Abstract
The purpose of this study is to establish the minimal injection doses of magnetic resonance imaging (MRI) contrast agents that can achieve optimized images while improving the safety of injectable MRI drugs. Gadolinium-diethylenetriamine penta-acetic acid (Gd-DTPA) and ferucarbotran, commonly used in clinical practice, were selected and evaluated with in vitro and in vivo experiments. MRI was acquired using T1-weighted (T1W) and T2-weighted (T2W) sequences, and the results were quantitatively analyzed. For in vitro experiments, results showed that T1W and T2W images were optimal when Gd-DTPA-bisamide (2-oxoethyl) (Gd-DTPA-BMEA) and ferucarbotran were diluted to a volume percentage of 0.6% and 0.05%; all comparisons were significant differences in grayscale statistics using one-way analysis of variance (ANOVA). For in vivo experiments, the contrast agent with optimal concentration percentages determined from in vitro experiments were injected into mice with an injection volume of 100 μL, and the images of brain, heart, liver, and mesentery before and after injection were compared. The statistical results showed that the p values of both T1W and T2W were less than 0.001, which were statistically significant. Under safety considerations for MRI contrast agent injection, optimized MRI images could still be obtained after reducing the injection concentration, which can provide a reference for the safety concentrations of MRI contrast agent injection in the future.
1. Introduction
Magnetic resonance imaging (MRI) was developed in 1973 for application to medical diagnosis; it since has become an indispensable technology [1,2,3,4,5,6,7]. According to the basis of imaging, the magnetic momentum generated by the spinning of hydrogen protons in organisms, when placed in an applied external static magnetic field, causes them to move longitudinally towards the direction of the static magnetic field and be arranged in a consistent direction with the magnetic field. At this time, when a radio frequency (RF) with appropriate energy is applied, hydrogen protons will absorb energy from RF pulse, and those that originally moved in the longitudinal direction will reduce the longitudinal moving magnetic momentum value in the direction of the static magnetic field and increase the 90-degree transverse moving magnetic momentum value. When the RF pulse stops, the hydrogen protons will release energy, and the magnetic moment direction will gradually return to the original. The transverse moving magnetic momentum value of the hydrogen protons will also gradually decrease. This action is called transverse or spin-spin relaxation time. The magnetic moment of protons will slowly return to the static magnetic field or longitudinal motion direction in the balanced state. This action is called longitudinal or spin lattice relaxation time. After the released energy is received by the receiver coil, and the signals are converted by the terminal, they will present different grayscale images [8]. Through the relaxation time, T1-weighted (T1W) and T2-weighted (T2W) pulse sequences enhance the difference in proton density between tissues, which is the most important physical basis for MRI in clinical diagnosis [9]. When hydrogen protons are placed in an applied external magnetic field, T1 relaxation time is defined as the time required for 63% of the longitudinal hydrogen protons to return to the direction of the static magnetic field. T2 relaxation time is defined as the time required for the transverse static magnetic moment to decay by 63%, i.e., the time required for the transverse magnetic moment to decay to 37% of the original value, which is also called transversal or spin-spin relaxation time [10].
MRI uses different important parameters in imaging. Taking water as an example, it is characterized by long T1 and long T2. Long T1 means that most of the longitudinal magnetization cannot be recovered during the interval of TR (repetition time), so weak magnetic resonance signals are generated, and the image is dark; long T2 means that after the radio frequency pulse is applied, a relatively long echo time (TE) is generated, so relatively strong MR signals are received and thus the image is bright. These parameters form an important basis for magnetic resonance imaging [11].
In order to strengthen the diagnosis of tissue recognition, by virtue of the development of magnetic nanoparticles, the application fields of MRI technology have also extended to the field of biomedical materials, from animals and human bodies at the cellular and molecular levels [12]. Magnetic resonance contrast agents are used to enhance the imaging recognition of soft tissues and body structures in medical images; contrast agents are also divided into two structures with different attributes, i.e., paramagnetism and superparamagnetism [13,14]. Through the paramagnetic substance of unpaired electrons in the contrast agent in tissues, the relaxation time value of spin-lattice T1 or T2 is reduced to accelerate the changes in signal intensity generated through the proton relaxation of water molecules in tissues in the magnetic environment, thereby presenting different signal intensities generated among tissues and organs and improving the sensitivity of contrast presentation accordingly [15]. Gadolinium-based paramagnetic contrast agent is a gadolinium-diethylenetriamine penta-acetic acid. Gd3+ has a large magnetic moment. When the contrast agent is injected into normal animals, it is distributed in the space of blood vessels and extracellular fluid, but cannot penetrate the intact blood-brain barrier (BBB). It requires metabolism through normal glomerular filtration to be discharged from the body [16,17]. According to literature, paramagnetic contrast agent still has potential toxicity in animals; after being injected with the dose required for clinical imaging (0.2 mL/kg), it increases the risk of nephrogenic systemic fibrosis (NSF) in patients with abnormal glomerular filtration, and even increases the degree of renal injury [18].
