MP100 [wt%] |
| Cp | concentration of palmitic acid from hydrolysis of phospholipid [mM] |
| Cp* | concentration of palmitic acid from hydrolysis of phospholipid per unit mass of PLA1 [mM/g] |
| Cs | concentration of soybean phospholipid [mM] |
| Hb | the supplemental amount of buffer solution in the W/O microemulsion phase [mL] (volume of the organic phase was set at 50 mL throughout this study) |
| RC | characteristic radius of the W/O microemulsion [Å] |
| t | reaction time [min] |
| Vi | initial reaction rate of hydrolysis of phospholipid [μM/s] |
| Vi* | initial reaction rate of hydrolysis of phospholipid per unit mass of PLA1 [mM/s/g] |
| W | molar ratio of water to phospholipid (as an amphiphilic component) [mol-water of aqueous buffer solution/mol-phosphatidylcholine as an amphiphilic substrate (phospholipid)] |
| Abbreviations | |
| AOT | abbreviation of commercial name of “Aerosol® OT”. It is a typical anionic amphiphilic reagent. The main component of AOT is sodium dioctyl sulfosuccinate |
| GA | glutaraldehyde |
| PLA1 | phospholipase A1 |
| SEM | scanning electron microscopy |
| W/O | water-in-oil |
References
- Zhao, T.T.; No, D.S.; Kim, B.H.; Garcia, H.S.; Kim, Y.; Kim, I.H. Immobilized phospholipase A1-catalyzed modification of phosphatidylcholine with n-3 polyunsaturated fatty acid. Food Chem. 2014, 157, 132–140. [Google Scholar] [CrossRef]
- Kidd, P.; Head, K.A. review of the bioavailability and clinical efficacy of mil thistle phytosome: A silybin-phosphatidylcoline complex (Siliphos). Altern. Med. Rev. 2005, 10, 193–203. [Google Scholar] [PubMed]
- Mustranta, A.; Forssell, P.; Poutanen, K. Comparison of lipases and phospholipases in the hydrolysis of phospholipids. Process Biochem. 1995, 30, 393–401. [Google Scholar] [CrossRef]
- Hama, S.; Onodera, K.; Yoshida, A.; Noda, H.; Kondo, A. Improved production of phospholipase A1 by recombinant Aspergillus oryzae through immobilization to control the fungal morphology under nutrient-limited conditions. Biochem. Eng. J. 2015, 96, 1–6. [Google Scholar] [CrossRef]
- Li, Z.; Liu, H.; Zhao, G.; Wang, P.; Wang, P.; Wang, L.; Wu, H.; Fang, X.; Sun, X.; Wu, X.; et al. Enhancing the performance of a phospholipase A1 for oil degumming by bio-imprinting and immobilization. J. Mol. Catal. B Enzym. 2016, 123, 122–131. [Google Scholar] [CrossRef]
- Csaki, K.F.; Sebestyen, E. Who will carry out the tests that would be necessary for proper safety evaluation of food emulsifiers? Food Sci. Hum. Wellness 2019, 8, 126–135. [Google Scholar] [CrossRef]
- Noae, K.; Ohsa, T.; Kawagoe, M.; Imai, M. Esterification by Rhizopus delemar lipase in organic solvent using ester reverse micelles. Biochem. Eng. J. 2001, 9, 67–72. [Google Scholar] [CrossRef]
- Meunier, S.M.; Rajabzadeh, A.R.; Williams, T.G.; Legge, R.L. Methyl Oleate Production in a Supported Sol-Gel Immobilized Lipase Packed Bed Reactor. Energy Fuels 2015, 29, 3168–3175. [Google Scholar] [CrossRef]
- Xu, Y.; Nordblad, M.; Nielsen, P.M.; Brask, J.; Woodley, J.M. In situ visualization and effect of glycerol in lipase-catalyzed ethanolysis of rapeseed oil. J. Mol. Catal. B Enzym. 2011, 72, 213–219. [Google Scholar] [CrossRef]
