Abstract
Polymers from renewable feedstocks are receiving increasing attention as the awareness about environmental issues derived from petroleum exploitation and waste accumulation is growing. With unsaturated polyester resins being one of the most used classes of polymers worldwide, the utilization of biobased monomers for manufacturing is more relevant than ever. In the present work, succinic acid, one of the most promising biobased building blocks, was incorporated in the structure of the resins in question to increase their biobased content. By reacting with ethylene glycol (EG) or poly(ethylene glycol) and maleic anhydride (MA) at several molar rations, unsaturated polyester resins (UPRs) were prepared. Their synthesis was evaluated by a variety of spectroscopical techniques, and their rheological properties made use of the reactive diluent mandatory for facilitating processing. Thus, in a second stage acrylic acid (AA) was used as cross-linking agent in the present of initiators and accelerators producing thermosetting resins. Differential scanning calorimetry (DSC) was employed to screen the cross-linking procedure, whereas with X-ray photoelectron spectroscopy (XPS) and Fourier transform infrared spectroscopy (FTIR) it was proven that thermosetting resins were prepared. The thermal stability of the cured materials was evaluated by thermogravimetric analysis (TGA).
1. Introduction
One of the most widely used classes of polymers today is unsaturated polyester resins (UPRs). Along with polyurethanes, they are the most important thermosetting materials [1]. A major advantage of these materials is their wide range of processing temperatures, including processing at room temperature and atmospheric conditions, a feature that renders them ideal for the fabrication of large structures. Combining that with properties such as low weight, high toughness, and low cost, UPRs are used for various commercial applications, for instance as coatings, adhesives, parts for the automobile industry, boat hulls, structural laminates, and composite applications [2]. Glass fiber-reinforced UP resins are the most common composite prepared from UPRs, and they are extensively used in building and construction, and in the transportation, electric, and electronic industries [3].
Despite their processability and versatile nature, the majority of commercial UPRs today present a significant drawback: They are obtained from petroleum resources [4]. The recent fluctuation of crude oil prices associated with depleting resources of fossil fuels and increasing public awareness of the ecological disaster derived from the exploitation of non-renewable feedstocks has generated an increasing interest in polymeric materials from renewable resources [5,6]. With this in mind, several biobased monomers have been screened as potential replacements for the petroleum derived homologues, with the US Department of Energy selecting the 12 most important in a recent report [7]. Since then, an all-increasing number of publications on polymeric materials based on these monomers has been amassed, suggesting their high potential in replacing traditional ones. Research on biobased UPRs is also on the rise, with many interesting reports depicting the ability to produce high-value polymeric materials derived from renewable resources [8,9,10,11]. It is clear that the potential commercialization of such products, with increased biobased content, would be a huge step towards a sustainable circular economy.
In this context, one of the most important biobased monomers that is already used in commercially available products is succinic acid. Commercial uses include thermoplastic polyesters such as poly(butylene succinate) (PBS), poly(ethylene succinate) (PES), and copolymers consisting of these materials [12]. In addition, succinic acid has been used effectively as a comonomer to synthesize biodegradable copolymers with biobased aromatic moieties such as 2,5 furan dicarboxylic acid [13]. Lately, a very interesting series of poly(ester amides) was examined by Kluge et al. [14,15,16], enhancing the properties of traditional polyesters derived from succinic acid via the incorporation of symmetrical amidodiols. Produced via the fermentation of sugars [17], this monomer is ideal for producing materials with high biobased content, or even fully biobased ones when combined with other biobased monomers [18]. However, its use in the field of thermosetting polymers has not received similar attention. Though a number of very interesting studies on biobased UPRs based on succinic acid are available in the literature [19,20,21,22,23,24], more fundamental research needs to be conducted to establish this monomer in the field of UPRs.
In this work, we aim to synthesize and characterize UPRs with high biobased content. To this end, biobased monomers were used, such as succinic acid, ethylene glycol, and poly(ethylene glycol). The structure of the prepared resins and their successful cross-linking were verified by nuclear magnetic resonance, Fourier transform infrared, and X-ray photoelectron spectroscopies. The molecular weight of the resins was determined by size exclusion chromatography and their rheological characteristics were also examined. Due to its increased viscosity, acrylic acid was used as the reactive diluent to increase processability, as it was recently found to able to be produced via green routes [25,26,27,28]. The thermally induced curing and the cross-linking characteristics were monitored by means of a differential scanning calorimetry study and the thermal stability of the cured samples was examined by means of thermogravimetry.
2. Materials and Methods
2.1. Materials
Succinic acid (SA, purum 99+%), maleic anhydride (MA, purum 99%), ethylene glycol (EG, purum 99+%), poly(ethylene glycol) molecular weight (M.W.):200 (PEG200, 99%), acrylic acid (AA, purum 99%) and 2,6-di-tert-butyl-4-methylphenol (BHT, 99%) were purchased from Alfa Aesar, Kandel, Germany. Methylethylketone peroxide (MEKP), benzoyl peroxide (BPO), and cobalt(II) octoate were purchased from Merck, Darmstadt, Germany. FASCAT 4101 catalyst was kindly donated by PMC Organometallix, through Safic Alcan HELLAS SA, Athens, Greece. Solvents were reagent or analytical grade and were purchased from Aldrich, Steinheim, Germany. All reagents were used without further purification.
2.2. Synthesis of UPRs and Cross-Linking Procedure
Typically, succinic acid and maleic anhydride (1 eq), along with EG or PEG200 (1.25 eq), 0.2 wt% FASCAT 4101, and BHT (400 ppm), were charged into a three-necked round-bottomed flask, which was equipped with a mechanical stirrer and a Dean–Stark apparatus. Using toluene as an entrainer, the mixture was heated gradually until homogenization, and then the temperature was set at 180 °C. The course of the reaction was tracked by the analysis of the acid value (AV). When the AV reached a value of less than 5 mg KOH/g, the toluene was removed under reduced pressure. The polyester was obtained without any further purification as a yellow viscous liquid. According to this procedure six unsaturated polyester resins were prepared and the ratio of monomers used for the synthesis of each resin is given in Table 1.

Table 1.
Composition of the unsaturated polyester resins synthesized in the study.