Ferucarbotran is a superparamagnetic iron oxide magnetic nanoparticle contrast agent [19]. Although it is a relatively safe drug, it still has the possibility of causing allergic reactions after being injected at the dose required for clinical imaging (0.08 mL/kg) [20].
Based on the risk of side effects caused by contrast agents and the concern about the quality of imaging after injection, we wonder whether it is possible to guarantee the quality of imaging while reducing the risk of drug side effects under the condition of injecting a smaller amount of contrast agent, in order to improve the medication safety. This study is designed with two types of clinically used contrast agents with different properties as the experimental materials, namely OptiMARK (a paramagnetic contrast agent of Gd-DTPA-bisamide (2-oxoethyl) (Gd-DTPA-BMEA)) and Resovist (a superparamagnetic contrast agent of ferucarbotran). Based on the characteristics of different responses of T1W and T2W pulse sequences on images, in vitro experimental contrast imaging with different drug dilution ratios was carried out, and the images were visually counted [21] in order to quantitatively analyze the optimal dilution ratio as well as the contrast intensity of image signals [22]. In vivo experiments were carried out with the optimal dilution ratio analyzed through the in vitro experiment, and the presented images were subjected to quantitative statistical analysis by visual counting, thereby comparing the differences before and after the injections of contrast agents. Quantification of the optimal injection doses of the contrast agents and their contrast intensities of image signals was also carried out. The research results were compared with the FDA report to establish a reference basis for the medication safety of MRI contrast agent injection in experimental animals and clinical application [23].
2. Materials and Methods
2.1. Contrast Agents
Two types of contrast agents with different properties were used to compare the differences in MRI images in this experiment.
The first was paramagnetic contrast agent Gadolinium-DTPA OptiMARK (OptiMARK is a paramagnetic contrast agent of Gd-DTPA-BMEA) (Gd-DTPA bisamide (2-oxoethyl)). In December 1999, this agent was approved by the Food and Drug Administration (FDA) of the United States for clinical MRI contrast examination. It is a gadolinium-based MRI contrast agent at 0.5 mmol/mL. It can form a large magnetic moment when it acts in a magnetic field, which can enhance the relaxation rate of protons in adjacent water and thus increase the signal intensity of tissues.
The second was superparamagnetic contrast agent ferucarbotran (superparamagnetic iron oxide, SPIO) Resovist (Resovist is a superparamagnetic iron oxide contrast agent of ferucarbotran) (SH U 555 A; Schering, Berlin, Germany). In 1999, it was approved by the Food and Drug Administration (FDA) of the United States for clinical MRI contrast examination. It is presented as 28 mg of iron oxide particles, 1.4 mL/syringe. The uneven magnetic field generated by the contrast agent enhances the transverse relaxation rate (relaxivity, r2), showing the darkness of the radiation field and highlighting the presence of the radiation target.
2.2. Dilution of Contrast Agents
The dilution dose of the contrast agent in this experiment was extremely small. In order to prepare and fix the correct dose and image signal purity and avoid the interference by multiple chemical shift signals generated by the experimental reagent, D.D. water (pure secondary water), which was filtered and impurity-free, was used as the solvent for dilution, and micro straws with different capacities (Gilson Wealtec Accupet 100–1000 μL, Autoclavable pipette, Japan; Michiryo Nichipet EX 20–200 μL, Autoclavable pipette, Japan; Gilson Pipetman 2–20 μL, Micro pipette, Japan) were used for quantitative configuration of contrast agents with different dilution ratios for experiments.
2.3. Deployment
In this experiment, the contrast agents were prepared with 1 mL total solution volume and a 24 well dish (Falcon tissue culture plate, Becton Dickinson Labware, USA) at different dilution ratios. The paramagnetic contrast agent bound to hydrogen protons in water and the relaxation of protons led to the changes in signal intensity, which showed the changes of Gd-DTPA-BMEA in the MRI images through different dilution ratios. In the same way, the superparamagnetic contrast agent interacted with hydrogen protons in water, showing the changes of ferucarbotran in MRI images through different dilution ratios.
2.4. MRI Instrument
The instrument used in this study was an open type permanent MRI scanner Hitachi AIRIS II Open type 0.3 T (Hitachi Medical Corporation, Tokyo, Japan) in the MRI room of Yuanpei University of Medical Technology Image Center established by Yanpei University of Medical Technology in Hsinchu, Taiwan, on 17 May 2017. The instrument had a vertical magnetic field with 0.35 Tesla permanent magnet. The version of the operating system was AIRIS2-1 Fo-dicom 4.0.0 (Airias, Hitachi-Medico, Japan). Standard and clear MRI images could be provided for different experimental requirements.
