Ca-Alginate-PEGMA Hydrogels for In Situ Delivery of TGF-β Neutralizing Antibodies in a Mouse Model of Wound Healing
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of Composite Hydrogels
2.2. HEK-Blue TGF-β SEAP Reporter Assay
2.3. Full-Thickness Excision Wound Model
2.3.1. Macroscopic Evaluation of Wound Healing
2.3.2. Histological Analysis
2.4. RNA Isolation, Reverse Transcription, and Real-Time PCR Analysis
2.5. Statistical Analysis
3. Results and Discussion
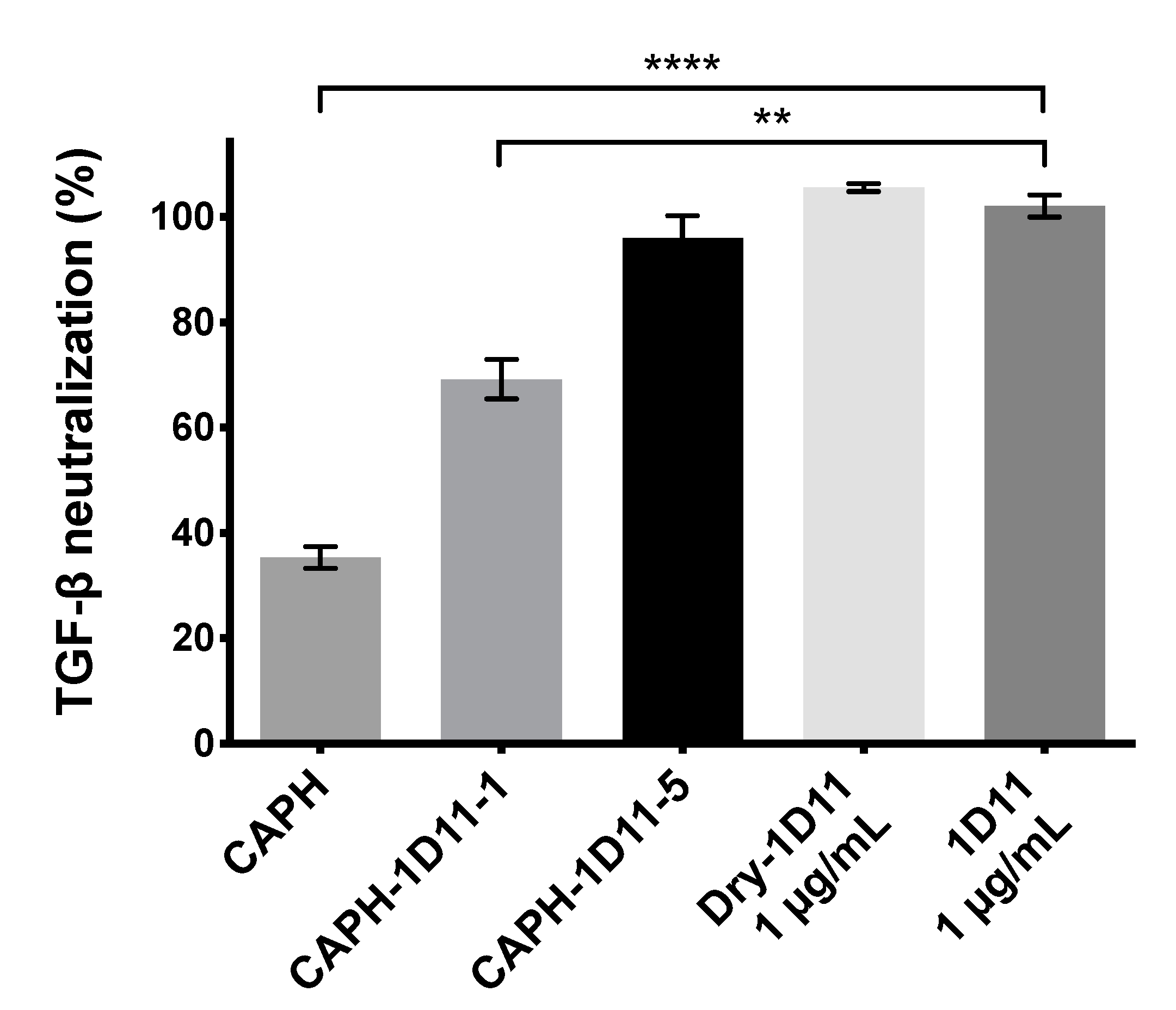
3.1. HEK-Blue TGF-β SEAP Reporter Cells Assay
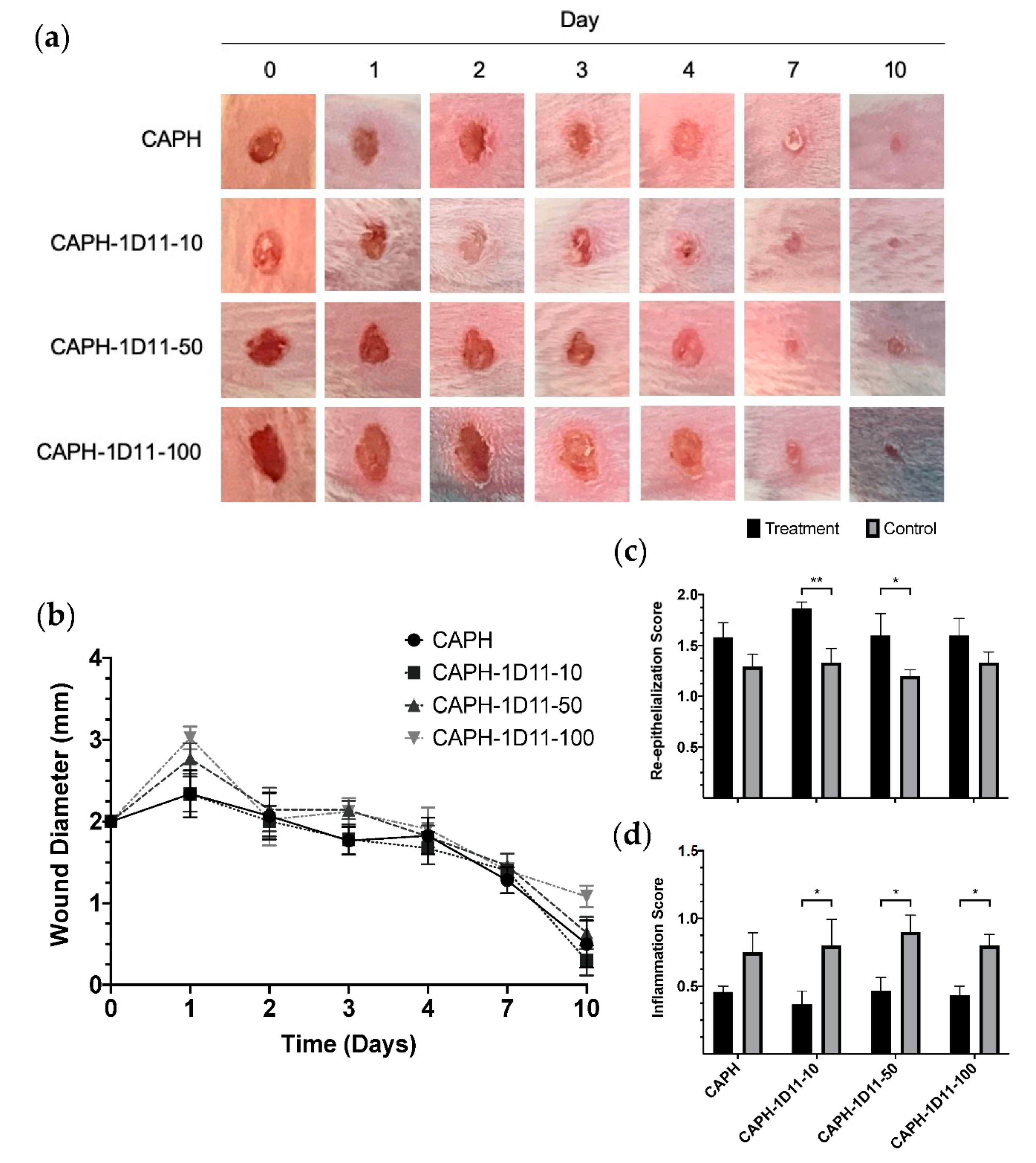
3.2. Wound Contraction
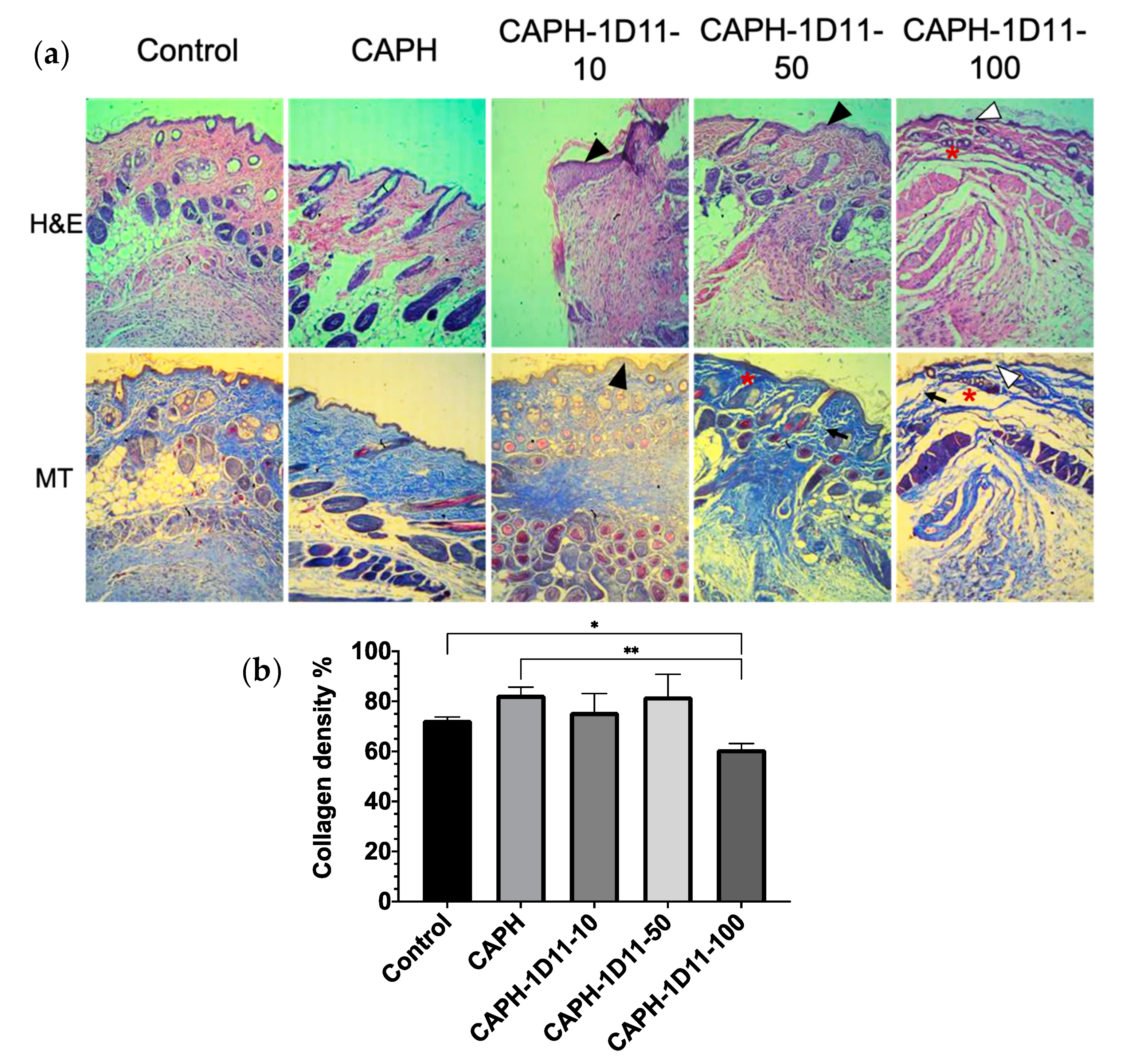
3.3. Histological Observations
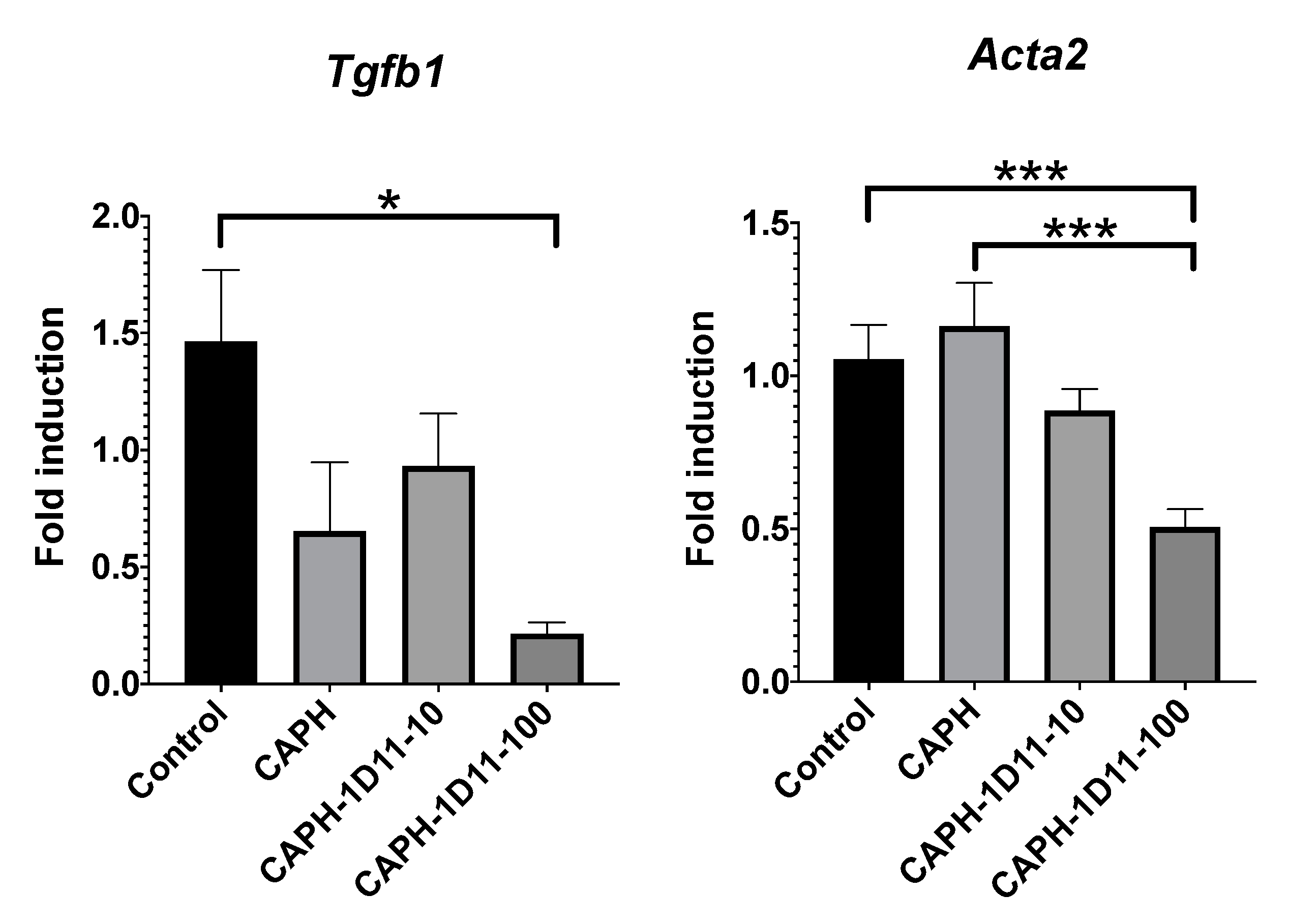
3.4. Reverse Transcription and Real-Time PCR Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Acknowledgments
Conflicts of Interest
References
- Kajdaniuk, D.; Marek, B.; Borgiel-Marek, H.; Kos-Kudła, B. Transforming growth factor beta1 (TGFbeta1) in physiology and pathology. Endokrynol. Pol. 2013, 64, 384–396. [Google Scholar] [CrossRef] [PubMed]
- Pakyari, M.; Farrokhi, A.; Maharlooei, M.K.; Ghahary, A. Critical Role of Transforming Growth Factor Beta in Different Phases of Wound Healing. Adv. Wound Care 2013, 2, 215–224. [Google Scholar] [CrossRef] [PubMed]
- Lichtman, M.K.; Otero-Vinas, M.; Falanga, V. Transforming growth factor beta (TGF-β) isoforms in wound healing and fibrosis. Wound Repair Regen. 2016, 24, 215–222. [Google Scholar] [CrossRef] [PubMed]
- Gilbert, R.W.D.; Vickaryous, M.K.; Viloria-Petit, A.M. Signalling by transforming growth factor beta isoforms in wound healing and tissue regeneration. J. Dev. Biol. 2016, 4, 21. [Google Scholar] [CrossRef]
- Le, M.; Naridze, R.; Morrison, J.; Biggs, L.C.; Rhea, L.; Schutte, B.C.; Kaartinen, V.; Dunnwald, M. Transforming Growth Factor Beta 3 Is Required for Excisional Wound Repair In Vivo. PLoS ONE 2012, 7, e48040. [Google Scholar] [CrossRef]
- Occleston, N.L.; O’Kane, S.; Laverty, H.G.; Cooper, M.; Fairlamb, D.; Mason, T.; Bush, J.A.; Ferguson, M.W.J. Discovery and development of avotermin (recombinant human transforming growth factor beta 3): A new class of prophylactic therapeutic for the improvement of scarring. Wound Repair Regen. 2011, 19, s38–s48. [Google Scholar] [CrossRef]