The acid value (AV) corresponded to the non-reacted acid groups. It was defined as the number of milligrams of potassium hydroxide required to neutralize 1 g of sample and was determined according to DIN EN ISO 2114 by titrating the remaining carboxylic acid groups of the sample with a solution of potassium hydroxide in methanol (0.3 mol/L).
To cross-link the synthesized resins, a mixture of the prepared UPR from Table 1 and AA in a mass ratio of 2:1 was mixed. For the preparation of the cross-linked resins, a mixture of UPR and AA in a mass ratio of 2:1 was mixed at room temperature. Curing of the materials was initiated using 3 wt% MEKP or BPO initiator and Co(II) octoate as the accelerator. After adding the initiating system, the mixtures were put in Teflon molds and heated at 100 °C for 4 h.
2.3. Characterization Methods
2.3.1. Nuclear Magnetic Resonance Spectroscopy (1H-NMR)
1H-NMR spectra were recorded in deuterated chloroform (CDCl3-d1) on an Agilent 500 spectrometer (Agilent Technologies, Santa Clara, CA, USA), at room temperature. Spectra were internally referenced with tetramethylsilane (TMS) and calibrated using the residual solvent peaks (chloroform-d1, 7.26 ppm for hydrogen atoms).
2.3.2. Fourier Transform Infrared Spectroscopy (FTIR)
FTIR measurements were performed with a Cary 670 spectroscope from Agilent Technologies (Palo Alto, CA, USA) equipped with a diamond attenuated total reflectance (ATR) accessory, (GladiATR, Pike Technologies, Madison, WI, USA). The spectra were collected in the mid IR area (4000–400 cm−1), with 16 scans and a resolution of 4 cm−1.
2.3.3. Size-Exclusion Chromatography (SEC)
SEC measurements were conducted to determine the molecular weight Mn, Mw and the dispersity index Ð of the synthesized resins. Chloroform at 25 °C was used as eluent, with a flow rate of 1 mL min-1. The SEC was composed of a Waters 600 high-performance liquid chromatographic pump, Waters Ultrastyragel columns HR-1, HR-2, HR-4E, HR-4, and HR-5, and a Shimadzu RID-10A (Kyoto, Japan) refractive index detector. Nine PS standards between 1 and 300 kg mol−1 were used for calibration. The prepared solutions had a concentration of 12 mg mL−1, the injection volume was 150 μL, and the total elution time was 60 min.
2.3.4. Rheological Properties
The rheological properties of the resins were studied on a Bohlin CVO 100 rheometer (Bohlin Instruments, Cirencester, UK) equipped with cone-plate geometry (CP 4°, 40 mm). For the table temperature scan, measurements were done with a shear rate of 100 s−1 for 10 s. Five measurements were carried out for each temperature (20 °C, 30 °C, 40 °C, 60 °C, and 80 °C), and the average value was calculated for each temperature.
2.3.5. Differential Scanning Calorimetry (DSC)
The curing reaction of UPR resins with AA were studied by means of DSC measurements using a Setaram instrument (Lyon, France) model DSC 141. Samples of 5–6 mg were placed in sealed stainless-steel crucibles and measured with a heating rate of 5 °C/min under N2 purge gas from room temperature up to 200 °C for dynamic measurements. In order to analyze the curing process, the same conditions were applied for isothermal measurements. In every case—especially for the isothermal measurements—the experimental UPRs along with AA in a 2:1 ratio were mixed with the accelerator and the initiator in ambient conditions, placed in the crucibles, and measured immediately (<2 min for the whole preparation procedure) [29]. For the cross-linking, MEKP and BPO were used as initiators, and cobalt(II) octoate as the accelerator.
2.3.6. X-ray Photoelectron Spectroscopy (XPS)
XPS was performed with a Kratos Analytical AXIS UltraDLD system (Kyoto, Japan), with an aluminum monochromatic X-ray beam (λKa = 8.3401 Å) and a hemispherical sector analyzer (HSA) under high vacuum conditions (10−6 Pa). The pass energy was 160 eV with one swap for the survey wide scans, and 20 eV with three swaps for the high-resolution spectra. The collected spectra were corrected in terms of charging at 284.6 eV on the peak of C-1s. After the subtraction of a Shirley background, the collected high-resolution spectra were curve-fitted by Gaussian–Lorentzian peaks.
2.3.7. Thermogravimetric Analysis (TGA)
The TGA analysis of the cured samples was performed with a Setaram (Lyon, France) SETSYS TG-DTA 16/18 instrument. Samples of (2 ± 0.5) mg were placed in alumina crucibles and measured in dynamic conditions in a temperature range from 25 to 600 °C with a heating rate of 20 °C/min and in a 50 mL/min N2 atmosphere. The continuous signals of the sample temperature, weight, and heat flow were recorded. For every measurement, the same conditions were applied to the empty alumina crucibles and subtracted from the experimental curves in order to eliminate the buoyancy effect.
3. Results and Discussion
3.1. Synthesis of Unsaturated Resins
Six unsaturated polyester resins of high biobased content—up to 78%—were successfully prepared by a direct esterification of SA and MA with the respective diols, such as ethylene glycol and PEG200. The polyesters were named PESM X-Y and PPEGSM X-Y, respectively, where X stands for the mol% of SA and Y for the mol% of MA contained in the resin. The synthesis of the resins is presented in Figure 1. After preliminary studies it was found that the best esterification temperature was 180 °C under nitrogen flow.
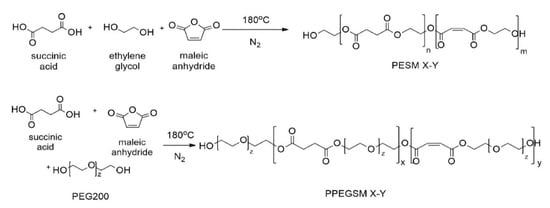
Figure 1.
Chemical structure of the synthesized UPRs.