- Kuroiwa, T.; Hamazaki, K.; Katayama, M.; Sato, S.; Matsui, T. Improvement of synthetic activity and stability of a commercial lipase in a low-water system via immobilization of hydrated lipase aggregates. Process Biochem. 2016, 51, 2047–2054. [Google Scholar] [CrossRef]
- Aguieiras, E.C.G.; Ribeiro, D.S.; Couteiro, P.P.; Bastos, C.M.B.; Queiroz, D.S.; Parreira, J.M.; Langone, M.A.P. Investigation of the reuse of immobilized lipases in biodiesel synthesis: Influence of different solvents in lipase activity. Appl. Biochem. Biotechnol. 2016, 179, 485–496. [Google Scholar] [CrossRef] [PubMed]
- Iso, M.; Chen, B.; Eguchi, M.; Kudo, T.; Shrestha, S. Production of biodiesel fuel from triglycerides and alcohol using immobilized lipase. J. Mol. Catal. B Enzym. 2001, 16, 53–58. [Google Scholar] [CrossRef]
- Ban, K.; Hama, S.; Nishizuka, K.; Kaieda, M.; Matsumoto, T.; Kondo, A.; Noda, H.; Fukuda, H. Repeated use whole-cell biocatalysis immobilized within biomass support particles for biodiesel fuel production. J. Mol. Catal. B Enzym. 2002, 17, 157–165. [Google Scholar] [CrossRef]
- Nascimento, M.G.; Silva, J.M.R.; Silva, J.C.; Alves, M.M. The use of organic solvents/ionic liquid mixtures in reactions catalyzed by lipase from Burkholderia cepacia immobilized in different support. J. Mol. Catal. B Enzym. 2015, 112, 1–8. [Google Scholar] [CrossRef]
- Koutinas, M.; Yiangou, C.; Osório, N.M.; Ioannou, K.; Canet, A.; Valero, F.; Diasd, S.F. Application of commercial non-commercial immobilized lipases for biocatalytic production of ethyl lactate in organic solvents. Bioresour. Technol. 2018, 247, 496–503. [Google Scholar] [CrossRef]
- Mukherjee, J.; Gupta, M.N. Lipase coated clusters of iron oxide nanoparticles for biodiesel synthesis in a solvent free medium. Bioresour. Technol. 2016, 209, 166–171. [Google Scholar] [CrossRef] [PubMed]
- Uehara, A.; Imai, M.; Suzuki, I. The most favorable condition for lipid hydrolysis by Rhizopus delemar lipase in combination with a sugar-ester and alcohol W/O microemulsion system. Colloids Surf. A: Physicochem. Eng. Asp. 2008, 324, 79–85. [Google Scholar] [CrossRef]
- Persson, M.; Wehtje, E.; Adlercreutz, P. Immobilisation of lipases by adsorption and deposition: High protein loading gives lower water activity optimum. Biotechnol. Lett. 2000, 22, 1571–1575. [Google Scholar] [CrossRef]
- Naya, M.; Imai, M. Regulation of the hydrolysis reactivity of immobilized Candida rugose lipase with the aid of a hydrophobic porous carrier. Asia-Pac. J. Chem. Eng. 2012, 7, S157–S165. [Google Scholar] [CrossRef]
- Nagesha, G.K.; Manohar, B.; Sankar, U. Enzymatic esterification of free fatty acids of hydrolyzed soy deodorizer distillate in supercritical carbon dioxide. J. Supercrit. Fluids. 2004, 32, 137–145. [Google Scholar] [CrossRef]
- Lozano, P.; Diego, T.D.; Sauer, T.; Vaultier, M.; Gmouh, S.; Iborra, J.L. On the importance of the supporting material for activity of immobilized Candida antarctica Lipase B in ionic liquid/hexane and ionic liquid/supercritical carbon dioxide biphasic media. J. Supercrit. Fluids. 2007, 40, 93–100. [Google Scholar] [CrossRef]
- Zappaterra, F.; Summa, D.; Semeraro, B.; Buzzi, R.; Trapella, C.; Ladero, M.; Costa, S.; Tamburini, E. Enzymatic esterification as potential strategy to enhance the sorbic acid behavior as food and beverage preservative. Fermentation 2020, 6, 96. [Google Scholar] [CrossRef]