- Finnson, K.W.; Arany, P.R.; Philip, A. Transforming Growth Factor Beta Signaling in Cutaneous Wound Healing: Lessons Learned from Animal Studies. Adv. Wound Care 2013, 2, 225–237. [Google Scholar] [CrossRef]
- Rice, L.M.; Padilla, C.M.; McLaughlin, S.R.; Mathes, A.; Ziemek, J.; Goummih, S.; Nakerakanti, S.; York, M.; Farina, G.; Whitfield, M.L.; et al. Fresolimumab treatment decreases biomarkers and improves clinical symptoms in systemic sclerosis patients. J. Clin. Investig. 2015, 125, 2795–2807. [Google Scholar] [CrossRef]
- Lacouture, M.E.; Morris, J.C.; Lawrence, D.P.; Tan, A.R.; Olencki, T.E.; Shapiro, G.I.; Dezube, B.J.; Berzofsky, J.A.; Hsu, F.J.; Guitart, J. Cutaneous keratoacanthomas/squamous cell carcinomas associated with neutralization of transforming growth factor β by the monoclonal antibody fresolimumab (GC1008). Cancer Immunol. Immunother. 2015, 64, 437–446. [Google Scholar] [CrossRef]
- Lee, K.Y.; Mooney, D.J. Alginate: Properties and biomedical applications. Prog. Polym. Sci. 2012, 37, 106–126. [Google Scholar] [CrossRef]
- Sakai, S.; Sato, K.; Tabata, Y.; Kishi, K. Local release of pioglitazone (a peroxisome proliferator-activated receptor γ agonist) accelerates proliferation and remodeling phases of wound healing. Wound Repair Regen. 2016, 24, 57–64. [Google Scholar] [CrossRef]
- D’Ayala, G.; Malinconico, M.; Laurienzo, P. Marine Derived Polysaccharides for Biomedical Applications: Chemical Modification Approaches. Molecules 2008, 13, 2069–2106. [Google Scholar] [CrossRef] [PubMed]
- Naghshineh, N.; Tahvildari, K.; Nozari, M. Preparation of Chitosan, Sodium Alginate, Gelatin and Collagen Biodegradable Sponge Composites and their Application in Wound Healing and Curcumin Delivery. J. Polym. Environ. 2019, 27, 2819–2830. [Google Scholar] [CrossRef]
- Ahmed, A.; Getti, G.; Boateng, J. Ciprofloxacin-loaded calcium alginate wafers prepared by freeze-drying technique for potential healing of chronic diabetic foot ulcers. Drug Deliv. Transl. Res. 2018, 8, 1751–1768. [Google Scholar] [CrossRef] [PubMed]