3.2. Characterisation of Unsaturated Resins
3.2.1. 1H NMR
The structure of the produced resins was examined by means of 1H NMR. The collected spectra are presented in Figure 2. The “a” protons from the methylene groups of succinic acid were visible at 2.63 ppm. Following the ring opening of maleic anhydride, maleic acid was the main unsaturated acid incorporated into the resins’ backbone, and the protons of the double bond appeared at 6.26 ppm, marked as “d.” Nevertheless, some isomerization from maleic to fumaric acid also took place, as the signal at 6.85 ppm clearly shows (“f” protons, not shown in the inset scheme) [30]. An example of integration of the signals can be found in Figure S2. As the acid-to-diol ratio in the synthesis protocol was 1:1.25, free –OH terminal groups were also visible that were derived from monoesterified diols. Free –OH groups of EG near the succinic acid appeared at 3.77 ppm—the protons marked “b,”—whereas those near the unsaturated acid appeared at 3.82 ppm—the protons marked “c” in Figure 2a. For the resins containing PEG200, a new signal appeared in the spectra. The signal came from protons marked “g” in the inset scheme, near the ether bond of PEG200, and appeared at 3.61 ppm. For the protons of the methyl groups near the ester bonds, the “e1” protons near the unsaturated acid appeared at 4.31 ppm and the “e2” protons near the succinic acid appeared at 4.2 ppm, shown in Figure 2b. Finally, the protons of free –OH groups, derived from monoesterified PEG200, appeared at 3.71 ppm when close to the unsaturated acid and at 3.66 ppm when near succinic acid (protons “b” and “c” in Figure 2b, respectively).
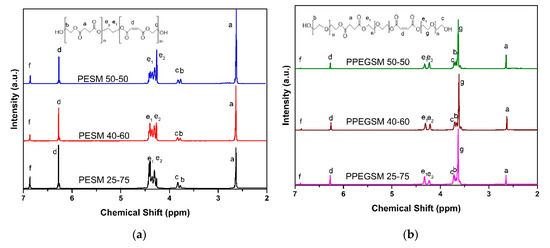
Figure 2.
1H NMR spectra of the synthesized resins (a) with ethylene glycol and (b) with PEG200.
3.2.2. FTIR
FTIR spectra of the synthesized resins are presented in Figure 3. The successful synthesis of the resins was verified by the presence of the ester band at 1720 cm−1, whereas the presence of the unsaturated C=C peak at 1635 cm−1 suggests that any unwanted side reactions were suppressed. The main differences between the two sets of resins concerned the peaks derived from the respective diol, mainly at 2900–2850 cm−1, where Csp3-H groups of the aliphatic chain appeared, and also at 1250–1100 cm−1 (C–C–O stretching). The relative intensities of the FTIR bands of the samples were a result of the ratios of reagents used during synthesis. Finally, as a 0.25 eq excess of diol was used during synthesis, free –OH groups appeared around 3500 cm−1 [31,32,33,34]. In all, FTIR spectra supported the 1H NMR findings, verifying the successful synthesis of the resins.
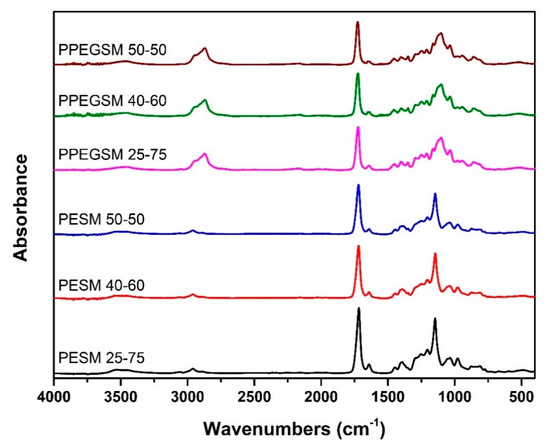
Figure 3.
FTIR collected spectra of the resins before curing.
3.2.3. Molecular Weight Determination by SEC
The molecular weights of synthesized resins were studied by SEC and the collected results are shown in Table 2, whereas the elugrams are given in Supporting Information (Figure S1). As the 1H NMR data suggested, no undesired cross-linking occurred during the synthesis of the resins, as no fraction of high molecular weight was found. The Mn of the resins was in the range of 1600 to 3600 g/mol and it increased with increasing MA content, regardless of the respective diol. It was also clear that the resins containing PEG200 increased in molecular weight compared to their EG counterparts as a direct result of the longer alkylene chain of the former diol. Dispersity (Đ) was found above 2, which is typical for products of polycondensation reactions, where the molecular weights are obtained in Gaussian distribution [10].

Table 2.
Size-exclusion chromatography (SEC) results for the produced resins.
3.2.4. Viscosity Measurements
The viscosity of unsaturated polyester resins is a key parameter that determines the possible applications of these materials. It is a function of the resins’ molecular weight [35], functional groups such as terminal –OH groups, or intermolecular –H bonds [11], and can be modified with the addition of reactive diluents [36,37]. In our work, the viscosity of the obtained resins was measured at five different temperatures, applying a constant shear rate of 100 s−1. The results are shown in Figure 4. For each group of resins, containing PEG200 and EG, respectively, viscosity increased with an increasing amount of MA incorporated in the polyester backbone. This was a direct consequence of the increased molecular weight of the materials, in accordance with the SEC measurements. In all, the resins exhibited high viscosities at ambient temperatures, drastically limiting their potential applications. Therefore, the use of reactive diluents is mandatory to facilitate processing or to increase the applied temperature to above 40 °C.
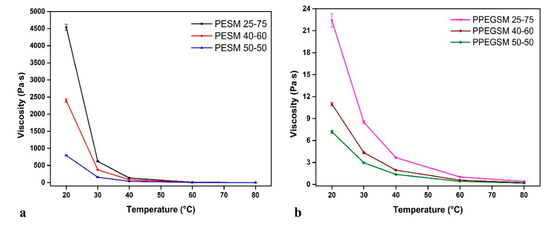
Figure 4.
Viscosity of the synthesized resins (a) containing EG, (b) containing PEG200 as a function of temperature.