2.5. Pulse Sequence
The 24 well dishes with contrast agents prepared at different dilution ratios were put into the receive frequency coil isocenter, and the experimental contrast examination was carried out on the Hitachi AIRIS II Open type MRI system with a magnetic field strength of 0.35 T. In vitro and in vivo (IRB: 106–008) experiments were conducted with consistent T1W and T2W pulse sequences, and the experimental variance factors were reduced accordingly (Field of view (FOV) = 200 mm × 200 mm, slice thickness = 2.0 mm, Matrix = 512, etc.) These parameters were all set consistently according to the different properties of the contrast agent. Parameter setting of the T1W pulse sequence was mainly adopted for the paramagnetic contrast agent, with TE = 23.3 ms and TR = 1000 ms; parameter setting of the T2W pulse sequence was mainly adopted for the superparamagnetic contrast agent, with TE = 100 ms and TR = 4000 ms. By setting consistent parameters and changing attribute parameters, the contrast images were different, and quantitative statistical analysis of images was carried out accordingly.
2.6. In Vivo Experiment (IRB: 106-008)
The two types of MRI contrast agents with different properties, according to the optimal dilution ratios determined in vitro, were prepared into diluted dosage forms with a total volume of 100 μL per dose, and these doses were injected into the mice. The T1W and T2W image visualization statistics of brain, cardiac, liver, and mesentery before and after injections of contrast agents were compared, and the differences among different organs in vivo before and after injections of contrast agents were validated accordingly.
2.7. Statistical Analysis
Medical imaging, all conducted according to Digital Imaging and Communications in Medicine (DICOM), presented multi-level grayscale images in a photometric interpretation of monochrome mode. Hydrogen proton signals in the material were presented in MRI contrast images, and the strength of image signals indicated the number of hydrogen protons in the material. The image presentation was the recognition and comparison of visual differences. After statistically analyzing the grayscale values in each pixel (picture element) to be measured in the images with matrix = 512 × 512 by visual quantitation, one-way ANOVA and linear regression statistics were carried out with Sigmaplot 12.5, which converted the visual recognition of differences into numbers for comparison.
3. Results
3.1. In Vitro Experiment Results
After using D.D. water for in vitro T1W and T2W pulse sequence imaging, visualized grayscale numerical statistics were conducted to verify the feasibility of applying T1W and T2W pulse sequences in the scheme, as shown in Figure 1. Figure 1A shows that with T1W and T2W pulse sequence D.D. water sample imaging, it could be clearly distinguished from the MRI DICOM grayscale image that T1W presented low signal images (dark) and T2W presented high signal images (bright). With the T1W pulse sequence setting, during the interval of the repetition time (TR), most of the longitudinal magnetization intensity could not be recovered, and the MR received signal was weak, showing dark images. In the MRI image on the left of Figure 1A, the richer the hydrogen atom signals, the darker the grayscale display of MRI images. Correspondingly, the grayscale values of the images were lower, as shown in the color gradation diagram on the left side of Figure 1B and the numerical visual diagram on the left side of Figure 1C. With the T2W pulse sequence setting, a relatively long receiving TE was generated, the MR received signals were relatively strong, and bright images were presented, as shown in the MRI images on the right side of Figure 1A. The richer the hydrogen atom signals, the brighter the grayscale display of the MRI images. Correspondingly, the grayscale values of the images were also higher, as shown in the color gradation diagram on the right side of Figure 1B and the numerical visual diagram on the right side of Figure 1C.
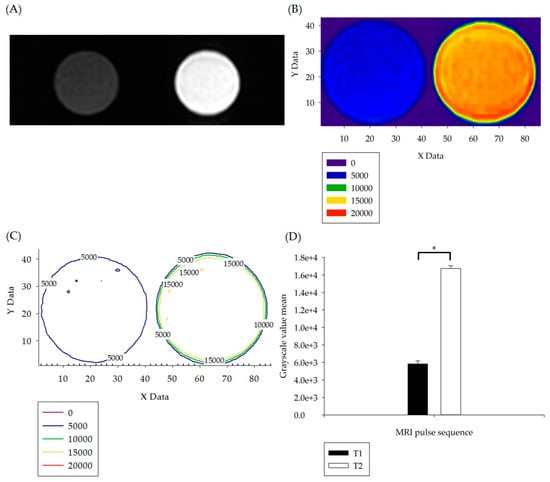
Figure 1.
(A) T1W (dark) and T2W (bright) pulse sequence imaging was carried out with D.D. water as the sample. (B) The images in Figure 1A were subjected to grayscale image visualization color gradation, and the lower left corner of Figure 1B is the grayscale value color distribution table of each image. (C) The layered numerical information in Figure 1B was converted into a graphic numerical visual diagram. (D) Statistical analysis and comparison were carried out on the numerical information in Figure 1C. The p values less than 0.05 are summarized with one asterisk.