- Stagnaro, P.; Schizzi, I.; Utzeri, R.; Marsano, E.; Castellano, M. Alginate-polymethacrylate hybrid hydrogels for potential osteochondral tissue regeneration. Carbohydr. Polym. 2018, 185, 56–62. [Google Scholar] [CrossRef]
- Boateng, J.; Burgos-Amador, R.; Okeke, O.; Pawar, H. Composite alginate and gelatin based bio-polymeric wafers containing silver sulfadiazine for wound healing. Int. J. Biol. Macromol. 2015, 79, 63–71. [Google Scholar] [CrossRef]
- Yeom, C.K.; Lee, K.H. Characterization of sodium alginate membrane crosslinked with glutaraldehyde in pervaporation separation. J. Appl. Polym. Sci. 1998, 67, 209–219. [Google Scholar] [CrossRef]
- Afjoul, H.; Shamloo, A.; Kamali, A. Freeze-gelled alginate/gelatin scaffolds for wound healing applications: An in vitro, in vivo study. Mater. Sci. Eng. C 2020, 113, 110957. [Google Scholar] [CrossRef]
- Santana, A.A.; Kieckbusch, T.G. Physical evaluation of biodegradable films of calcium alginate plasticized with polyols. Braz. J. Chem. Eng. 2013, 30, 835–845. [Google Scholar] [CrossRef]
- Rubio-Elizalde, I.; Bernáldez-Sarabia, J.; Moreno-Ulloa, A.; Vilanova, C.; Juárez, P.; Licea-Navarro, A.; Castro-Ceseña, A.B. Scaffolds based on alginate-PEG methyl ether methacrylate-Moringa oleifera-Aloe vera for wound healing applications. Carbohydr. Polym. 2019, 206, 455–467. [Google Scholar] [CrossRef]
- Dasch, J.R.; Pace, D.R.; Waegell, W.; Inenaga, D.; Ellingsworth, L. Monoclonal antibodies recognizing transforming growth factor-beta. Bioactivity neutralization and transforming growth factor beta 2 affinity purification. J. Immunol. 1989, 142, 1536–1541. [Google Scholar] [PubMed]
- Shah, M.; Foreman, D.M.; Ferguson, M.W. Neutralising antibody to TGF-beta 1,2 reduces cutaneous scarring in adult rodents. J. Cell Sci. 1994, 107 Pt 5, 1137–1157. [Google Scholar]
- Huang, J.S.; Wang, Y.-H.; Ling, T.-Y.; Chuang, S.-S.; Johnson, F.E.; Huang, S.S. Synthetic TGF-b antagonist accelerates wound healing and reduces scarring. FASEB J. 2002, 16, 1269–1270. [Google Scholar] [CrossRef]