3.3. Synthesis and Characterization of Cross-Linked Resins
3.3.1. DSC Results
From the above studies it was proven that UPR resins were successfully prepared and should be further cross-linked using AA in the presence of different initiators and accelerators. In order to better understand the curing procedure, DSC investigation was implemented. Typically, the two most frequently chosen initiators for thermal-induced cross-linking are BPO and MEKP, with their main difference being the temperature of homolytic dissociation [38,39]. For the measurements, UPRs were mixed with AA at a 2:1 ratio, as well as the respective initiator, along with 1% Co(II) octoate, which served as the accelerator [40]. In Figure 5, the influence of the accelerator and both the two initiators is presented, regarding the thermal curing evaluation of the UPRs by means of DSC measurements, using the PESM 50-50 resin as representative. The strong influence of the initiator type was noticed in the curing process, as is shown by the two thermographs of Figure 5 with the indication “Initiator only.” The cross-linking of the resins presented two exothermic peaks with maxima at 110.9 and 126.5 °C when using BPO, and at 98.8 and 157.3 °C when using MEKP as the initiator. These two exothermic peaks show that the cross-linking is a two-stage reaction, as also reported for the cross-linking of UPRs with the use of accelerator and initiator in previous studies [41,42,43]. These two stages are attributed either to redox decomposition of the initiator initially, followed by its thermal decomposition [41,44], or to the radical copolymerization of AA with the resin followed by the radical homopolymerization of AA [45,46]. Nevertheless, the attribution of these two peaks is a matter still under consideration [40]. After the addition of the accelerator, the two stages of cross-linking using BPO (Figure 5a) were shifted to 103.7 and 121.4 °C, whereas in the case of MEKP (Figure 5b) the two stages were difficult to separate, as the first stage at 90.5 °C was the dominant one.
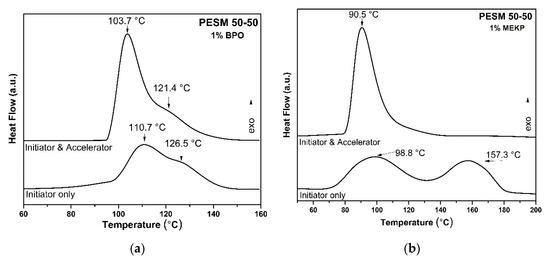
Figure 5.
Thermographs of the homolytic dissociation of the two initiators (a) BPO and (b) MEKP.
MEKP and BPO are among the most commonly used peroxides for the cross-linking of monomers in the wood industry [47], whereas it has been reported that polyester resins present better thermal stability when using MEKP as the initiator, instead of BPO [48,49]. On the other hand, although BPO has been reported as more efficient than MEKP for the polymerization of the UPR of PEG-MA with styrene [50], the UPRs of the present study presented better cross-linking behavior with MEKP. Regarding the use of accelerator, the addition of cobalt(II) octoate enhances the curing, adding better control of the reaction as it provides a higher cross-linking rate at lower temperatures and also shortens the duration of the reaction. On the other hand, the use of MEKP as the initiator provides the cross-linking of the resin at more than 10 °C lower than BPO, and the whole phenomenon is also completed faster. For the abovementioned reasons, MEKP was exclusively used as the initiator for the cross-linking of the experimental resins, with the additional use of cobalt(II) octoate as the accelerator.
The influence of the amounts of both the accelerator and the initiator were examined. In Figure 6, the curing of PPEGSM 25-75 is presented, with the use of 3 wt% Co(II) octoate. Once again, the cross-linking was described as a two-stage reaction. The radical copolymerization of AA with the UPR was initiated at 61, 55, and 48 °C for the three different amounts of MEKP, with exothermic peak maxima at 80, 68, and 66 °C, respectively, showing that the temperature dependence of the reaction with the initiator stabilized after the use of 3 wt% of MEKP.
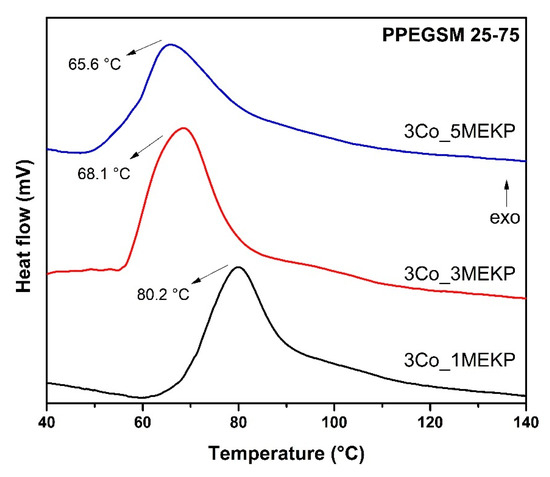
Figure 6.
DSC thermographs in dynamic conditions of the influence of the amount of accelerator in the cross-linking of PPEGSM 25-75.
The cross-linking process is the most crucial stage of the UPRs manufacturing, since during that procedure, a three-dimensional network structure is created, urging the resin to be transformed into a solid. The curing dependence of the experimental resins on the curing temperature and the accelerator amount influence were studied by means of isothermal DSC measurements for low temperatures. In Table 3, the isothermal curing study for the experimental UPRs is presented for representative samples. It is clear that by increasing the reaction temperature and the amount of initiator, the two-stage reaction of the cross-linking was completed in less time.

Table 3.
Times for the completion of curing during isothermal conditions under different temperatures for representative experimental resins.
3.3.2. Spectroscopies Studies
In Figure 7, the FTIR spectra of the cured samples are presented. When compared with Figure 3, alternations of the signals in the areas of 3600–3500 cm−1 and 1635 cm−1 can be seen, suggesting differences in the structure of the materials after the incorporation of acrylic acid. These are direct indications supporting the proposed chemical structure for the cross-linked resins, as depicted in Figure 8. Nevertheless, a more detailed analysis is given in the following XPS section.
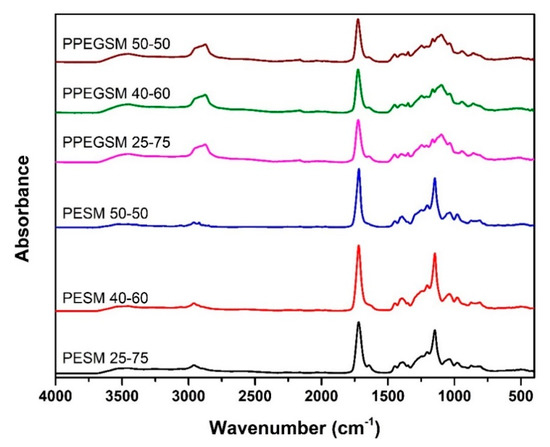
Figure 7.
