Effects of Simulated Nitrogen Deposition on the Bacterial Community of Urban Green Spaces
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Sites and Experimental Design
2.2. Soil Sampling and Analysis
2.3. DNA Extraction
2.4. Statistical Analysis
3. Results
3.1. Effects of N Deposition on Soil Properties
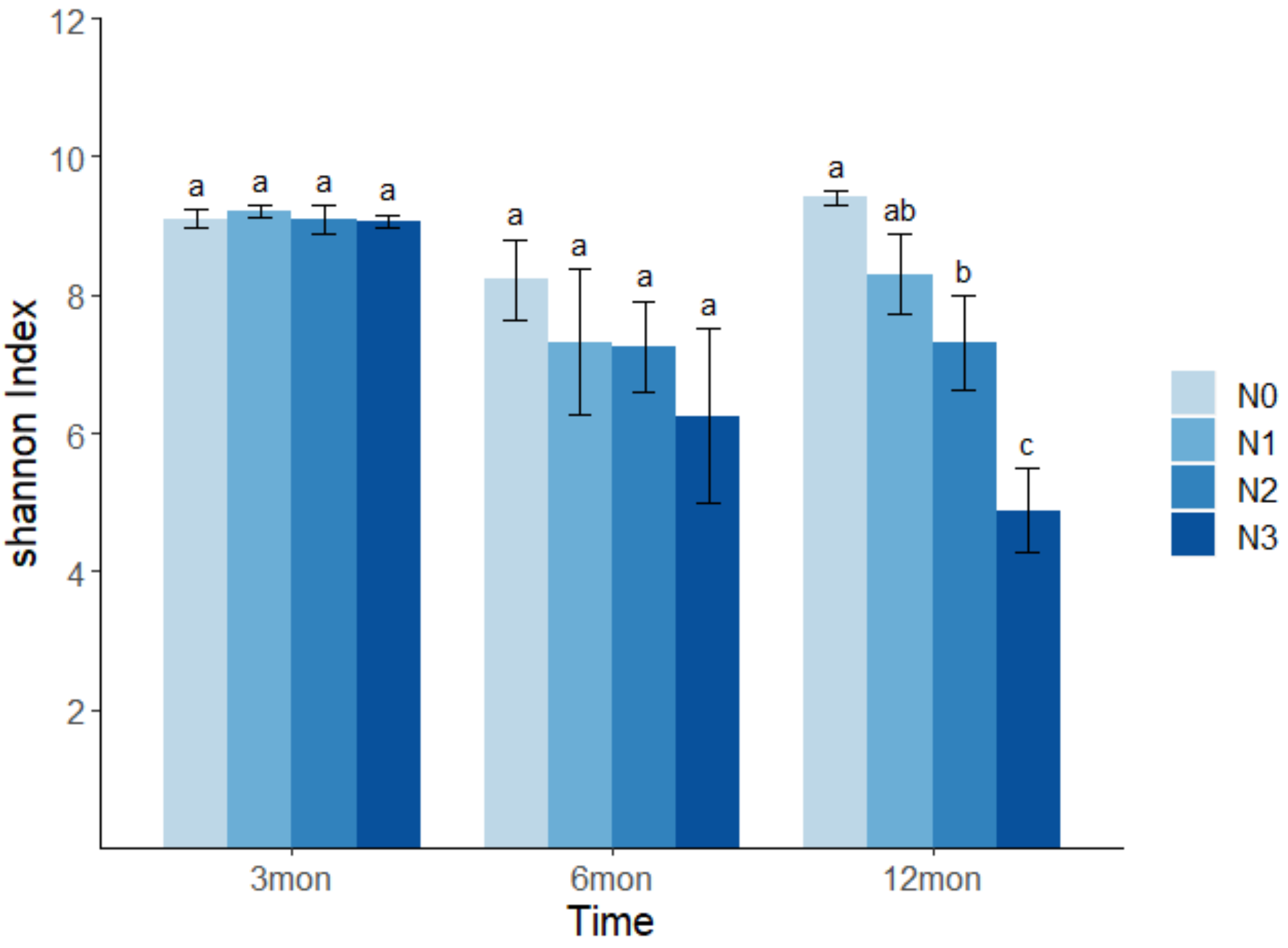
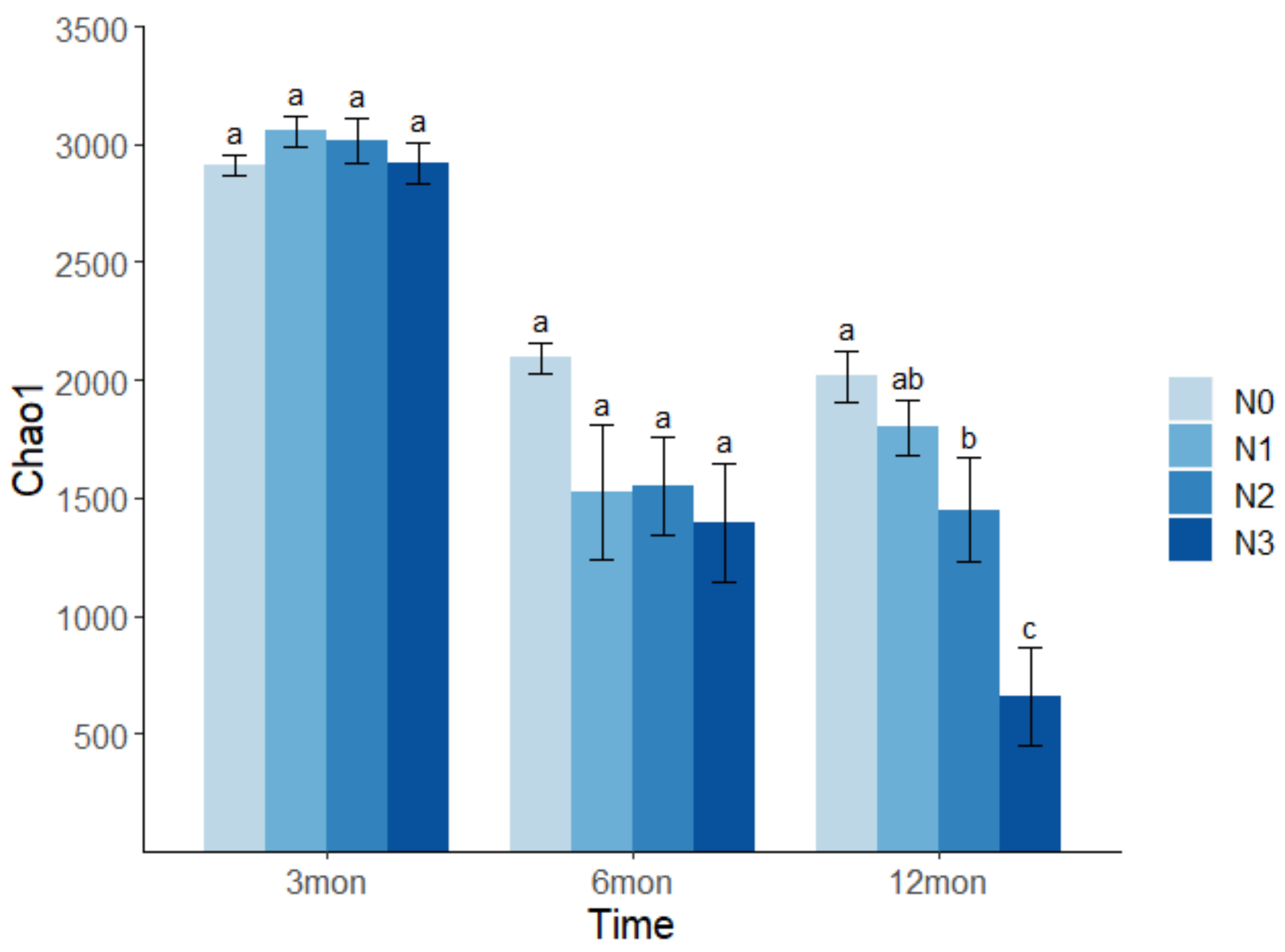
3.2. Effects of N Deposition on Soil Bacterial Richness and Diversity
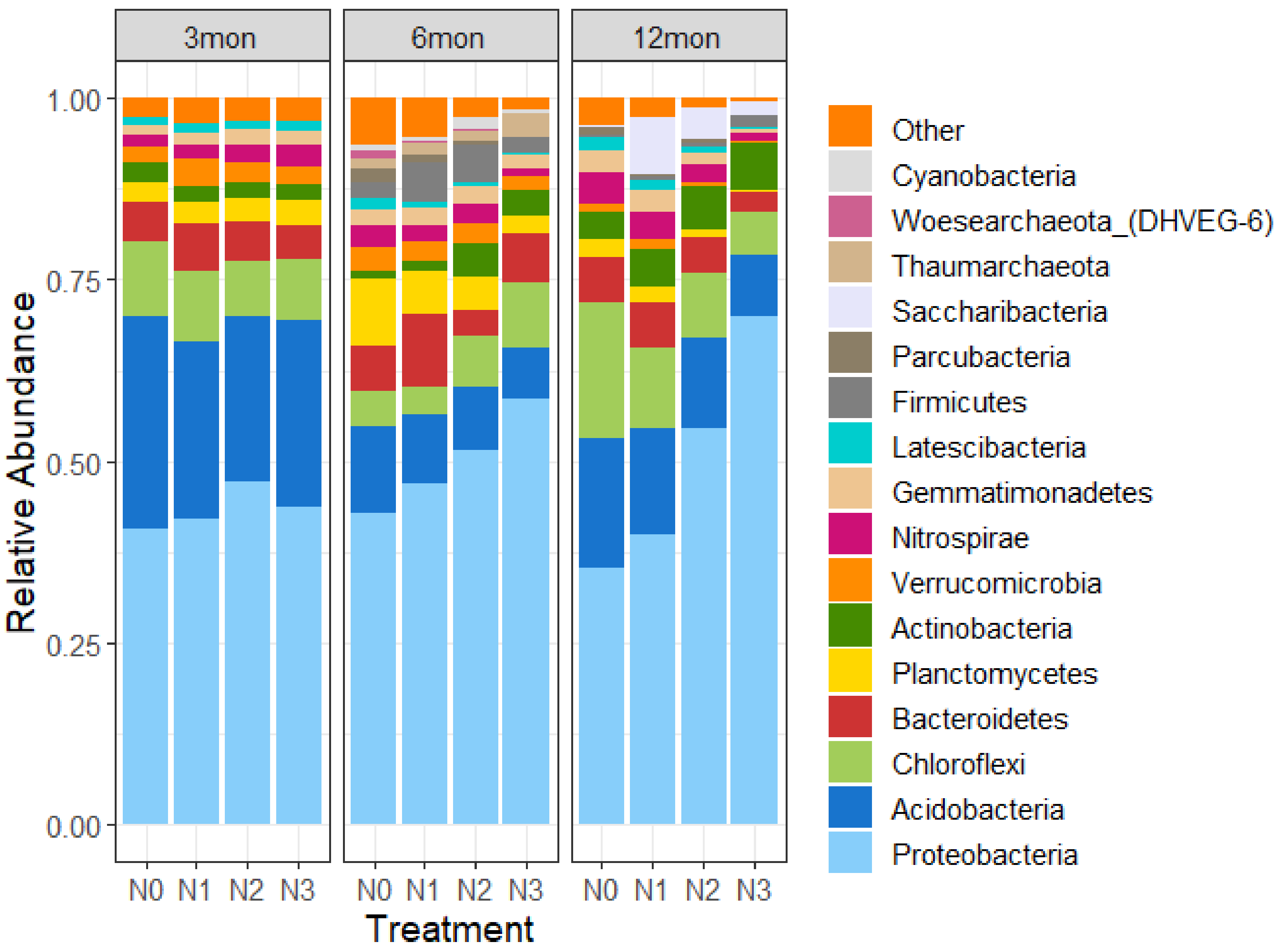
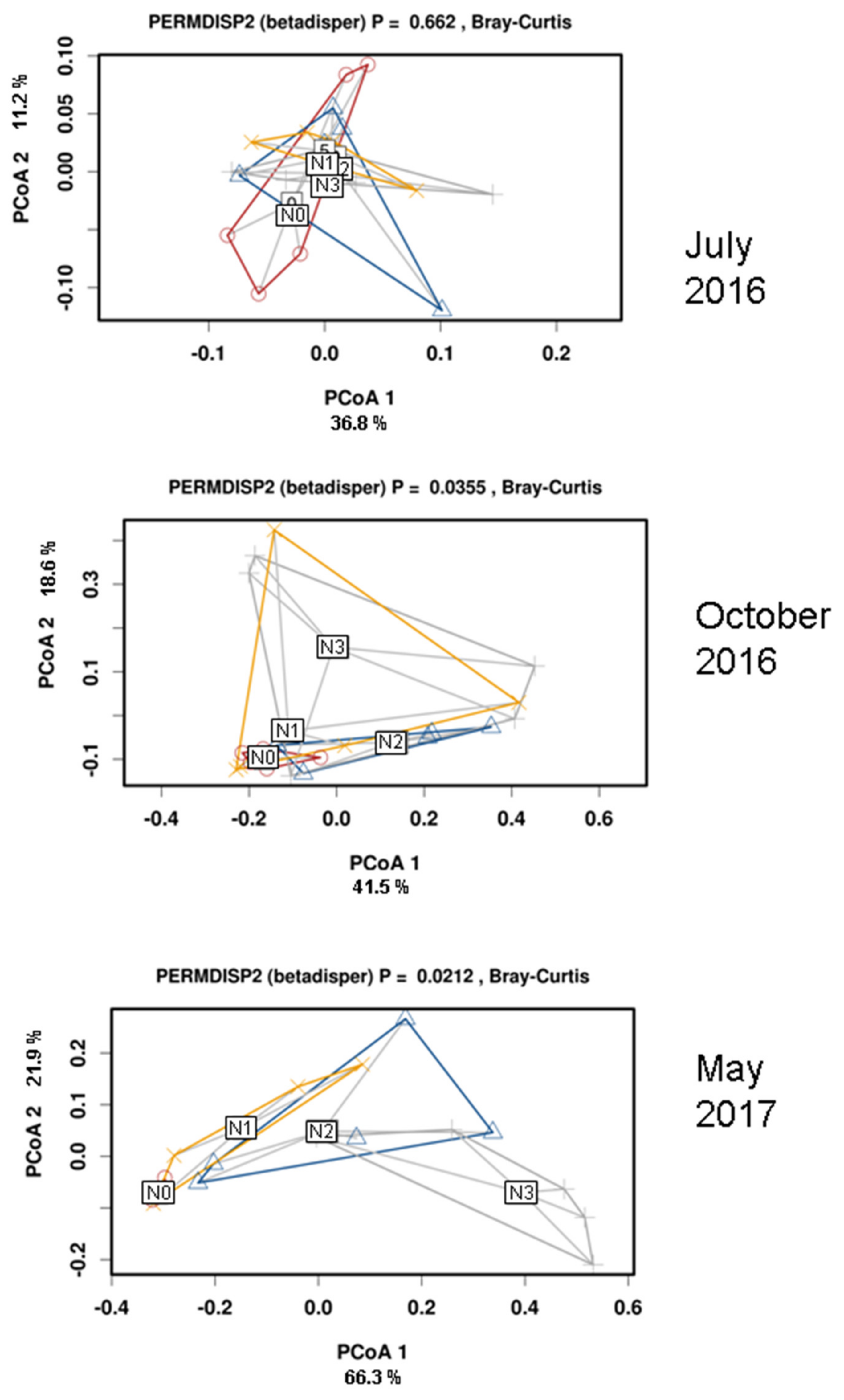
3.3. Effects of N Deposition on Soil Bacterial Community Composition
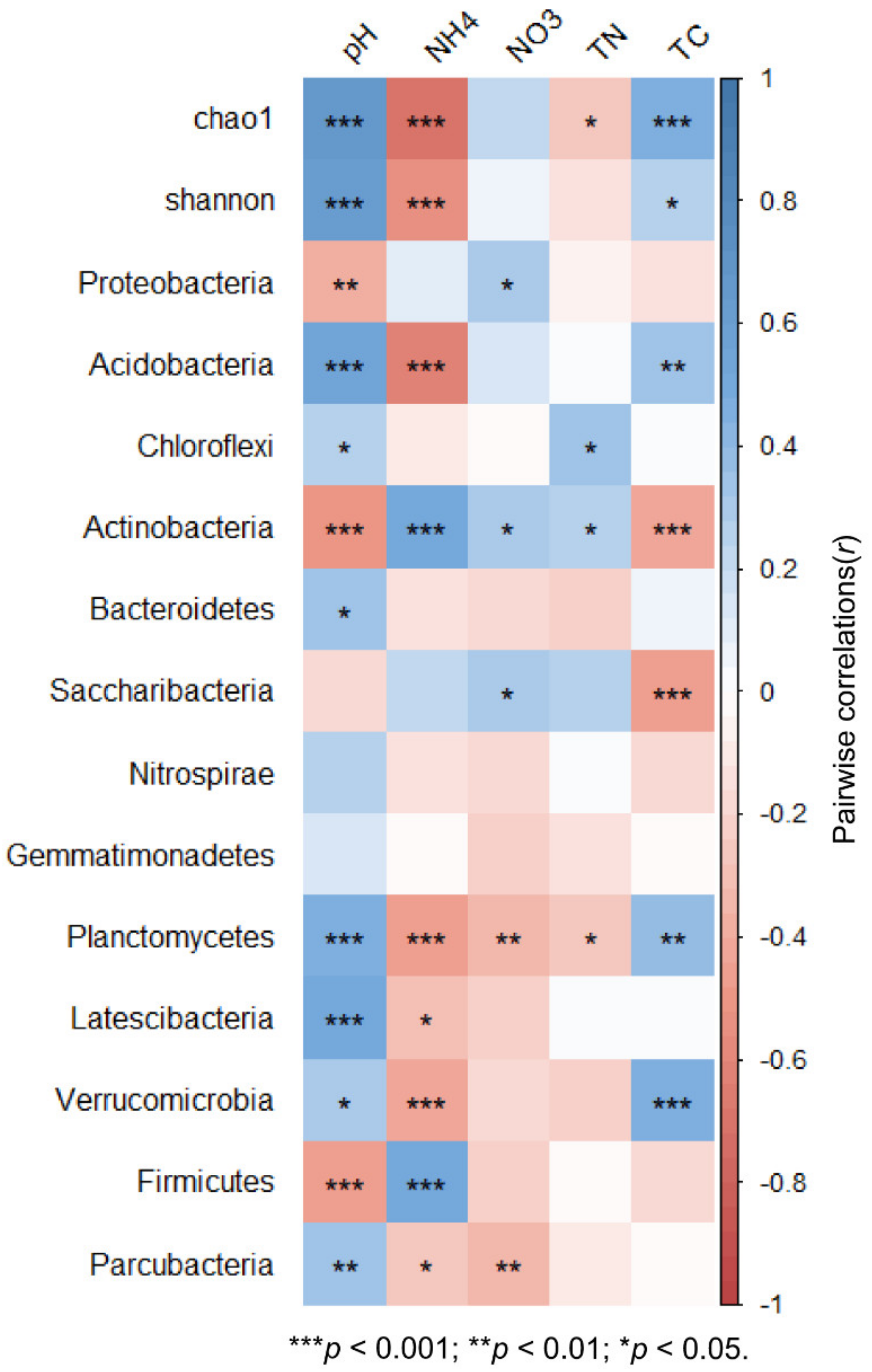
3.4. Effects of Soil Chemical Properties on the Soil Bacterial Diversity and Community Composition
4. Discussion
4.1. Effects of Soil Chemical Properties on the Soil Bacterial Diversity and Community Composition
4.2. Temporal Trends and Potential Consequences of N Deposition
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Liu, X.; Duan, L.; Mo, J.; Du, E.; Shen, J.; Lu, X.; Zhang, Y.; Zhou, X.; He, C.; Zhang, F. Nitrogen deposition and its ecological impact in China: An overview. Environ. Pollut. 2011, 159, 2251–2264. [Google Scholar] [CrossRef]
- Bobbink, R.; Hicks, K.; Galloway, J.; Spranger, T.; Alkemade, R.; Ashmore, M.; Bustamante, M.; Cinderby, S.; Davidson, E.; Dentener, F.; et al. Global assessment of nitrogen deposition effects on terrestrial plant diversity: A synthesis. Ecol. Appl. 2010, 20, 30–59. [Google Scholar] [CrossRef] [PubMed]
- Boutin, M.; Corcket, E.; Alard, D.; Villar, L.; Jiménez, J.J.; Blaix, C.; Lemaire, C.; Corriol, G.; Lamaze, T.; Pornon, A. Nitrogen deposition and climate change have increased vascular plant species richness and altered the composition of grazed subalpine grasslands. J. Ecol. 2017, 105, 1199–1209. [Google Scholar] [CrossRef]
- Galloway, J.N.; Dentener, F.J.; Capone, D.G.; Boyer, E.W.; Howarth, R.W.; Seitzinger, S.P.; Asner, G.P.; Cleveland, C.C.; Green, P.A.; Holland, E.A. Nitrogen cycles: Past, present, and future. Biogeochemistry 2004, 70, 153–226. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, Y.; Han, W.; Tang, A.; Shen, J.; Cui, Z.; Vitousek, P.; Erisman, J.W.; Goulding, K.; Christie, P. Enhanced nitrogen deposition over China. Nature 2013, 494, 459. [Google Scholar] [CrossRef]