- Lu, L.; Saulis, A.S.; Liu, W.R.; Roy, N.K.; Chao, J.D.; Ledbetter, S.; Mustoe, T.A. The Temporal Effects of Anti-TGF-β1, 2, and 3 Monoclonal Antibody on Wound Healing and Hypertrophic Scar Formation. J. Am. Coll. Surg. 2005, 201, 391–397. [Google Scholar] [CrossRef]
- Shah, M.; Foreman, D.M.; Ferguson, M.W. Neutralisation of TGF-beta 1 and TGF-beta 2 or exogenous addition of TGF-beta 3 to cutaneous rat wounds reduces scarring. J. Cell Sci. 1995, 108, 985–1002. [Google Scholar] [PubMed]
- Tavares Pereira, D.D.S.; Lima-Ribeiro, M.H.M.; De Pontes-Filho, N.T.; Carneiro-Leão, A.M.D.A.; Correia, M.T.D.S. Development of animal model for studying deep second-degree thermal burns. J. Biomed. Biotechnol. 2012, 2012, 460841. [Google Scholar] [CrossRef] [PubMed]
- Bloemen, M.C.T.; Boekema, B.K.H.L.; Vlig, M.; Van Zuijlen, P.P.M.; Middelkoop, E. Digital image analysis versus clinical assessment of wound epithelialization: A validation study. Burns 2012, 38, 501–505. [Google Scholar] [CrossRef] [PubMed]
- Sardari, K.; Kakhki, E.G.; Mohri, M. Evaluation of wound contraction and epithelialization after subcutaneous administration of Theranekron® in cows. Comp. Clin. Path. 2007, 16, 197–200. [Google Scholar] [CrossRef]
- Perez, D.; Bramkamp, M.; Exe, C.; von Ruden, C.; Ziegler, A. Modern wound care for the poor: A randomized clinical trial comparing the vacuum system with conventional saline-soaked gauze dressings. Am. J. Surg. 2010, 199, 14–20. [Google Scholar] [CrossRef]
- Kadoyama, K.; Takenokuchi, M.; Matsuura, K.; Shichiri, H.; Watanabe, A.; Yamaguchi, H.; Takahashi, H.; Takano-Ohmuro, H.; Taniguchi, T. Therapeutic effects of shikonin on skin lesions in mouse models of allergic dermatitis and wound. Tradit. Kampo Med. 2019, 6, 62–70. [Google Scholar] [CrossRef]
- Shin, D.Y.; Park, J.-U.; Choi, M.-H.; Kim, S.; Kim, H.-E.; Jeong, S.-H. Polydeoxyribonucleotide-delivering therapeutic hydrogel for diabetic wound healing. Sci. Rep. 2020, 10, 16811. [Google Scholar] [CrossRef] [PubMed]
- Jones, J.A.; Novo, N.; Flagler, K.; Pagnucco, C.D.; Carew, S.; Cheong, C.; Kong, X.Z.; Burke, N.A.D.; Stöver, H.D.H. Thermoresponsive copolymers of methacrylic acid and poly(ethylene glycol) methyl ether methacrylate. J. Polym. Sci. Part A Polym. Chem. 2005, 43, 6095–6104. [Google Scholar] [CrossRef]