FTIR collected spectra of UPR resins after curing reactions with AA.
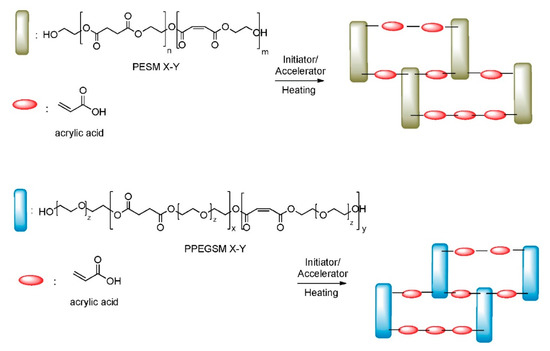
Figure 8.
Chemical structure of the cross-linked materials, after the procedure of curing UPRs with AA.
In Figure 9, the high-resolution XPS spectra of the C–1s and O–1s orbitals are presented for two representative resins of the PESM and PPEGSM groups after thermal cross-linking. Regarding the C–1s orbital of PESM 25-75 (Figure 9a), the applied curve-fitting resulted in different carbonyl components. In particular, the peak at 284.6 eV was attributed to aliphatic C–C and C–H bonds, together with adventitious carbon attributed to pollution [51,52,53]. The peaks at 286.5 and 289.0 eV were attributed to C–O and O–C=O of carbonyl and ester, respectively [54,55,56]. The peak at 287.5 eV was assigned to the stretching vibration of C=O bonds of maleic acid [57]. Finally, the peak at 285.5 eV was assigned to the vibration C–C of carbon chain branching, which supports that the cross-linking was successful, according to similar results obtained for poly(acrylic acid) by Louette et al. [58]. In the same direction, the curve-fitting of the C–1s spectrum of PPEGSM 25–75 (Figure 9c) presented similar results, differentiating only in the enhanced participation of the peak at 286.3 eV of C–O bonds because of the PEG incorporation [57]. In both cases, the two peaks at 285.5 and 287.5 eV of the carbon chain branching attributed to the incorporation of AA and the C=O bond of MA, respectively, prove that the resins were successfully cross-linked after the addition of AA [53,55,57,58,59].
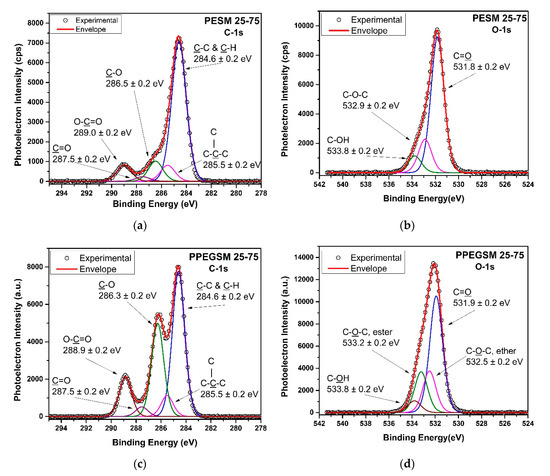
Figure 9.
XPS high-resolution spectra from C–1s and O–1s orbitals of the cured resins PESM 25-75 UPR/AA 1/0.5 (a,b) and PPEGSM 25-75 UPR/AA 1/0.5 (c,d).
In the case of the O–1s spectra of both the cross-linked experimental resins, their curve-fitting was performed conservatively, as there were expected participations of the same bonds attributed to different monomers. In this direction, the curve-fitting of the O–1s high-resolution core level spectrum of PESM 25–75 (Figure 9b) resulted in three separate peaks: at 531.8 eV of ester and carboxylic C=O bonds, at 532.9 of ester C–O–C bonds, and finally, at 533.8 eV, attributed to carboxylic C–OH bonds [53,55,58,59]. Similarly for the PPEGSM 25–75 (Figure 9d), the same attributions applied for the peaks at 531.9, 533.2, and 533.8 eV, with an additional peak at 532.5 eV because of the additional ether C–O–C bonds of PEG [53,58].
3.3.3. Thermal Stability of Cross-Linked Resins
The thermal degradation of the experimental resins after their cross-linking was studied by means of thermogravimetry. In Figure 10, the weight loss and dTG curves of the PESM and PPEGSM groups is presented, for a representative content of 50% in AA. The thermal decomposition of the cross-linked materials occurred in two distinct temperature stages for both groups of experimental resins. The first thermal decomposition of the experimental resins, with a maximum decomposition rate at ~320 °C and 280 °C for the PESM and the PPEGSM groups, respectively, was attributed to AA oligomers, which decomposed first. These AA oligomers were attributed to remaining AA molecules that did not participate in the cross-linking reaction and were polymerized together. The main degradation step—with a maximum decomposition rate at ~430 °C for both groups of experimental resins—was attributed to the decomposition of the cross-linked polyesters. Temperatures corresponding to key parameters for the evaluation of the TGA measurements are presented in Table 4.
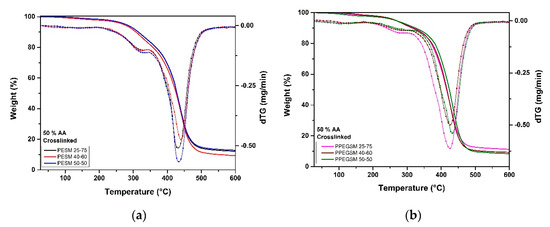
Figure 10.
Thermogravimetric curves (weight loss and dTG) of the cross-linked PESM (a) and PPEGSM (b) resins.

Table 4.
Characteristic temperatures for the thermal decomposition of the materials.