- Li-Hua, T.; Hong-Ling, H.; Ting-Xing, H.; Zhang, J.; Li, L.; Ren-Hong, L.; Hong-Zhong, D.; Shou-Hua, L. Decomposition of different litter fractions in a subtropical bamboo ecosystem as affected by experimental nitrogen deposition. Pedosphere 2011, 21, 685–695. [Google Scholar]
- Vitousek, P.M.; Aber, J.D.; Howarth, R.W.; Likens, G.E.; Matson, P.A.; Schindler, D.W.; Schlesinger, W.H.; Tilman, D.G. Human alteration of the global nitrogen cycle: Sources and consequences. Ecol. Appl. 1997, 7, 737–750. [Google Scholar] [CrossRef]
- Enloe, H.A.; Lockaby, B.G.; Zipperer, W.C.; Somers, G.L. Urbanization effects on soil nitrogen transformations and microbial biomass in the subtropics. Urban Ecosyst. 2015, 18, 963–976. [Google Scholar] [CrossRef]
- Templer, P.H.; Weathers, K.C.; Lindsey, A.; Lenoir, K.; Scott, L. Atmospheric inputs and nitrogen saturation status in and adjacent to Class I wilderness areas of the northeastern US. Oecologia 2015, 177, 5–15. [Google Scholar] [CrossRef]
- Russell, A.G.; Winner, D.A.; Harley, R.A.; McCue, K.F.; Cass, G.R. Mathematical modeling and control of the dry deposition flux of nitrogen-containing air pollutants. Environ. Sci. Technol. 1993, 27, 2772–2782. [Google Scholar] [CrossRef]
- Schaefer, S.C.; Hollibaugh, J.T.; Alber, M. Watershed nitrogen input and riverine export on the west coast of the US. Biogeochemistry 2009, 93, 219–233. [Google Scholar] [CrossRef]
- Elser, J.J.; Bracken, M.E.; Cleland, E.E.; Gruner, D.S.; Harpole, W.S.; Hillebrand, H.; Ngai, J.T.; Seabloom, E.W.; Shurin, J.B.; Smith, J.E. Global analysis of nitrogen and phosphorus limitation of primary producers in freshwater, marine and terrestrial ecosystems. Ecol. Lett. 2007, 10, 1135–1142. [Google Scholar] [CrossRef] [PubMed]
- Pierik, M.; Van Ruijven, J.; Bezemer, T.M.; Geerts, R.H.; Berendse, F. Recovery of plant species richness during long-term fertilization of a species-rich grassland. Ecology 2011, 92, 1393–1398. [Google Scholar] [CrossRef] [PubMed]
- Kabisch, N. Ecosystem service implementation and governance challenges in urban green space planning—The case of Berlin, Germany. Land Use Policy 2015, 42, 557–567. [Google Scholar] [CrossRef]
- Escobedo, F.J.; Kroeger, T.; Wagner, J.E. Urban forests and pollution mitigation: Analyzing ecosystem services and disservices. Environ. Pollut. 2011, 159, 2078–2087. [Google Scholar] [CrossRef]