- Hussain, H.; Mya, K.Y.; He, C. Self-assembly of brush-like poly[poly(ethylene glycol) methyl ether methacrylate] synthesized via aqueous atom transfer radical polymerization. Langmuir 2008, 24, 13279–13286. [Google Scholar] [CrossRef]
- Feng, W.; Hu, Y.; An, N.; Feng, Z.; Liu, J.; Mou, J.; Hu, T.; Guan, H.; Zhang, D.; Mao, Y. Alginate Oligosaccharide Alleviates Monocrotaline-Induced Pulmonary Hypertension via Anti-Oxidant and Anti-Inflammation Pathways in Rats. Int. Heart J. 2020, 61, 160–168. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.R.; Park, J.H.; Kim, K.H.; Kim, S.J.; Park, D.H.; Chae, M.H.; Suh, S.H.; Jeong, S.W.; Park, K.K. The biological effects of topical alginate treatment in an animal model of skin wound healing. Wound Repair Regen. 2009, 17, 505–510. [Google Scholar] [CrossRef]
- Xia, Z.; Ding, L.; Zheng, J.; Xu, Y.; Jin, W.; Sheng, X.; Wu, J. Alginate suppresses liver fibrosis through the inhibition of nuclear factor-κB signaling. Drug Des. Dev. Ther. 2020, 14, 1295–1305. [Google Scholar] [CrossRef]
- Organista-Juárez, D.; Carretero-Ortega, J.; Vicente-Fermín, O.; Vázquez-Victorio, G.; Sosa-Garrocho, M.; Vázquez-Prado, J.; Macías-Silva, M.; Reyes-Cruz, G. Calcium-sensing receptor inhibits TGF-β-signaling by decreasing Smad2 phosphorylation. IUBMB Life 2013, 65, 1035–1042. [Google Scholar] [CrossRef]
- Dunn, L.; Prosser, H.C.G.; Tan, J.T.M.; Vanags, L.Z.; Ng, M.K.C.; Bursill, C.A. Murine model of wound healing. J. Vis. Exp. 2013, 2013, 1–6. [Google Scholar] [CrossRef]
- Grada, A.; Mervis, J.; Falanga, V. Research Techniques Made Simple: Animal Models of Wound Healing. J. Investig. Dermatol. 2018, 138, 2095–2105.e1. [Google Scholar] [CrossRef]
- Rhea, L.; Dunnwald, M. Murine excisional wound healing model and histological morphometric wound analysis. J. Vis. Exp. 2020, 2020, 1–18. [Google Scholar] [CrossRef]
- Zomer, H.D.; Trentin, A.G. Skin wound healing in humans and mice: Challenges in translational research. J. Dermatol. Sci. 2018, 90, 3–12. [Google Scholar] [CrossRef] [PubMed]