Grayscale value visual matrix image color layer analysis was carried out on the DICOM grayscale images, and statistical analysis was performed by one-way ANOVA. The results are shown in Figure 1D, with p < 0.05 (p = 0.022), indicating that there was statistically a significant difference between T1W and T2W, which also verified the consistency between the qualitative comparison of MRI images and quantitative analysis of grayscale values.
The analysis of in vitro experiments is shown in Figure 2. Figure 2A and Figure 2C are the T1W and T2W images of paramagnetic contrast agent Gd-DTPA-BMEA and super-paramagnetic contrast agent ferucarbotran, respectively. The tables in the lower parts of Figure 2A,C show the contrast agents in different dilutions, sorted from large (upper left well) to small (lower right well). In Figure 2A, the percentages range from 2.3% to 0%, with a decreasing step of 0.1%, and the dilution percentages of Gd-DTPA-BMEA were optimized by investigating the highest signal among the 24 well dishes. The highest signal represents that the optimized concentration of Gd solutions attained the maximum increase of the relaxation rates of nearby water protons. In Figure 2C, the non-uniform magnetic field generated by iron oxide particles caused a marked decline (dark) in MRI signal intensity and an increase in the T2 relaxation rate. Different dilution ratios were deployed in a staggered 24 well dish in a multiple-decreasing manner, with the volume percentage concentration decreasing from 1% to 0.00025% over a total of 12 images. As the dilution volume percentage increased, the effect of iron oxide particles on the field of low-signal was weakened. The low-signal image (darkness) also gradually turned into a high-signal image (brightness). Figure 2B,C convert the grayscale images in Figure 2A,C into color images for convenient visualization. However, it is difficult to find the best dilution percentage from Figure 2 alone. Thus, we further performed image quantification and determined the optimized conditions using one-way ANOVA.
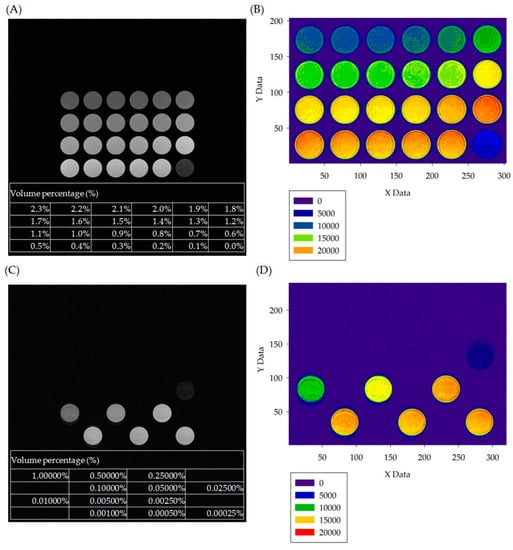
Figure 2.
(A) T1W pulse sequence imaging with samples of paramagnetic contrast agent of Gd-DTPA-BMEA diluted to different volume percentages (%). (B) Color layer images of MRI imaging in the visualized grayscale numerical statistics; the lower left corner of the figure is the grayscale value color distribution table of each figure. (C) T2W pulse sequence imaging with samples of superparamagnetic contrast agent of ferucarbotran diluted to different volume percentages. (D) Color layer images of MR imaging in the visualized grayscale numerical statistics; the lower left corner of the figure is the grayscale value color distribution table of each figure.
The statistical analysis results are shown in Figure 3. Figure 3A,B show the curves of visualized grayscale numerical statistics of paramagnetic contrast agent Gadolinium-DTPA and superparamagnetic contrast agent ferucarbotran samples, respectively, diluted to different volume percentages under T1W and T2W pulse sequence imaging. One-way ANOVA grayscale statistics comparison was carried out between each sample and D.D. water (white dot), and all results showed statistically significant differences (p < 0.001). In the figure, the optimal volume percentage was compared with the corresponding optimal dilution grayscale value (red dot). The quantitative value of the optimal dilution volume percentage in Figure 3A is 0.6%, and the quantitative value of the optimal dilution volume percentage in Figure 3B is 0.05%. These quantitative values provide the basis for the preparation of contrast agents for in vivo injection.
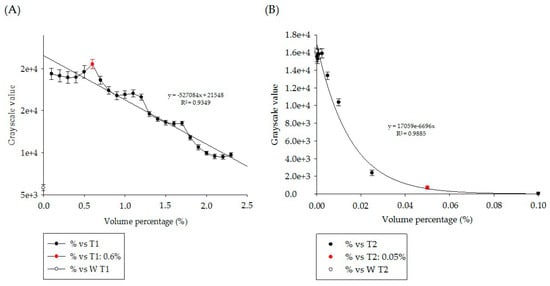
Figure 3.