- Cusack, D.F.; Silver, W.L.; Torn, M.S.; Burton, S.D.; Firestone, M.K. Changes in microbial community characteristics and soil organic matter with nitrogen additions in two tropical forests. Ecology 2011, 92, 621–632. [Google Scholar] [CrossRef]
- Yang, L.; Zhang, L.; Li, Y.; Wu, S. Water-related ecosystem services provided by urban green space: A case study in Yixing City (China). Landsc. Urban Plan. 2015, 136, 40–51. [Google Scholar] [CrossRef]
- Chiesura, A. The role of urban parks for the sustainable city. Landsc. Urban Plan. 2004, 68, 129–138. [Google Scholar] [CrossRef]
- Balestrini, R.; Lumini, E. Focus on mycorrhizal symbioses. Appl. Soil Ecol. 2018, 123, 299–304. [Google Scholar] [CrossRef]
- Bernier, N. Hotspots of biodiversity in the underground: A matter of humus form? Appl. Soil Ecol. 2018, 123, 305–312. [Google Scholar] [CrossRef]
- Blouin, M. Chemical communication: An evidence for co-evolution between plants and soil organisms. Appl. Soil Ecol. 2018, 123, 409–415. [Google Scholar] [CrossRef]
- Centenaro, G.; Hudek, C.; Zanella, A.; Crivellaro, A. Root-soil physical and biotic interactions with a focus on tree root systems: A review. Appl. Soil Ecol. 2018, 123, 318–327. [Google Scholar] [CrossRef]
- Geisen, S.; Bonkowski, M. Methodological advances to study the diversity of soil protists and their functioning in soil food webs. Appl. Soil Ecol. 2018, 123, 328–333. [Google Scholar] [CrossRef]
- Zhang, T.A.; Chen, H.Y.; Ruan, H. Global negative effects of nitrogen deposition on soil microbes. ISME J. 2018, 12, 1817–1825. [Google Scholar] [CrossRef]
- Artursson, V.; Finlay, R.D.; Jansson, J.K. Interactions between arbuscular mycorrhizal fungi and bacteria and their potential for stimulating plant growth. Environ. Microbiol. 2006, 8, 1–10. [Google Scholar] [CrossRef]
- Morris, S.J.; Blackwood, C.B. The ecology of the soil biota and their function. In Soil Microbiology, Ecology and Biochemistry, 4th ed.; Academic Press: San Diego, CA, USA, 2015; pp. 273–309. [Google Scholar]
- Philippot, L.; Andersson, S.G.; Battin, T.J.; Prosser, J.I.; Schimel, J.P.; Whitman, W.B.; Hallin, S. The ecological coherence of high bacterial taxonomic ranks. Nat. Rev. Microbiol. 2010, 8, 523. [Google Scholar] [CrossRef]