- Ansurudeen, I.; Sunkari, V.G.; Grünler, J.; Peters, V.; Schmitt, C.P.; Catrina, S.B.; Brismar, K.; Forsberg, E.A. Carnosine enhances diabetic wound healing in the db/db mouse model of type 2 diabetes. Amino Acids 2012, 43, 127–134. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, D.; Pinto, F.; Senoo, M. Inhibition of TGF-β signaling promotes expansion of human epidermal keratinocytes in feeder cell co-culture. Wound Repair Regen. 2017, 25, 526–531. [Google Scholar] [CrossRef] [PubMed]
- Liarte, S.; Bernabé-García, Á.; Nicolás, F.J. Role of TGF-β in Skin Chronic Wounds: A Keratinocyte Perspective. Cells 2020, 9, 306. [Google Scholar] [CrossRef] [PubMed]
- Mandapalli, P.K.; Labala, S.; Jose, A.; Bhatnagar, S.; Janupally, R.; Sriram, D.; Venuganti, V.V.K. Layer-by-Layer Thin Films for Co-Delivery of TGF-β siRNA and Epidermal Growth Factor to Improve Excisional Wound Healing. AAPS PharmSciTech 2017, 18, 809–820. [Google Scholar] [CrossRef]
- Li, H.L.; Chen, L.P.; Hu, Y.H.; Qin, Y.; Liang, G.; Xiong, Y.X.; Chen, Q.X. Crocodile oil enhances cutaneous burn wound healing and reduces scar formation in rats. Acad. Emerg. Med. 2012, 19, 265–273. [Google Scholar] [CrossRef]
- Pastar, I.; Stojadinovic, O.; Krzyzanowska, A.; Barrientos, S.; Stuelten, C.; Zimmerman, K.; Blumenberg, M.; Brem, H.; Tomic-Canic, M. Attenuation of the transforming growth factor β-signaling pathway in chronic venous ulcers. Mol. Med. 2010, 16, 92–101. [Google Scholar] [CrossRef]
- Kim, B.C.; Kim, H.T.; Park, S.H.; Cha, J.S.; Yufit, T.; Kim, S.J.; Falanga, V. Fibroblasts from chronic wounds show altered TGF-β-signaling and decreased TGF-β type II receptor expression. J. Cell. Physiol. 2003, 195, 331–336. [Google Scholar] [CrossRef]
- Sanjabi, S.; Oh, S.A.; Li, M.O. Regulation of the immune response by TGF-β: From conception to autoimmunity and infection. Cold Spring Harb. Perspect. Biol. 2017, 9, a022236. [Google Scholar] [CrossRef]
- Doyle, J.W.; Roth, T.P.; Smith, R.M.; Li, Y.Q.; Dunn, R.M. Effect of calcium alginate on cellular wound healing processes modeled in vitro. J. Biomed. Mater. Res. 1996, 32, 561–568. [Google Scholar] [CrossRef]