4. Conclusions
In this work, we reported the successful synthesis of unsaturated polyester resins with high biobased content, based on monomers from renewable resources such as ethylene glycol and succinic acid. The structure of the materials was verified by the means of 1H-NMR and FTIR spectroscopies. SEC measurements revealed that the molecular weight of the resins was between 1600 and 3600 g/mol, which is adequate for applications in the adhesives or coatings industry. Rheological examination of the resins showed increased viscosity at ambient temperatures, which drastically decreased at temperatures higher than 40 °C. For this reason, acrylic acid was used as a potential reactive diluent and cross-linked resins were successfully prepared in the presence of initiators and Co(II) octoate as the accelerator. Additionally, between BPO and MEKP for their properties as initiators, the latter presented better control of the cross-linking and in lower temperatures, achieving curing times down to ~4–5 min at a curing temperature of 90 °C. Apart from DSC studies of the curing of the systems, the cross-linked resins were thoroughly studied by means of FTIR and XPS, and the thermal stability of the materials was also examined via TGA. Overall, the resins presented in this study were prepared for the first time, showing potential as replacements of their fossil-based counterparts. It is apparent that the utilization of biobased monomers can lead to valuable materials. In addition, combining the correct monomers can lead to different attributes, as evidenced by the diol selection and the resulting viscosity of the resins. Nevertheless, more fundamental research needs to be conducted in order to establish such biobased products in the coatings and adhesives industry.
Supplementary Materials
The following are available online at https://www.mdpi.com/2076-3417/11/3/896/s1, Figure S1: SEC elugrams of the resins synthesized in this study, Figure S2: Example of 1H NMR integration of the signals.
Author Contributions
L.P.: conceptualization, methodology, investigation, writing—original draft preparation; L.M.: investigation, writing—original draft preparation; D.P., A.M., E.P. (Eleni Psochia), Z.T., and E.P. (Electra Papadopoulou): investigation; K.C., C.M., and D.N.B.: conceptualization, methodology, formal analysis, funding, supervision, writing—review and editing. All authors have read and agreed to the published version of the manuscript.
Funding
This research was co-financed by the European Union and Greek national funds through the Operational Program Competitiveness, Entrepreneurship and Innovation, under the call RESEARCH—CREATE—INNOVATE (project code: T1EDK-01413).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Acknowledgments
L.P. would like to acknowledge Tobias Robert for the performance of the viscosity measurements.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Penczek, P.; Czub, P.; Pielichowski, J. Unsaturated polyester resins: Chemistry and technology. Adv. Polym. Sci. 2005, 184, 1–95. [Google Scholar] [CrossRef]
- Jones, F.R. Unsaturated Polyester Resins. In Brydson’s Plastics Materials, 8th ed.; Elsevier: Amsterdam, The Netherlands, 2017; pp. 743–772. ISBN 9780323358248. [Google Scholar]
- Malik, M.; Choudhary, V.; Varma, I.K. Current Status of Unsaturated Polyester Resins. J. Macromol. Sci. Part C Polym. Rev. 2000, 40, 139–165. [Google Scholar] [CrossRef]
- Li, Q.; Ma, S.; Xu, X.; Zhu, J. Bio-based Unsaturated Polyesters. In Unsaturated Polyester Resins; Elsevier: Amsterdam, The Netherlands, 2019; pp. 515–555. ISBN 9780128161296. [Google Scholar]
- Zhu, Y.; Romain, C.; Williams, C.K. Sustainable polymers from renewable resources. Nature 2016, 540, 354–362. [Google Scholar] [CrossRef] [PubMed]
- Schneiderman, D.K.; Hillmyer, M.A. 50th Anniversary Perspective: There Is a Great Future in Sustainable Polymers. Macromolecules 2017, 50, 3733–3749. [Google Scholar] [CrossRef]
- Werpy, T.; Petersen, G. Top Value Added Chemicals from Biomass: Volume I—Results of Screening for Potential Candidates from Sugars and Synthesis Gas. Off. Sci. Tech. Inf. 2004, 69. [Google Scholar] [CrossRef]
- Schoon, I.; Kluge, M.; Eschig, S.; Robert, T. Catalyst influence on undesired side reactions in the polycondensation of fully bio-based polyester itaconates. Polymers 2017, 9, 693. [Google Scholar] [CrossRef]
- Robert, T.; Eschig, S.; Biemans, T.; Scheifler, F. Bio-based polyester itaconates as binder resins for UV-curing offset printing inks. J. Coatings Technol. Res. 2019, 16, 689–697. [Google Scholar] [CrossRef]
- Papadopoulos, L.; Kluge, M.; Bikiaris, D.N.; Robert, T. Straightforward synthetic protocol to bio-based unsaturated poly(ester amide)s from itaconic acid with thixotropic behavior. Polymers 2020, 12, 980. [Google Scholar] [CrossRef]
- Ouhichi, R.; Pérocheau Arnaud, S.; Bougarech, A.; Abid, S.; Abid, M.; Robert, T. First Example of Unsaturated Poly(Ester Amide)s Derived From Itaconic Acid and Their Application as Bio-Based UV-Curing Polymers. Appl. Sci. 2020, 10, 2163. [Google Scholar] [CrossRef]
- Terzopoulou, Z.; Tsanaktsis, V.; Bikiaris, D.N.; Exarhopoulos, S.; Papageorgiou, D.G.; Papageorgiou, G.Z. Biobased poly(ethylene furanoate-co-ethylene succinate) copolyesters: Solid state structure, melting point depression and biodegradability. RSC Adv. 2016, 6, 84003–84015. [Google Scholar] [CrossRef]