- Mardis, E.R. The impact of next-generation sequencing technology on genetics. Trends Genet. 2008, 24, 133–141. [Google Scholar] [CrossRef]
- Handelsman, J. Metagenomics: Application of genomics to uncultured microorganisms. Microbiol. Mol. Biol. Rev. 2004, 68, 669–685. [Google Scholar] [CrossRef]
- Handelsman, J.; Rondon, M.R.; Brady, S.F.; Clardy, J.; Goodman, R.M. Molecular biological access to the chemistry of unknown soil microbes: A new frontier for natural products. Chem. Biol. 1998, 5, R245–R249. [Google Scholar] [CrossRef]
- He, Z.; Xu, M.; Deng, Y.; Kang, S.; Kellogg, L.; Wu, L.; Van Nostrand, J.D.; Hobbie, S.E.; Reich, P.B.; Zhou, J. Metagenomic analysis reveals a marked divergence in the structure of belowground microbial communities at elevated CO2. Ecol. Lett. 2010, 13, 564–575. [Google Scholar] [CrossRef]
- Monteiro, J.M.; Vollú, R.E.; Coelho, M.R.R.; Fonseca, A.; Neto, S.C.G.; Seldin, L. Bacterial communities within the rhizosphere and roots of vetiver (Chrysopogon zizanioides (L.) Roberty) sampled at different growth stages. Eur. J. Soil Biol. 2011, 47, 236–242. [Google Scholar] [CrossRef]
- Cederlund, H.; Wessén, E.; Enwall, K.; Jones, C.M.; Juhanson, J.; Pell, M.; Philippot, L.; Hallin, S. Soil carbon quality and nitrogen fertilization structure bacterial communities with predictable responses of major bacterial phyla. Appl. Soil Ecol. 2014, 84, 62–68. [Google Scholar] [CrossRef]
- Lv, X.; Ma, B.; Yu, J.; Chang, S.X.; Xu, J.; Li, Y.; Wang, G.; Han, G.; Bo, G.; Chu, X. Bacterial community structure and function shift along a successional series of tidal flats in the Yellow River Delta. Sci. Rep. 2016, 6, 36550. [Google Scholar] [CrossRef] [PubMed]
- Fierer, N.; Strickland, M.S.; Liptzin, D.; Bradford, M.A.; Cleveland, C.C. Global patterns in belowground communities. Ecol. Lett. 2009, 12, 1238–1249. [Google Scholar] [CrossRef]
- Zanardo, M.; Rosselli, R.; Meneghesso, A.; Sablok, G.; Stevanato, P.; Engel, M.; Altissimo, A.; Peserico, L.; Dezuani, V.; Concheri, G.; et al. Response of Bacterial Communities upon Application of Different Innovative Organic Fertilizers in a Greenhouse Experiment Using Low-Nutrient Soil Cultivated with Cynodon dactylon. Soil Syst. 2018, 2, 52. [Google Scholar] [CrossRef]