- Caetano, G.F.; Frade, M.A.C.; Andrade, T.A.M.; Leite, M.N.; Bueno, C.Z.; Moraes, Â.M.; Ribeiro-Paes, J.T. Chitosan-alginate membranes accelerate wound healing. J. Biomed. Mater. Res. Part B Appl. Biomater. 2015, 103, 1013–1022. [Google Scholar] [CrossRef] [PubMed]
- Varga, J.; Rosenbloom, J.; Jimenez, S.A. Transforming growth factor β (TGFβ) causes a persistent increase in steady-state amounts of type I and type III collagen and fibronectin mRNAs in normal human dermal fibroblasts. Biochem. J. 1987, 247, 597–604. [Google Scholar] [CrossRef] [PubMed]
- Jiang, D.; Christ, S.; Correa-Gallegos, D.; Ramesh, P.; Kalgudde Gopal, S.; Wannemacher, J.; Mayr, C.H.; Lupperger, V.; Yu, Q.; Ye, H.; et al. Injury triggers fascia fibroblast collective cell migration to drive scar formation through N-cadherin. Nat. Commun. 2020, 11, 5653. [Google Scholar] [CrossRef] [PubMed]
- Meng, X.; Nikolic-Paterson, D.J.; Lan, H.Y. TGF-β: The master regulator of fibrosis. Nat. Rev. Nephrol. 2016, 12, 325–338. [Google Scholar] [CrossRef] [PubMed]
- Roberts, A.B.; McCune, B.K.; Sporn, M.B. TGF-β: Regulation of extracellular matrix. Kidney Int. 1992, 41, 557–559. [Google Scholar] [CrossRef] [PubMed]
- Bedinger, D.; Lao, L.; Khan, S.; Lee, S.; Takeuchi, T.; Mirza, A.M. Development and characterization of human monoclonal antibodies that neutralize multiple TGF β isoforms. MAbs 2016, 8, 389–404. [Google Scholar] [CrossRef]
- Van Obberghen-Schillin, E.; Roche, N.S.; Flanders, K.C.; Sporn, M.B.; Roberts, A.B. Transforming growth factor beta 1 positively regulates its own expression in normal and transformed cells. J. Biol. Chem. 1988, 263, 7741–7746. [Google Scholar] [CrossRef]
- Desmouliere, A.; Geinoz, A.; Gabbiani, F.; Gabbiani, G. Transforming growth factor-β1 induces α-smooth muscle actin expression in granulation tissue myofibroblasts and in quiescent and growing cultured fibroblasts. J. Cell Biol. 1993, 122, 103–111. [Google Scholar] [CrossRef]
- Goffin, J.M.; Pittet, P.; Csucs, G.; Lussi, J.W.; Meister, J.J.; Hinz, B. Focal adhesion size controls tension-dependent recruitment of α-smooth muscle actin to stress fibers. J. Cell Biol. 2006, 172, 259–268. [Google Scholar] [CrossRef]