The curves of visualized grayscale numerical statistics of (A) paramagnetic contrast agent gadolinium-DTPA and (B) superparamagnetic contrast agent ferucarbotran samples diluted to different volume percentages under T1W and T2W pulse sequence imaging. Note that only nine points (from 0.1% to 0.00025%) in (B) were shown in order to clearly display the exponential distribution.
3.2. In Vivo Experiment Results
In this study, the optimal dilution volume percentages of Gd-DTPA-BMEA and ferucarbotran, which are two types of contrast agents with different properties, were quantified in in vitro experiments and were prepared into reagents at doses for injection experiments with a total volume of 100 μL to be used for mice in in vivo experiments (IRB: 106-008). Two different MRI pulse sequences, T1W and T2W, were selected for contrast imaging. The differences in visualized grayscale numerical quantitative statistics of brain, cardiac, liver and mesentery images of mice before and after injection were statistically analyzed. Figure 4A shows the DICOM 3.0 images of mice under T1W pulse sequence, marking the brain, cardiac, liver, and mesentery regions. These regions correspond to the visualized grayscale imaging of the brain (Figure 4B) and the visualized grayscale imaging of the heart, liver and mesentery (Figure 4C). The lower left corners of Figure 4B,C show the color distribution table of grayscale values of each figure.
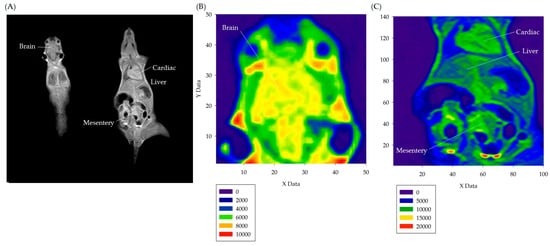
Figure 4.
(A) The DICOM 3.0 image of a mouse under T1W pulse sequence. (B) The visualized grayscale imaging of the mouse brain. (C) The visualized grayscale imaging of the mouse heart, liver, and mesentery.
Figure 5 compares the organs subjected to the visualized grayscale numerical analysis of the mice in the in vivo experiment. The visualized grayscale numerical statistics, before and after the injection of Gd-DTPA-BMEA at a volume percentage of 0.6% and ferucarbotran at a volume percentage of 0.05%, were subjected to imaging statistical analysis with one-way ANOVA. It can be seen from the results in Figure 5 that there were statistically significant differences (***, p < 0.001) in the visualized grayscale numerical statistics of the brain, heart, liver and mesentery before (−C) and after (+C) injection of diluted contrast agents under T1W and T2W pulse sequence imaging.
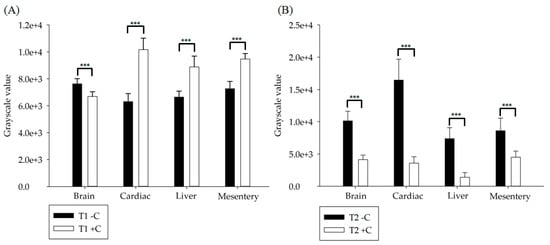
Figure 5.
(A) T1W pulse sequence imaging. (B) T2W pulse sequence imaging. The visualized grayscale numerical statistics of the brain, heart, liver and mesentery before and after injection of diluted paramagnetic contrast agent Gd-DTPA-BMEA and superparamagnetic contrast agent iron oxide were analyzed and compared, and one-way ANOVA grayscale statistical analysis was conducted. All p values less than 0.001 are summarized with three asterisks.
4. Discussion
The results of Figure 3A,B show that there were different curve distributions between dilution volume percentage and image grayscale. The distribution presented the interaction between the contrast agent and the hydrogen nucleus, possibly relating to the chemical exchange saturation transfer [24,25]. To understand the differences clearly, the statistical data of Gd-DTPA-BMEA and ferucarbotran with different attributes were subjected to linear and exponential regression analysis. The volume percentage of Gd-DTPA-BMEA contrast agent with D.D. water decreased from the highest weight percentage of 2.3% to 0% pure water, with 24 dilution points in total. The results of linear regression statistical analysis showed that the coefficient of determination R2 = 0.9349, with 93.49% reliability, and the grayscale of the image was negatively correlated with the dilution concentration of the contrast agent, showing a trend of linear normal probability. There was a total of 12 dilution points in the dilution ratio of iron oxide contrast agent to D.D. water, starting from the highest dilution weight percentage of 0.1% and decreasing progressively to 0.00025%. The results of exponential regression analysis showed that R2 = 0.9885, with a reliability of only 98.9%, and the grayscale of the image showed an exponential distribution relationship with contrast agent dilution concentration. The results suggested that the two types of contrast agents with different properties have completely different presentation characteristics [26,27,28].