- Huang, L.; Zhu, W.; Ren, H.; Chen, H.; Wang, J. Impact of atmospheric nitrogen deposition on soil properties and herb-layer diversity in remnant forests along an urban–rural gradient in Guangzhou, southern China. Plant Ecol. 2012, 213, 1187–1202. [Google Scholar] [CrossRef]
- Lu, R.-K. Soil Argrochemistry Analysis Protocoes; China Agriculture Science Press: Beijing, China, 1999; Volume 106. [Google Scholar]
- Wang, C.; Liu, D.; Bai, E. Decreasing soil microbial diversity is associated with decreasing microbial biomass under nitrogen addition. Soil Biol. Biochem. 2018, 120, 126–133. [Google Scholar] [CrossRef]
- Zeng, J.; Liu, X.; Song, L.; Lin, X.; Zhang, H.; Shen, C.; Chu, H. Nitrogen fertilization directly affects soil bacterial diversity and indirectly affects bacterial community composition. Soil Biol. Biochem. 2016, 92, 41–49. [Google Scholar] [CrossRef]
- Ding, J.; Zhu, D.; Chen, Q.L.; Zheng, F.; Wang, H.T.; Zhu, Y.G. Effects of long-term fertilization on the associated microbiota of soil collembolan. Soil Biol. Biochem. 2019, 130, 141–149. [Google Scholar] [CrossRef]
- Ndour, N.Y.B.; Achouak, W.; Christen, R.; Heulin, T.; Brauman, A.; Chotte, J.L. Characteristics of microbial habitats in a tropical soil subject to different fallow management. Appl. Soil Ecol. 2008, 38, 51–61. [Google Scholar] [CrossRef]
- Rousk, J.; Bååth, E.; Brookes, P.C.; Lauber, C.L.; Lozupone, C.; Caporaso, J.G.; Knight, R.; Fierer, N. Soil bacterial and fungal communities across a pH gradient in an arable soil. ISME J. 2010, 4, 1340. [Google Scholar] [CrossRef] [PubMed]
- Treseder, K.K. Nitrogen additions and microbial biomass: A meta-analysis of ecosystem studies. Ecol. Lett. 2008, 11, 1111–1120. [Google Scholar] [CrossRef] [PubMed]
- Serna-Chavez, H.M.; Fierer, N.; Van Bodegom, P.M. Global drivers and patterns of microbial abundance in soil. Glob. Ecol. Biogeogr. 2013, 22, 1162–1172. [Google Scholar] [CrossRef]
- Lauber, C.L.; Hamady, M.; Knight, R.; Fierer, N. Soil pH as a predictor of soil bacterial community structure at the continental scale: A pyrosequencing-based assessment. Appl. Environ. Microbiol. 2009, 75, 5111–5120. [Google Scholar] [CrossRef] [PubMed]