- Madoery, R.; Gattone, C.G.; Fidelio, G. Bioconversion of phospholipids by immobilized phospholipase A2. J. Biotechnol. 1995, 40, 145–153. [Google Scholar] [CrossRef]
- Xi, X.; Feng, X.; Shi, N.; Ma, X.; Lin, H.; Han, Y. Immobilized phospholipase A1-catalyzed acidolysis of phosphatidylcholine from Antarctic krill (Euphausia superba) for docosahexaenoic acid enrichment under supercritical conditions. J. Mol. Catal. B Enzym. 2016, 126, 46–55. [Google Scholar] [CrossRef]
- Naya, M.; Imai, M. Advantages of supercritical carbon dioxide for lipid hydrolysis by immobilized lipase with higher reaction rate and reproducible repeated use. J. Chem. Technol. Biotechnol. 2016, 91, 2620–2630. [Google Scholar] [CrossRef]
- Lowry, R.R.; Tinsley, I.J. Rapid colorimetric determination of free fatty acids. J. Am. Oil Chem. Soc. 1976, 53, 470–472. [Google Scholar] [CrossRef] [PubMed]
- Bradford, M.M. A rapid and sensitive method for the quantification of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Splittgerber, A.G.; Sohl, J. Nonlinearity in protein assays by the coomassie blue dye-binding method. Anal. Biochem. 1989, 179, 198–201. [Google Scholar] [CrossRef]
- Naoe, K.; Noda, K.; Kawagoe, M.; Imai, M. Higher order structure of proteins solubilized in AOT reverse micelles. Colloids Surf. B Biointerfaces 2004, 38, 179–185. [Google Scholar] [CrossRef]
- Nagayama, K.; Karaiwa, K.; Doi, T.; Imai, M. Esterification activity and stability of Candida rugose lipase in AOT microemulsion-based organogels. Biochem. Eng. J. 1998, 2, 121–126. [Google Scholar] [CrossRef]
- Nagayama, K.; Nishimura, R.; Doi, T.; Imai, M. Enhanced recovery and catalytic activity of Rhizopus delemar lipase in an AOT microemulsion system with guanidine hydrochloride. J. Chem. Technol. Biotechnol. 1999, 74, 227–230. [Google Scholar] [CrossRef]
- Yamazaki, K.; Imai, M.; Suzuki, I. Soybean lecithin hydrolysis using hog pancreas phospholipase A2 influenced by the hydrophobic character of W/O microemulsion systems. Biochem. Eng. J. 2004, 19, 171–179. [Google Scholar] [CrossRef]
- Pileni, M.P.; Zemb, T.; Petit, C. Solubilization by reverse micelles: Solute localization and structure perturbation. Chem. Phys. Lett. 1985, 118, 414–420. [Google Scholar] [CrossRef]
- Yamazaki, K.; Imai, M.; Suzuki, I. Water solubilizatoin capacity and mean emulsion size of phospholipid-based isooctane-alcohol W/O microemulsion. Colloids Surf. A: Physicochem. Eng. Asp. 2007, 293, 241–246. [Google Scholar] [CrossRef]
- Luisi, P.L.; Magid, L.J.; Fendler, J.H. Solubilization of enzymes and nucleic acids in hydrocarbon micellar solution. CRC Crit. Rev. Biochem. 1986, 20, 409–474. [Google Scholar] [CrossRef]
- Kumar, V.V.; Raghunathan, P. Proton NMR studies of the interaction water with lecithin in non-polar organic media. Chem. Phys. Lipids 1986, 41, 159–171. [Google Scholar] [CrossRef]
- Wong, M.; Thomas, J.K.; Nowak, T. Structure and state of H2O in Reversed Micelles. J. Am. Chem. Soc. 1977, 99, 4730–4736. [Google Scholar] [CrossRef]
- Han, D.; Rhee, J.S. Characteristics of lipase-catalyzed hydrolysis of olive oil in AOT-isooctane reversed micelles. Biotechnol. Bioeng. 1986, 28, 1250–1255. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).