OptiMARK is a paramagnetic MRI contrast agent, which belongs to Gd-DTPA-BMEA metal ion chelating agent [29]. Gd3+ has a large magnetic moment, which is distributed in the space of blood vessels and extracellular fluids after injection into animals, but cannot penetrate the intact BBB under normal circumstances. The statistical results in Figure 5A are consistent with the literature [30]. Figure 5A shows that although there are significant differences in grayscale numerical statistics after injection of diluted contrast agent in normal mice (***, p < 0.001), only the brain grayscale value before injection was greater than that after injection, while other values were smaller. This confirmed the result in the literature that Gd-DTPA-BMEA could not penetrate the intact BBB. Resovist is an iron oxide microparticle preparation of ferucarbotran and is a superparamagnetic contrast agent for MRI in organ-specific imaging [31]. Comparison of statistical results in Figure 5B also shows this phenomenon, i.e., the grayscale numerical statistics before injection were all higher than those after injection, especially in the liver. The results verified that the optimal dilution ratios of Gd-DTPA-BMEA and ferucarbotran contrast agents can be quantified by visualized grayscale numerical statistics and applied in in vivo experiments.
Paramagnetic contrast agent Gd-DTPA-BMEA and superparamagnetic contrast agent ferucarbotran are two types of contrast agents with different properties commonly used in clinical practice. Although Gd-DTPA-BMEA and Ferucarbotran contrast agents have different pharmacokinetics (PK) responses, both of them are suitable for liver indications [32]. Comparing grayscale statistics of brain, heart, liver and mesentery before and after the contrast agent injection in Figure 5, the ratios are 1.14, 0.62, 0.75 and 0.77 for Gd-DTPA-BMEA and 2.45, 4.60, 5.32 and 1.91 for iron oxide, respectively. The results indicate that the image contrast of iron oxide is superior to Gd-DTPA-BMEA, and iron oxide is not only suitable for the liver, but also applicable to other organs and tissues [33,34,35,36].
According to the recommendations of the Food and Drug Administration (FDA) of the United States, the clinical injection doses are 0.2 mL/kg for Gd-DTPA-BMEA and 0.08 mL/kg for ferucarbotran, which are 0.2 µL/g and 0.08 µL/g, respectively. If injected into mice with 20 g weights according to the ratios, 4 µL and 1.6 µL should be injected according to the recommended doses for clinical injection. If the total injection dose for mice is 100 μL, the dilution ratios are 4% and 1.6%, respectively. Compared with the experimental dilution ratios of 0.6% and 0.05%, the clinically recommended injection doses are 6.7 times and 32 times the experimental injection doses. This result represents a balance between using a lower injection dose of contrast agent and achieving good diagnostic image quality. According to this result, the injection dose of MRI contrast agent can still be adjusted, and there is still room to reduce the injection dose of MRI contrast agent. It can be seen from the results that the two types of contrast agents with different properties also show different outcomes in the organism characteristics.
5. Conclusions
In the present study, the injection dose of MRI contrast is optimized by analyzing how image values vary with dilution ratios. When Gd-DTPA-BMEA and ferucarbotran were diluted to a volume percentage of 0.6% and 0.05%, respectively, the images could achieve the optimal values. The clinically recommended injection doses are 6.7 times and 32 times of the optimized injection doses. Considering the safety of MRI contrast agent injection, it is suggested that the injection concentration can be reduced. The recommended injection concentration in this study can provide a reference for the safety concentration of MRI contrast agent injection in the future.
Author Contributions
Conceptualization, W.-T.H. and J.-W.T.; methodology, W.-T.H.; validation, W.-T.H. and L.-H.L.; formal analysis, L.-H.L.; writing—original draft preparation, W.-T.H. and L.-H.L.; writing—review and editing, L.-H.L.; supervision and project administration, Y.-H.C. and A.-Y.W. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Ministry of Science and Technology of Taiwan (Project Nos. 109-2622-8-264-001-TB1).
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki and approved by the Ethics Committee of Yuanpei University of Medical Technology (protocol code 106-008 and approved 08/2018).
Informed Consent Statement
Not applicable for studies not involving humans.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.