- Ramirez, K.S.; Lauber, C.L.; Knight, R.; Bradford, M.A.; Fierer, N. Consistent effects of nitrogen fertilization on soil bacterial communities in contrasting systems. Ecology 2010, 91, 3463–3470. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Guan, D.; Zhou, B.; Zhao, B.; Ma, M.; Qin, J.; Jiang, X.; Chen, S.; Cao, F.; Shen, D.; et al. Influence of 34-years of fertilization on bacterial communities in an intensively cultivated black soil in northeast China. Soil Biol. Biochem. 2015, 90, 42–51. [Google Scholar] [CrossRef]
- Xun, W.; Zhao, J.; Xue, C.; Zhang, G.; Ran, W.; Wang, B.; Shen, Q.; Zhang, R. Significant alteration of soil bacterial communities and organic carbon decomposition by different long-term fertilization management conditions of extremely low-productivity arable soil in South China. Environ. Microbiol. 2016, 18, 1907–1917. [Google Scholar] [CrossRef]
- Wang, Q.; Jiang, X.; Guan, D.; Wei, D.; Zhao, B.; Ma, M.; Li, J. Long-term fertilization changes bacterial diversity and bacterial communities in the maize rhizosphere of Chinese Mollisols. Appl. Soil Ecol. 2018, 125, 88–96. [Google Scholar] [CrossRef]
- Fan, H.B.; Liu, W.F.; Li, Y.Y.; Liao, Y.C.; Yuan, Y.H.; Xu, L. Tree growth and soil nutrients in response to nitrogen deposition in a subtropical Chinese fir plantation. Acta Ecol. Sin. 2007, 27, 4630–4642. [Google Scholar]
- Wang, Q.; Wang, C.; Yu, W.; Turak, A.; Chen, D.; Huang, Y.; Ao, J.; Jiang, Y.; Huang, Z. Effects of nitrogen and phosphorus inputs on soil bacterial abundance, diversity, and community composition in Chinese Fir plantations. Front. Microbiol. 2018, 9, 1543. [Google Scholar] [CrossRef]
- Nie, Y.; Wang, M.; Zhang, W.; Ni, Z.; Hashidoko, Y.; Shen, W. Ammonium nitrogen content is a dominant predictor of bacterial community composition in an acidic forest soil with exogenous nitrogen enrichment. Sci. Total Environ. 2018, 624, 407–415. [Google Scholar] [CrossRef] [PubMed]
- Fierer, N.; Lauber, C.L.; Ramirez, K.S.; Zaneveld, J.; Bradford, M.A.; Knight, R. Comparative metagenomic, phylogenetic and physiological analyses of soil microbial communities across nitrogen gradients. ISME J. 2012, 6, 1007. [Google Scholar] [CrossRef] [PubMed]
- Shen, C.; Xiong, J.; Zhang, H.; Feng, Y.; Lin, X.; Li, X.; Liang, W.; Chu, H. Soil pH drives the spatial distribution of bacterial communities along elevation on Changbai Mountain. Soil Biol. Biochem. 2013, 57, 204–211. [Google Scholar] [CrossRef]