- Wu, L.; Mincheva, R.; Xu, Y.; Raquez, J.; Dubois, P. High Molecular Weight Poly(butylene succinate-co-butylene furandicarboxylate) Copolyesters: From Catalyzed Polycondensation Reaction to Thermomechanical Properties. Biomacromolecules 2012, 13, 2973–2981. [Google Scholar] [CrossRef] [PubMed]
- Kluge, M.; Rennhofer, H.; Lichtenegger, H.C.; Liebner, F.W.; Robert, T. Poly(ester amide)s from poly(alkylene succinate)s and rapid crystallizing amido diols: Synthesis, thermal properties and crystallization behavior. Eur. Polym. J. 2020, 129, 109622. [Google Scholar] [CrossRef]
- Klonos, P.A.; Kluge, M.; Robert, T.; Kyritsis, A.; Bikiaris, D.N. Molecular dynamics, crystallization and hydration study of Poly(Propylene succinate) based Poly(Ester amide)s. Polymer (Guildf.) 2020, 186, 122056. [Google Scholar] [CrossRef]
- Kluge, M.; Bikiaris, D.N.; Robert, T. Enhancing the properties of poly(propylene succinate) by the incorporation of crystallizable symmetrical amido diols. Eur. Polym. J. 2019, 120, 109195. [Google Scholar] [CrossRef]
- Jacquel, N.; Freyermouth, F.; Fenouillot, F.; Rousseau, A.; Pascault, J.P.; Fuertes, P.; Saint-Loup, R. Synthesis and properties of poly(butylene succinate): Efficiency of different transesterification catalysts. J. Polym. Sci. Part A Polym. Chem. 2011, 49, 5301–5312. [Google Scholar] [CrossRef]
- Jiang, M.; Ma, J.; Wu, M.; Liu, R.; Liang, L.; Xin, F.; Zhang, W.; Jia, H.; Dong, W. Progress of succinic acid production from renewable resources: Metabolic and fermentative strategies. Bioresour. Technol. 2017, 245, 1710–1717. [Google Scholar] [CrossRef] [PubMed]
- Jasinska-Walc, L.; Koning, C.E. Unsaturated, Biobased Polyesters and Their Cross-Linking via Radical Copolymerization. J. Polym. Sci. 2010. [Google Scholar] [CrossRef]
- Mehta, L.B.; Wadgaonkar, K.K.; Jagtap, R.N. Synthesis and characterization of high bio-based content unsaturated polyester resin for wood coating from itaconic acid: Effect of various reactive diluents as an alternative to styrene. J. Dispers. Sci. Technol. 2019, 40, 756–765. [Google Scholar] [CrossRef]
- Waig, S.; De Caro, P.; Pennarun, P.; Vaca-Garcia, C.; Thiebaud-Roux, S. Synthesis and characterization of new polyesters based on renewable resources. Ind. Crop. Prod. 2013, 43, 398–404. [Google Scholar] [CrossRef][Green Version]
- Noordover, B.A.J.; van Staalduinen, V.G.; Duchateau, R.; Koning, C.E.; van Benthem, R.A.T.M.; Mak, M.; Heise, A.; Frissen, A.E.; van Haveren, J. Co- and terpolyesters based on isosorbide and succinic acid for coating applications: Synthesis and characterization. Biomacromolecules 2006, 7, 3406–3416. [Google Scholar] [CrossRef]
- Brännström, S.; Malmström, E.; Johansson, M. Biobased UV-curable coatings based on itaconic acid. J. Coatings Technol. Res. 2017, 14, 851–861. [Google Scholar] [CrossRef]
- Farmer, T.J.; Castle, R.L.; Clark, J.H.; Macquarrie, D.J. Synthesis of unsaturated polyester resins from various bio-derived platform molecules. Int. J. Mol. Sci. 2015, 16, 14912–14932. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Zhang, Y. Oxidative Dehydration of Glycerol to Acrylic Acid over Vanadium-Substituted Cesium Salts of Keggin-Type Heteropolyacids. ACS Catal. 2016, 6, 2785–2791. [Google Scholar] [CrossRef]
- Pestana, C.F.M.; Guerra, A.C.O.; Ferreira, G.B.; Turci, C.C.; Mota, C.J.A. Oxidative dehydration of glycerol to acrylic acid over vanadium-impregnated zeolite beta. J. Braz. Chem. Soc. 2013, 24, 100–105. [Google Scholar] [CrossRef]
- Witsuthammakul, A.; Sooknoi, T. Direct conversion of glycerol to acrylic acid via integrated dehydration–oxidation bed system. Appl. Catal. A Gen. 2012, 413–414, 109–116. [Google Scholar] [CrossRef]
- Deleplanque, J.; Dubois, J.-L.; Devaux, J.-F.; Ueda, W. Production of acrolein and acrylic acid through dehydration and oxydehydration of glycerol with mixed oxide catalysts. Catal. Today 2010, 157, 351–358. [Google Scholar] [CrossRef]
- Fava, R.A. Differential scanning calorimetry of epoxy resins. Polymer (Guildf.) 1968, 9, 137–151. [Google Scholar] [CrossRef]
- Takenouchi, S.; Takasu, A.; Inai, Y.; Hirabayashi, T. Effects of Geometric Structure in Unsaturated Aliphatic Polyesters on Their Biodegradability. Polym. J. 2001, 33, 746–753. [Google Scholar] [CrossRef]
- Matynia, T.; Worzakowska, M.; Tarnawski, W. Synthesis of unsaturated polyesters of increased solubility in styrene. J. Appl. Polym. Sci. 2006, 101, 3143–3150. [Google Scholar] [CrossRef]
- Hernández-Moreno, D.; de la Casa Resino, I.; Soler-Rodríguez, F. Maleic Anhydride. In Encyclopedia of Toxicology, 3rd ed.; Academic Press: Cambridge, MA, USA, 2014; Volume 3, pp. 138–141. [Google Scholar] [CrossRef]
- Lohbeck, K.; Haferkorn, H.; Fuhrmann, W.; Fedtke, N. Maleic and fumaric acids. In Ullmann’s Encyclopedia of Industrial Chemistry; Wiley: Hoboken, NJ, USA, 2000. [Google Scholar]
- Worzakowska, M. Chemical modification of unsaturated polyesters influence of polyester’s structure on thermal and viscoelastic properties of low styrene content copolymers. J. Appl. Polym. Sci. 2009, 114, 720–731. [Google Scholar] [CrossRef]
- Pérocheau Arnaud, S.; Hashemi, P.; Mischnick, P.; Robert, T. Optimized synthesis of highly reactive UV-curable hyperbranched polyester acrylates. J. Coatings Technol. Res. 2019. [Google Scholar] [CrossRef]