Acknowledgments
We would like to thank the Ministry of Science and Technology of Taiwan, Hitachi Medical corporation, Promed Instrument Co., Ltd., and En Chu Kong Hospital, New Taipei City for their assistance.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Lauterbur, P.C. Image formation by induced local interactions: Examples employing nuclear magnetic resonance. Nature 1973, 242, 190–191. [Google Scholar] [CrossRef]
- Fox, N.C.; Freeborough, P.A. Brain atrophy progression measured from registered serial MRI: Validation and application to Alzheimer’s disease. J. Magn. Reson. Imaging 1997, 7, 1069–1075. [Google Scholar] [CrossRef] [PubMed]
- Darge, K.; Jaramillo, D.; Siegel, M.J. Whole-body MRI in children: Current status and future applications. Eur. J. Radiol. 2008, 68, 289–298. [Google Scholar] [CrossRef] [PubMed]
- Townsend, K.A.; Wollstein, G.; Schuman, J.S. Clinical application of MRI in ophthalmology. NMR Biomed 2008, 21, 997–1002. [Google Scholar] [CrossRef]
- Atlas, S.W. Magnetic Resonance Imaging of the Brain and Spine, 4th ed.; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2009. [Google Scholar]
- Cadotte, D.W.; Wilson, J.R.; Mikulis, D.; Stroman, P.W.; Brady, S.; Fehlings, M.G. Conventional MRI as a diagnostic and prognostic tool in spinal cord injury: A systemic review of its application to date and an overview on emerging MRI methods. Expert Opin. Med. Diagn. 2011, 5, 121–133. [Google Scholar] [CrossRef] [PubMed]
- Vargas, M.I.; Delattre, B.M.A.; Boto, J.; Gariani, J.; Dhouib, A.; Fitsiori, A.; Dietemann, J.L. Advanced magnetic resonance imaging (MRI) techniques of the spine and spinal cord in children and adults. Insights Imaging 2018, 9, 549–557. [Google Scholar] [CrossRef] [PubMed]
- Hashemi, R.H.; Bradley, W.G.; Lisanti, C.J. MRI: The Basics, 3rd ed.; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2012. [Google Scholar]
- Murray, R.C. Equine MRI, 1st ed.; John Wiley & Sons: West Sussex, UK, 2010. [Google Scholar]
- Brown, M.A.; Semelka, R.C. MRI: Basic Principles and Applications, 4th ed.; John Wiley & Sons: Philadelphia, PA, USA, 2011. [Google Scholar]
- Weishaupt, D.; Köchli, V.D.; Marincek, B. How Does MRI Work? An Introduction to the Physics and Function of Magnetic Resonance Imaging, 2nd ed.; Springer Science & Business Media: Philadelphia, PA, USA, 2008. [Google Scholar]
- Wei, H.; Bruns, O.T.; Kaul, M.G.; Hansen, E.C.; Barch, M.; Wiśniowska, A.; Cordero, J.M. Exceedingly small iron oxide nanoparticles as positive MRI contrast agents. Proc. Natl. Acad. Sci. USA 2017, 114, 2325–2330. [Google Scholar] [CrossRef]
- Strijkers, G.J.; Mulder, W.J.M.; van Tilborg, G.A.F.; Nicolay, K. MRI contrast agents: Current status and future perspectives. Anticancer Agents Med. Chem. 2007, 7, 291–305. [Google Scholar] [CrossRef]
- Wang, Y.X.J. Superparamagnetic iron oxide based MRI contrast agents: Current status of clinical application. Quant. Imaging Med. Surg. 2011, 1, 35–40. [Google Scholar]
- Santra, S.; Jativa, S.D.; Kaittanis, C.; Normand, G.; Grimm, J.; Perez, J.M. Gadolinium-encapsulating iron oxide nanoprobe as activatable NMR/MRI contrast agent. ACS Nano 2012, 6, 7281–7294. [Google Scholar] [CrossRef]
- Serlin, Y.; Shelef, I.; Knyazer, B.; Friedman, A. Anatomy and physiology of the blood–brain barrier. Semin. Cell Dev. Biol. 2015, 38, 2–6. [Google Scholar] [CrossRef] [PubMed]
- Runge, V.M.; Schoerner, W.; Niendorf, H.P.; Laniado, M.; Koehler, D.; Claussen, C.; James, A.E., Jr. Initial clinical evaluation of gadolinium DTPA for contrast-enhanced magnetic resonance imaging. Magn. Reson. Imaging 1985, 3, 27–35. [Google Scholar] [CrossRef]
- Greenberger, P.A.; Patterson, R. The prevention of immediate generalized reactions to radiocontrast media in high-risk patients. J. Allergy Clin. Immunol. 1991, 87, 867–872. [Google Scholar] [CrossRef]
- Reimer, P.; Balzer, T. Ferucarbotran (Resovist): A new clinically approved RES-specific contrast agent for contrast-enhanced MRI of the liver: Properties, clinical development, and applications. Eur. Radiol. 2003, 13, 1266–1276. [Google Scholar] [CrossRef]
- Valdiglesias, V.; Fernández-Bertólez, N.; Kiliç, G.; Costa, C.; Costa, S.; Fraga, S.; Laffon, B. Are iron oxide nanoparticles safe? Current knowledge and future perspectives. J. Trace Elem. Med. Biol. 2016, 38, 53–63. [Google Scholar] [CrossRef]
- Tesileanu, T.; Conte, M.M.; Briguglio, J.J.; Hermundstad, A.M.; Victor, J.D.; Balasubramanian, V. Efficient coding of natural scene statistics predicts discrimination thresholds for grayscale textures. Elife 2020, 9, e54347. [Google Scholar] [CrossRef]
- Misaki, M.; Savitz, J.; Zotev, V.; Phillips, R.; Yuan, H.; Young, K.D.; Bodurka, J. Contrast enhancement by combining T1-and T 2-weighted structural brain MR Images. Magn. Reson. Med. 2015, 74, 1609–1620. [Google Scholar] [CrossRef]
- US Food and Drug Administration. FDA Identifies No Harmful Effects to Date with Brain Retention of Gadolinium-Based Contrast Agents for MRIs; Review to Continue. 2017. Available online: https://www.fda.gov/drugs/fda-drug-safety-podcasts/fda-drug-safety-podcast-fda-identifies-no-harmful-effects-date-brain-retention-gadolinium-based (accessed on 26 January 2021).