- Jones, R.T.; Robeson, M.S.; Lauber, C.L.; Hamady, M.; Knight, R.; Fierer, N. A comprehensive survey of soil Acidobacterial diversity using pyrosequencing and clone library analyses. ISME J. 2009, 3, 442. [Google Scholar] [CrossRef]
- Yu, W.T.; Jiang, C.M.; Ma, Q.; Xu, Y.G.; Zou, H.; Zhang, S.C. Observation of the nitrogen deposition in the lower Liaohe River Plain, Northeast China and assessing its ecological risk. Atmos. Res. 2011, 101, 460–468. [Google Scholar] [CrossRef]
- Campbell, B.J.; Polson, S.W.; Hanson, T.E.; Mack, M.C.; Schuur, E.A. The effect of nutrient deposition on bacterial communities in Arctic tundra soil. Environ. Microbiol. 2010, 12, 1842–1854. [Google Scholar] [CrossRef]
- Lin, L.W.; Xiao, H.L.; Liu, T. Dynamics of wet atmospheric nitrogen deposition and the relation to acid rain in the northeast suburb of Guangzhou. Ecol. Environ. Sci. 2013, 22, 293–297. [Google Scholar]
- Geisseler, D.; Scow, K.M. Long-term effects of mineral fertilizers on soil microorganisms—A review. Soil Biol. Biochem. 2014, 75, 54–63. [Google Scholar] [CrossRef]
- Dai, Z.; Su, W.; Chen, H.; Barberán, A.; Zhao, H.; Yu, M.; Yu, L.; Brookes, P.; Schadt, C.W.; Chang, S.X.; et al. Long-term nitrogen fertilization decreases bacterial diversity and favors the growth of Actinobacteria and Proteobacteria in Agro-ecosystems across the globe. Glob. Chang. Biol. 2018, 24, 3452–3461. [Google Scholar] [CrossRef]
- Báez, S.; Fargione, J.; Moore, D.I.; Collins, S.L.; Gosz, J.R. Atmospheric nitrogen deposition in the northern Chihuahuan desert: Temporal trends and potential consequences. J. Arid Environ. 2007, 68, 640–651. [Google Scholar] [CrossRef]
- Torsvik, V.; Øvreås, L. Microbial diversity and function in soil: From genes to ecosystems. Curr. Opin. Microbiol. 2002, 5, 240–245. [Google Scholar] [CrossRef]
- Van Der Heijden, M.G.; Bardgett, R.D.; Van Straalen, N.M. The unseen majority: Soil microbes as drivers of plant diversity and productivity in terrestrial ecosystems. Ecol. Lett. 2008, 11, 296–310. [Google Scholar] [CrossRef] [PubMed]
| Time | Treatment | pH | NH4+-N (mg kg−1) | NO3−-N (mg kg−1) | TN (g kg−1) | TC (g kg−1) |
|---|---|---|---|---|---|---|
| 3 months | N0 | 7.89 ± 0.18 a | 2.95 ± 0.54 a | 27.712 ± 18.84 a | 0.78 ± 0.09 a | 12.77 ± 1.39 a |
| N1 | 7.77 ± 0.29 a | 3.50 ± 0.40 a | 11.05 ± 5.37 a | 0.82 ± 0.14 a | 13.96 ± 1.23 a | |