- Cousinet, S.; Ghadban, A.; Fleury, E.; Lortie, F.; Pascault, J.P.; Portinha, D. Toward replacement of styrene by bio-based methacrylates in unsaturated polyester resins. Eur. Polym. J. 2015, 67, 539–550. [Google Scholar] [CrossRef]
- Lima, M.S.; Costa, C.S.M.F.; Coelho, J.F.J.; Fonseca, A.C.; Serra, A.C. A simple strategy toward the substitution of styrene by sobrerol-based monomers in unsaturated polyester resins. Green Chem. 2018, 20, 4880–4890. [Google Scholar] [CrossRef]
- Lee, M.-H.; Chen, J.-R.; Das, M.; Hsieh, T.-F.; Shu, C.-M. Thermokinetic parameter evaluation by DSC and TAM III along with accountability of mass loss by TG from the thermal decomposition analyses of benzoyl peroxide. J. Therm. Anal. Calorim. 2015, 122, 1143–1150. [Google Scholar] [CrossRef]
- Tseng, J.-M.; Chang, Y.-Y.; Su, T.-S.; Shu, C.-M. Study of thermal decomposition of methyl ethyl ketone peroxide using DSC and simulation. J. Hazard. Mater. 2007, 142, 765–770. [Google Scholar] [CrossRef]
- Martin, J.L. Kinetic analysis of two DSC peaks in the curing of an unsaturated polyester resin catalyzed with methylethylketone peroxide and cobalt octoate. Polym. Eng. Sci. 2007, 47, 62–70. [Google Scholar] [CrossRef]
- Martín, J.L. Kinetic analysis of an asymmetrical DSC peak in the curing of an unsaturated polyester resin catalysed with MEKP and cobalt octoate. Polymer (Guildf.) 1999, 40, 3451–3462. [Google Scholar] [CrossRef]
- Dupuy, J.; Adami, J.; Maazouz, A.; Sobotka, V.; Delaunay, D. Kinetic modeling of an unsaturated polyester resin using two complementary techniques: Near infrared spectroscopy and heat flux sensors. Polym. Eng. Sci. 2005, 45, 846–856. [Google Scholar] [CrossRef]
- Hong, C.M.; Wang, X.J.; Kong, P.; Pan, Z.G.; Wang, X.Y. Effect of succinic acid on the shrinkage of unsaturated polyester resin. J. Appl. Polym. Sci. 2015, 132, 1–9. [Google Scholar] [CrossRef]
- Salla, J.M.; Ramis, X. A kinetic study of the effect of three catalytic systems on the curing of an unsaturated polyester resin. J. Appl. Polym. Sci. 1994, 51, 453–462. [Google Scholar] [CrossRef]
- Avella, M.; Martuscelli, E.; Mazzola, M. Kinetic study of the cure reaction of unsaturated polyester resins. J. Therm. Anal. 1985, 30, 1359–1366. [Google Scholar] [CrossRef]
- Ganglani, M.; Carr, S.H.; Torkelson, J.M. Influence of cure via network structure on mechanical properties of a free-radical polymerizing thermoset. Polymer (Guildf.) 2002, 43, 2747–2760. [Google Scholar] [CrossRef]
- Li, Y. Wood-Polymer Composites. In Advances in Composite Materials: Analysis of Natural and Man-Made Materials; Tesinova, P., Ed.; InTech: Rijeka, Croatia, 2011; p. 285. ISBN 978-953-307-449-8. [Google Scholar]
- Bansal, R.K.; Mittal, J.; Singh, P. Thermal stability and degradation studies of polyester resins. J. Appl. Polym. Sci. 1989, 37, 1901–1908. [Google Scholar] [CrossRef]
- Penczek, P.; Czub, P.; Pielichowski, J. Unsaturated polyester resins: Chemistry and technology. In Crosslinking in Materials Science; Advances in Polymer Science; Springe: Berlin/Heidelberg, Germany, 2005; Volume 184, pp. 1–95. [Google Scholar]
- Rodriguez, E.L. The effect of free radical initiators and fillers on the cure of unsaturated polyester resins. Polym. Eng. Sci. 1991, 31, 1022–1028. [Google Scholar] [CrossRef]
- Rouxhet, P.G.; Misselyn-Bauduin, A.M.; Ahimou, F.; Genet, M.J.; Adriaensen, Y.; Desille, T.; Bodson, P.; Deroanne, C. XPS analysis of food products: Toward chemical functions and molecular compounds. Surf. Interface Anal. 2008, 40, 718–724. [Google Scholar] [CrossRef]
- Biesinger, M. X-ray Photoelectron Spectroscopy (XPS) Reference Pages. Available online: http://www.xpsfitting.com (accessed on 28 February 2020).
- Beamson, G.; Briggs, D. High Resolution XPS of Organic Polymers: The Scienta ESCA300 Database; Wiley: New York, NY, USA, 1992; ISBN 0471935921. [Google Scholar]
- Louette, P.; Bodino, F.; Pireaux, J.-J. Poly(ethylene succinate) (PESU) XPS Reference Core Level and Energy Loss Spectra. Surf. Sci. Spectra 2005, 12, 144–148. [Google Scholar] [CrossRef]
- Patel, D.I.; O’Tani, J.; Bahr, S.; Dietrich, P.; Meyer, M.; Thißen, A.; Linford, M.R. Ethylene glycol, by near-ambient pressure XPS. Surf. Sci. Spectra 2019, 26, 024007. [Google Scholar] [CrossRef]
- Nerantzaki, M.; Filippousi, M.; Van Tendeloo, G.; Terzopoulou, Z.; Bikiaris, D.; Goudouri, O.M.; Detsch, R.; Grüenewald, A.; Boccaccini, A.R. Novel poly(butylene succinate) nanocomposites containing strontium hydroxyapatite nanorods with enhanced osteoconductivity for tissue engineering applications. Express Polym. Lett. 2015, 9, 773–789. [Google Scholar] [CrossRef]
- Fu, B.; Yuan, J.; Qian, W.; Shen, Q.; Sun, X.; Hannig, M. Evidence of chemisorption of maleic acid to enamel and hydroxyapatite. Eur. J. Oral Sci. 2004, 112, 362–367. [Google Scholar] [CrossRef]
- Louette, P.; Bodino, F.; Pireaux, J.-J. Poly(acrylic acid) (PAA) XPS Reference Core Level and Energy Loss Spectra. Surf. Sci. Spectra 2005, 12, 22–26. [Google Scholar] [CrossRef]
- Ferreira, J.M.; Trindade, G.F.; Tshulu, R.; Watts, J.F.; Baker, M.A. Dicarboxylic acids analysed by x-ray photoelectron spectroscopy, Part II—Butanedioic acid anhydrous. Surf. Sci. Spectra 2017, 24, 011102. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).