- Ali, M.M.; Liu, G.; Shah, T.; Flask, C.A.; Pagel, M.D. Using two chemical exchange saturation transfer magnetic resonance imaging contrast agents for molecular imaging studies. Acc. Chem. Res. 2009, 42, 915–924. [Google Scholar] [CrossRef]
- Chen, Z.; Han, Z.; Liu, G. Repurposing Clinical Agents for Chemical Exchange Saturation Transfer Magnetic Resonance Imaging: Current Status and Future Perspectives. Pharmaceuticals 2021, 14, 11. [Google Scholar] [CrossRef]
- Burton, M.E. Applied Pharmacokinetics & Pharmacodynamics: Principles of Therapeutic Drug Monitoring, 4th ed.; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2006. [Google Scholar]
- Dieckhoff, J.; Kaul, M.G.; Mummert, T.; Jung, C.; Salamon, J.; Adam, G.; Ittrich, H. In vivo liver visualizations with magnetic particle imaging based on the calibration measurement approach. Phys. Med. Biol. 2017, 62, 3470. [Google Scholar] [CrossRef]
- Mørkenborg, J.; Pedersen, M.; Jensen, F.T.; Stødkilde-Jørgensen, H.; Djurhuus, J.C.; Frøkiær, J. Quantitative assessment of Gd-DTPA contrast agent from signal enhancement: An in vitro study. Magn. Reson. Imaging 2003, 21, 637–643. [Google Scholar] [CrossRef]
- Runge, V.M. Safety of approved MR contrast media for intravenous injection. J. Magn. Reson. Imaging JMRI 2000, 12, 205–213. [Google Scholar] [CrossRef]
- Runge, V.M.; Clanton, J.A.; Price, A.C.; Wehr, C.J.; Herzer, W.A.; Partain, C.L.; James, A.E., Jr. The use of Gd DTPA as a perfusion agent and marker of blood-brain barrier disruption. Magn. Reson. Imaging 1985, 3, 43–55. [Google Scholar] [CrossRef]
- Kopp, A.F.; Laniado, M.; Dammann, F.; Stern, W.; Grönewäller, E.; Balzer, T.; Claussen, C.D. MR imaging of the liver with Resovist: Safety, efficacy, and pharmacodynamic properties. Radiology 1997, 204, 749–756. [Google Scholar] [CrossRef]
- Lee, J.S.; Goo, E.H.; Park, C.S.; Lee, S.Y.; Choi, Y.S. A Study on Usefulness of Specific Agents with Liver Disease at MRI Imaging: Comparison with Ferucarbotran and Gd-EOB-DTPA Contrast Agents. Korean J. Med. Phys. 2009, 20, 235–243. [Google Scholar]
- Bulte, J.W. In vivo MRI cell tracking: Clinical studies. Am. J. Roentgenol. 2009, 193, 314–325. [Google Scholar] [CrossRef]
- Cho, E.S.; Yu, J.S.; Kim, M.J.; Kim, J.H.; Chung, J.J.; Kim, K.W. Focal Eosinophilic Necrosis on Superparamagnetic Iron Oxide–Enhanced MRI. Am. J. Roentgenol. 2010, 194, 1296–1302. [Google Scholar] [CrossRef]
- Lee, S.M.; Lee, S.H.; Kang, H.Y.; Baek, S.Y.; Kim, S.M.; Shin, M.J. Assessment of musculoskeletal infection in rats to determine usefulness of SPIO-enhanced MRI. Am. J. Roentgenol. 2007, 189, 542–548. [Google Scholar] [CrossRef]
- Neuwelt, A.; Sidhu, N.; Hu, C.A.A.; Mlady, G.; Eberhardt, S.C.; Sillerud, L.O. Iron-based superparamagnetic nanoparticle contrast agents for MRI of infection and inflammation. Am. J. Roentgenol. 2015, 204, 302–313. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).