- Ibrahim, M.M.; Chen, L.; Bond, J.E.; Medina, M.A.; Ren, L.; Kokosis, G.; Selim, A.M.; Levinson, H. Myofibroblasts contribute to but are not necessary for wound contraction. Lab. Investig. 2015, 95, 1429–1438. [Google Scholar] [CrossRef]
| Gene | Name | Primer (5′-3′) |
|---|---|---|
| Tgfb1 | Transforming growth factor beta | Forward: CACTCCCGTGGCTTCTAGTG Reverse: GGACTGGCGAGCCTTAGTTT |
| Acta2 | Alpha-smooth muscle actin | Forward: TCAGCGCCTCCAGTTCCT Reverse: AAAAAAAACCACGAGTAACAAATCAA |
| Hprt | Hypoxanthine phosphoribosyltransferase | Forward: GTAATGATCAGTCAACGGGGGAC Reverse: CCAGCAAGCTTGCAACCTTAACCA |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gasperin-Bulbarela, J.; Castro-Ceseña, A.B.; Camacho-Villegas, T.; Lugo-Fabres, P.H.; Díaz-Martínez, N.E.; Padilla-Camberos, E.; Echavarría, R.; Licea-Navarro, A.F. Ca-Alginate-PEGMA Hydrogels for In Situ Delivery of TGF-β Neutralizing Antibodies in a Mouse Model of Wound Healing. Appl. Sci. 2021, 11, 1164. https://doi.org/10.3390/app11031164
Gasperin-Bulbarela J, Castro-Ceseña AB, Camacho-Villegas T, Lugo-Fabres PH, Díaz-Martínez NE, Padilla-Camberos E, Echavarría R, Licea-Navarro AF. Ca-Alginate-PEGMA Hydrogels for In Situ Delivery of TGF-β Neutralizing Antibodies in a Mouse Model of Wound Healing. Applied Sciences. 2021; 11(3):1164. https://doi.org/10.3390/app11031164
Chicago/Turabian StyleGasperin-Bulbarela, Jahaziel, Ana B. Castro-Ceseña, Tanya Camacho-Villegas, Pavel H. Lugo-Fabres, Nestor Emmanuel Díaz-Martínez, Eduardo Padilla-Camberos, Raquel Echavarría, and Alexei F. Licea-Navarro. 2021. "Ca-Alginate-PEGMA Hydrogels for In Situ Delivery of TGF-β Neutralizing Antibodies in a Mouse Model of Wound Healing" Applied Sciences 11, no. 3: 1164. https://doi.org/10.3390/app11031164
APA StyleGasperin-Bulbarela, J., Castro-Ceseña, A. B., Camacho-Villegas, T., Lugo-Fabres, P. H., Díaz-Martínez, N. E., Padilla-Camberos, E., Echavarría, R., & Licea-Navarro, A. F. (2021). Ca-Alginate-PEGMA Hydrogels for In Situ Delivery of TGF-β Neutralizing Antibodies in a Mouse Model of Wound Healing. Applied Sciences, 11(3), 1164. https://doi.org/10.3390/app11031164