| N2 | 7.58 ± 0.29 a | 2.23 ± 0.57 a | 42.04 ± 15.71 a | 0.72 ± 0.07 a | 12.93 ± 1.14 a | |
| N3 | 7.50 ± 0.43 a | 3.03 ± 0.24 a | 19.68 ± 5.79 a | 0.67 ± 0.05 a | 12.14 ± 1.25a | |
| p * | 0.81 | 0.31 | 0.40 | 0.66 | 0.78 | |
| 6 months | N0 | 7.92 ± 0.13 a | 7.49 ± 0.12 b | 0.59 ± 0.03 d | 0.64 ± 0.12 a | 10.88 ± 0.45 a |
| N1 | 7.22 ± 0.45 a,b | 15.40 ± 6.28 b | 4.22 ± 0.32 c | 0.78 ± 0.18 a | 12.61 ± 1.66 a | |
| N2 | 6.56 ± 0.23 b | 31.61 ± 3.80 b | 9.29 ± 1.04 b | 0.82 ± 0.10 a | 11.34 ± 0.84 a | |
| N3 | 6.23 ± 0.47 b | 75.26 ± 17.67 a | 15.29 ± 1.81 a | 0.94 ± 0.09 a | 10.76 ± 0.41 a | |
| p * | <0.05 | <0.01 | <0.01 | 0.45 | 0.54 | |
| 12 months | N0 | 7.79 ± 0.08 a | 19.34 ± 0.74 b | 1.48 ± 0.38 b | 0.82 ± 0.08 b | 8.78 ± 0.81 a |
| N1 | 7.08 ± 0.47 a,b | 37.34 ± 9.41 b | 6.49 ± 0.99 b | 0.88 ± 0.07 b | 8.90 ± 0.74 a | |
| N2 | 6.23 ± 0.47 b,c | 78.94 ± 11.71 a | 17.13 ± 2.45 a | 1.12 ± 0.23 a,b | 8.66 ± 1.31 a | |
| N3 | 5.73 ± 0.47 c | 108.69 ± 18.90 a | 23.18 ± 4.13 a | 1.33 ± 0.12 a | 8.60 ± 0.34 a | |
| p * | <0.05 | <0.01 | <0.01 | 0.08 | 0.99 |
| Soil Properties | r | p |
|---|---|---|
| pH | 0.2273 | 0.004 |
| NH4+-N | 0.1799 | 0.037 |
| NO3--N | −0.1457 | 0.986 |
| TN | 0.0066 | 0.401 |
| TC | −0.0815 | 0.848 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mo, L.; Zanella, A.; Chen, X.; Peng, B.; Lin, J.; Su, J.; Luo, X.; Xu, G.; Squartini, A. Effects of Simulated Nitrogen Deposition on the Bacterial Community of Urban Green Spaces. Appl. Sci. 2021, 11, 918. https://doi.org/10.3390/app11030918
Mo L, Zanella A, Chen X, Peng B, Lin J, Su J, Luo X, Xu G, Squartini A. Effects of Simulated Nitrogen Deposition on the Bacterial Community of Urban Green Spaces. Applied Sciences. 2021; 11(3):918. https://doi.org/10.3390/app11030918
Chicago/Turabian StyleMo, Lingzi, Augusto Zanella, Xiaohua Chen, Bin Peng, Jiahui Lin, Jiaxuan Su, Xinghao Luo, Guoliang Xu, and Andrea Squartini. 2021. "Effects of Simulated Nitrogen Deposition on the Bacterial Community of Urban Green Spaces" Applied Sciences 11, no. 3: 918. https://doi.org/10.3390/app11030918
APA StyleMo, L., Zanella, A., Chen, X., Peng, B., Lin, J., Su, J., Luo, X., Xu, G., & Squartini, A. (2021). Effects of Simulated Nitrogen Deposition on the Bacterial Community of Urban Green Spaces. Applied Sciences, 11(3), 918. https://doi.org/10.3390/app11030918








